Abstract
Background:
Prognosis of patients affected by metastatic esophageal–gastric junction (EGJ) or gastric cancer (GC) remains dismal. Trastuzumab, an anti-HER2 monoclonal antibody, is the only targeted agent approved for the first-line treatment of patients with HER2-overexpressing advanced EGJ or GC in combination with chemotherapy. However, patients invariably become resistant during this treatment. We recently identified the overexpression of fibroblast growth factor (FGF) receptor 3 (FGFR3) as a molecular mechanism responsible for trastuzumab resistance in GC models, providing the rationale for the inhibition of this receptor as a potential second-line strategy in this disease. Pemigatinib is a selective, potent, oral inhibitor of FGFR1, 2, and 3.
Methods:
The FiGhTeR trial is a phase II, single-arm, open-label study to assess safety and activity of the FGFR inhibitor pemigatinib as second-line treatment strategy in metastatic EGJ/GC patients progressing under trastuzumab-containing therapies. The primary endpoint is the 12-week progression-free survival rate. Plasma and tumor tissue samples will be collected for translational research analyses at baseline, during treatment, and at progression on pemigatinib.
Discussion:
Co-alterations in genes coding for different tyrosine-kinase receptors are emerging as relevant mechanisms of acquired resistance to anti-HER2 therapeutic strategies in GC. In particular, our group has recently identified that in GC models the overexpression of FGFR3 sustains the acquired resistance to trastuzumab. This trial aims to assess the safety, tolerability and activity of the FGFR inhibitor pemigatinib as a second-line treatment in metastatic EGJ/GC patients refractory to first-line trastuzumab-containing therapies. Furthermore, this study offers the opportunity to prospectively study mechanisms and pathways involved in trastuzumab resistance.
Protocol number:
CRC2017_02
EudraCT Number:
2017-004522-14
Keywords: esophageal–gastric junction cancer, FGFR3, gastric cancer, HER-2, pemigatinib, trastuzumab resistance
Background
Gastric cancer (GC) constitutes a major global health problem as it remains the fifth most-frequently diagnosed cancer and the third leading cause of cancer-related deaths worldwide.1 Systemic chemotherapeutic and targeted agents provide a significant improvement in survival for patients with metastatic GC. However, this disease inevitably progresses under these treatments, so that the prognosis of metastatic GC patients remains unfavorable with a 5-year overall survival (OS) rate around 5–20%.2
Human epidermal growth factor receptor 2 (HER2), also known as p185 or ErbB-2, is a transmembrane tyrosine kinase receptor of 185 kDa and is encoded by the proto-oncogene HER2/neu mapped on chromosome 17q21; it belongs to the epidermal growth factor receptor family.3,4 In GC, HER2 overexpression is present in a percentage that varies between 7% and 34% of the cases.5
Trastuzumab, a recombinant humanized monoclonal antibody directed against HER2, is the only targeted agent approved for the first-line treatment of patients with HER2-overexpressing metastatic GC in combination with chemotherapy. The ToGA trial demonstrated that the addition of trastuzumab to a doublet chemotherapeutic regimen with cisplatin and fluoropyrimidine improves OS, progression-free survival (PFS), and response rate (RR) in HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.6
If primary resistance to trastuzumab is quite rare in patients with HER2 overexpression, almost all patients become resistant after a certain period of therapy. It has been shown that the selection of HER2 not amplified clones is a common mechanism of trastuzumab resistance.7,8 In particular, patients with uncertain basal immunohistochemistry positivity (IHC 2+) seem to frequently lose amplified HER2 clones. Furthermore, in the same patient the loss of amplified HER2 clones may be due to tumor progression or tumor heterogeneity between metastatic and primary tumor.9 Additional mutations in receptor tyrosine kinases, RAS and PI3K pathways are emerging as relevant mechanisms of both intrinsic and acquired resistance to anti-HER2 therapies. Patients with these co-alterations have a lower benefit from receiving trastuzumab and are characterized by a shorter PFS.10
Fibroblast growth factor (FGF) receptor 3 (FGFR3) is a member of a family of four tyrosine kinase receptors that are implicated in cellular growth and survival and tumor angiogenesis.11 FGF family includes heparin-binding polypeptide ligands, including FGF9, that has a unique affinity for FGFR3.12 We recently demonstrated in GC that the overexpression of FGFR3 and FGF9 activating the PI3K/AKT/mTOR signaling pathway sustains acquired resistance to trastuzumab. In our study, GC models selected for resistance to trastuzumab were shown to be associated with overexpression of FGFR3, FGF9, and phosphorylated AKT, in addition to losing HER2 expression. In vivo, trastuzumab-resistant murine models treated with dovitinib, a selective inhibitor of FGFR3, showed a higher reduction in tumor burden and longer OS compared with the control group. The relevance of FGFR3 overexpression has been demonstrated in a clinical setting, comparing biopsies at baseline and after progression to trastuzumab in three patients. In all these patients, biopsies collected at trastuzumab progression exhibited significantly higher FGFR3 expression than their matched pretreatment samples.13
This preclinical evidence led to the design of the FiGhTeR trial, which aims to assess the safety, tolerability and activity of pemigatinib (INCB54828), an inhibitor of FGFR1, 2, and 3, in metastatic esophageal–gastric junction (EGJ)/GC patients refractory to first-line trastuzumab-containing therapies.
Many studies have provided evidence for an increasing role of FGFR signaling as a key mediator of resistance to anti-HER2 therapies. Pemigatinib (INCB054828) is a potent and selective inhibitor of the FGFR family members. The FiGhTeR trial aims to assess the safety and activity of the FGFR inhibitor pemigatinib in HER2 trastuzumab-resistant GC patients. Translational research analyses on plasma and tissue specimens collected in this study will be used to identify novel molecular mechanisms responsible for trastuzumab resistance and predictive biomarkers of pemigatinib in GC patients.
Methods/design
Study setting
The FiGhTeR trial is a phase II, single-arm, open-label study to assess safety, tolerability, and activity of pemigatinib as second-line treatment strategy in metastatic EGJ/GC patients refractory to first-line trastuzumab-containing therapies. Patients will be screened for eligibility by research staff to ensure that all inclusion and exclusion criteria are met. Main eligibility criteria include disease progression within 3 months of the last dose of first-line trastuzumab-containing therapy, fresh biopsy available, and adequate bone marrow, liver, and renal function (Table 1). Exclusion criteria include corneal disorders, serious cardiovascular disease, elevated serum phosphate and calcium levels (Table 2).
Table 1.
Eligibility criteria.
| 1. Histologically confirmed advanced or metastatic adenocarcinoma of the stomach and the gastroesophageal junction. 2. Disease progression within 3 months of the last dose of first-line trastuzumab-containing therapy. 3. Patients must have performed a fresh biopsy (at least 8–10 slides with >20% tumor content) at the baseline of study enrollment. 4. At least one measurable and evaluable disease site based on response evaluation criteria in solid tumors (RECIST v1.1). 5. Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–1. 6. Age ⩾18, no age upper limit unless patient would be unable to tolerate chemotherapy. 7. An expected survival of more than 3 months. 8. Duration from the last therapy is more than 4 weeks for other cytotoxic agents, surgery, or radiotherapy. 9. Major organ function has to meet the following criteria hemoglobin (Hb)>8 g/dl, platelet count >75 × 109/l, neutrophil count >1.5 × 109/L 10. Absence of pregnancy or breastfeeding. 11. Pregnancy test (serum or urine) must be performed for woman of childbearing age within 7 days before enrolment and the test result must be negative. They shall take appropriate methods for contraception during the study until the 8th week after the last administration of study drug. For men, shall agree to take appropriate methods of contraception during the study until the 8th week after the last administration of study drug. 12. Patients must be accessible to follow-up and management in the treatment center. 13. Patient must voluntarily join the study and sign the informed consent form for the study |
Table 2.
Exclusion criteria.
| 1. Current evidence of corneal disorder/keratopathy, including but not limited to bullous/band keratopathy, corneal abrasion, inflammation/ulceration, keratoconjunctivitis, etc., confirmed by ophthalmologic examination. 2. Any active malignancies except cured basal cell carcinoma of skin and carcinoma in situ of uterine cervix. 3. Poorly controlled arterial hypertension (systolic blood pressure >140 mmHg and diastolic blood pressure >90 mmHg) despite standard medical management. 4. Serious cardiovascular disease: II level myocardial ischemia or myocardial infarction, arrhythmia (including QT interval prolongation, for man >450 ms, for woman >470 ms); III–IV level cardiac function insufficiency, or echocardiography showed that left ventricular ejection fraction (LVEF) <50%. 5. Positive urinary protein (urine protein detection of 2 or more, or 24-hour urine protein > 1.0 g). 6. Total bilirubin ⩾1.5 × upper limit of normal (ULN; ⩾2.5 × ULN if Gilbert syndrome or metastatic disease involving liver). 7. AST and ALT >2.5 × ULN (AST and ALT >5 × ULN in the presence of liver metastases). 8. Creatinine clearance ⩽30 ml/min based on Cockcroft–Gault. 9. Factors that could have an effect on oral medication (such as inability to swallow, chronic diarrhea, and intestinal obstruction). 10. Serum phosphate > institutional ULN. 11. Serum calcium outside of the institutional normal range or serum albumin-corrected calcium outside of the institutional normal range when serum albumin is outside of the institutional normal range. 12. History of calcium/phosphate homeostasis disorder. 13. History and/or current evidence of ectopic mineralization/calcification, including but not limited to soft tissue, kidneys, intestine, myocardia, or lung, excepting calcified lymph nodes and asymptomatic arterial or cartilage/tendon calcification. 14. Current evidence of significant corneal disorder/keratopathy or retinal disorder, confirmed by ophthalmologic examination. 15. Factors of high gastrointestinal bleeding risk, including the following conditions: local active ulcer lesions with positive fecal occult blood test (++); history of black stool, or vomiting blood in the past 2 months; unresected primary lesion in stomach with positive fecal occult blood test (+), ulcerated gastric carcinoma with massive alimentary tract bleeding risk judged by Pis based on gastric endoscopy result. 16. Abnormal Coagulation (INR > 1.5 APTT > 1.5 UNL), with tendency of bleed. 17. Known hypersensitivity or severe reaction to INCB054828 or excipients of INCB054828 study drug 18. Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule; those conditions should be discussed with the patient before registration in the trial. |
This is a single-institution trial conducted at the early phase clinical trial unit “Centro Ricerche Cliniche di Verona” at the University Hospital of Verona, Italy.
All reporting will adhere to the Consolidated Standards of Reporting Trials (CONSORT) guideline.14 The study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting (see Supplemental Figure S1).15
Study treatment
All patients receive self-administered, oral pemigatinib at a dose of 13.5 mg once daily (21-day cycle; 2 weeks on, 1 week off), until radiologic disease progression, unacceptable toxicity, withdrawal of consent, or patient/physician choice. This dose regimen was supported by pharmacokinetic/pharmacodynamic results from a phase I/II study of pemigatinib for metastatic malignancies.16 In cases where study drug should be restarted at a next lower dose, the new doses can be 9 mg and then 6 mg (Table 3). Concomitant potent CYP3A4 inhibitors and inducers are prohibited. Other anticancer medications and study drugs are prohibited. Compliance with the study treatments is emphasized to the participant by the site personnel. Participants are instructed to bring the study drug with them to the study visits so that site personnel can conduct tablet counts to assess study drug accountability.
Table 3.
Guidelines for interruption and restarting of study drug.
| Adverse event | Action taken |
|---|---|
| Chemistry | |
| AST and/or ALT is >5.0 × ULN |
Step 1: interrupt study drug up to 14 days until the toxicity has resolved to ⩽Grade 1 except by approval of the medical monitor Step 2: restart study drug at same dose. If assessed related to study drug, restart study drug at next lower dose; monitor as clinically indicated |
| Other toxicities | |
| Any Grade 1 or Grade 2 toxicity | Continue study drug treatment and treat the toxicity; monitor as clinically indicated |
| Any Grade 3 toxicity if clinically significant and not manageable by supportive care |
Step 1: interrupt study drug up to 14 days until the toxicity has resolved to ⩽Grade 1 Step 2: restart study drug at same dose. If assessed related to study drug, restart study drug at next lower dose; monitor as clinically indicated |
| Any recurrent Grade 3 toxicity after two dose reductions | Discontinue study drug administration and follow-up per protocol |
| Any other Grade 4 toxicity | Discontinue study drug administration and follow-up per protocol |
Follow-up and study assessment
The patients at the screening undergo a physical exam, a 12-lead ECG, blood count, serum chemistry and a CT or MRI radiological tumor assessment. Before every cycle of treatment, the patients undergo a physical exam, blood count, serum chemistry, and adverse event evaluation. Disease assessment is performed every 6 weeks for the first 4 cycles, and then every 9 weeks by using CT or MRI scan. All study assessments are performed as indicated in the schedule of assessments (Table 4).
Table 4.
Schedule of assessments.
| Screening |
Treatment |
EOT | Follow-up |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 |
Cycle 2 |
Safety |
Disease status |
Survival |
|||||
| Procedures | Days −28 to −1 |
Day 1 | Day 8 (±3 days) |
Day 15 (±3 days) |
Day 1 (±3 days) |
EOT+30– 35 Days |
Every 9 weeks | Every 12 weeks | |
| Pemigatinib | D1–D14 | D1–D14 | |||||||
| Informed consent | X | ||||||||
| Demographics | X | ||||||||
| Medical history | X | ||||||||
| Concurrent meds | X | X | X | X | X | X | |||
| Physical exam | X | X | X | X | X | X | X | ||
| Vital signs | X | X | X | X | X | X | X | ||
| Height | X | ||||||||
| Weight | X | X | X | X | X | X | X | ||
| ECOG Performance status |
X | X | X | X | X | X | X | ||
| Blood count | X | X | X | X | X | X | X | ||
| Serum chemistry | X | X | X | X | X | X | X | ||
| Coagulation panel | X | ||||||||
| Urinalysis | X | ||||||||
| 12- lead ECG | X | X | X | X | X | ||||
| Adverse event evaluation | X | X | X | X | X | X | |||
| Radiological tumor assessment | X | X (every 2 cycles through Cycle 4, every 3 cycles thereafter) |
X (only for participant who discontinue for a reason other than PD) | ||||||
Endpoints
The primary endpoint is the 12-week PFS rate, which is the probability of being without progression (loco-regional or distant) or death due to any cause at 12 weeks from trial enrollment. The secondary endpoints are OS, disease control rate (DCR), and safety profile (incidence of ⩾grade 3 adverse events using Common Terminology Criteria for Adverse Events - CTCAE - version 4.0).
Other exploratory objectives include collecting and banking serial blood and tumor tissue specimens from participants at baseline and at the time of pemigatinib progression for correlative biomarker studies.
Statistical design and sample size
Simon’s two-stage design is used. The null hypothesis that the 12-weeks PFS rate is 20% is tested against a one-sided alternative. The optimal two-stage design to test the null hypothesis that p ⩽ 0.200 versus the alternative that p ⩾ 0.50 has an expected sample size of 10.03 and a probability of early termination of 0.797. If the drug is actually ineffective, there is a 0.039 probability of concluding that it is (the target for this value was 0.05). If the drug is actually effective, there is a 0.2 probability of concluding that it is not (the target for this value was 0.2). After testing the drug on 8 patients in the first stage, the trial will be terminated if there are 2 or fewer patients progression free at 12 weeks. If the trial goes on to the second stage, a total of 18 patients will be studied. If the total number of progression-free patients at 12 weeks is less than or equal to six, the drug is rejected.
Safety assessment
All adverse events observed must be reported in the patients’ medical records and in electronic case report forms (eCRFs). All serious adverse events (SAEs) occurring during the study treatment period must be reported within 24 h.
The inhibition of FGFR is responsible for specific on-target adverse events of pemigatinib. Preliminary results from the phase I/II FIGHT-101 [INCB 54828-101, ClinicalTrials.gov identifier: NCT02393248] study have been reported. In parts 1 and 2 combined, in which pemigatinib was used alone, the most frequent adverse events were hyperphosphatemia (61%; all at doses ⩾6 mg), fatigue (39%), dry mouth (31%), alopecia (28%), constipation (23%), and stomatitis (21%).16 Most frequent CTCAE grade ⩾3 adverse events were fatigue (10%), pneumonia (8%), and hyponatremia (7%).
Hyperphosphatemia is an expected on-target pharmacologic effect of FGFR inhibition. It should be managed with diet modifications, phosphate-binding therapy, dose reductions, or interruptions (Table 5). Liver chemistries, including serum levels of ALT, AST, and bilirubin, are assessed on a regular basis. A baseline comprehensive ophthalmology examination is obtained at baseline and on a regular basis while receiving pemigatinib. The plasmatic levels of phosphate, calcium, vitamin D3, and parathyroid hormone are monitored regularly.
Table 5.
Recommended approach for hyperphosphatemia management.
| Serum phosphate level | Supportive care | Guidance for interruption/discontinuation of pemigatinib | Guidance for restarting pemigatinib |
|---|---|---|---|
| >5.5 mg/dl and ⩽7 mg/dl | Initiate a low-phosphate diet | No action | Not applicable |
| >7 mg/dl and ⩽10 mg/dl | Initiate/continue a low-phosphate diet and initiate phosphate binding. Monitor serum phosphate at least twice a week and adjust the dose of binders as needed; continue to monitor serum phosphate at least twice a week until return to normal range | If serum phosphate level continues to be >7 mg/dl and ⩽10 mg/dl with concomitant phosphate-binding therapy for 2 weeks, or if there is recurrence of serum phosphate level in this range, interrupt pemigatinib for up to 2 weeks | Restart at the same dose when serum phosphate is <7 mg/dl. If serum phosphate level recurs at >7 mg/dl, restart study drug with dose reduction |
| >10 mg/dl | Continue to maintain a low-phosphate diet, adjust phosphate-binding therapy, and start/continue phosphaturic agent. Continue to monitor serum phosphate at least twice a week until return to normal range | If serum phosphate level is >10 mg/dl for 1 week following phosphate-binding therapy and low-phosphate diet, interrupt study drug. If there is recurrence of serum phosphate level in this range following two dose reductions, permanently discontinue pemigatinib | Restart study drug at reduced dose with phosphate binders when serum phosphate is <7 mg/dl. |
Data management, control of data consistency, quality control, monitoring, and audits
The investigator is responsible for ensuring data quality. All information required the protocol are entered in the eCRF. Periodic monitoring visits at the center are planned. Monitoring procedures will be adapted to the study-specific risks for patients. Standard operating procedures (SOPs) will be interpreted to ensure patient safety and the integrity of the clinical data. The investigator or a designated representative is obliged to provide clarification or respond to queries. If no further corrections are to be made in the database, it will be locked and used for statistical analysis.
Ethics approval and consent to participate
This study is conducted in accordance with the 1964 Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects. The study received approval on May 7, 2019 by Ethics Committee of Verona and Rovigo (protocol number CRC2017_02, v.1.1). All patients will provide written informed consent before enrollment. The investigator will inform patients that participation in the trial is voluntary and that they can withdraw at any time. They will be informed that the investigator will maintain their records over the long-term follow-up period and that their records may be viewed by management officers, within the limits of regulations. The privacy of patients will be protected. The written consent will include the permission of collecting and use data and biological data. This study is carried out in accordance with International Conference on Harmonization Good Clinical Practice (ICH-GCP). Every amendment in the research protocol will be first submitted to Research Ethics Committee approval. To avoid breaking data confidentiality only Principal Investigator will have access to the final study database, which will be typed into online (REDCap) with restrictive access passwords.
Translational research analyses
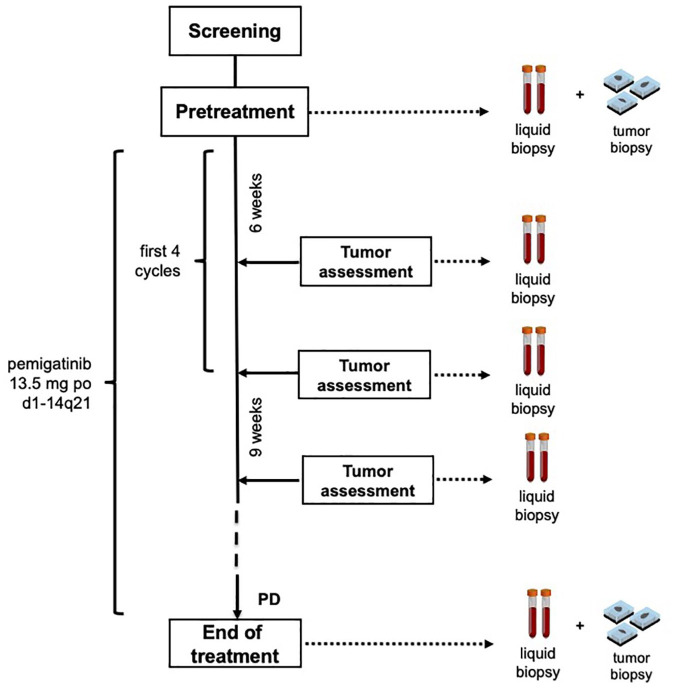
An extensive program of translational research is planned. In order to identify novel potential mechanisms of resistance to trastuzumab and pemigatinib, tissue and blood samples from enrolled patients are collected at baseline after progression under treatment with trastuzumab, along the treatment, and at disease progression under treatment with pemigatinib (Figure 1).
Figure 1.
Schema of study treatment and timeline of blood and tumor samples collection for translational analyses.
To prospectively evaluate new potential biomarkers and study the dynamic nature of therapeutic resistance in metastatic GC, immunohistochemistry (IHC) and next-generation sequencing (NGS) on tumor samples are performed. In order to correlate pemigatinib response to genomic and epigenomic profiling of patients with trastuzumab-resistant GC, biopsies are analyzed for mutational and epigenetic status through NGS analysis and chromatin immunoprecipitation (ChIP) sequencing, respectively. To identify potential biomarkers of response to pemigatinib, genomic findings are correlated with treatment response and patient outcomes.
To overcome a possible limitation depending on tumor heterogeneity, NGS analysis of free circulating tumor DNA (ctDNA) is performed on liquid biopsies of plasma from enrolled patients.
Discussion
GC remains one of the most lethal solid tumors worldwide.17 Trastuzumab contributed to improve prognosis of patients with HER2-positive GC; however, resistance to this monoclonal antibody remains one of the major challenges in this setting. To date, no specific targeted therapies are available after failing trastuzumab-containing regimens.18–20 Our group recently identified the FGFR3/AKT axis as an escape pathway responsible for trastuzumab resistance in GC, laying the basis for testing FGFR3 inhibition as a potential strategy to modulate this resistance.13
The mammalian FGFR family includes four highly conserved receptors (FGFR1, FGFR2, FGFR3, and FGFR4) that have an extracellular ligand binding domain, a single transmembrane domain and an intracellular tyrosine kinase domain. Eighteen FGF ligands bind to FGFRs leading to receptor dimerization, activation of the kinase domain and transphosphorylation of the receptors. FGFRs are involved in the regulation of cell survival, proliferation, differentiation and motility during embryogenesis, adult-tissue homeostasis, and carcinogenesis. There is a strong genetic and functional evidence that dysregulation of FGFR can lead to the establishment and progression of cancer. Genetic alterations in FGFR1, FGFR2, and FGFR3 have been described in many tumor types.11 Further studies have provided evidence for an increasing role of FGFR signaling as a key mediator of resistance to several drugs, including anti-HER2 therapies.21–23
Recently, signals of activity from targeting FGFR pathway in GC has been reported. The monoclonal antibody bemarituzumab showed activity in high FGFR2b-overexpressing advanced gastroesophageal junction adenocarcinoma in a phase I study.24
Pemigatinib (INCB054828) is a potent and selective inhibitor of the FGFR family members. In vitro, pemigatinib inhibits the kinase activity of FGFR1, FGFR2, and FGFR3 with IC50 values that ranged from 0.39 to 1.2 nM, while it has a minor inhibitory potency against FGFR4. Safety and activity of pemigatinib have been assessed in a number of complete and ongoing clinical trials. FIGHT-101 was a three-part, phase I/II study that showed preliminary safety and efficacy data of pemigatinib in patients with metastatic solid tumors (parts 1 and 3) or tumors with FGF/FGFR alteration (part 2), of which the most common were cholangiocarcinoma, breast, esophageal, ovarian, and head and neck cancer. In part 1, no patients had a complete response (CR) or partial response (PR), while in part 2 three patients showed PR. Most frequent adverse events were hyperphosphatemia, anemia, diarrhea, fatigue, dehydration, constipation, nausea, and vomiting.16
Two phase II studies tested pemigatinib in solid tumors in which FGFR alterations seem to have an important pathogenetic role. FIGHT-201 [INCB 54828-201, ClinicalTrials.gov identifier: NCT02872714] is an ongoing phase II study evaluating the efficacy and safety of pemigatinib in participants with metastatic or surgically unresectable urothelial carcinoma failing at least one therapy or platinum ineligible and harboring FGF/FGFR alterations. Interim results showed that the drug was well-tolerated and preliminary data of efficacy have been presented. In cohort A (patients with FGFR3 mutations/fusions) overall response rate (ORR) was 25%.25
FIGHT-202 [INCB 54828-202, ClinicalTrials.gov identifier: NCT029243769] was a phase II study that aimed to test efficacy and safety of pemigatinib in participants with advanced or metastatic or surgically unresectable cholangiocarcinoma who failed at least one previous therapy. In cohort A, which included patients with FGFR2 fusions or rearrangements, ORR was 35.5% (95% CI 26.5−45.4%), with 3 complete responses and 32.7% of partial responses, and DCR was 82% (95% CI 74%−89%); median PFS and OS reported were respectively 6.9 (95% CI 6.2–9.6) and 21.1 (14.8 not reached; OS not mature at cutoff) months.26 On the basis of these results, FIGHT-302 trial [ClinicalTrials.gov identifier: NCT03656536] has been started. It is a phase III study comparing pemigatinib versus gemcitabine plus cisplatin chemotherapy in first-line treatment of patients with unresectable or metastatic cholangiocarcinoma with FGFR2 rearrangement.
FiGhTeR trial aims to assess safety and activity of the FGFR inhibitor pemigatinib in HER2 trastuzumab-resistant GC patients. Achieving the primary endpoint of 12-week PFS would lay the basis for a practice changing GC treatment strategy.
One major open area for discussion is the lack of patients’ selection based on FGFR3 genetic alterations in tumor or liquid biopsy at baseline. While several other studies with pemigatinib have among the inclusion criteria the detection of FGFR alterations in the tumor tissue, we decided not to include this criterion in our study. In our preclinical study, indeed, FGFR3 overexpression in GC models selected for resistance to trastuzumab was not due to a gene amplification or rearrangement. Thus, selecting patients using a genomic profiling assay could exclude patients that may benefit from this therapeutic strategy. Moreover, recent studies demonstrated that FGFR3 genetic alterations such as mutations or copy-number variation could be identified only in 6–7% of GC patients at baseline. Circulating tumor DNA profiling in pre- versus post-progression plasma samples from patients treated with trastuzumab demonstrated no difference in FGFR3 alterations rates.27 In this regard, we hypothesize that the overexpression of FGFR3 and its ligand FGF9 could be a dynamic compensatory mechanism independent from preexistent of acquired genetic alterations. Thus, patients are enrolled in the FiGhTeR trial without any genetic selection. Patients will be adequately informed at the screening phase about the lack of evidence of a certain FGFR3 expression in all trastuzumab resistant patients. Translational research analyses on plasma and tissue specimens collected in this study will be used to identify novel molecular mechanisms responsible for trastuzumab resistance and predictive biomarkers of response to pemigatinib in GC patients.28 Overall, we believe that this study could offer an additional therapeutic option to trastuzumab resistant GC patients, for whom no many other effective therapies are actually available.
Supplemental Material
Supplemental material, Fig_S1 for A phase II trial of the FGFR inhibitor pemigatinib in patients with metastatic esophageal–gastric junction/gastric cancer trastuzumab resistant: the FiGhTeR trial by Valeria Merz, Camilla Zecchetto, Francesca Simionato, Alessandro Cavaliere, Simona Casalino, Michele Pavarana, Simone Giacopuzzi, Maria Bencivenga, Anna Tomezzoli, Raffaela Santoro, Vita Fedele, Serena Contarelli, Irene Rossi, Serena Giacomazzi, Martina Pasquato, Cristiana Piazzola, Stefano Milleri, Giovanni de Manzoni and Davide Melisi in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: DM is the principal investigator for this study. He ideated, initiated, hold the intellectual property of the project, and was a major contributor in writing the protocol. VM, CZ, and FS contributed to the study design and writing the protocol. VM, CZ, FS, AC, SC, MP, SG, MB, AT, SG, MP, CP, and GdM contributed to conducting the study. IR and SM were involved in protocol writing and approval. RS, VF, and SC contributed to conducting the translational studies. VM, CZ, FS, AC, SC, MP, SG, MB, AT, RS, VF, SC, IR, SG, MP, CP, SM, GdM, and DM read and approved the final manuscript.
Availability of data and materials: The datasets generated and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Conflict of interest statement: DM declares research funding from Shire, Incyte, Evotec, Celgene, and iOnctura and consulting roles with Eli Lilly, Shire, Evotec, Baxter, Incyte, iOnctura. The remaining authors have no conflicts of interest to declare.
Consent for publication: All authors have approved the submission of this manuscript for publication. No restriction of future publication of data is made by any of the study partners.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study has been provided by an unrestricted research grant by Incyte. Incyte did not have any role in the design of the study and will not have any role in collection, analysis, and interpretation of data and in writing the manuscript. Work in the DM unit is supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC; Investigator Grant number 19111) and 5 per mille (grant number 12182).
ORCID iD: Davide Melisi  https://orcid.org/0000-0002-4031-7585
https://orcid.org/0000-0002-4031-7585
Supplemental material: Supplemental material for this article is available online.
Trial registration: FiGhTeR is registered at EudraCT 2017-004522-14 (7 November 2017).
Trial sponsor: This is an investigator-initiated, non-profit trial supported by an unrestricted grant by Incyte.
Contributor Information
Valeria Merz, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Camilla Zecchetto, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Francesca Simionato, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Alessandro Cavaliere, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Simona Casalino, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Michele Pavarana, Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Simone Giacopuzzi, Esophageal and Gastric Surgery Unit, Department of Surgery, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Maria Bencivenga, Esophageal and Gastric Surgery Unit, Department of Surgery, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Anna Tomezzoli, Anatomical Pathology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Raffaela Santoro, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy.
Vita Fedele, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy.
Serena Contarelli, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, Università degli studi di Verona, Verona, Italy.
Irene Rossi, Centro Ricerche Cliniche di Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Serena Giacomazzi, Centro Ricerche Cliniche di Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Martina Pasquato, Centro Ricerche Cliniche di Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Cristiana Piazzola, Centro Ricerche Cliniche di Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Stefano Milleri, Centro Ricerche Cliniche di Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Giovanni de Manzoni, Esophageal and Gastric Surgery Unit, Department of Surgery, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Davide Melisi, Digestive Molecular Clinical Oncology unit, Section of Medical Oncology, Department of Medicine, University of Verona, AOUI Verona - Policlinico “G.B. Rossi”, Piazzale L.A. Scuro,10, Verona, 37134, Italy; Medical Oncology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017; 8: CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986; 232: 1644–1646. [DOI] [PubMed] [Google Scholar]
- 4. Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984; 312: 513–516. [DOI] [PubMed] [Google Scholar]
- 5. Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol 2015; 28: 1481–1491. [DOI] [PubMed] [Google Scholar]
- 6. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 7. Piro G, Carbone C, Santoro R, et al. Predictive biomarkers for the treatment of resectable esophageal and esophago-gastric junction adenocarcinoma: from hypothesis generation to clinical validation. Expert Rev Mol Diagn 2018; 18: 357–370. [DOI] [PubMed] [Google Scholar]
- 8. Cavaliere A, Merz V, Zecchetto C, et al. Novel biomarkers for prediction of response to preoperative systemic therapies in gastric cancer. J Gastric Cancer 2019; 19: 375–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pietrantonio F, Caporale M, Morano F, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int J Cancer 2016; 139: 2859–2864. [DOI] [PubMed] [Google Scholar]
- 10. Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018; 8: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Touat M, Ileana E, Postel-Vinay S, et al. Targeting FGFR signaling in cancer. Clin Cancer Res 2015; 21: 2684–2694. [DOI] [PubMed] [Google Scholar]
- 12. Hecht D, Zimmerman N, Bedford M, et al. Identification of fibroblast growth factor 9 (FGF9) as a high affinity, heparin dependent ligand for FGF receptors 3 and 2 but not for FGF receptors 1 and 4. Growth Factors 1995; 12: 223–233. [DOI] [PubMed] [Google Scholar]
- 13. Piro G, Carbone C, Cataldo I, et al. An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin Cancer Res 2016; 22: 6164–6175. [DOI] [PubMed] [Google Scholar]
- 14. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; 355: i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013; 158: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saleh M, Gutierrez ME, Subbiah V, et al. Preliminary results from a phase 1/2 study of INCB054828, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced malignancies. Mol Cancer Ther 2018; 17(Suppl. 1): Abstract A098. [Google Scholar]
- 17. Fornaro L, Fanotto V, Musettini G, et al. Selecting patients for gastrectomy in metastatic esophago-gastric cancer: clinics and pathology are not enough. Future Oncol 2017; 13: 2265–2275. [DOI] [PubMed] [Google Scholar]
- 18. Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer 2017; 20: 825–833. [DOI] [PubMed] [Google Scholar]
- 19. Fanotto V, Fornaro L, Bordonaro R, et al. Second-line treatment efficacy and toxicity in older vs. non-older patients with advanced gastric cancer: a multicentre real-world study. J Geriatr Oncol. Epub ahead of print 11 December 2018. DOI: 10.1016/j.jgo.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 20. Fanotto V, Uccello M, Pecora I, et al. Outcomes of advanced gastric cancer patients treated with at least three lines of systemic chemotherapy. Oncologist 2018; 23: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveras-Ferraros C, Cufí S, Queralt B, et al. Cross-suppression of EGFR ligands amphiregulin and epiregulin and de-repression of FGFR3 signalling contribute to cetuximab resistance in wild-type KRAS tumour cells. Br J Cancer 2012; 106: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azuma K, Tsurutani J, Sakai K, et al. Switching addictions between HER2 and FGFR2 in HER2-positive breast tumor cells: FGFR2 as a potential target for salvage after lapatinib failure. Biochem Biophys Res Commun 2011; 407: 219–224. [DOI] [PubMed] [Google Scholar]
- 23. Hanker AB, Garrett JT, Estrada MV, et al. HER2-overexpressing breast cancers amplify FGFR signaling upon acquisition of resistance to dual therapeutic blockade of HER2. Clin Cancer Res 2017; 23: 4323–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catenacci DVT, Rasco D, Lee J, et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J Clin Oncol 2020: JCO1901834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Necchi A, Pouessel D, Leibowitz-Amit R, et al. Interim results of fight-201, a phase 2, open-label, multicenter study of INCB054828 in patients (pts) with metastatic or surgically unresectable urothelial carcinoma (UC) harboring fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations (GA). Ann Oncol 2018; 29(Suppl. 8): viii303–viii331. [Google Scholar]
- 26. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 2020; 21: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang DS, Liu ZX, Lu YX, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2019; 68: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 28. Melisi D, Piro G, Tamburrino A, et al. Rationale and clinical use of multitargeting anticancer agents. Curr Opin Pharmacol 2013; 13: 536–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Fig_S1 for A phase II trial of the FGFR inhibitor pemigatinib in patients with metastatic esophageal–gastric junction/gastric cancer trastuzumab resistant: the FiGhTeR trial by Valeria Merz, Camilla Zecchetto, Francesca Simionato, Alessandro Cavaliere, Simona Casalino, Michele Pavarana, Simone Giacopuzzi, Maria Bencivenga, Anna Tomezzoli, Raffaela Santoro, Vita Fedele, Serena Contarelli, Irene Rossi, Serena Giacomazzi, Martina Pasquato, Cristiana Piazzola, Stefano Milleri, Giovanni de Manzoni and Davide Melisi in Therapeutic Advances in Medical Oncology