Abstract
Emerging evidence highlights the substantial role of the kynurenine pathway in various physiological systems and pathological conditions. Physical exercise has been shown to impact the kynurenine pathway in response to both single (acute) and multiple (chronic) exercise training stimuli. In this perspective article, we briefly outline the current knowledge concerning exercise-induced modulations of the kynurenine pathway and discuss underlying mechanisms. Furthermore, we expose the potential involvement of exercise-induced kynurenine pathway modulations on energy homeostasis (eg, through de novo synthesis of NAD+) and finally suggest how these modulations may contribute to exercise-induced benefits in the prevention and treatment of chronic diseases.
Keywords: Exercise, kynurenine pathway, energy homeostasis, inflammation, chronic diseases, immunometabolism
Introduction
The kynurenine (KYN) pathway represents the major route of tryptophan (TRP) degradation and generates several bioactive components. While more than 90% of the essential amino acid TRP is catabolised via the KYN pathway, only minor amounts are used for protein biosynthesis and serotonin/melatonin production.1 Under normal physiological conditions, the KYN pathway is mainly regulated by the enzyme tryptophan 2,3 dioxygenase (TDO), which mediates the conversion of tryptophan to KYN (representing the initial step of the KYN pathway). However, in the presence of local or systemic inflammatory conditions, the activity of the TDO isoenzymes indoleamine 2,3 (IDO) 1 and 2 can increase substantially, thereby leading to an activation of the KYN pathway.2 Following the initial step of the pathway, the metabolic flux can yield different end products. The most studied KYN derivative is kynurenic acid (KA). Aside from immunological aspects, KA is known to prevent neuronal excitotoxicity within the central nervous system (CNS). However, only a small amount of the metabolic flux yields KA. The vast majority of the KYN pathway is metabolized – via several intermediate products such as 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) – towards NAD+.1 Both metabolites, 3-HK and QA, are known to induce neuronal excitotoxicity within the CNS.3 The enzyme kynurenine 3-monooxygenase (KMO) catalyses the conversion of KYN to 3-HK and therefore represents a key regulator of the KYN pathway branch yielding NAD+. Detailed functional and regulatory aspects of the KYN pathway have been reviewed elsewhere.1
The KYN pathway is deregulated in various chronic diseases, such as different internal (eg, chronic obstructive pulmonary disease,4 diabetes,2 cancer5) or neurological (eg, multiple sclerosis,6 Alzheimer and Parkinson disease7) disorders. When compared with healthy controls, most of these diseases show a persistent overactivation of the KYN pathway as indicated by elevated levels of KYN and the KYN/TRP ratio. While previously introduced as a marker of IDO activity,8 the KYN/TRP ratio now represents a general indicator of KYN pathway activation, independent of the enzyme mediating the conversion of TRP to KYN. Predominantly in neurological disorders, a pathological disbalance characterized by an increased flux through the neurotoxic metabolites 3-HK and QA concomitant with less KA production within the CNS has been indicated to play a key role in neurodegenerative processes.9 In this context, central enzymes of the KYN pathway have become a current and promising therapeutic drug target in a variety of chronic diseases, aiming to modulate the metabolic flux and to induce/prevent associated consequences for the immune system and CNS.5
Recently, physical exercise has been shown to impact the KYN pathway regulation in response to both single bouts of (acute) exercise and long-term interventions of repetitive exercise bouts (chronic training). Existing studies mostly focused on the measurement of TRP and KYN to examine a possible activation and on the metabolic flux towards KA, including considering kynurenine aminotransferase (KAT) enzyme expression. Overall, a broad variety of samples (animal models, healthy subjects, athletes, clinical populations) have been investigated, thereby providing different perspectives and also limiting collective interpretation due to heterogeneity. Acute and chronic exercise-induced effects on the KYN pathway have recently been summarized in detail10 and will be briefly presented in the following.
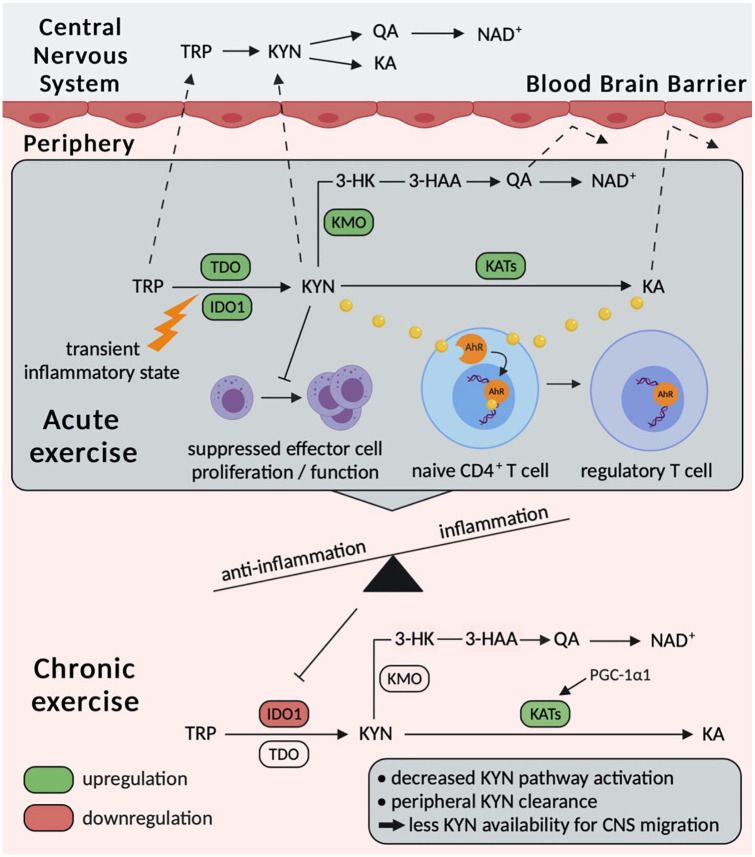
Several studies showed an initial activation of the KYN pathway in response to acute exercise as indicated by a decrease in TRP and an increase in KYN measured in human serum/plasma.10-14 Most of these studies investigated severe aerobic exercise as intervention in the form of an incremental exercise test until exhaustion.11-14 Furthermore, several studies reported an increased metabolic flux towards KA following acute exercise,10,15-17 which could possibly be due to an elevation in KAT enzyme expression. Not only the metabolic flux towards KA, but also the major branch of the KYN pathway towards QA can apparently be upregulated after acute exercise.10,16,18 However, our understanding of the impact of different exercise modalities (eg, frequency, intensity, type, time) and the kinetics of metabolites is limited. Recent results suggest that alterations of KYN pathway metabolites measured in blood serum are of transient nature, and levels return to baseline within 1 hour after exercise cessation.10,15
Regarding the effects of chronic exercise training on the KYN pathway, existing evidence from human studies is more contradictive compared with effects of acute exercise. Many studies do not show statistical effects on circulating metabolites,19-21 but the design and/or description of the interventions is questionable due to low training frequency/intensity or lacking information on the applied exercise modalities and adherence. However, evidence from an animal model as well as results from a randomized controlled trial in patients with breast cancer reported promising effects. Agudelo et al22 showed elevated KAT expression and protein levels in skeletal muscle concomitant with reduced plasma levels in KYN following 8 weeks of aerobic exercise in rodents with stress-induced depression. Zimmer et al investigated the effects of chronic resistance exercise training on KYN pathway metabolites in patients with breast cancer undergoing chemotherapy. Indeed, serum levels of KYN and KYN/TRP ratio were reduced after exercise training compared with the control group. As an initial KYN pathway activation was observed in the control group, the results of this study indicate that exercise can counteract a treatment-related activation of the KYN pathway.23
When interpreting and comparing the effects of acute or chronic exercise on the KYN pathway, the time of sample collection needs to be considered carefully. To examine effects of acute exercise samples are usually collected immediately after completion of the exercise bout, ideally with additional follow-up measurement time points during the recovery period to depict outcome kinetics. To examine effects of chronic exercise samples are typically collected in a resting condition, mostly 24 hours or longer after the completion of the last exercise bout. However, without detailed knowledge of the outcome kinetics after acute exercise, it is possible that acute effects ‘bias’ chronic effects due to incomplete recovery/adaptation processes. For example, KA has been shown to increase after acute exercise10,16,18 and to return to baseline levels within few hours after exercise completion in humans.10 Therefore, it is not surprising that no effects after chronic exercise are observed – if samples are collected in a resting condition.
Despite heterogeneous study populations, exercise modalities, and measurement time points, several studies indicate the overarching impact of exercise on the KYN pathway by assessing pre- and post-differences in various metabolites in blood plasma or serum. Although a large body of evidence indicates profound involvements of the KYN pathway in different physiological and pathological processes (eg, immune regulation or brain health), current knowledge about the exercise-induced consequences of KYN pathway modulations is lacking. Also, the underlying mechanisms of exercise-induced KYN pathway modulations are insufficiently investigated but are of major relevance to develop tailored exercise interventions. Here, we briefly outline potential underlying mechanisms of exercise-induced alterations of the KYN pathway and expose possible consequences for the immune system, the CNS, and cellular energy homeostasis. Finally, we propose how exercise as KYN pathway modulator might be involved in the well-known beneficial effects on different diseases and the associated symptoms.
Underlying Mechanisms of Exercise-Induced Alterations in KYN Pathway Regulation
There is a strong rationale suggesting that the observed initial activation of the KYN pathway as well as the increase in the metabolic flux towards QA is driven by immune activation as they appear transiently during and after single bouts of exercise. Immune activation during and after acute exercise has especially been described as an elevation in inflammatory cytokines such as interleukin-6 (IL-6).24,25 Indeed, primarily interferon-γ (IFN-γ) and also other inflammatory cytokines (eg, IL-6) are known to induce IDO1, IDO2, and KMO,5 thereby creating a solid link to an IDO-mediated upregulation of the KYN pathway and an increased degradation of KYN to QA in response to acute exercise. However, this hypothesis has not yet been investigated in detail and needs to be addressed in future research.
Aside from the elevation in inflammatory cytokines, acute exercise has also been described to increase levels of cortisol.26,27 Although it was previously assumed that TDO is mainly constant, more recent research indicates that changes in TDO activity are also strongly involved in KYN pathway regulation.28 Therefore, exercise-induced elevations in circulating cortisol levels represent another potential link to the observed initial activations of the KYN pathway after acute exercise, which also needs further investigation.
Another potential causative mechanism of the initial activation of the KYN pathway driven by acute exercise could be due to hypoxic conditions that appear in response to the increased energy demand during exercise. As both IDO and TDO are dioxygenases, it seems to be conceivable that their activity might be affected by exercise through differing oxygen levels in tissues that are required for or stimulated by physical movement. Especially skeletal muscle29 (but possibly also other cells) shows hypoxic conditions during acute exercise, thereby potentially affecting the activity of IDO and TDO. However, no study has yet investigated this link between hypoxic conditions and IDO or TDO activity in any cell type.
Furthermore, a link between IDO activity and expression, nitric oxide, and exercise appears to be worth investigating. Although not yet examined in the context of exercise, nitric oxide has been shown to inhibit IDO activity.30 Moreover, the impact of exercise on nitric oxide has been described in different tissues, for example, in skeletal muscle,31 endothelial cells,32 and erythrocytes.33 Thus, a strong rationale suggests that exercise-induced changes in nitric oxide may mediate an inhibition of IDO activity, possibly leading to a chronic downregulation/stabilization of the KYN pathway as reported by Zimmer et al.23
The mechanisms underlying both acute and chronic exercise-induced elevations in the metabolic flux towards KA could be driven by KAT expression in different tissues or cell types. While investigated merely for acute effects, KYN degradation to KA represents a well-examined mechanism for chronic exercise training. Agudelo et al22 revealed coactivator PGC-1α1-mediated increases in KAT expression in skeletal muscle after chronic aerobic exercise, which were accompanied by decreases in KYN in blood plasma. As PGC-1α1 has also been described to increase in skeletal muscle after single bouts of aerobic exercise,34,35 the observed acute increased metabolic flux towards KA might be due to this mechanism. As a transcriptional coactivator, PGC-1α is strongly dependent on DNA-binding transcription factors that are collectively known as peroxisome proliferator–activated receptor (PPAR) family.36,37 In skeletal muscle, PPAR-α and PPAR-δ closely interact with PGC-1α1,22 whereas PPAR-γ is mainly expressed in immune cells and regulates distinct processes of immune response.38 As the PPAR expression is ligand-dependent and some of these ligands can be generated by exercise stimuli,39 the PPAR family might play a pivotal role in PGC-1α1-mediated KAT expressions in response to acute exercise – not only regarding skeletal muscle. However, an elevated KAT enzyme expression has only been shown in peripheral mononuclear immune cells after acute exercise so far (KAT4 only), whereas further examination in skeletal muscle is warranted. Regarding the reported effects of acute resistance exercise, there might be a connection to PGC-1α4 isoform worth investigating, because not PGC-1α1 but PGC-1α4 has been linked to resistance training and skeletal muscle hypertrophy regulation.40
In summary, several underlying mechanisms for acute exercise-induced effects on the KYN pathway have been hypothesized and need further investigation. Concerning underlying mechanisms of chronic effects, the PGC-1α1-KAT axis in skeletal muscle has been well-described for aerobic exercise training. However, potential explanations for chronic effects on the initial KYN pathway activation/suppression are sparse and statistical effects are not reported consistently, which could be due to inadequate exercise intervention prescription or adherence. Nevertheless, building upon the vast body of evidence summarizing biologically relevant aspects of KYN pathway regulation and driven by the promising results recently reported by Zimmer et al,23 we propose distinct immuno- and neuromodulatory consequences of exercise-induced KYN pathway alterations. These consequences might be due to frequently occurring acute effects that lead to important chronic adaptations of the immune system and CNS (see Figure 1) and further contribute to several well-described improvements in clinical outcomes caused by exercise in various chronic diseases.
Figure 1.
Schematic illustration of potential acute and chronic exercise-induced modulations of the KYN pathway and possible consequences for the immune system and central nervous system. CNS indicates central nervous system; HK, hydroxykynurenine; IDO, isoenzymes indoleamine; KA, kynurenic acid; KMO, kynurenine 3-monooxygenase; KYN, kynurenine; QA, quinolinic acid; TDO, tryptophan 2,3 dioxygenases; TRP, tryptophan.
Potential Immunomodulatory and Neuroprotective Implications of Exercise-Induced KYN Pathway Alterations
Aside from the initial activation of the KYN pathway, further interactions with the immune system seem to be plausible. In line with the hypothesis that the initial activation of the KYN pathway is provoked by an increased IDO activity, IDO-mediated conversion to KYN is known to suppress the activity of different immune cells, whereas primarily T cells41,42 and natural killer (NK) cells42 have been described to be inhibited by KYN and further downstream metabolites.43 Furthermore, we recently showed an association between the KYN/TRP ratio and NK cell subsets in young healthy men at rest, suggesting novel immunoregulatory properties that need further examination. Interestingly, the cytokine-producing NK cell subset characterized by CD56bright correlated positively with the KYN/TRP ratio, whereas the more cytotoxic NK cell subset characterized by CD56dim correlated negatively.10 However, as the existing evidence mostly indicates acute effects of exercise on the KYN pathway that are of transient nature, it remains questionable whether these effects are sufficient to induce immunological adaptations. On the contrary, transient increases may decrease rapidly because KYN and KA serve as ligands of the aryl hydrocarbon receptor (AhR) in different immune cells, representing a major mechanism for the immunomodulatory effects of TRP degradation via the KYN pathway.5,44,45 Interestingly, Martin et al46 recently highlighted in a review article that not only KYN and KA but also cinnabarinic acid (CA), another KYN pathway metabolite, may represent an exercise-sensitive endogenous ligand of the AhR. However, to the best of our knowledge, no study has yet investigated the impact of acute or chronic exercise on CA levels.
In the context of acute effects, elevated KYN and especially KA levels have frequently been reported.10,13,15,16 Both metabolites are well-known endogenous ligands of the AhR, which is involved in cell differentiation processes and represents a promising drug target in cancer therapy aiming to inhibit tumour-mediated suppression of endogenous tumour defence by NK and cytotoxic T cells.5,45 As the AhR is a ligand-dependent receptor, acute increased levels of the ligands KYN and KA might activate the AhR and consequently decrease in the plasma. This cascade could be initiated by acute exercise-induced upregulation of the KYN pathway, although the following effects of AhR activation are possibly prolonged compared with the observed alterations in KYN and KA. Interestingly, the most described role of AhR signalling is the T-cell differentiation. Evidence suggests that AhR signalling mediates the differentiation of TH-17 cells to regulatory T cells (Tregs).47 In line with our hypothesis that the AhR is activated in response to acute exercise, numbers and proportions of Tregs are associated with the level of cardiorespiratory fitness.48 Therefore, repetitive elevations in endogenous AhR ligands KYN and KA in response to acute exercise might be involved in the enhanced T-cell differentiation towards the Tregs subsets, thereby enhancing the body’s anti-inflammatory potential.
It is worth mentioning that not only acute exercise induces elevated levels of KA, but also chronic exercise interventions can result in increased KA resting levels,22 albeit human studies are lacking. Thus, permanently elevated ligand availability for AhR in the form of elevated KA level activation can be also assumed through chronic exercise, which is why the KYN pathway-AhR axis in response to long-term exercise interventions seems to be a promising subject for upcoming investigations.
However, exercise-induced modulations of the KYN pathway are possibly strongly dependent on the investigated population. Especially in diseased populations with a chronically upregulated KYN pathway (eg, due to chronic inflammatory conditions), effects of exercise might be more pronounced as a ‘normalization’ can occur. Indeed, particularly chronic exercise interventions might reveal effects on the KYN pathway regulation only when an (pathological) upregulation is present at baseline. To investigate this hypothesis, future studies comparing the effects of chronic exercise between diseased populations that exhibit an upregulation in the KYN pathway and healthy matched controls are warranted.
The described immunosuppressive adaptations could – probably together with other well-known anti-inflammatory effects of chronic exercise – be responsible for a chronic reduction of KYN pathway activation. Hence, the interaction of repetitively occurring transient increases in KYN pathway metabolites after acute exercise and the chronic downregulation of the KYN pathway may represent a counterregulatory mechanism. The immunological adaptations described could be of major relevance for several chronic diseases, including neuroinflammatory (eg, multiple sclerosis) and metabolic (eg, diabetes mellitus) diseases.
Besides potential immunological adaptations, neuroprotective effects were observed due to exercise-induced KYN clearance in blood plasma/serum.22 Increased metabolic fluxes towards QA and KA, respectively, in response to acute and chronic exercise (shown towards KA only) are suspected to protect the CNS from KYN accumulation by peripheral KYN clearance.10 Interestingly, solely TRP and KYN can pass the blood-brain barrier, whereas KA and QA cannot. Within the CNS, TRP and KYN can be degraded to neurotoxic metabolites along the KYN pathway, such as 3-HK and QA. Therefore, ‘peripheral’ clearance of TRP and KYN might prevent pathological accumulation within the CNS, which appears in different neurological diseases, such as Alzheimer disease or multiple sclerosis.6,49 Agudelo et al22 showed a decrease in plasma KYN levels mediated by PGC-1α1-induced KATs in skeletal muscle in mice, which protected from stress-induced depression. However, neuroinflammatory conditions that are associated with several neurological diseases (eg, multiple sclerosis, depression) have also been described to exaggerate KMO activity, which leads to an increase in QA and thereby mediates neurotoxicity.6,50 Although the peripheral KYN clearance prevents an accumulation within the CNS, a pathological disbalance between the metabolic fluxes towards KA and QA within the CNS should not be discounted. Similar to the results of Agudelo et al, Allison et al51 investigated the impact of 12 weeks of combined exercise in elderly persons and revealed elevations in KAT expressions in skeletal muscle, whereas no effects on plasma KYN, KA, or QA levels were observed. Possibly, (pathological) elevations of the KYN pathway at baseline are also relevant for considerable exercise-mediated KYN clearance in blood plasma as a minimum demand of KYN is always covered under basal physiological conditions.
Currently, little attention has been drawn on the potential impact of exercise on the metabolic flux towards QA. Some acute studies suggest increases in QA following single bouts of exercise,10,16,18 thereby potentially also indicating transient peripheral KYN clearance. Regarding chronic studies, Herrstedt et al52 investigated the impact of a 12-week aerobic and resistance exercise programme in patients with gastroesophageal junction cancer on KMO expression in skeletal muscle and found significant lower expression levels in the exercise group compared with the control group after the intervention. Although only post-measurements were compared and the study was non-randomized, the results provide a first hint that KMO expression may also be influenceable by exercise. However, there is a need for research investigating the effects of acute and chronic exercise on the metabolic flux towards QA and its potential contribution to peripheral KYN clearance.
Importantly, to the best of our knowledge, there are no human studies that assessed exercise-induced KYN pathway changes in the cerebrospinal fluid. Serum and plasma measurements can determine TRP and KYN availability and provide information on the metabolic flux downstream in the peripheral blood, but conclusions on changes in the KYN pathway regulation within the CNS should be treated with caution. However, as mediators of the KYN pathway, such as inflammatory cytokines (or immune cells as cytokine producers), are also impacted/mobilized by exercise, changes within the CNS seem probable. Especially in the context of neurological and mental disorders, future studies on exercise-induced KYN pathway alterations measured in the cerebrospinal fluid are of major relevance to investigate neuroprotective consequences.
Do Exercise-Induced Modulations of the KYN Pathway Occur to Improve Energy Homeostasis?
Considering the effects and consequences of acute and chronic exercise-induced alterations of the KYN pathway, the question why different cell types or tissues (eg, immune cells, skeletal muscle) respond with alterations in KYN pathway regulation has not yet been sufficiently addressed. Currently, growing research interest focuses on the contribution of the KYN pathway to maintain cellular energy demands in different cell types.
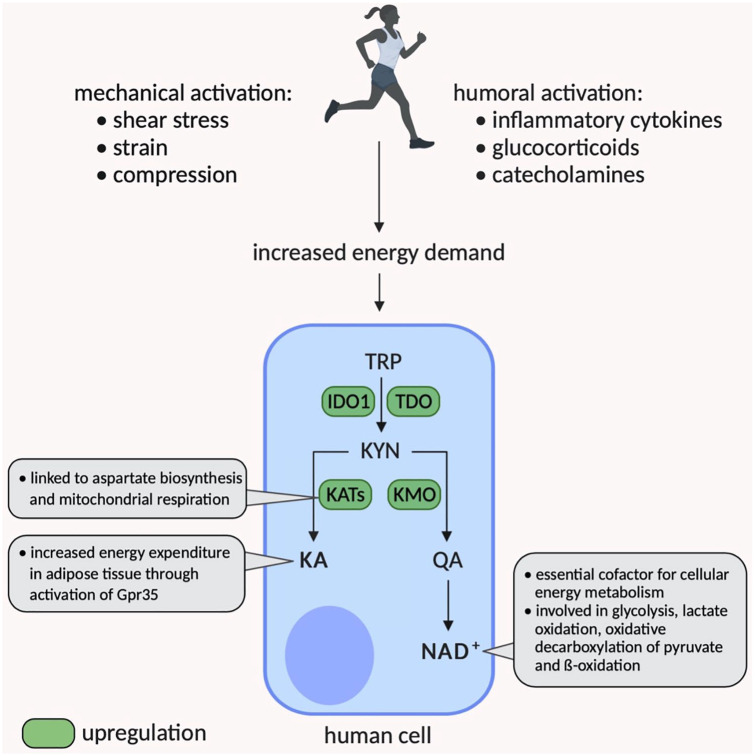
Research beyond exercise medicine is increasingly investigating the role of the NAD+ de novo synthesis via the KYN pathway. NAD+ as the preferred end product of the KYN pathway plays a pivotal role as cofactor in cellular energy metabolism, is known to decrease with ageing, and is reduced in various chronic diseases.53,54 To investigate the role of the KYN pathway in maintaining intracellular NAD+ levels in astrocytes and neurons, Braidy et al55 inhibited the KYN pathway and consequently detected reduced NAD+ levels, which correlated with a decrease in cell viability. Moreover, IFN-γ-activated macrophages showed elevated IDO activity and increased NAD+ production, whereas the NAD+ catabolism was also augmented.56 Acute exercise is known to be accompanied by higher demands of energy (mainly for skeletal muscle) and is also described as humoral (eg, cytokines, hormones) and mechanical stimuli for different tissues,57 potentially leading to cell activation. For example, the exercise-induced increase in epinephrine has been well-described to mediate NK cell mobilization.58 Together with the reported acute exercise-induced increases in QA as direct precursor of NAD+, there appears to be a solid rationale that acute exercise-induced KYN pathway alterations occur to maintain cellular energy homeostasis. However, to the best of our knowledge, no study has examined NAD+ de novo synthesis during or following acute exercise intracellularly or in the peripheral bloodstream. Concomitant with an acute exercise-induced activation of the KYN pathway that increases the NAD+ biosynthesis to meet intracellular energy demands, KYN and other metabolites might migrate to the peripheral bloodstream, thereby enabling further physiological processes, such as increased AhR activity. Thus, acute exercise-induced alterations of the KYN pathway could mediate the communication between different types of immune cells, overall attempting to prevent an overactivation of specific immune components (eg, T-cell compartment) and to enhance cellular energy homeostasis by reducing energy demand due to functional suppression. More far-reaching than solely focussing on the immune system, it is plausible that the de novo synthesis of NAD+ can appear in all cells that express IDO1, IDO2, or TDO and therefore potentially be influenced by exercise. Future research is highly warranted to investigate the impact of acute and chronic exercise on intracellular NAD+ de novo synthesis in different cell types, on functional consequences for distinct tissues or physiological systems, and on extracellular NAD+ levels measured in the peripheral bloodstream.
Furthermore, other important mechanisms in different cell types (adipocytes and skeletal muscle) contributing to cellular energy homeostasis via the KYN pathway have recently been discovered. Agudelo et al59 showed that increased circulating levels of KA, which are well-described after exercise, activate the G protein–coupled receptor (Gpr)35 in adipose tissue, thereby regulating energy homeostasis through stimulating lipid metabolism and thermogenic and anti-inflammatory gene expression in adipocytes. More precisely, the KA/Gpr35 network signalling actions comprise intracellular Ca2+ release, ERK1/2 phosphorylation, and PGC-1α1 activation. The authors showed that KA and Gpr35 actions suppressed weight gain in mice fed with a high-fat diet, increased systemic energy expenditure, and mediated adipose tissue beiging,59 overall indicating the profound involvement of the KYN pathway regulation for energy homeostasis. In skeletal muscle, Agudelo et al revealed the contribution of KYN pathway metabolism to improve energy utilization by increasing the efficiency of glucose oxidation through the expression of malate-aspartate shuttle. Interestingly, this mechanism is also PGC-1α1-dependent and has been described to delay the use of fatty acid oxidation due to greater glucose oxidation efficiency. Therefore, regular aerobic exercise could be proposed to enhance these adaptations.60
In summary, evidence with and without directly investigating exercise indicates the extended involvement of the KYN pathway in the maintenance or even improvement of energy metabolism (Figure 2) through differing mechanisms and tissue types. A potential overarching explanation could be of evolutionary nature. Cells have a constant energy demand which is necessary to survive. In conditions of increased energy demands, such as during or after metabolic or mechanical stimulation, strategies to provide more energy and to prepare for potentially upcoming conditions of energy deficiency may be initiated. Thus, the different actions described to maintain/improve energy homeostasis might, at least in part, represent self-protective mechanisms. Concerning immune cells, the potential acute exercise-induced increase in NAD+ de novo synthesis may represent a reaction to maintain energy demand, whereas the immunosuppressive properties associated with KYN degradation potentially prevent cellular exhaustion that could result in decreased viability or even cell death in the long term.
Figure 2.
Potential impact of acute exercise on cellular energy homeostasis mediated by the kynurenine pathway. IDO indicates isoenzymes indoleamine; KA, kynurenic acid; KAT, kynurenine aminotransferase; QA, quinolinic acid; TRP, tryptophan.
Contribution of KYN Pathway Modulations to Exercise-Induced Benefits for Chronic Diseases?
Considering the KYN pathway involvement in the pathogenesis and progression of different diseases together with the described exercise-induced consequences for the immune system and CNS, the impact on different steps of disease appears to be a major topic for future research. The anti-inflammatory properties (eg, increase in Tregs) might be of high relevance already in disease prevention, as conditions of low-grade systemic inflammation represent a major risk factor for several different chronic diseases, including internal (eg, cardiovascular diseases,61 cancer62) and neurological (eg, Alzheimer disease63) disorders. Furthermore, exercise seems to have the potential to counteract pathological KYN accumulation in the CNS mediated by peripheral KYN clearance, thereby preventing neurodegenerative and neuroinflammatory conditions. Consequently, these mechanisms could represent a comprehensive explanation for the well-described improvements in CNS-related symptoms in several chronic diseases which are known to be alleviated by exercise, such as cognitive impairments or fatigue.64 Aside from the potentially beneficial effects on different symptoms, current research increasingly investigates the impact of exercise on the progression of the above-mentioned diseases. As KYN pathway metabolites represent markers of progression and severity, exercise might also influence the disease itself, which could be due to the described immunosuppressive and neuroprotective effects. However, solely the preclinical animal model by Agudelo et al22 provided substantial evidence indicating that exercise-induced KYN pathway modulations can directly impact chronic diseases. As KYN pathway metabolites and ratios represent prognostic or progression biomarkers in many different chronic diseases,65-67 exercise-induced KYN pathway modulations may represent a global underlying mechanism that, at least in part, is involved in the well-described benefits of exercise in a broad range of medical conditions.64
Conclusion
During the past decade, a rising number of publications dealt with the acute and chronic effects of exercise on the KYN pathway in animal models and various human populations. Overall, promising mechanistic insights have been described in different cell types and the circulation. As the KYN pathway is ubiquitous, a major challenge for future research will be to reveal the interaction between different tissues and cells. However, it should be kept in mind that most cell types are evolutionarily formed to survive, even in conditions of overactivation, for example, in response to metabolic stress (eg, due to immune activation). Therefore, the frequently disregarded de novo synthesis of NAD+ also appears to be of high interest for future research approaches and might be, at least in part, underlying for other biological consequences of the KYN pathway. Currently, to the best of our knowledge, no study exists investigating the impact of acute or chronic exercise (representing acute or repetitive metabolic stressors that may result in long-term adaptations) on the de novo synthesis of NAD+. Finally, future studies should also consider the contribution of exercise-induced KYN pathway modulations and associated effects on the immune system and CNS to potential changes in clinical outcomes. Research approaches with translational orientation are warranted to fill the gap between underlying mechanisms, different exercise modalities as intervention, biological consequences, and clinical relevance.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NJ and PZ developed the concept of this work. NJ and PZ drafted the article. WB, DW, and AJM revised the article critically for important intellectual content. DW created figures. All authors approved the final version of the article.
References
- 1. Badawy AA-B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 3. Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64-82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 4. Gosker HR, Clarke G, Theije CC, de Cryan JF, Schols AMWJ. Impaired skeletal muscle kynurenine metabolism in patients with chronic obstructive pulmonary disease. J Clin Med. 2019;8:915. doi: 10.3390/jcm8070915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379-401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 6. Lovelace MD, Varney B, Sundaram G, et al. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorgdrager FJH, Vermeiren Y, van Faassen M, et al. Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J Neurochem. 2019;151:656-668. doi: 10.1111/jnc.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424-2426. doi: 10.1093/clinchem/43.12.2424. [DOI] [PubMed] [Google Scholar]
- 9. Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237-247. doi: 10.1016/j.neuropharm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joisten N, Kummerhoff F, Koliamitra C, et al. Exercise and the kynurenine pathway: current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc Immunol Rev. 2020;26:24-42. [PubMed] [Google Scholar]
- 11. Bansi J, Koliamitra C, Bloch W, et al. Persons with secondary progressive and relapsing remitting multiple sclerosis reveal different responses of tryptophan metabolism to acute endurance exercise and training. J Neuroimmunol. 2018;314:101-105. doi: 10.1016/j.jneuroim.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 12. Koliamitra C, Javelle F, Joisten N, et al. Do acute exercise-induced activations of the kynurenine pathway induce regulatory T-cells on the long-term? A theoretical frame work supported by pilot data. J Sports Sci Med. 2019;18:669-673. [PMC free article] [PubMed] [Google Scholar]
- 13. Strasser B, Geiger D, Schauer M, Gatterer H, Burtscher M, Fuchs D. Effects of exhaustive aerobic exercise on tryptophan-kynurenine metabolism in trained athletes. PLoS ONE. 2016;11:e0153617. doi: 10.1371/journal.pone.0153617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strasser B, Geiger D, Schauer M, et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8:752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joisten N, Schumann M, Schenk A, et al. Acute hypertrophic but not maximal strength loading transiently enhances the kynurenine pathway towards kynurenic acid. Eur J Appl Physiol. 2020;120:1429-1436. 10.1007/s00421-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mudry JM, Alm PS, Erhardt S, et al. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab Res Rev. 2016;32:754-761. doi: 10.1002/dmrr.2798. [DOI] [PubMed] [Google Scholar]
- 18. Schlittler M, Goiny M, Agudelo LZ, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310:C836-C840. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 19. Hennings A, Schwarz MJ, Riemer S, Stapf TM, Selberdinger VB, Rief W. Exercise affects symptom severity but not biological measures in depression and somatization – results on IL-6, neopterin, tryptophan, kynurenine and 5-HIAA. Psychiatry Res. 2013;210:925-933. doi: 10.1016/j.psychres.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 20. Küster OC, Laptinskaya D, Fissler P, et al. Novel blood-based biomarkers of cognition, stress, and physical or cognitive training in older adults at risk of dementia: preliminary evidence for a role of BDNF, irisin, and the kynurenine pathway. J Alzheimers Dis. 2017;59:1097-1111. doi: 10.3233/JAD-170447. [DOI] [PubMed] [Google Scholar]
- 21. Millischer V, Erhardt S, Ekblom Forsell ÖY, Lavebratt C. Twelve-week physical exercise does not have a long-lasting effect on kynurenines in plasma of depressed patients. Neuropsychiatr Dis Treat. 2017;13:967-972. doi: 10.2147/NDT.S131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agudelo LZ, Femenía T, Orhan F, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33-45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 23. Zimmer P, Schmidt ME, Prentzell MT, et al. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front Oncol. 2019;9:962. doi: 10.3389/fonc.2019.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6-63. [PubMed] [Google Scholar]
- 25. Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18-31. [PubMed] [Google Scholar]
- 26. Hötting K, Schickert N, Kaiser J, Röder B, Schmidt-Kassow M. The effects of acute physical exercise on memory, peripheral BDNF, and cortisol in young adults. Neural Plast. 2016;2016:6860573. doi: 10.1155/2016/6860573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Leary CB, Hackney AC. Acute and chronic effects of resistance exercise on the testosterone and cortisol responses in obese males: a systematic review. Physiol Res. 2014;63:693-704. [DOI] [PubMed] [Google Scholar]
- 28. Badawy AA-B, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int J Tryptophan Res. 2019;12:1178646919868978. doi: 10.1177/1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916-1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas SR, Terentis AC, Cai H, et al. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778-23787. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]
- 31. Suhr F, Gehlert S, Grau M, Bloch W. Skeletal muscle function during exercise-fine-tuning of diverse subsystems by nitric oxide. Int J Mol Sci. 2013;14:7109-7139. doi: 10.3390/ijms14047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol (London). 2004;561:1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baskurt OK, Ulker P, Meiselman HJ. Nitric oxide, erythrocytes and exercise. Clin Hemorheol Microcirc. 2011;49:175-181. doi: 10.3233/CH-2011-1467. [DOI] [PubMed] [Google Scholar]
- 34. Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879-1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 35. Silvennoinen M, Ahtiainen JP, Hulmi JJ, et al. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep. 2015;3:e12563. doi: 10.14814/phy2.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hondares E, Rosell M, Díaz-Delfín J, et al. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112-43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hondares E, Pineda-Torra I, Iglesias R, Staels B, Villarroya F, Giralt M. PPARdelta, but not PPARalpha, activates PGC-1alpha gene transcription in muscle. Biochem Biophys Res Commun. 2007;354:1021-1027. doi: 10.1016/j.bbrc.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 38. Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748-759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361-370. [DOI] [PubMed] [Google Scholar]
- 40. Ruas JL, White JP, Rao RR, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319-1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459-468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021-22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 44. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190-3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197-203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 46. Martin KS, Azzolini M, Lira Ruas J. The kynurenine connection: how exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am J Physiol Cell Physiol. 2020;318:C818-C830. doi: 10.1152/ajpcell.00580.2019. [DOI] [PubMed] [Google Scholar]
- 47. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184-197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 48. Weinhold M, Shimabukuro-Vornhagen A, Franke A, et al. Physical exercise modulates the homeostasis of human regulatory T cells. J Allergy Clin Immunol. 2016;137:1607.e8-1610.e8. doi: 10.1016/j.jaci.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 49. Lovelace MD, Varney B, Sundaram G, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373-388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 50. Parrott JM, O’Connor JC. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry. 2015;6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Allison DJ, Nederveen JP, Snijders T, et al. Exercise training impacts skeletal muscle gene expression related to the kynurenine pathway. Am J Physiol Cell Physiol. 2019;316:C444-C448. doi: 10.1152/ajpcell.00448.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herrstedt A, Bay ML, Simonsen C, et al. Exercise-mediated improvement of depression in patients with gastro-esophageal junction cancer is linked to kynurenine metabolism. Acta Oncol. 2019;58:579-587. doi: 10.1080/0284186X.2018.1558371. [DOI] [PubMed] [Google Scholar]
- 53. Katsyuba E, Auwerx J. Modulating NAD+ metabolism, from bench to bedside. EMBO J. 2017;36:2670-2683. doi: 10.15252/embj.201797135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020;2:9-31. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 55. Braidy N, Guillemin GJ, Grant R. Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int J Tryptophan Res. 2011;4:29-37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grant RS. Indoleamine 2,3-dioxygenase activity increases NAD+ production in IFN-γ-stimulated human primary mononuclear cells. Int J Tryptophan Res. 2018;11:1178646917751636. doi: 10.1177/1178646917751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738-749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 58. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184:503-511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- 59. Agudelo LZ, Ferreira DMS, Cervenka I, et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27:378.e5-392.e5. doi: 10.1016/j.cmet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 60. Agudelo LZ, Ferreira DMS, Dadvar S, et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat Commun. 2019;10:2767. doi: 10.1038/s41467-019-10712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739-2748. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 62. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deleidi M, Jäggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:172. doi: 10.3389/fnins.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pedersen BK, Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1-72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 65. Lim CK, Bilgin A, Lovejoy DB, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lund A, Nordrehaug JE, Slettom G, et al. Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE. 2020;15:e0227365. doi: 10.1371/journal.pone.0227365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suzuki Y, Suda T, Furuhashi K, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67:361-365. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]