Abstract
Mast cells are multifunctional immune cells that are found most abundantly at host-environment interfaces, such as the skin, respiratory tract, and oral/gastrointestinal mucosa. Not surprisingly, mast cells act as sentinel cells that sense microbial attacks and initiate a protective immune response and promote healing. Although mast cells share many features with other innate immune effector cells, such as neutrophils and macrophages, they uniquely interact closely with blood vessels and release an extensive set of mediators for the recruitment of innate and adaptive immune cells. A novel human G protein-coupled receptor (GPCR), known as Mas-related GPCR-X2 (MRGPRX2, mouse ortholog, MrgprB2), has recently been identified, which is expressed on mast cells but not neutrophils and macrophages. Interestingly, activation of MrgprB2 by bacteria-derived quorum-sensing peptides inhibits bacterial growth, prevents biofilm formation, and leads to the recruitment of neutrophils to effectively clear bacteria. Furthermore, host defense antimicrobial peptides and small-molecule peptide mimetics also activate mast cells via MRGPRX2/B2. MrgprB2-mediated activation of local mast cells also clears cutaneous bacterial infection, promotes healing, and protects against reinfection. In addition to their role in host defense, mast cells contribute to a number of chronic inflammatory diseases such as periodontitis, neurogenic inflammation, and inflammatory pain likely via the activation of MRGPRX2. In this review, we discuss the roles of MRGPRX2/B2 in the clearance of bacterial infection, wound healing, periodontal disease, neurogenic inflammation, and inflammatory pain. We propose that harnessing mast cells’ host defense and immunomodulatory properties via the activation of MRGPRX2 may lead to novel approaches for the treatment of drug-resistant bacterial infections. On the other hand, increased MRGPRX2 expression on mast cells and their inappropriate activation may contribute to periodontitis, neurogenic inflammation, and inflammatory pain. Thus, targeting MRGPRX2 could provide novel approaches to modulate these conditions.
Keywords: mast cells, Mas-related G protein-coupled receptor X2/B2, bacterial infection, wound healing, periodontal disease, neurogenic inflammation
Introduction
Mast cells are multifunctional tissue-resident granulocytes of hematopoietic origin. While best known as key effectors in IgE-mediated allergic reactions, mast cells have recently been recognized for their protective role in host defense against bacterial pathogens (Abraham and St John 2010; Piliponsky et al. 2010; Matsuguchi 2012; Trivedi et al. 2013; Zimmermann et al. 2019). Studies using mast cell–deficient mice demonstrated that their absence is associated with enhanced bacterial burden, decreased neutrophil recruitment, decreased immune responses, and increased mortality (Echtenacher et al. 1996; Malaviya et al. 1996; Gendrin et al. 2015; Arifuzzaman et al. 2019; Zimmermann et al. 2019). For example, in the cecal ligation and puncture (CLP) model of sepsis, mast cells are crucial for initiating innate immune responses to enterobacteria, particularly through their ability to recruit neutrophils to promote bacterial clearance (Echtenacher et al. 1996).
Mast cells possess several unique properties that make them ideal for host defense function. First, they are widely distributed throughout the body and are particularly found at the host-environment interfaces, such as the skin (Fig. 1A), respiratory tract, and oral/gastrointestinal mucosa. In the oral cavity, mast cells are found in the gingiva, periodontal ligament, and dental pulp (Gaje et al. 2016). Given their strategic location, mast cells ideally serve as professional sentinel cells and one of the first responders to encounter bacterial invasion (Metz and Maurer 2007; Abraham and St John 2010; Piliponsky et al. 2010; Trivedi et al. 2013; Cardamone et al. 2016; Zimmermann et al. 2019). Second, mast cells express a variety of receptors that enable them to recognize and respond to a broad range of pathogenic stimuli (Abraham and St John 2010; Trivedi et al. 2013). Third, upon activation, mast cells rapidly release a plethora of preformed mediators such as histamine, proteases, and prestored tumor necrosis factor–α (TNF-α) from their cytoplasmic granules (Fig. 1B), followed by generation and secretion of de novo synthesized mediators, including cytokines, chemokines, and growth and angiogenic factors (Metz and Maurer 2007; Abraham and St John 2010; Cardamone et al. 2016). These mast cell–derived mediators exert potent antimicrobial and immunomodulatory properties, which facilitate both bacterial clearance and promote healing.
Figure 1.
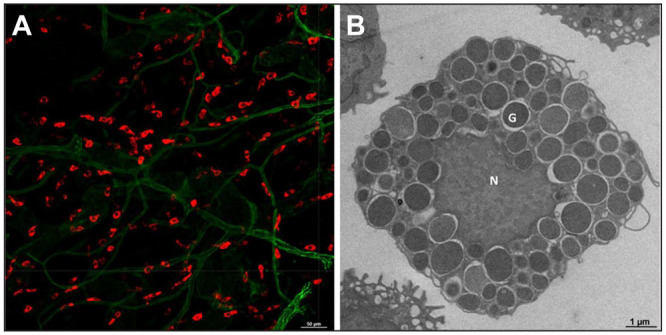
Mast cell localization in the skin and its ultrastructure. (A) Whole-mount immunofluorescence confocal image of adult mouse ear skin stained with Texas Red Avidin (red, mast cells) and Alexa Fluor 488–conjugated CD31 (green, endothelial cells). Bar = 50 µm. (B) Freshly isolated peritoneal lavage cells from mice were used for transmission electron microcopy to visualize mast cell granules. “N” is depicted as mast cell nucleus and “G” is depicted as mast cell granules. Bar = 1 μm.
Periodontitis is a chronic inflammatory disease that affects the tooth-supporting structures, resulting in progressive destruction of the periodontal ligament and alveolar bone (Darveau 2010). Bacterial plaque has been implicated as the primary etiological factor in the pathogenesis of periodontitis. A subset of specific gram-negative periodontal bacteria, so-called red complex pathogens, including Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, are frequently isolated together in disease sites and strongly associated with disease (Holt and Ebersole 2005). Of these, P. gingivalis is considered the “keystone pathogen” of periodontitis (Hajishengallis et al. 2012). However, while the presence of specific pathogenic bacteria can trigger microbial dysbiosis characteristics and play a crucial role in the initiation and progression of periodontitis, emerging evidence shows that the host response to bacterial challenge is central to tissue destruction and alveolar bone resorption, which is the hallmark of periodontitis (Darveau 2010; Hajishengallis et al. 2012; Hajishengallis 2014). Among the cells found in the periodontal tissues, mast cells have been detected in both healthy and inflamed gingiva. Mast cell numbers are increased in gingiva of patients with chronic periodontitis, and the degree of degranulation and proinflammatory cytokine production from these cells correlates with disease severity (Huang et al. 2013; Agrawal et al. 2016; Malcolm et al. 2016; Gupta et al. 2017; Tada et al. 2019). Furthermore, mast cell–deficient mice are protected from P. gingivalis–induced bone loss when compared to wild-type (WT) mice (Malcolm et al. 2016), indicating a potential role of mast cells in the pathogenesis of periodontitis.
In addition to periodontitis, inappropriate activation of mast cells has been implicated in neurogenic inflammation and pain. Mast cells are found in close proximity to nerve endings and express receptors for a range of neurotransmitters (Forsythe 2019). Upon activation by neuropeptides such as substance P (SP), mast cells release a broad range of proinflammatory cytokines and chemokines, including TNF-α, CCL2, and CCL3, that alter vascular permeability and promote the immune cell recruitment to the affected site (Green et al. 2019). The recruitment of immune cells, including neutrophils and monocytes, mediated by mast cells amplifies local inflammatory responses and facilitates peripheral nociceptor sensitization, contributing to neurogenic inflammation and inflammatory pain (Ren and Dubner 2010; Green et al. 2019).
One of the most recent developments in mast cell biology research has been the discovery of a novel G protein-coupled receptor (GPCR) known as Mas-related GPCR-X2 (MRGPRX2; mouse counterpart MrgprB2). MRGPRX2/B2 is expressed predominantly in connective tissue mast cells (Tatemoto et al. 2006; Motakis et al. 2014; McNeil et al. 2015; Varricchi et al. 2019). Emerging evidence suggests that the activation of mast cells via MRGPRX2/B2 is pivotal to controlling bacterial infection and promoting wound healing (Arifuzzaman et al. 2019; Pundir et al. 2019). On the other hand, inappropriate mast cell activation contributes to chronic inflammatory diseases and inflammatory pain, likely via MRGPRX2/B2 (Subramanian et al. 2016). Our recent study demonstrated that the number of MRGPRX2-expressing mast cells is increased in patients with chronic periodontitis (Gupta et al. 2017). Thus, the main objectives of this article are to discuss the recent advances in our understanding of the roles of MRGPRX2/B2 expressed on mast cells in host defense to bacterial infection, periodontitis, and inflammatory pain.
Discovery of a Novel Mast Cell Receptor MRGPRX2
Unlike other immune cells, mast cells display considerable heterogeneity (Welle 1997; Galli et al. 2011). In humans, mast cells are generally categorized into 2 subtypes based on the protease contents in their granules: mast cells that contain both tryptase and chymase (MCTC) are predominantly found in connective tissues such as the skin and gingiva, while those that contain only tryptase (MCT) are mostly found in the lungs (Welle 1997; Gurish and Austen 2012; Gupta et al. 2017). MCTC contain abundant heparin in their granules, but MCT do not.
Mast cells are sometimes referred to as constitutive (innate) and mucosal (adaptive) not only based on their tissue distribution and granularity but also on their development patterns and expression of cell surface receptors. MCTC are considered innate mast cells as they are present constitutively in connective tissues and are generally unaffected by T cells. By contrast, adaptive mast cells are found predominantly in intraepithelial tissues such as the lungs and are induced by T-cell-dependent inflammation (Dwyer et al. 2016). Furthermore, MCTC express MRGPRX2 on the cell surfaces, whereas MCT do not (Subramanian et al. 2016) (Table). Thus, while both human mast cell subtypes can be activated via high-affinity IgE receptors (FcεRI), only MCTC are responsive to a diverse range of cationic substances, collectively called basic secretagogues, through the activation of MRGPRX2 (Tatemoto et al. 2006; Fujisawa et al. 2014).
Table.
Different Properties of Mast Cell Subtypes.
| Characteristics | Mast Cell Subtypes | |
|---|---|---|
| Human | MCTC (tryptase/chymase) | MCT |
| Rodent | Connective tissue-type mast cells | Mucosal-type mast cells |
| Tissue | Skin, gingiva | Lungs |
| Type | Innate | Adaptive |
| T cell | Independent | Dependent |
| Function | Homeostasis/host defense | Immunologic |
| Receptors | FcεRI and MRGPRX2/B2 | FcεRI |
Initial identification of MRGPRX2 as a mast cell–specific receptor for basic secretagogues was established from the study by Tatemoto et al. (2006). They demonstrated that a number of basic secretagogues, such as neuropeptide substance P, pituitary adenylate cyclase–activating polypeptide, and compound 48/80, activate CD34+ cell-derived mast cells through this receptor. Subsequent studies showed that MRGPRX2 is also expressed in human skin and gingival mast cells (Fujisawa et al. 2014; Gupta et al. 2017). The presence of 22 potential MRG coding genes in the mouse genome initially made it difficult to identify the putative MRGPRX2 ortholog in mice. However, in 2015, a seminal article by McNeil et al. (2015) demonstrated that mouse peritoneal mast cells solely express MrgprB2 messenger RNA (mRNA), and that MrgprB2 serves as the murine ortholog of human MRGPRX2. Emerging evidence now suggests that activation of mast cells via MRGPRX2/B2 plays a major role in the immune response to bacterial infection (Pundir et al. 2019).
MRGPRX2/B2 Detects Bacterial Quorum Sensing Molecules and Mediates Antibacterial Immunity
Several Gram-positive and Gram-negative bacteria secrete an extracellular chemical signal, termed an autoinducer, which, upon reaching a threshold concentration, leads to an increase in bacterial cell population. In a particular environment, these autoinducers, collectively called quorum-sensing molecules (QSMs), synchronize the bacterial behavior to function as a multicellular organism (Whiteley et al. 2017). Bacteria use QSMs to communicate and modulate their behavior changes in antibiotic resistance, biofilm formation, and virulence (Whiteley et al. 2017; Pundir et al. 2019). A recent study by Pundir et al. (2019) showed that a number of Gram-positive bacterial QSMs such as competence-stimulating peptide-1 (CSP-1) from Streptococcus pneumoniae, Entf metabolite from Enterococcus faecium, and Streptin-1 from Streptococcus pyogenes are recognized by mast cells through MRGPRX2/B2. Of these, CSP-1 is the most potent candidate to induce antibacterial host response via MRGPRX2/B2-mediated mast cell activation (Pundir et al. 2019). Mast cell activation by QSMs leads to neutrophil recruitment and production of reactive oxygen species (ROS), which eliminates the bacterial infection and reduces biofilm biomass. These antibacterial effects are mediated through MRGPRX2 as the levels of ROS and cytokine/chemokine production are reduced upon knocking down MRGPRX2 in human mast cells. Similarly, WT mouse peritoneal mast cells (PMCs) but not MrgprB2MUT PMCs induced a similar antibacterial effect (Pundir et al. 2019). CSP-precursor Com is required for virulence during nasopharyngeal colonization. Mutant D39 strain devoid of either comC (dComC) or autoinducer 2 (dLuxS) is resistant to mast cell (MRGPRX2/B2)–mediated killing. Intranasal inoculation with S. pneumoniae, intraperitoneal infection with E. faecium, or skin infection by S. pyogenes cause enhanced bacterial clearance in WT mice compared to MrgprB2MUT mice (Pundir et al. 2019). Finally, injection of MRGPRX2/B2 agonist CSP-1 or compound 48/80 locally at the site of bacterial infection significantly reduce in vivo local bacterial counts, establishing MRGPRX2/B2 as a pharmacological candidate for the modulation of bacterial infection (Fig. 2).
Figure 2.
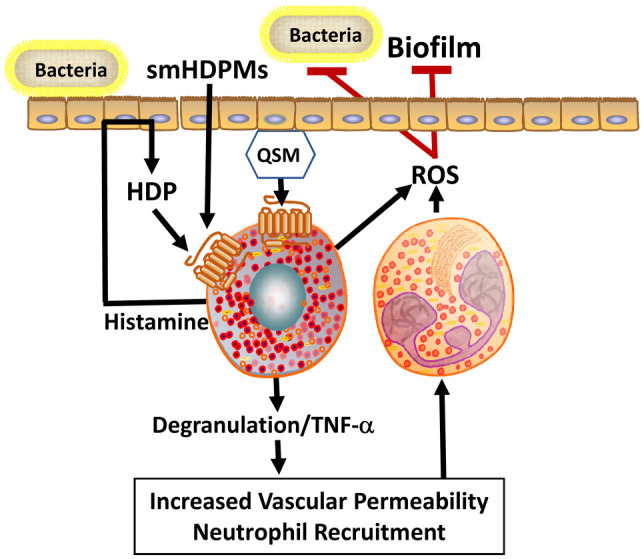
MRGPRX2/B2 act as receptors for quorum-sensing molecules (QSMs) and host defense peptides to mediate host defense. QSMs act as an extracellular signal for bacteria to facilitating cell-cell communication. Mast cells detect QSMs by MRGPRX2/B2 and induce degranulation, which releases mediators such as histamine and tumor necrosis factor–α (TNF-α). These mediators increase local vascular permeability to recruit neutrophils in the infection site, which in turn produces reactive oxygen species (ROS). ROS act as effector molecules by eliminating biofilm formation and inhibiting bacterial growth. Moreover, during infection, epithelial cells secrete host defense peptides (HDPs), which can also activate mast cells by MRGPRX2/B2 and induce degranulation to release histamine. Histamine further activates epithelial cells to produce more HDPs in a feedback loop. Small-molecule host defense peptide mimetics (smHDPMs), which have direct antimicrobial activity, also activate mast cells by MRGPRX2/B2.
Although the QSMs investigated by Pundir et al. (2019) are of nonoral organisms, oral bacteria also produce a number of QSMs to enable communication between mixed bacterial communities of the oral biofilm (Miller and Lamont 2019). Biofilm formation is one of the crucial virulence factors of oral pathogens for nutrient acquisition and processing, protection from immune responses, and increased tolerance to antimicrobial agents (Mira et al. 2017; Jiao et al. 2019). Periodontal pathogens, such as P. gingivalis, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans, as well as oral streptococci have been shown to form biofilms and possess the autoinducer 2 quorum-sensing system for signal exchange (Frias et al. 2001; Basavaraju et al. 2016; Miller and Lamont 2019). Compared to commensal bacteria, periodontal pathogens produce higher levels of autoinducer 2, which facilitate the development and maturation of biofilm, subsequently leading to periodontitis (Kolenbrander et al. 2010). For instance, autoinducer 2 produced by F. nucleatum stimulates the coaggregation and expression of adhesion molecules of P. gingivalis (Jang et al. 2013). Autoinducer 2 inhibition by quorum-sensing inhibitors, BMK-Q101 (a synthesized furanone analogue) and D-ribose, reduces alveolar bone loss induced by P. gingivalis/F. nucleatum coinfection in vivo (Ben Amara et al. 2018). It is therefore possible that oral bacterial QSMs activate mast cells via MRGPRX2/B2 to reduce biofilm mass, thus promoting gingival homeostasis.
Host Defense Peptides Contribute to Mast Cell–Mediated Bacterial Killing
Host defense peptides (HDPs) are positively charged amphipathic peptides that act as broad-spectrum antibiotics against bacteria, fungi, and viruses by interacting with their negatively charged phospholipid moieties and disrupting their membranes (Hazlett and Wu 2011). Mast cells display direct bactericidal activity through the expression and production of HDPs, such as cathelicidin LL-37, human β-defensins (hBDs), and a recently described lipocalin 2 (Di Nardo et al. 2003; Di Nardo et al. 2008; Chang et al. 2019). Secretion of cathelicidins by mast cells leads to protective antibacterial effects to prevent and control group A Streptococcus skin infection (Di Nardo et al. 2003; Di Nardo et al. 2008). A study by Di Nardo et al. (2008) demonstrated that mast cell–deficient mice developed larger skin lesions with higher numbers of bacteria and delayed recovery after group A Streptococcus infection compared to the WT mice. Reconstitution of those mice with WT mast cells, but not cathelicidin-deficient mast cells, significantly reduced edema formation and wound extension, indicating the importance of cathelicidin in controlling bacterial infection (Di Nardo et al. 2008). Furthermore, Chang et al. (2019) recently identified a novel HDP, lipocalin 2, expressed in human and mouse mast cells. Lipocalin 2 released from mast cells inhibits Escherichia coli growth, suggesting that it plays an important role in protection against E. coli infection (Chang et al. 2019).
Apart from the direct bactericidal effect, HDPs also demonstrate indirect antimicrobial activities through mast cell–dependent recruitment and activation of other immune cells (Hancock et al. 2016). These immunomodulatory features likely contribute to their effectiveness as antimicrobial agents. HDPs such as LL-37 are potent chemoattractants for mast cells and can induce mast cell degranulation (Niyonsaba et al. 2007; Babolewska and Brzezinska-Blaszczyk 2015). They also increase the expression of Toll-like receptor 4 on the mast cell surface, which may enhance the ability of mast cells to detect invading pathogens (Yoshioka et al. 2008).
MRGPRX2 Is a Novel Mast Cell Receptor for Host Defense Peptides
Using transfected cell lines and short hairpin RNA (shRNA)–mediated silencing in primary human mast cells, our lab was the first to demonstrate that LL-37 induces degranulation, chemokine generation, and chemotaxis via the activation of MRGPRX2 (Subramanian et al. 2011). hBDs are produced from skin and gingival epithelial cells upon microbial assault and consist of 4 members, hBD1–4 (Weinberg et al. 2012; Greer et al. 2013). Of these, hBD1–3 are expressed and secreted in the human gingival tissue. In vitro studies have demonstrated that P. gingivalis induces the expression of hBD1 (Vankeerberghen et al. 2005) and hBD2 (Taguchi and Imai 2006) in cultured human gingival epithelial cells, while F. nucleatum and A. actinomycetemcomitans stimulate the production of hBD2 and hBD3 (Feucht et al. 2003; Vankeerberghen et al. 2005). We demonstrated that hBD2 and hBD3 induce mast cell degranulation via MRGPRX2 (Subramanian et al. 2011; Subramanian et al. 2013). HDP-induced mast cell degranulation during bacterial infection likely provides critical antimicrobial host defense by enhancing vascular permeability and initiating the recruitment of neutrophils at the site of infection (Chen et al. 2007; Subramanian et al. 2016). Moreover, mast cell–derived mediators such as histamine can, in turn, induce hBD2 and hBD3 secretion from human epithelial cells (Ishikawa et al. 2009), which contributes to further mast cell degranulation, indicating the biphasic innate function of mast cells. Thus, QSMs and HDPs, which activate MCTC-type mast cells via MRGPRX2/B2, may provide a general mechanism for host defense in the skin and gingiva (Table and Fig. 2).
Small-Molecule Host Defense Peptide Mimetic Antibacterial Agents Activate Mast Cells via MRGPRX2/B2
Although HDPs have an important therapeutic potential against microbial infection, their metabolic instability and cellular cytotoxicity have limited their utility. To overcome these limitations, a series of small-molecule HDP mimetics (smHDPMs) have been developed (Choi et al. 2009; Scott and Tew 2017). These compounds are relatively inexpensive to synthesize and have distinct advantages over HDPs in terms of stability, bioavailability, and low toxicity. Furthermore, these compounds exhibit potent antibacterial activity (Beckloff et al. 2007). Recently, we tested the effects of 5 smHDPMs on cytotoxicity, antimicrobial activity, and mast cell degranulation. None of these smHDPMs displayed cytotoxicity against mouse 3T3 fibroblasts or human-transformed liver HepG2 cells, but all compounds induced degranulation in human and murine mast cells via MRGPRX2 and MrgprB2, respectively (Alkanfari et al. 2019). These findings suggest that smHDPMs could serve as novel targets for the treatment of bacterial infection because of their ability to harness mast cells’ host defense functions (Fig. 2).
Mast Cell Activation via MrgprB2 Leads to Neutrophil Recruitment and Accelerated Bacterial Clearance
Mastoparan, a peptide toxin from wasp venom, displays antibacterial activity and induces mast cell degranulation via MRGPRX2/B2 (McNeil et al. 2015). In a Staphylococcus aureus–induced skin infection mouse model, topical application of mastoparan promotes neutrophil recruitment, and this action is highly dependent on the presence of MrgprB2-expressing mast cells (Arifuzzaman et al. 2019). A mastoparan derivative (MP-6I), which does not activate mast cells, failed to clear S. aureus infection despite having potent antibacterial property. By contrast, Duke Mast F, a mastoparan derivative with mast cell–degranulating capacity without any antibacterial activity, reduced the lesion size significantly compared to vehicle (Arifuzzaman et al. 2019). These results suggest that the therapeutic benefit of mastoparan depends on its mast cell–activating capacity rather than antibacterial property. Given that mastoparan activates mast cells via MRGPRX2/B2, it is likely that its host defense function is mediated via the activation of these receptors.
Mast Cell Activation via MrgprB2 Promotes Regenerative Healing
Wound healing is a coordinated, multicomponent, progressive series of events intended to restore the barrier function and mechanical integrity of the skin breached by mechanical or microbial assault (Schultz et al. 2011). It is well documented that persistence of bacteria at wound sites delays natural wound healing and increases scarring (Singer and McClain 2002). To determine if mastoparan-mediated acceleration in bacterial clearance, as described above, results in faster skin regeneration and reduced scarring, Arifuzzaman et al. (2019) allowed infected lesions to close completely and proliferative processes in the skin to reach steady-state levels. Analysis of skin sections with trichrome staining demonstrated reduced scarring in mastoparan-treated mice when compared with untreated mice (Arifuzzaman et al. 2019). Dermal CD301b+ dendritic cells play critical roles in promoting reepithelialization of sterile wounds (Yang et al. 2017), and this population is significantly decreased in S. aureus–infected skin but restored in later stages of infection. Furthermore, depletion of MrgprB2-expressing mast cells reduces CD301b+ cells, indicating that mast cells contribute to restoring the skin CD301b+ cell population to homeostatic levels (Arifuzzaman et al. 2019). Based on these findings, Arifuzzaman et al. (2019) proposed that mast cells contribute to the healing of infectious wounds not only by clearing bacteria via neutrophil recruitment but also by restoring the CD301b+ cell population, which promotes reepithelialization (Fig. 3). In addition, in a Pseudomonas aeruginosa–infected murine skin wound model, mast cell–deficient mice exhibited significantly delayed wound closure, which is associated with impaired bacterial clearance. However, reconstitution of mast cells restored both bacterial clearance and wound closure (Zimmermann et al. 2019). Thus, mast cell activation via MRGPRX2/B2 may contribute to the accelerated clearance of bacterial infection and regenerative healing.
Figure 3.
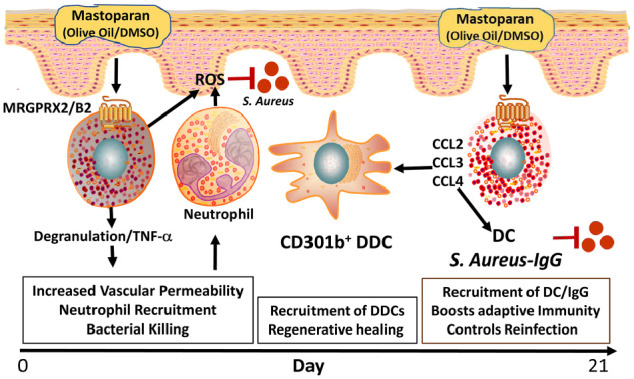
Mastoparan activates mast cells through MRGPRX2/B2 and generates innate and adaptive immunity against Staphylococcus aureus infection. Application of mastoparan in olive oil/DMSO mixture activates mast cells via MRGPRX2/B2 during S. aureus–mediated skin infection and results in mast cell degranulation and release of mediators. This increases vascular permeability and recruits neutrophils. Mastoparan-mediated neutrophil recruitment eliminates bacterial infection by reactive oxygen species (ROS) generation. Mast cells also release cytokines such as CCL2, CCL3, CCL4, and tumor necrosis factor–α (TNF-α), which facilitate the recruitment of CD301b+ dermal dendritic cells (DDCs) in the infection and mediate regenerative healing. Moreover, mastoparan treatment also produces S. aureus–specific IgG. These altogether boost antibacterial adaptive immunity and controls reinfection.
Mast Cell Activation via MrgprB2 Promotes Adaptive Immunity and Controls Bacterial Reinfection
In addition to neutrophil and dendritic CD301b+ cell recruitment, mast cells are responsible for recruiting antigen-presenting dendritic cells to the site of infection and promoting their migration from the inflamed site to the draining lymph nodes (Shelburne and Abraham 2011). Mastoparan induces the generation of chemokines such as CCL2, CCL3, and CCL4, which contribute to the mobilization of dendritic cells. Topical application of mastoparan into S. aureus–infected wound results in a significantly higher level of S. aureus–specific total IgG compared to vehicle control group. Furthermore, this increased IgG is associated with reduced lesion size following reinfection (Arifuzzaman et al. 2019). Based on these findings, it has been proposed that mastoparan not only accelerates bacterial clearance and promotes regenerative healing but is also capable of enhancing adaptive immunity, which provides protection against reinfection (Fig. 3).
Potential Roles of MRGPRX2 in Periodontal Tissue Homeostasis and Periodontitis
Mast cells of the MCTC type, which express MRGPRX2, are found in healthy gingiva and have been implicated in periodontal tissue homeostasis (Steinsvoll et al. 2004; Gupta et al. 2017). Mast cells are activated by HDPs generated from gingival epithelium (hBD3) and infiltrating neutrophils (LL-37) via the activation of MRGPRX2 (Subramanian et al. 2011; Subramanian et al. 2013). Furthermore, mast cells may sense QSMs produced from periodontal pathogens in bacterial plaque via MRGPRX2, as discussed earlier. Upon activation, mast cells release several mediators, including cytokines and chemokines, that promote host defense by facilitating neutrophil recruitment through the periodontal tissue, resulting in maintenance of tissue homeostasis (Steinsvoll et al. 2004) (Fig. 4).
Figure 4.
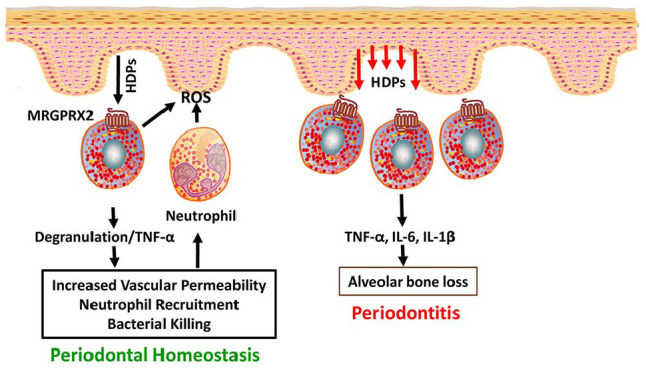
MRGPRX2 contributes to both periodontal tissue homeostasis and periodontitis. During infection, host defense peptides (HDPs) produced from gingival epithelium activate mast cells through MRGPRX2, which undergo degranulation to release preformed mediators and tumor necrosis factor–α (TNF-α). This causes increased vascular permeability and increased migration of neutrophils in the infected tissue. Neutrophils clear microbial infection through effector molecule (reactive oxygen species [ROS]) secretion and maintain tissue homeostasis. In contrast, during chronic infections such as periodontitis, overproduction of HDPs from gingival epithelium leads to recruitment of more mast cells, and its activation by MRGPRX2 releases more proinflammatory cytokines such as interleukin (IL)–6, IL-1β, and TNF-α. This results in periodontal tissue breakdown, a major characteristic feature of periodontitis.
While MRGPRX2-mediated low-level inflammation may contribute to periodontal homeostasis, sustained inflammation due to MRGPRX2 dysregulation may, however, promote periodontal disease. The number of MRGPRX2-expressing mast cells is increased in gingiva obtained from chronic periodontitis patients compared with those with normal gingiva (Gupta et al. 2017). Of note, an increased number and expression of MRGPRX2 have been reported in skin mast cells from patients with chronic urticaria and have been linked to the pathogenesis of the disease (Fujisawa et al. 2014). It is noteworthy that expression of HDPs is upregulated in patients with periodontitis when compared to healthy individuals (Turkoglu et al. 2009; Pereira et al. 2013; Wang et al. 2015). Following activation by HDPs, mast cells produce and release an array of proinflammatory mediators, including TNF-α and interleukin (IL)–1, IL-6, and IL-31, which have been shown to induce gingival epithelial barrier dysfunction and alveolar bone loss (Malcolm et al. 2016; Tada et al. 2019). Thus, enhanced HDP/MRGPRX2-mediated mast cell degranulation and cytokine generation may lead to periodontal tissue breakdown, which is the characteristic feature of periodontitis (Fig. 4).
Novel Roles of MRGPRX2 on Neurogenic Inflammation and Pain
Mast cells are often found in close proximity to sensory nerve endings in most tissues and appear to serve as a functional homeostatic regulatory unit (Subramanian et al. 2016). However, dysregulation of mast cell–nerve interaction has been suggested as a major contributor in the pathogenesis of neurogenic inflammation and pain (Ren and Dubner 2010; Forsythe 2019). Mast cell–derived mediators such as histamine, leukotrienes, and tryptase interact with their specific receptors on sensory nerve endings, resulting in the release of neuropeptides, including SP (Steinhoff et al. 2000; Shim and Oh 2008; Taylor-Clark et al. 2008). In turn, SP released from nerve endings can evoke further mast cell degranulation, subsequently resulting in the release of a wide array of proinflammatory cytokines and chemokines, as well as the recruitment of innate immune cells (Kulka et al. 2008; Green et al. 2019).
SP has been well recognized for its roles in inflammation and pain. SP-induced neurogenic inflammation has been implicated in the pathogenesis of many chronic inflammatory conditions, including orofacial pain and inflammatory processes in pulpitis and periodontal diseases (Sacerdote and Levrini 2012; de Avila et al. 2014). Neurokinin-1 receptor (NK-1R) was previously thought to be responsible for SP-mediated pain and neurogenic inflammation due to its close anatomical distribution and functional role in pain pathways (Garcia-Recio and Gascon 2015). However, the failure of NK-1R antagonists as potential anti-inflammatory and analgesic therapeutics in human clinical studies raises the possibility that nociceptive and proinflammatory effects of SP may be mediated via alternative mechanisms rather than the interaction with NK-1R (Navratilova and Porreca 2019).
Recently, a landmark study by Green et al. (2019) provided strong support that the inflammatory effects of SP are mediated via the activation of mast cells through MRGPRX2/B2, independent of NK-1R. Activation of MRGPRX2/B2 is required for inflammatory and thermal hyperalgesia; release of proinflammatory cytokines and chemokines such as TNF-α, CCL2, and CCL3; and recruitment of immune cells at the injury site (Green et al. 2019). This immune cell recruitment reinforces the pathophysiology of neurogenic inflammation and inflammatory pain as it further facilitates inflammatory responses and peripheral nerve sensitization (Navratilova and Porreca 2019) (Fig. 5). Preventing the activation of mast cells from released SP either by blocking the MrgprB2 receptor or by ablation of mast cells greatly diminishes pain responses (Green et al. 2019). Thus, targeting MRGPRX2/B2 may lead to novel effective treatment modalities for inflammatory pain.
Figure 5.

The neuropeptide substance P (SP) contributes to neurogenic inflammation and pain through the activation of mast cells via MRGPRX2. Mast cells are found in close proximity to peripheral nerve endings. During tissue damage, SP released from peripheral nerve endings activates mast cells via MRGPRX2. Activated mast cells release several proinflammatory cytokines and chemokines such as CCL2 and CCL3, and they recruit immune cells in the injury site. These lead to the pathophysiology of neurogenic inflammation and peripheral neuron sensitization, resulting in pain sensation.
Therapeutic Prospects and Future Directions
The emergence of antibiotic resistance poses a tremendous public health concern globally and requires an urgent need to develop a novel therapy for the treatment of infections caused by drug-resistant microbes (Laxminarayan et al. 2013). Brilacidin, a defensin mimetic antibiotic, is being developed as a novel treatment for drug-resistant bacterial infections (Kowalski et al. 2016). It is also being investigated for possible application in the prevention of mucositis in patients undergoing chemoradiation for treatment of head and neck cancer (Sonis and Villa 2018). Based on the recent findings on mastoparan (Arifuzzaman et al. 2019) and smHDPMs (Alkanfari et al. 2019), it is possible that the potential clinical utility of brilacidin may be mediated by harnessing immunomodulatory properties through the activation of MRGPRX2, in addition to its direct antimicrobial activity. Taken together, these findings raise the interesting possibility that exploiting MRGPRX2-mediated mast cell activation by host defense peptidomimetics could provide a novel approach for the treatment of drug-resistant bacterial infections.
Although MRGPRX2-mediated mast cell activation could contribute to periodontal homeostasis, its inappropriate activation may lead to chronic inflammatory diseases, including periodontitis and inflammatory pain. The expression of MRGPRX2 and its ligands is increased in patients with chronic periodontitis (Gupta et al. 2017). Enhanced activation of MRGPRX2 by HDPs and neuropeptides may contribute to the inflammatory processes responsible for periodontal breakdown and inflammatory pain, respectively. Thus, development of MRGPRX2 antagonists targeting the receptor might therefore represents a novel and effective therapeutics approach for the prevention/treatment of periodontitis and inflammatory pain.
Author Contributions
C. Chompunud Na Ayudhya, S. Roy, M. Thapaliya, contributed to data acquisition and analysis, drafted and critically revised the manuscript; H. Ali, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by National Institutes of Health grants R01-AI124182 and R01-AI149487 to H. Ali.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: H. Ali  https://orcid.org/0000-0001-9190-1960
https://orcid.org/0000-0001-9190-1960
References
- Abraham SN, St John AL. 2010. Mast cell–orchestrated immunity to pathogens. Nat Rev Immunol. 10(6):440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Gupta J, Gupta KK, Kumar V. 2016. Correlation of mast cells in different stages of human periodontal diseases: pilot study. J Oral Maxillofac Pathol. 20(1):91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkanfari I, Freeman KB, Roy S, Jahan T, Scott RW, Ali H. 2019. Small-molecule host-defense peptide mimetic antibacterial and antifungal agents activate human and mouse mast cells via mas-related GPCRs. Cells. 8(4). pii: E311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arifuzzaman M, Mobley YR, Choi HW, Bist P, Salinas CA, Brown ZD, Chen SL, Staats HF, Abraham SN. 2019. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci Adv. 5(1):eaav0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babolewska E, Brzezinska-Blaszczyk E. 2015. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response. Cell Immunol. 293(2):67–73. [DOI] [PubMed] [Google Scholar]
- Basavaraju M, Sisnity VS, Palaparthy R, Addanki PK. 2016. Quorum quenching: signal jamming in dental plaque biofilms. J Dent Sci. 11(4):349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN, et al. 2007. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 51(11):4125–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amara H, Song HY, Ryu E, Park JS, Schwarz F, Kim BM, Choi BK, Koo KT. 2018. Effects of quorum-sensing inhibition on experimental periodontitis induced by mixed infection in mice. Eur J Oral Sci. 126(6):449–457. [DOI] [PubMed] [Google Scholar]
- Cardamone C, Parente R, Feo GD, Triggiani M. 2016. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett. 178:10–14. [DOI] [PubMed] [Google Scholar]
- Chang YL, Wang Z, Igawa S, Choi JE, Werbel T, Di Nardo A. 2019. Lipocalin 2: a new antimicrobial in mast cells. Int J Mol Sci. 20(10). pii: E2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, et al. 2007. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 37(2):434–444. [DOI] [PubMed] [Google Scholar]
- Choi S, Isaacs A, Clements D, Liu D, Kim H, Scott RW, Winkler JD, DeGrado WF. 2009. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc Natl Acad Sci USA. 106(17):6968–6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 8(7):481–490. [DOI] [PubMed] [Google Scholar]
- de Avila ED, de Molon RS, de Godoi Gonçalves DA, Camparis CM. 2014. Relationship between levels of neuropeptide substance P in periodontal disease and chronic pain: a literature review. J Investig Clin Dent. 5(2):91–97. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Vitiello A, Gallo RL. 2003. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide.J Immunol. 170(5):2274–2278. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. 2008. Mast cell cathelicidin antimicrobial peptide prevents invasive group a streptococcus infection of the skin. J Immunol. 180(11):7565–7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DF, Barrett NA, Austen KF; Immunological Genome Project Consortium. 2016. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 17(7):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 381(6577):75–77. [DOI] [PubMed] [Google Scholar]
- Feucht EC, DeSanti CL, Weinberg A. 2003. Selective induction of human beta-defensin mRNAs by Actinobacillus actinomycetemcomitans in primary and immortalized oral epithelial cells. Oral Microbiol Immunol. 18(6):359–363. [DOI] [PubMed] [Google Scholar]
- Forsythe P. 2019. Mast cells in neuroimmune interactions. Trends Neurosci. 42(1):43–55. [DOI] [PubMed] [Google Scholar]
- Frias J, Olle E, Alsina M. 2001. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 69(5):3431–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, et al. 2014. Expression of mas-related gene x2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 134(3):622–633.e9. [DOI] [PubMed] [Google Scholar]
- Gaje PN, Amalia Ceausu R, Jitariu A, Stratul SI, Rusu LC, Popovici RA, Raica M. 2016. Mast cells: key players in the shadow in oral inflammation and in squamous cell carcinoma of the oral cavity. Biomed Res Int. 2016:9235080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. 2011. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 12(11):1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Recio S, Gascon P. 2015. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int. 2015:495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrin C, Vornhagen J, Ngo L, Whidbey C, Boldenow E, Santana-Ufret V, Clauson M, Burnside K, Galloway DP, Adams Waldorf KM, et al. 2015. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 1(6):e1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. 2019. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 101(3):412–420.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer A, Zenobia C, Darveau RP. 2013. Defensins and LL-37: a review of function in the gingival epithelium. Periodontol 2000. 63(1):67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Idahosa C, Roy S, Lee D, Subramanian H, Dhingra A, Boesze-Battaglia K, Korostoff J, Ali H. 2017. Differential regulation of mas-related g protein-coupled receptor x2-mediated mast cell degranulation by antimicrobial host defense peptides and Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 85(10). pii: e00246-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Austen KF. 2012. Developmental origin and functional specialization of mast cell subsets. Immunity. 37(1):25–33. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. 2014. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. 2012. The keystone-pathogen hypothesis. Nat Rev Microbiol. 10(10):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Haney EF, Gill EE. 2016. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 16(5):321–334. [DOI] [PubMed] [Google Scholar]
- Hazlett L, Wu M. 2011. Defensins in innate immunity. Cell Tissue Res. 343(1):175–188. [DOI] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex,” a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 38:72–122. [DOI] [PubMed] [Google Scholar]
- Huang S, Lu F, Chen Y, Huang B, Liu M. 2013. Mast cell degranulation in human periodontitis. J Periodontol. 84(2):248–255. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kanda N, Hau CS, Tada Y, Watanabe S. 2009. Histamine induces human beta-defensin-3 production in human keratinocytes. J Dermatol Sci. 56(2):121–127. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Choi YJ, Lee SH, Jun HK, Choi BK. 2013. Autoinducer 2 of fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol. 58(1):17–27. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Tay FR, Niu LN, Chen JH. 2019. Advancing antimicrobial strategies for managing oral biofilm infections. Int J Oral Sci. 11(3):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. 2010. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 8(7):471–480. [DOI] [PubMed] [Google Scholar]
- Kowalski RP, Romanowski EG, Yates KA, Mah FS. 2016. An independent evaluation of a novel peptide mimetic, brilacidin (pmx30063), for ocular anti-infective. J Ocul Pharmacol Ther. 32(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. 2008. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 123(3):398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, et al. 2013. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 13(12):1057–1098. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 381(6577):77–80. [DOI] [PubMed] [Google Scholar]
- Malcolm J, Millington O, Millhouse E, Campbell L, Adrados Planell A, Butcher JP, Lawrence C, Ross K, Ramage G, McInnes IB, et al. 2016. Mast cells contribute to Porphyromonas gingivalis–induced bone loss. J Dent Res. 95(6):704–710. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T. 2012. Mast cells as critical effectors of host immune defense against gram-negative bacteria. Curr Med Chem. 19(10):1432–1442. [DOI] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 519(7542):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Maurer M. 2007. Mast cells—key effector cells in immune responses. Trends Immunol. 28(5):234–241. [DOI] [PubMed] [Google Scholar]
- Miller DP, Lamont RJ. 2019. Signaling systems in oral bacteria. Adv Exp Med Biol. 1197:27–43. [DOI] [PubMed] [Google Scholar]
- Mira A, Simon-Soro A, Curtis MA. 2017. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 44(Suppl 18):S23–S38. [DOI] [PubMed] [Google Scholar]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, et al. 2014. Redefinition of the human mast cell transcriptome by deep-cage sequencing. Blood. 123(17):e58–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Porreca F. 2019. Substance P and inflammatory pain: getting it wrong and right simultaneously. Neuron. 101(3):353–355. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. 2007. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 127(3):594–604. [DOI] [PubMed] [Google Scholar]
- Pereira AL, Franco GC, Cortelli SC, Aquino DR, Costa FO, Raslan SA, Cortelli JR. 2013. Influence of periodontal status and periodontopathogens on levels of oral human beta-defensin-2 in saliva. J Periodontol. 84(10):1445–1453. [DOI] [PubMed] [Google Scholar]
- Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. 2010. Mast cell–derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 176(2):926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundir P, Liu R, Vasavda C, Serhan N, Limjunyawong N, Yee R, Zhan Y, Dong X, Wu X, Zhang Y, et al. 2019. A connective tissue mast-cell-specific receptor detects bacterial quorum-sensing molecules and mediates antibacterial immunity. Cell Host Microbe. 26(1):114–122.e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. 2010. Interactions between the immune and nervous systems in pain. Nat Med. 16(11):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P, Levrini L. 2012. Peripheral mechanisms of dental pain: the role of substance P. Mediators Inflamm. 2012:951920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. 2011. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 19(2):134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RW, Tew GN. 2017. Mimics of host defense proteins: strategies for translation to therapeutic applications. Curr Top Med Chem. 17(5):576–589. [DOI] [PubMed] [Google Scholar]
- Shelburne CP, Abraham SN. 2011. The mast cell in innate and adaptive immunity. Adv Exp Med Biol. 716:162–185. [DOI] [PubMed] [Google Scholar]
- Shim WS, Oh U. 2008. Histamine-induced itch and its relationship with pain. Mol Pain. 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, McClain SA. 2002. Persistent wound infection delays epidermal maturation and increases scarring in thermal burns. Wound Repair Regen. 10(6):372–377. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Villa A. 2018. Phase II investigational oral drugs for the treatment of radio/chemotherapy induced oral mucositis. Expert Opin Investig Drugs. 27(2):147–154. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, et al. 2000. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 6(2):151–158. [DOI] [PubMed] [Google Scholar]
- Steinsvoll S, Helgeland K, Schenck K. 2004. Mast cells—a role in periodontal diseases? J Clin Periodontol. 31(6):413–419. [DOI] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Ali H. 2016. Roles of Mas-related G protein-coupled receptor x2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 138(3):700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Guo Q, Price R, Ali H. 2011. Mas-related gene x2 (MRGX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 286(52):44739–44749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. 2013. Beta-defensins activate human mast cells via Mas-related gene x2. J Immunol. 191(1):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Nishioka T, Takase A, Numazaki K, Bando K, Matsushita K. 2019. Porphyromonas gingivalis induces the production of interleukin-31 by human mast cells, resulting in dysfunction of the gingival epithelial barrier. Cell Microbiol. 21(3):e12972. [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Imai H. 2006. Expression of beta-defensin-2 in human gingival epithelial cells in response to challenge with Porphyromonas gingivalis in vitro. J Periodontal Res. 41(4):334–339. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, et al. 2006. Immunoglobulin E–independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 349(4):1322–1328. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Nassenstein C, Undem BJ. 2008. Leukotriene D4 increases the excitability of capsaicin-sensitive nasal sensory nerves to electrical and chemical stimuli. Br J Pharmacol. 154(6):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi NH, Guentzel MN, Rodriguez AR, Yu JJ, Forsthuber TG, Arulanandam BP. 2013. Mast cells: multitalented facilitators of protection against bacterial pathogens. Expert Rev Clin Immunol. 9(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkoglu O, Emingil G, Kutukculer N, Atilla G. 2009. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 80(6):969–976. [DOI] [PubMed] [Google Scholar]
- Vankeerberghen A, Nuytten H, Dierickx K, Quirynen M, Cassiman JJ, Cuppens H. 2005. Differential induction of human beta-defensin expression by periodontal commensals and pathogens in periodontal pocket epithelial cells. J Periodontol. 76(8):1293–1303. [DOI] [PubMed] [Google Scholar]
- Varricchi G, Pecoraro A, Loffredo S, Poto R, Rivellese F, Genovese A, Marone G, Spadaro G. 2019. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front Cell Neurosci. 13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Duan D, Zhou X, Li X, Yang J, Deng M, Xu Y. 2015. Relationship between expression of human gingival beta-defensins and levels of periodontopathogens in subgingival plaque. J Periodontal Res. 50(1):113–122. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Jin G, Sieg S, McCormick TS. 2012. The yin and yang of human beta-defensins in health and disease. Front Immunol. 3:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle M. 1997. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell–specific proteases chymase and tryptase. J Leukoc Biol. 61(3):233–245. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Diggle SP, Greenberg EP. 2017. Progress in and promise of bacterial quorum sensing research. Nature. 551(7680):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Suwanpradid J, Sanchez-Lagunes R, Choi HW, Hoang P, Wang D, Abraham SN, MacLeod AS. 2017. IL-27 facilitates skin wound healing through induction of epidermal proliferation and host defense. J Invest Dermatol. 137(5):1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Fukuishi N, Kubo Y, Yamanobe H, Ohsaki K, Kawasoe Y, Murata M, Ishizumi A, Nishii Y, Matsui N, et al. 2008. Human cathelicidin cap18/LL-37 changes mast cell function toward innate immunity. Biol Pharm Bull. 31(2):212–216. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Troeltzsch D, Gimenez-Rivera VA, Galli SJ, Metz M, Maurer M, Siebenhaar F. 2019. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc Natl Acad Sci USA. 116(41):20500–20504. [DOI] [PMC free article] [PubMed] [Google Scholar]


