Abstract
Objective
Cognitive impairment is a common feature of Parkinson disease (PD), for which age is a major contributing factor. Insulin‐like growth factor‐1 (IGF‐1) declines with age and contributes to age‐related cognitive impairment in PD. Cyclic glycine‐proline (cGP) is a metabolite of IGF‐1 and normalizes bioavailable IGF‐1. Plasma cGP/IGF‐1 molar ratio that represents bioactive IGF‐1 in circulation, may associate with the cognitive status in PD.
Methods
We examined the association of plasma cGP/IGF‐1 molar ratio with the cognitive scores or age in PD patients with normal cognition (PD‐N, n = 74), mild cognitive impairment (PD‐MCI, n = 71), or dementia (PD‐D, n = 33), and with the cognitive scores in 23 age‐matched healthy controls. Plasma concentrations of IGF‐1, IGF binding protein‐3, and cGP were evaluated using enzyme‐linked immunosorbent assay (ELISA) and high‐performance liquid chromatography‐mass spectrometry (HPLC‐MS), respectively.
Results
The cGP/IGF‐1 molar ratio was positively correlated with the age of PD‐N group, negatively correlated with the age of PD‐D group, and not associated with the age of PD‐MCI group. Independent of age, the cGP/IGF‐1 molar ratio was positively correlated with the cognitive scores of healthy controls, but not in PD groups.
Conclusion
Old healthy people with a higher cGP/IGF‐1 molar ratio showed better preserved cognition, possibly due to improved IGF‐1 function. Increased cGP/IGF‐1 molar ratio with age may contribute to cognitive retention in the PD‐N group. The absence or reversal of such association with age in the PD‐MCI and PD‐D groups may indicate the conversion of cognitive status in PD, if confirmed through longitudinal investigations within the individuals with advancing cognitive impairment.
Keywords: cGP/IGF‐1 molar ratio, plasma biomarker, Parkinson disease, and cognitive status
1. BACKGROUND
Vascular health is critical for maintaining normal cognition in humans. 1 , 2 Functional plasma insulin‐like growth factor‐1 (IGF‐1) plays an essential role in vascular remodeling of the adult brain 3 and supports the retention of normal cognition. 4 Plasma IGF‐1 declines with age, 5 which is a major contributing factor to cognitive impairment in old people those are 60 and older. 4 , 5 , 6 , 7 Given that Parkinson disease (PD) is an age‐related neurological condition, the decline of IGF‐1 with age might contribute to cognitive decline in PD. 5 , 8 , 9 , 10 Compared with the normal aging population, patients with PD have a higher risk of developing cognitive impairment, 11 and those with mild cognitive impairment (PD‐MCI) have a higher risk of developing dementia (PD‐D) than PD patients with normal cognition (PD‐N). 12 , 13 Poor cognition in patients with PD is a recognized primary problem affecting patient and carer well‐being. 14 , 15 A reliable plasma biomarker for predicting and tracking the changes in cognitive status may assist in identifying patients at greater risk of developing dementia and the window of opportunity for suitable intervention.
Plasma concentration of IGF‐1 has been under clinical evaluation as a potential biomarker for IGF‐1 function and its association with cognitive status of PD. 8 , 16 However, the findings have been mixed and difficult to interpret 8 , 16 because the majority of plasma IGF‐1 is not bioavailable. The majority of plasma IGF‐1 is bound to IGF‐binding proteins (IGFBP)‐3. This high‐affinity binding prevents IGF‐1 from activating its functional receptors. 17 Alternatively, the ratio of IGF‐1/IGFBP‐3 has been evaluated as a marker of the amount of unbound bioactive IGF‐1. 17 Although the IGF‐1/IGFBP‐3 ratio may help to identify functional plasma IGF‐1, its does not have clear association with cognitive function. 18
Our recent clinical and experimental studies suggest that the relative concentration of cyclic glycine‐proline (cGP) to IGF‐1 (cGP/IGF‐1 molar ratio) represents the amount of bioavailable IGF‐1 in plasma and has been shown to be a reliable biomarker of functional IGF‐1 in circulation. 19 , 20 , 21 , 22 As a stable metabolite from a major binding site of IGF‐1, 23 cGP retains the binding affinity to IGFBP‐3 and effectively competes with IGF‐1 to bind to plasma IGFBP‐3, and it does so, in a concentration‐dependent manner. 19 A higher concentration of plasma cGP would occupy a greater proportion of IGFBP‐3 and increase the amount of free, bioavailable IGF‐1. 19 , 20 , 21 , 22 To determine the potential association between plasma cGP/IGF‐1 molar ratio and cognitive status, this cross‐sectional study examined plasma concentrations of IGF‐1, cGP, and IGFBP‐3, and their ratios in PD patients with normal cognitive function, MCI, and dementia.
RESEARCH IN CONTEXT
Systematic review: We reviewed (1) insulin‐like growth factor‐1 (IGF‐1) function in vascular health and its association with cognitive impairment and age; (2) biomarkers representing plasma IGF‐1 function and their link to cognitive status in Parkinson disease (PD); (3) cyclic glycine‐proline (cGP) function in memory and its role in regulating bioavailable IGF‐1 in plasma; and (4) the association of the cGP/IGF‐1 molar ratio with stroke recovery, obesity, and hypertension.
Interpretation: The changes in the cGP/IGF‐1 molar ratio with age may assist in predicting cognitive status, and the risk and conversions of mild cognitive impairment (MCI) to dementia in PD.
Future directions: This article proposes future studies: (1) confirmation of the age‐related changes in cGP/IGF‐1 molar ratio and its association with cognitive status through longitudinal studies; (2) evaluation of improved vascular remodelling mediates cGP effect on cognitive function in a clinical setting; 3) evaluation of improved cerebral circulation may aid the reduction of the pathology of dementia.
2. METHODS
2.1. Study population and design
The study was approved by the Southern Health and Disability Ethics Committee (URB‐09‐08‐037). A convenience sample of patients with PD (n = 204) was recruited through a movement disorders clinic (Figure 1). Healthy control participants (n = 24) were recruited from the local community. Twenty‐six PD patients were excluded from the study due to incomplete cognitive assessments (n = 24) and the subsequent diagnosis of atypical symptoms (n = 2). One control participant was excluded due to an MCI classification. Plasma samples and neurological and cognitive assessments were available for 23 control participants, 74 PD with normal cognitive (PD‐N), 71 PD with mild cognitive impairment (PD‐MCI), and 33 PD with dementia (PD‐D, Figure 1). All participants took their usual medications on the day of testing to allow for optimal performance. All testing was performed in the morning.
FIGURE 1.
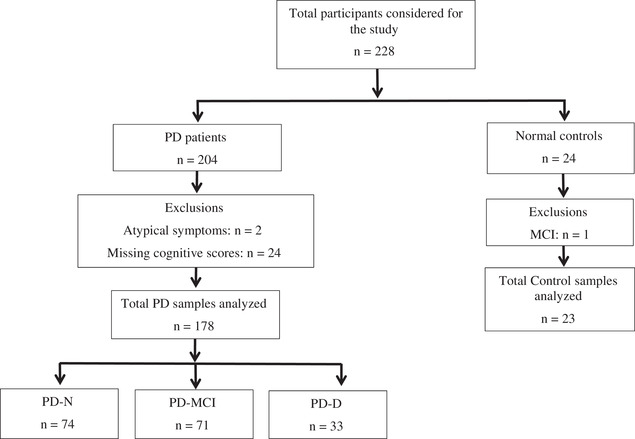
Shows the study population
2.2. Inclusion criteria
Patients with PD included in the study were between age 50 and 90 years of age and meeting UK Brain Bank criteria 24 for idiopathic PD as confirmed by a movement disorders neurologist (T.J.A.). Patients with cognitive impairment exhibited motor features of PD for at least 12 months before the onset of cognitive impairment.
2.3. Exclusion criteria
Participants were excluded if there was a history of central nervous system (CNS) disorders other than PD (eg, stroke or head injury), any other explanation for parkinsonism or cognitive impairment, major medical illness, major depression in the last 6 months, and other major psychiatric disorders, dementia with Lewy bodies, and inability to speak and read English.
2.4. Assessments of cognitive status
Cognitive status was determined using a battery of assessments meeting the requirements of the movement disorder society‐task force (MDS‐TF) Level II criteria. 24 , 25 Five cognitive domains were examined, with the tests conducted over two sessions. 26 , 27 Executive function was assessed using Stroop interference, letter fluency, category fluency, and category switching (from the Delis‐Kaplan Executive Function System 45), action fluency, and Trails B. Attention, working memory, and processing speed were evaluated using digits forward/backward, digit ordering, map search task (from the Test of Everyday Attention), Stroop colour reading, Stroop word reading, and Trails A. Memory was measured with the California Verbal Language Test‐II Short Form (acquisition, short and long delays), and the Rey Complex Figure Test (short and long delays); impairment in either or both delay components of each memory test counted as one impairment. Visuoperceptual/visuospatial performance was determined using the judgment of line orientation, fragmented letters test, the picture completion test, and the Rey Complex Figure Test‐Copy. Language was assessed using the Boston Naming Test, Dementia Rating Scale‐2 similarities sub‐test, and the language component of the Alzheimer's Dementia Assessment Cognitive Scale (object and finger naming, commands, comprehension, spoken language, and word‐finding difficulties). Scoring of the neuropsychological tests was conducted using age‐ and education‐adjusted normative data. Participants also completed the Montreal Cognitive Assessment (MoCA). Global cognitive ability is expressed as an aggregate score (Global Z score) derived from averaging performance from tests conducted in four cognitive domains.
Classification of PD‐MCI required two deficits of 1.5 standard deviations (SD) or more below age‐ and education‐adjusted norms within one cognitive domain, but with the maintenance of everyday functioning. 26 , 27 A PD‐D classification required cognitive impairments of 2 SD below normative data in each of two measures within one or more cognitive domains as well as impairments in everyday functioning not attributed to motor deficits. Everyday functioning was assessed through interviews with a significant other using the Clinical Dementia Rating, the Global Deterioration Scale, and the Reisberg Instrumental Activities of Daily Living‐Scale. 26 , 27 , 28 The motor function of the PD patients was assessed using the part III of the MDS‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS III) and Hoehn and Yahr staging (H&Y).
2.5. Plasma sample collection and preparation
Non‐fasting venous blood was collected in the morning using lithium heparin tubes (Becton Dickinson) and gently inverted as per manufacture directions. Filled tubes were placed on wet ice and immediately transferred to the processing laboratory. The samples were spun in a cooled centrifuge (4°C) for 10 minutes at 2000g and plasma stored as 250 µL aliquots at −80ۤ°C until assayed.
2.5.1. Plasma extraction of cGP
The methodology of extraction of cGP from plasma has been described previously. 20 , 21 The cGP‐1,5,6,7,8‐13C, 4‐15N (cGP‐4x13C, 1 × 15N) provided an internal standard for the cGP assay. Briefly, cGP‐4x13C, 1 × 15 N (50 µL of 500 ng/mL) was mixed with 100 µL of plasma, and then transferred to a Phree phospholipid removal cartridge (Phenomenex, Auckland, New Zealand). Five hundred milliliters of 1% formic acid in acetonitrile was added to the cartridge and centrifuged at 284g for 5 minutes at 4°C. The filtrate was dried using a vacuum concentrator. The dried samples were reconstituted in 100 µL 10% methanol/water (v/v) and transferred to an ultrapressure liquid chromatography vial for quantitation, and then centrifuged at 142g for 4 minutes at 4°C to sediment any remaining particulates. Standards were prepared by spiking cGP into charcoal‐stripped human plasma. Quality control samples, with cGP at two different concentrations (Bachem, G‐1720), were utilized and then subjected to the same extraction procedure as the samples.
2.5.2. High‐performance liquid chromatography mass spectrometry (HPLC‐MS)
The chromatography conditions consisted of a Synergy Hydro 2.5 µm column (Phenomenex) 100 × 2 mm with an initial mobile phase composition of 10% methanol/90% water at a flow rate of 200 µL/minute and a column temperature of 35°C, as described previously. 20 , 21 The mass spectrometry conditions consisted of electrospray ionization in positive mode with a voltage of 4000 V, a sheath gas flow of 30 psi, an auxiliary gas flow of 2 psi, and a capillary temperature of 250°C. Fragmentation was achieved with argon at 1.2 mTorr as the collision gas and a dissociation voltage of 35 V. The mass spectrometer ran in selective reaction monitoring (SRM) mode with the following two transitions 155.1 → 70.2 m/z and 161 → 75.1 m/z utilized for cGP and cGP‐4x13C, 1 × 15 N, respectively. The retention time for both peaks was 3.6 minutes. Unknown samples were quantitated using the peak area ratio of cGP/cGP‐4x13C, 1 × 15 N compared with the standard curve of known concentrations.
2.5.3. Measurement of plasma concentrations of IGF‐1 and IGFBP‐3 using ELISA
Plasma concentration of IGF‐1 and IGFBP‐3 were measured using commercial enzyme‐linked immunosorbent assay (ELISA) kits (Crystal Chem, Chicago, IL) according to the manufacturer's instructions. 20 , 21
2.6. Statistical analysis
SPSS (IBM SPSS Statistics 24, Chicago, IL) was used for statistical analysis. The differences between the control and PD‐N groups were analyzed using t test or Mann‐Whitney U test. The differences between PD groups were analyzed using one‐way analysis of variance (ANOVA) with Tukey post hoc tests or Kruskal‐Wallis test with Dunn‐Bonferroni post hoc test. The chi‐square test was used to compare gender differences. The associations between biological measures and cognitive scores were analyzed in control and PD groups using multiple linear regression analysis after being adjusted for motor scores and age. The association between biological measures with age were analyzed in the controls and PD groups using multiple linear regression after being adjusted for motor and/or cognitive scores. As a secondary analysis, the association of biological measures with age was compared between the PD groups using multiple linear regression analysis. The significance level was set at P < 0.05. Data are presented as mean ± standard error of the mean (SEM) unless otherwise stated.
3. RESULTS
Table 1 shows the demographic, clinical, and biological differences between the control and PD‐N groups and within the PD groups. There was no difference in the age between the controls and combined PD groups, although the PD‐N group was younger than the control (P < 0.05), PD‐MCI (P < 0.05), and PD‐D (P < 0.05) groups. The function of cGP in improving learning and memory has been documented previously in rats. 22 , 29 , 30 Thus we compared the scores of learning and memory domain (LMD), MoCA, and Global Z between the control and PD groups. The MoCA (P = 0.01), Global Z (P < 0.001), and LMD scores (P = 0.001) were lower in PD‐N compared to controls, and as expected, these scores were further reduced in PD‐MCI (P < 0.05) and PD‐D groups (P < 0.05) compared to PD‐N and PD‐MCI groups, respectively. Plasma IGF‐1 was significantly lower in the PD‐D group compared to the PD‐N and PD‐MCI groups (P < 0.05). IGF‐1/IGFBP‐3 ratio was higher in the PD‐N group than the control group (P < 0.05). Biological measures remained the same when the analysis being adjusted for age.
Table 1.
Demographic, clinical, and biological data of control participants and patients with PD
| Controls (n = 23) | PD‐N (n = 74) | PD‐MCI (n = 71) | PD‐D (n = 33) | P‐value Control versus PD‐N | P‐value within PD groups | |
|---|---|---|---|---|---|---|
| Age (years) | 74.53 (1.37) | 70.91 (0.82) | 72.89 (0.76) | 76.17 * , † (0.93) | 0.03 | 0.001 |
| Female/male | 8/15 | 28/46 | 19/52 | 5/28 | ns | 0.049 |
| Education (years) Median (range) | 13 (10‐18) | 13 (9‐20) | 11 *(8‐19) | 11 (9‐20) | ns | 0.005 |
| Years since symptom duration | 11.56 (0.61) | 11.60 (0.57) | 11.40 (1.19) | ns | ||
| Years since diagnosis | 8.96 (0.53) | 10.04 (0.56) | 10.07 (1.17) | ns | ||
| LED Median (range) | 845.37 (0‐3893) | 914.38 (0‐3162) | 798.00 (0‐3140) | ns | ||
| BMI | 25.85 (0.89) | 26.30 (0.52) | 26.35 (0.63) | 25.35 (0.50) | ns | ns |
| MoCA | 27.57 (0.32) | 26.51 (0.27) | 22.53 * (0.34) | 16.85 * , † (0.86) | 0.01 | <0.001 |
| Global Z score | 0.87 (0.08) | 0.33 (0.06) | −0.85 * (0.06) | −1.79 * , † (0.14) | <0.001 | <0.001 |
| LMD score | 1.43 (0.19) | 0.67 (0.10) | −0.72 * (0.10) | −1.80 * , † (0.20) | 0.001 | <0.001 |
| H&Y Median (range) | 2.50 (1‐3) | 2.50 ** (1‐5) | 3.00 *** (2‐5) | <0.001 | ||
| MDS‐UPDRS III | 35.20 (1.68) | 43.73 * (1.84) | 48.95 * (2.95) | <0.001 | ||
| IGF‐1 (ng/mL) | 146.27 (10.41) | 159.50 (6.03) | 160.30 (5.95) | 135.20 * , † (8.15) | ns | 0.03 |
| IGFBP‐3 (ng/mL) | 3332.92 (154.22) | 3128.31 (74.55) | 3166.86 (85.41) | 2865.40 (122.71) | ns | ns |
| cGP (ng/mL) | 7.44 (1.69) | 7.41 (0.54) | 7.56 (0.61) | 6.24 (0.74) | ns | Ns |
| cGP/IGF‐1 molar ratio | 2.32 (0.61) | 2.07 (0.17) | 2.11 (0.20) | 2.10 (0.31) | ns | Ns |
| IGF‐1/IGFBP‐3 molar ratio | 0.18 (0.009) | 0.21 (0.007) | 0.21 (0.007) | 0.19 (0.008) | 0.03 | ns |
PD‐N, Parkinson disease with normal cognitive function; PD‐MCI, Parkinson disease with mild cognitive impairment; PD‐D, Parkinson disease with dementia; LED, levodopa equivalent dose; BMI, body mass index; H&Y, Hoehn and Yahr score; MDS‐UPDRS III, Movement Disorder Society—Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment; LMD, Learning Memory Domain scores; Global Z score, global measure of cognitive function derived from comprehensive neuropsychological testing.
Parametric data were analyzed using t test and one‐way ANOVA with Tukey post hoc tests for comparison between controls and PD‐N and within PD groups, respectively, and presented as mean (SEM). Non‐parametric data were analyzed using Mann‐Whitney U test and Kruskal‐Wallis test with Dunn‐Bonferroni post hoc test accordingly and presented as median (range). The gender effects were analyzed using chi‐square test.
* P < 0.05, ** P < 0.01, *** P < 0.001 indicating the differences from PD‐N group;
† P < 0.05, †† P < 0.01 indicating the difference from PD‐MCI.
Table 2 presents the association of biological measures with cognitive scores (MoCA, Global Z, and LMD) in both healthy control and PD groups after being adjusted for age and/or MDS‐UPDRS III scores. The results showed that the cGP concentration was positively associated with MoCA (B = 0.1, P = 0.006), Global Z (B = 0.02, P = 0.009), and LMD scores (B = 0.05, P = 0.01) of the normal control group; as was cGP/IGF‐1 molar ratio (MoCA: B = 0.25, P = 0.016; Global Z: B = 0.07, P = 0.02; and LMD: B = 0.16, P = 0.01). The IGF‐1 and IGFBP‐3 concentrations were not associated with the cognitive scores of control group. The analysis did not show any relationship between biological measures and cognitive scores in PD groups after being adjusted for age and/or MDS‐UPDRS III scores. The data suggest that the healthy participants with higher plasma cGP and cGP/IGF‐1 molar ratio have better preserved cognitive function. Such association was absent in PD.
Table 2.
Association between biological measures and cognitive scores after being adjusted for age and/or motor scores in the control and PD groups
| Groups | Dependent variable (independent variable) | IGF‐1 (ng/mL) | cGP (ng/mL) | cGP/IGF‐1 molar ratio | IGFBP‐3 (ng/mL) | IGF‐1/IGFBP‐3 molar ratio | |
|---|---|---|---|---|---|---|---|
| Control | MoCA (Age) n = 23 | B | 0.005 | 0.10 | 0.25 | 0.001 | 12.09 |
| P | 0.42 | 0.006 ** | 0.01 * | 0.35 | 0.07 | ||
| Global Z score (Age) n = 23 | B | 0.000 | 0.02 | 0.07 | 0.000 | 1.86 | |
| P | 0.99 | 0.009 ** | 0.02 * | 0.27 | 0.35 | ||
| LMD (Age) n = 23 | B | −0.002 | 0.05 | 0.16 | 0.000 | 3.04 | |
| P | 0.59 | 0.01 * | 0.01 * | 0.08 | 0.47 | ||
| PD‐N | MoCA (Age and MDS‐UPDRS III) n = 70 | B | −0.007 | −0.04 | −0.16 | 0.001 | −8.57 |
| P | 0.24 | 0.52 | 0.44 | 0.22 | 0.07 | ||
| Global Z score (Age and MDS‐UPDRS III) n = 67 | B | −0.001 | −0.01 | −0.03 | 0.00 | 0.02 | |
| P | 0.70 | 0.32 | 0.46 | 0.70 | 0.98 | ||
| LMD (Age and MDS‐UPDRS III) n = 67 | B | 0.00 | −0.02 | −0.11 | 0.00 | 1.55 | |
| P | 0.65 | 0.35 | 0.18 | 0.52 | 0.43 | ||
| PD‐MCI | MoCA (Age and MDS‐UPDRS III) n = 63 | B | 0.00 | −0.11 | −0.23 | 0.00 | −3.30 |
| P | 0.99 | 0.14 | 0.40 | 0.78 | 0.63 | ||
| Global Z score (Age and MDS‐UPDRS III) n = 61 | B | 0.00 | −0.003 | −0.001 | 0.00 | 0.01 | |
| P | 0.71 | 0.82 | 0.98 | 0.36 | 0.99 | ||
| LMD (Age and MDS‐UPDRS III) n = 61 | B | 0.00 | 0.02 | 0.05 | 0.00 | 1.71 | |
| P | 0.81 | 0.29 | 0.36 | 0.33 | 0.38 | ||
| PD‐D | MoCA (Age and MDS‐UPDRS III) n = 22 | B | 0.008 | 0.44 | 0.12 | 0.001 | −0.09 |
| P | 0.71 | 0.15 | 0.59 | 0.29 | 0.63 |
PD‐N, Parkinson disease with normal cognitive function; PD‐MCI, Parkinson disease with mild cognitive impairment; PD‐D, Parkinson disease with dementia; MDS‐UPDRS III, Movement Disorder Society—Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment; LMD, Learning Memory Domain scores; Global Z score, global measure of cognitive function derived from comprehensive neuropsychological testing; B, unstandardized co‐efficiency.
* P < 0.05.
** P < 0.01.
Table 3 shows the association of biological measures with age after the adjustment for motor (MDS‐UPDRS III) and/or cognitive scores (MoCA, Global Z, and LMD) in the control and PD groups. The cGP concentration (B = 0.39, P = 0.02) and cGP/IGF‐1 molar ratio (B = 0.96, P = 0.04) were positively associated with the age of the control group after being adjusted for MoCA, but not for Global Z score and LMD scores. Plasma IGF‐1 and IGFBP‐3 concentrations were not associated with the age of control group after being adjusted for the cognitive scores.
Table 3.
Association between the biological measures and age after being adjusted for cognitive and/or motor scores in the control and PD groups
| Groups | Dependent variable (Independent variable) | IGF‐1 (ng/mL) | cGP (ng/mL) | cGP/IGF‐1 molar ratio | IGFBP‐3 (ng/mL) | IGF‐1/IGFBP‐3 molar ratio | |
|---|---|---|---|---|---|---|---|
| Control | Age (MoCA) n = 23 | B | −0.01 | 0.39 | 0.96 | −0.003 | 22.83 |
| P | 0.53 | 0.02 * | 0.04 * | 0.12 | 0.46 | ||
| Age (Global Z score) n = 23 | B | −0.02 | 0.16 | 0.38 | −0.002 | −4.61 | |
| P | 0.35 | 0.43 | 0.50 | 0.27 | 0.88 | ||
| Age (LMD) n = 23 | B | −0.02 | 0.12 | 0.27 | −0.002 | −4.49 | |
| P | 0.42 | 0.54 | 0.63 | 0.37 | 0.88 | ||
| PD‐N | Age (MoCA & MDS‐UPDRS III) n = 70 | B | −0.03 | 0.29 | 1.57 | −0.001 | −26.47 |
| P | 0.01 * | 0.09 | 0.004 ** | 0.55 | 0.052 | ||
| Age (Global Z score & MDS‐UPDRS III) n = 67 | B | −0.03 | 0.36 | 1.77 | −0.002 | −19.57 | |
| P | 0.02 * | 0.05 | 0.002 ** | 0.25 | 0.17 | ||
| Age (LMD & MDS‐UPDRS III) n = 67 | B | −0.03 | 0.37 | 1.84 | −0.002 | −21.00 | |
| P | 0.02 * | 0.04 * | 0.001 ** | 0.21 | 0.13 | ||
| PD‐MCI | Age (MoCA & MDS‐UPDRS III) n = 63 | B | −0.02 | 0.28 | 1.13 | −0.001 | −15.85 |
| P | 0.17 | 0.12 | 0.06 | 0.36 | 0.32 | ||
| Age (Global Z score & MDS‐UPDRS III) n = 61 | B | −0.02 | 0.21 | 0.56 | −0.001 | −11.79 | |
| P | 0.24 | 0.20 | 0.26 | 0.40 | 0.45 | ||
| Age (LMD & MDS‐UPDRS III) n = 61 | B | −0.02 | 0.18 | 0.50 | −0.001 | −13.94 | |
| P | 0.21 | 0.26 | 0.32 | 0.40 | 0.37 | ||
| PD‐D | Age (MoCA & MDS‐UPDRS III) n = 22 | B | 0.003 | −0.70 | −3.56 | 0.001 | −1.64 |
| P | 0.90 | 0.04 * | 0.003 ** | 0.65 | 0.95 |
PD‐N, Parkinson disease with normal cognitive function; PD‐MCI, Parkinson disease with mild cognitive impairment; PD‐D, Parkinson disease with dementia; MDS‐UPDRS III, Movement Disorder Society—Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment; LMD, learning memory domain scores; Global Z score, global measure of cognitive function derived from comprehensive neuropsychological testing; B, unstandardized co‐efficiency.
* P < 0.05; ** P < 0.01 indicate significant correlation of age and biological measures.
IGF‐1 concentration was negatively associated with the age of the PD‐N group after being adjusted for MDS‐UPDRS III and MoCA (B = −0.03, P = 0.02) or Global Z (B = −0.03, P = 0.01) or LMD (B = −0.03, P = 0.02) scores, respectively. cGP concentration was positively associated with the age of PD‐N group after being adjusted for MDS‐UPDRS III and Global Z (B = 0.36, P = 0.05) or LMD (B = 0.37, P = 0.04) scores, respectively. cGP/IGF‐1 molar ratio was positively associated with the age of PD‐N group after being adjusted for MDS‐UPDRS III and MoCA (B = 1.57, P = 0.004) or Global Z (B = 1.77, P = 0.002) or LMD (B = 1.84, P = 0.001) scores, respectively. There was no association between the biological measures and the age in the PD‐MCI group after being adjusted for motor and cognitive scores. In contrast, cGP (B = −0.70, P = 0.04) and cGP/IGF‐1 molar ratio (B = ‐3.56, P = 0.003) was negatively associated with the age of PD‐D group after being adjusted for MDS‐UPDRS III and MoCA scores. There were no Global Z and LMD data in PD‐D group. The data suggested that the changes of cGP/IGF‐1 molar ratio with age were different between PD‐N and PD with cognitive impairments.
The comparison between the PD groups with normal and impaired cognitive status showed that the age of the PD‐N group was positively associated with cGP concentration (B = 0.16, P = 0.04, Figure 2D) and cGP/IGF‐1 molar ratio (B = 0.09, P < 0.001, Figure 2G), whereas negatively associated with IGF‐1 concentration (B = −2.85, P < 0.001, Figure 2A) and IGF‐1/IGFBP‐3 molar ratio (B = −0.003, P < 0.01, Figure 2M). The age of PD‐MCI (Figure 2B, E, H, K, and N) and PD‐D groups (Figure 2 C, F, I, L, and O) was not associated with the biological measures. Compared to the PD‐N, the association slope of cGP/IGF‐1 molar ratio was reversed in the PD‐D group (B = −0.18, P < 0.001, Table 4), but not in PD‐MCI group. The data further support that the age effects on the biological measures were differently associated with the cognitive status of PD and that the cGP/IGF‐1 molar ratio increases with age in PD‐N, whereas it decreases in PD‐D.
FIGURE 2.
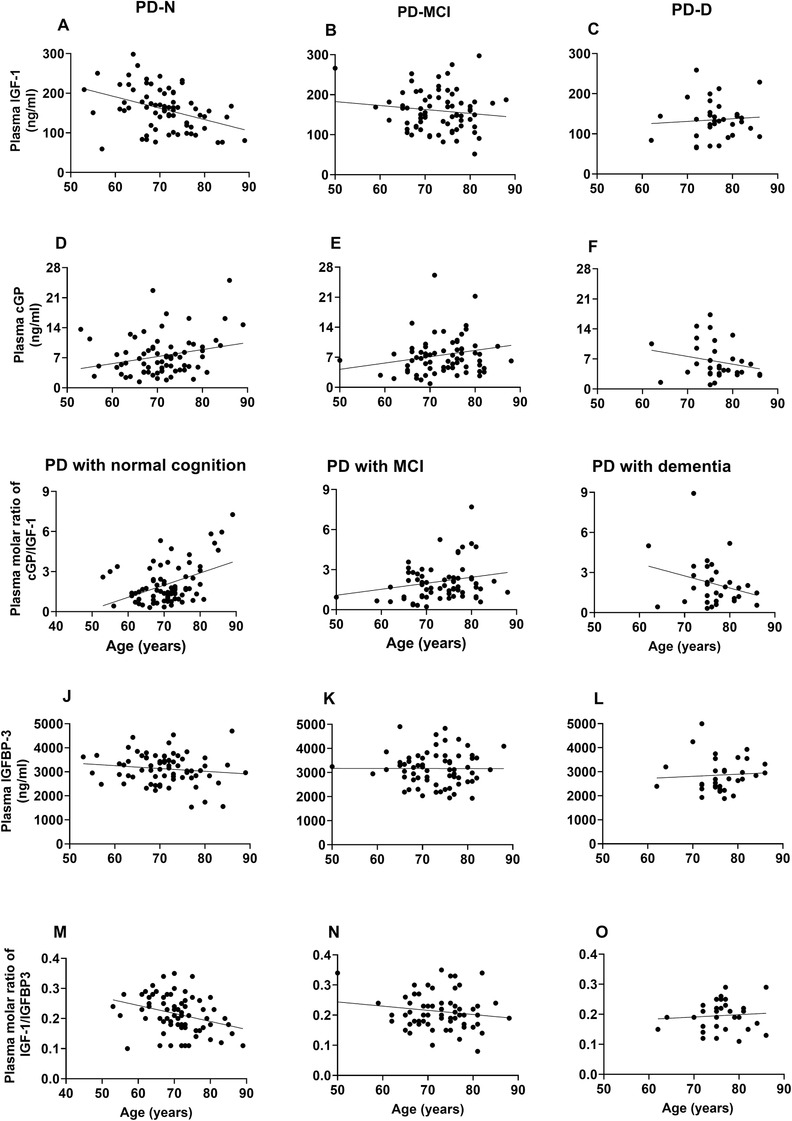
Compares the age effects on the concentrations of IGF‐1 (A‐C), cGP (D‐F), and IGFBP‐3 (J‐L); and cGP/IGF‐1 molar ratio (G‐I) and IGF‐1/IGFBP‐3 molar ratio (M‐O) between the PD‐N, PD‐MCI, and PD‐D groups. The IGF‐1 concentration and IGF‐1/IGFBP‐3 molar ratio were negatively associated with age, whereas the cGP concentration and cGP/IGF‐1 molar ratio were positively associated with age in the PD‐N group. Compared to the changes in the PD‐N group, the biological measures were differently associated with age in the PD‐MCI and PD‐D groups, with strong interactions between the PD groups
Table 4.
Changes of association slope between biological measures and age in PD groups with cognitive impairment
| PD‐N versus PD‐MCI | PD‐N versus PD‐D | |
|---|---|---|
| IGF‐1 (ng/mL) | B = 1.87 P = 0.12 | B = 3.45 P = 0.06 |
| cGP (ng/mL) | B = −0.02, P = 0.86 | B = −0.33 P = 0.06 |
| cGP/IGF‐1 molar ratio | B = −0.05 P = 0.23 | B = −0.18 P < 0.001 ** |
| IGFBP‐3 (ng/mL) | B = 11.36 P = 0.5 | B = 18.64 P = 0.45 |
| IGF‐1/IGFBP‐3 molar ratio | B = 0.001 P = 0.37 | B = 0.003 P = 0.10 |
PD‐N, Parkinson's disease with normal cognitive function; PD‐MCI, Parkinson's disease with mild cognitive impairment; PD‐D, Parkinson's disease with dementia; B, unstandardized co‐efficiency, PD‐N: n = 74; PD‐MCI: n = 71; and PD‐D: n = 33.
4. DISCUSSIONS
Our findings suggest that old healthy people with a higher cGP/IGF‐1 molar ratio have better preserved cognition independent of age, possibly due to more bioavailable IGF‐1 in circulation. The cGP/IGF‐1 molar ratio increased with age in the PD‐N. However, such an association with age was absent in the PD‐MCI and reversed to a decrease in the cGP/IGF‐1 molar ratio with age in the PD‐D. These age‐related variations in cGP/IGF‐1 molar ratio may assist in identifying cognitive status and the risk of advancing cognitive impairment in PD. This interpretation, if further confirmed by longitudinal studies, has the potential to be developed for clinical use as a prognostic biomarker for cognitive status of PD.
The changes of circulating IGF‐1 and IGFBP‐3 have been under clinical evaluations for their potential association with PD and their cognitive status with inclusive and inconsistent outcomes. 8 , 16 , 31 , 32 It is known that plasma IGF‐1 concentration decreases with age, 5 , 18 whereas it increases in PD. 31 , 32 As a neurodegenerative condition, PD may confound the age effect on plasma concentration of IGF‐1, and the subsequent changes in plasma cGP and IGFBP‐3. The collective effects of aging and PD led to a moderate decrease in IGF‐1 concentration of the PD‐D group, with cGP and IGFBP‐3 concentrations remaining similar between the groups. Multiple linear regression analysis after the adjustment for confounding factors revealed that these biological measures were associated with normal cognitive function in healthy controls independent of age (Table 2), whereas they were associated with age in the PD groups independent of cognitive and motor function (Table 3).
Better preserved cognitive function of old healthy people was associated with higher plasma concentration of cGP and/or cGP/IGF‐1 molar ratio, which was independent of age. The data support a role for cGP in maintaining cognitive function during brain aging. The effects of cGP in memory have been reported previously in rats. 22 , 29 , 30 For example, maternal administration of cGP improves learning and memory in young male rats. 22 The plasma concentration of IGF‐1 and molar ratio of IGF‐1/IGFBP‐3 have been under clinical evaluation for predicting the cognitive function of old people with inconsistent outcomes. 4 , 6 , 7 , 18 Our data did not suggest the relationship between cognitive scores and IGF‐1 concentration and IGF‐1/IGFBP‐3 molar ratio in normal controls (Table 3). Thus cGP concentration and cGP/IGF‐1 molar ratio may be more closely related to the cognitive function of healthy old people.
Such an association with cognitive function was absent in PD groups, possibly due to their lower cognitive scores and abnormal cognitive function. Although the cognitive function of PD‐N was “normal,” the group had significant lower scores when compared to the healthy control group. This may suggest an initial decline of cognition in the PD‐N due to accelerated brain aging in PD. Age‐related decline of IGF‐1 is a major contributor to cognitive impairment, including in PD. 4 , 5 , 6 , 7 , 8 , 16 Our results suggest that the biological measures of PD groups changed with age, which were dependent on the cognitive status (Tables 3 and 4, Figure 2).
Plasma IGF‐1 decreased with age of the PD‐N group. As an autocrine response to improve the amount of bioavailable IGF‐1 in plasma, 19 , 20 , 21 , 22 cGP correspondingly increased with age, leading to an age‐associated increase of cGP/IGF‐1 molar ratio in the PD‐N group (Table 3 and Figure 2G). The increase of the cGP/IGF‐1 molar ratio with age is a compensatory response to the age‐related decline of IGF‐1 function in circulation that may contribute to the preserved cognitive function in PD‐N patients.
This age‐related increase in cGP/IGF‐1 ratio in the PD‐N group was absent in the PD‐MCI group (Table 3, Figure 2H), whereas it was statistically reversed to a decrease with age in the PD‐D group (ie, lower ratio with greater age). This negative association was driven clearly by a progressive decline of cGP with age (Tables 3 and 4, Figure 2F). Low plasma concentration of cGP and/or the cGP/IGF‐1 molar ratio has been suggested to be associated with impairment of the autocrine regulation of IGF‐1 in hypertension 21 and stroke patients, 20 and may also contribute to cognitive impairment in PD. Indeed, the administration of cGP can prevent ischemic brain injury, 19 , 33 normalize blood pressures, 34 and improve memory in rats. 22 A longitudinal study shows that the cGP/IGF‐1 molar ratio increases while patients recover from stroke and that the cGP/IGF‐1 molar ratio at hospital admission (<3 days of stroke) predicts 3 months recovery and clinical outcome of stroke. 20 These age‐related observations in PD need to be validated through longitudinal studies in individuals with advancing cognitive impairments. If confirmed, the changes of cGP/IGF‐1 molar ratio with age may potentially be used as a biomarker for assisting the diagnosis and/or prognosis of cognitive risk and conversions of PD.
Poor vascular health, like hypertension, is a common risk factor contributing to cognitive impairment in PD and stroke patients. 35 , 36 , 37 The function of plasma IGF‐1 is essential for cerebral vascular remodeling of mature brains and is mediated through the blood face of cerebral vessels. 3 The administration of cGP protects neuronal and, more so, vascular injury from ischemia through promoting IGF‐1‐mediated vascular protection/remodeling in rats. 19 Given the nature of cerebral vascular degeneration/dysfunction in age‐related cognitive impairment, 38 the changes in the plasma cGP/IGF‐1 molar ratio may represent IGF‐1‐mediated cerebral vascular function/remodeling and its association with cognitive function. Given the role of cGP in memory, 22 we speculate that intervention with cGP may restore the cGP/IGF‐1 molar ratio and improve the cognitive function in PD patients with progressive decline of plasma cGP and/or cGP/IGF‐1 molar ratio.
The small number of control participants and PD‐D patients used for analysis is a limitation of the study. However, the publication of this hypothesis‐generating finding will promote research for confirming the hypothesis through larger and longitudinal studies.
5. CONCLUSION
An increase in plasma cGP/IGF‐1 molar ratio with age may contribute to the preserved cognitive function in the PD‐N group. In contrast, the decrease in the ratio with age may be associated with dementia in the PD‐D group. The static relationship between cGP/IGF‐1 molar ratio and age in the PD‐MCI group could be a transition phase before dementia. These observations raise the possibility that the association between cGP/IGF‐1 ratio and age may assist prediction of cognitive status and risk of advancing cognitive impairment in patients with PD if further confirmed in longitudinal research.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Dawei Fan: biological assays, statistical analysis, and manuscript preparation; Toni Pitcher: sample collection and management and manuscript revision; Michael MacAskill: data management and statistical analysis; John Dalrymple‐Alford: cohort PI and manuscript revision; Tim Anderson: cohort PI and manuscript revision; and Jian Guan: project PI, conception, and writing of the manuscript.
ACKNOWLEDGMENTS
We would like to acknowledge the Cancer Society Tissue Bank for providing the bio banking services, and Leslie Livingston, Sophie Grenfell, and Bob Young from New Zealand Brain Research Institute, New Zealand, for completing the clinical and cognitive assessments. The clinical investigation and blood collection was funded by Health Research Council New Zealand and Lottery Health New Zealand. The biological analysis was funded by Auckland Medical Research Foundation, New Zealand.
Fan D, Pitcher T, Dalrymple‐Alford J, MacAskill M, Anderson T, Guan J. Changes of plasma cGP/IGF‐1 molar ratio with age is associated with cognitive status of Parkinson disease. Alzheimer's Dement. 2020;12:e12025 10.1002/dad2.12025
REFERENCES
- 1. Lim SL, Gao Q, Nyunt MS, et al. Vascular health indices and cognitive domain function: Singapore Longitudinal Ageing Studies. J Alzheimers Dis. 2016;50:27‐40.. [DOI] [PubMed] [Google Scholar]
- 2. Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle‐aged and older adults. Neuropsychology. 2007;21:149‐157.. [DOI] [PubMed] [Google Scholar]
- 3. Lopez‐Lopez C, LeRoith D, Torres‐Aleman I. Insulin‐like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833‐9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aleman A, Torres‐Aleman I. Circulating insulin‐like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89:256‐265. [DOI] [PubMed] [Google Scholar]
- 5. Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres‐Aleman I. Reduced brain insulin‐like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9‐12. [DOI] [PubMed] [Google Scholar]
- 6. Okereke O, Kang JH, Ma J, Hankinson SE, Pollak MN, Grodstein F. Plasma IGF‐I levels and cognitive performance in older women. Neurobiol Aging. 2007;28:135‐142. [DOI] [PubMed] [Google Scholar]
- 7. Okereke OI, Kang JH, Ma J, Gaziano JM, Grodstein F. Midlife plasma insulin‐like growth factor I and cognitive function in older men. J Clin Endocrinol Metab. 2006;91:4306‐4312. [DOI] [PubMed] [Google Scholar]
- 8. Pellecchia MT, Santangelo G, Picillo M, et al. Insulin‐like growth factor‐1 predicts cognitive functions at 2‐year follow‐up in early, drug‐naive Parkinson's disease. Eur J Neurol. 2014;21:802‐807. [DOI] [PubMed] [Google Scholar]
- 9. Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin‐like growth factor‐1 (IGF‐1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195‐212. [DOI] [PubMed] [Google Scholar]
- 10. Dore S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF‐I, [125I]IGF‐II and [125I]insulin receptor binding sites in the hippocampus of aged memory‐unimpaired and ‐impaired rats. Neuroscience. 1997;80:1033‐1040. [DOI] [PubMed] [Google Scholar]
- 11. Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson's disease: a 20‐year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry. 2011;82:1033‐1037. [DOI] [PubMed] [Google Scholar]
- 12. Wood K‐L, Myall DJ, Livingston L, et al. Different PD‐MCI criteria and risk of dementia in Parkinson's disease: four year longitudinal study. NPJ Parkinson Dis. 2016;2:15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoogland J, Boel JA, de Bie RMA, et al. Mild cognitive impairment as a risk factor for Parkinson's disease dementia. Mov Disord. 2017;32:1056‐1065. [DOI] [PubMed] [Google Scholar]
- 14. Jones AJ, Kuijer RG, Livingston L, et al. Caregiver burden is increased in Parkinson's disease with mild cognitive impairment (PD‐MCI). Transl Neurodegener. 2017;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawson RA, Yarnall AJ, Duncan GW, et al. Severity of mild cognitive impairment in early Parkinson's disease contributes to poorer quality of life. Parkinsonism Relat Disord. 2014;20:1071‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Picillo M, Pivonello R, Santangelo G, et al. Serum IGF‐1 is associated with cognitive functions in early, drug‐naive Parkinson's disease. PLoS One. 2017;12:e0186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duan C, Xu Q. Roles of insulin‐like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44‐52. [DOI] [PubMed] [Google Scholar]
- 18. Wennberg AMV, Hagen CE, Machulda MM, et al. The association between peripheral total IGF‐1, IGFBP‐3, and IGF‐1/IGFBP‐3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol Aging. 2018;66:68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan J, Gluckman P, Yang P, et al. Cyclic glycine‐proline regulates IGF‐1 homeostasis by altering the binding of IGFBP‐3 to IGF‐1. Sci Rep. 2014;4:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan D, Krishnamurthi R, Harris AP, Barber PA, Guan J. Plasma cyclic glycine proline/IGF‐1 ratio predicts clinical outcome and recovery in stroke patients. Ann Clin Trans Neurol. 2019;210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan J, Singh‐Mallah G, Liu K, et al. The role for cyclic Glycine‐Proline, a biological regulator of insulin‐like growth factor‐1 in pregnancy‐related obesity and weight changes. J Biol Regul Homeost Agents. 2018;32:465‐478. [PubMed] [Google Scholar]
- 22. Singh‐Mallah G, Singh K, McMahon CD, et al. Maternally administered cyclic glycine‐proline increases insulin‐like growth factor‐1 bioavailability and novelty recognition in developing offspring. Endocrinology. 2016;157:3130‐3139. [DOI] [PubMed] [Google Scholar]
- 23. Guan J, Harris P, Brimble M, et al. The role for IGF‐1‐derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin Ther Targets. 2015;19:785‐794. [DOI] [PubMed] [Google Scholar]
- 24. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689‐1707;quiz 1837. [DOI] [PubMed] [Google Scholar]
- 25. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalrymple‐Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson's disease. Mov Disord. 2011;26:629‐636. [DOI] [PubMed] [Google Scholar]
- 28. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. [DOI] [PubMed] [Google Scholar]
- 29. Ostrovskaya RU, Gruden MA, Bobkova NA, et al. The nootropic and neuroprotective proline‐containing dipeptide noopept restores spatial memory and increases immunoreactivity to amyloid in an Alzheimer's disease model. J Psychopharmacol. 2007;21:611‐619. [DOI] [PubMed] [Google Scholar]
- 30. Guan J, Zhang R, Dale‐Gandar L, Hodgkinson S, Vickers M. NNZ‐2591, a novel diketopiperazine, prevented scopolamine‐induced acute memory impairment in the adult rat. Behav Brain Res. 2010;210:221‐228. [DOI] [PubMed] [Google Scholar]
- 31. Godau J, Knauel K, Weber K, et al. Serum insulinlike growth factor 1 as possible marker for risk and early diagnosis of Parkinson disease. Arch Neurol. 2011;68:925‐931. [DOI] [PubMed] [Google Scholar]
- 32. Godau J, Herfurth M, Kattner B, Gasser T, Berg D. Increased serum insulin‐like growth factor 1 in early idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:536‐538. [DOI] [PubMed] [Google Scholar]
- 33. Guan J, Mathai S, Harris P, et al. Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory‐motor function following hypoxia‐ischemia in adult rats. Neuropharmacology. 2007;53:749‐762. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Liu K, Gray C, et al. Cyclic glycine‐proline normalizes systolic blood pressure in high‐fat diet‐induced obese male rats. Nutr Metab Cardiavasc Dis. 2020;30:339‐346. [DOI] [PubMed] [Google Scholar]
- 35. Williams IC, Park MH, Tsang S, Sperling SA, Manning C. Cognitive function and vascular risk factors among older African American adults. J Immigr Minor Health. 2018;20:612‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doiron M, Langlois M, Dupre N, Simard M. The influence of vascular risk factors on cognitive function in early Parkinson's disease. Int J Geriatr Psychiatry. 2018;33:288‐297. [DOI] [PubMed] [Google Scholar]
- 37. Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang R, Kadar T, Sirimanne E, MacGibbon A, Guan J. Age‐related memory decline is associated with vascular and microglial degeneration in aged rats. Behav Brain Res. 2012;235:210‐217. [DOI] [PubMed] [Google Scholar]


