Abstract
Cell-cell adhesion is a key mechanism to control tissue integrity and migration. In head and neck squamous cell carcinoma (HNSCC), cell migration facilitates distant metastases and is correlated with poor prognosis. RAP1, a ras-like protein, has an important role in the progression of HNSCC. RAC1 is an integrin-linked, ras-like protein that promotes cell migration. Here we show that loss of cell-cell adhesion is correlated with inactivation of RAP1 confirmed by 2 different biochemical approaches. RAP1 activation is required for cell-matrix adhesion confirmed by adhesion to fibronectin-coated plates with cells that have biochemically activated RAP1. This effect is reversed when RAP1 is inactivated. In addition, RAP1GTP-mediated adhesion is only facilitated through α5β1 integrin complex and is not a function of either α5 or β1 integrin alone. Moreover, the inside-out signaling of RAP1 activation is coordinated with RAC1 activation. These findings show that RAP1 has a prominent role in cell-matrix adhesion via extracellular matrix molecule fibronectin-induced α5β1 integrin and supports a critical role for the RAP1/RAC1 signaling axis in HNSCC cell migration.
Keywords: integrin α5β1, fibronectin, extracellular matrix, cal adhesion kinase, small GTPases, squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) is the sixth most common cancer in the world. Despite appropriate treatment, the survival rate (~50%) is poorer than breast cancer and melanoma (Siegel et al. 2016). Invasion is an oncogenic phenotype that is essential for development and progression of HNSCC. Discovering mechanisms that regulate invasion and metastasis of HNSCC could enable the development of novel treatment approaches. RAP1 is a ras-like, small guanosine triphosphate (GTP) binding protein that has an important role in progression of HNSCC. Guanine nucleotide-exchange factors (GEFs) mediate RAP1 activation by promoting guanosine diphosphate (GDP) to GTP exchange. Inactivation is affected by specific GTPase-activating proteins (GAPs), which enhance RAP1’s intrinsic GTPase activity.
RAP1 has an important role in epithelial cell adhesion; in Drosophila, inactivating mutations of RAP1 redistributed adherens junctions to one side of the cell, whereas wild-type epithelial cells showed uniform distribution of adherens junctions around their circumference (Knox and Brown 2002). Importantly, mutant cell clones randomly invaded the surrounding tissues, suggesting a role in cell motility (Knox and Brown 2002). Consistent with these studies in Drosophila, osteosarcoma cells with reduced active RAP1 do not form adherens junctions and are invasive (Yajnik et al. 2003).
Integrins comprise a family of heterodimeric transmembrane receptors that mediate cell adhesion to the extracellular matrix (ECM); integrins may influence cancer progression, via effects on polarity, proliferation, and migration (Hamidi and Ivaska 2018). Integrins are bidirectional signaling molecules; extracellular ligand-induced signaling (outside-in) induces recruitment and activation of a multiprotein complex (adhesome) and ultimately activates FAK, RAS-MAPK, and PI3K-Akt. Intracellular signals (inside-out), particularly activation of RAP1A, can induce recruitment and activation of cytosolic integrin-binding proteins (e.g., talin, kindlin) that can modulate signaling (Shattil et al. 2010).
Overexpression of α5β1 integrin is observed in aggressive HNSCC and has been proposed as a biomarker (Chang et al. 2018). Moreover, α5β1 integrin mediates hypoxia-associated invasion and transforming growth factor β (TGFβ)–induced migration and invasion of HNSCC in vitro (Lyle et al. 2008). Activation of α5β1 integrin and its downstream signaling cascade may be regulated by interactions with other membrane-bound receptors; in pancreatic cancer, RAP1 bridges the interaction between receptor tyrosine kinase and α5β1 integrin to mediate invasion and metastasis (Ricono et al. 2009).
Interaction of epithelial cells with the extracellular environment has a crucial role in their phenotype. Disruption of integrin signaling transforms normal cells to neoplastic cells and can reverse the malignant phenotype of tumor cells (Weaver et al. 1997). Thus, components of integrin signaling have a fundamental role in tumor invasion. Among these, RAC1, another small GTPase, is dysregulated in cancer (Hudson et al. 2018). Increased RAC1GTP regulates various aspects of tumor development and progression, including actin remodeling, cell adhesion, migration, invasion, and epithelial-to-mesenchymal transition (EMT) (Maldonado and Dharmawardhane 2018). RAC1 has been implicated in tongue cancer (Zhang et al. 2017), and increased activity is associated with enhanced anchorage-independent growth of HNSCC cells (Su et al. 2014). Crosstalk between RAP1 and RAC1 (Maillet et al. 2003) involves Epac, a GEF that specifically activates RAP1 (Enserink et al. 2002). Here we investigated how inside-out signaling by RAP1 to α5β1 integrin and RAC1 affects cell-fibronectin adhesion and migration of HNSCC cells.
Materials and Methods
Cell Culture
UM-SCC-1 and UM-SCC-17B HNSCC cell lines (T. Carey, University of Michigan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)–penicillin/streptomycin (100,000 IU/mL; 100 µg/mL) and 10% Hi–fetal bovine serum (FBS). Cells were genotyped for authenticity and tested for mycoplasma. Cell lines were stably transfected with pcDNA or FLAG-RAP1GAP (gift from P. Stork, Oregon Health Sciences University) and maintained in 250 µg/mL G418. Integrin α5 and β1 were downregulated using RNAiMAX (Invitrogen 13778150) with ON-TARGET-plus (Dharmacon) small interfering RNAs (siRNAs) to human-ITGA5 (J-008003-10-0020, J-008003-11-0020) and human-ITGB1 (J-004506-07-0020, J-004506-08-0020). RAP1 was activated using 8-pCPT-2′O-Me-cAMP (BioLog C041) (8-CPT), a specific activator of RAP1 (Enserink et al. 2002; Holz et al. 2008).
Western Blot Analysis
Whole-cell lysates were immunoblotted as described (Mitra et al. 2003). Primary antibodies were anti-RAP1, anti-α5-integrin, anti-β-integrin, anti-phospho-FAK(Y397), and anti-FAK (Cell Signaling, #2399, 1:1,000; #4705, 1:2,000; #4706, 1:2,000; #2556, 1:1,000; #13009, 1:1,000, respectively); anti-FLAG (Sigma-Aldrich, #HPA003373; 1:5,000); and anti-RAC1 and anti-GAPDH (Millipore, #05-389, 1:1,000, #MAB374, 1:10,000). Signals were detected using horseradish peroxidase (HRP)–conjugated secondary antibodies (Jackson Immuno-Research) and Super-Signal West-Pico Chemiluminescent system (Pierce #PI34080). Signal intensity was quantified using ImageJ software (http://rsbweb.nih.gov/ij/) and expressed as percentage of control.
Adhesion Assay
Flat-bottomed, 96-well plates were coated with freshly prepared fibronectin (10 µg/mL; Millipore, #FC010) or phosphate-buffered saline (PBS) for 3.5 h at 37°C followed by blocking with bovine serum albumin (BSA) (Sigma, #A0281) for 2 h. Cells (30,000) were incubated for 3.5 h at 37°C, fixed in methanol, and stained with crystal violet (0.1%) for 30 min. After incubation with 2% sodium dodecyl sulfate (SDS), optical density was read at 595 nm (Softmax Pro 4.8). Anti-α5, anti-β1, and anti-α5β1 integrin (Millipore MAB1956Z, MAB2253Z, and MAB1969, respectively) were used for inhibition studies. Cells (2.4 × 105 cells in 100 µL) were incubated with antibody (final concentration 0.025 mg/mL) for 1 h at 37°C.
Active RAP1 Pulldown Assay
RAP1GTP was assayed as described (Mitra et al. 2003) using ralGDS protein (gift from Dr. J Bos, University Medical Centre Utrecht).
Active RAC-1 Pulldown Assay
RAC1GTP was assayed using GST-PBD expressed in BL21-DE3 (Das et al. 2011). Cells were washed with ice-cold PBS, lysed in RIPA-Z buffer (50 mM Tris pH 7.4, 0.5% sodium-deoxycholate, 100 mM NaCl, 10 mM MgCl2, 10% glycerol, 1 mM dithiothreitol [DTT], 1% NP-40 with protease inhibitor cocktail; Roche #11697498001), and precleared with beads. The supernatant was incubated with GST-PBD beads, washed with lysis buffer, and boiled with SDS–polyacrylamide gel electrophoresis (PAGE) loading buffer. The supernatant was immunoblotted with anti-RAC1 antibody.
Immunofluorescence
Cells were seeded on poly-L-lysine coated coverslips, incubated with EDTA or phenylarsine oxide (PAO), and fixed in 4% paraformaldehyde. Cells were permeabilized with 0.3% Triton X100 followed by incubation with 5% donkey serum for 1 h, then E-cadherin antibody (BD, #610182; 1:400) and goat anti-mouse secondary antibody (Invitrogen, #A-11001; 1:2,000), and mounted with ProLong-Gold Antifade (Invitrogen, #P36931).
Cell Migration Assays
Cells were serum starved in blank DMEM and incubated with mitomycin C (14.35 µM for UM-SCC-1; 30.51 µM for UM-SCC-17B) for 2 h in serum-free conditions. After a scratch, cells were incubated in 10 µM 8-CPT or DMEM (control) in serum-free DMEM for 30 min, then in 10% FBS-DMEM. To understand the progression of cell migration, similarly treated plates were incubated in an IncuCyte incubator (Essen Bioscience) for 24 h with photographs taken at 2-h intervals (10× objective). Images were processed through IncuCyte-Zoom software and a video file was created.
Statistical analysis was performed using a Student’s t test or 1-way analysis of variance (ANOVA).
Results
Disruption of Cell Adhesion Inactivates RAP1
Cell-cell adhesion was disrupted by 2 biochemical approaches to investigate the outside-in impact on RAP1 activation. Cell-cell junctions were disrupted with the calcium chelator EGTA or PAO. PAO is a specific tyrosine phosphatase inhibitor that decreases cadherin-mediated adhesion by phosphorylating constituents of the adherens junctions. UM-SCC-1, serum-starved for 3 h, was treated with 5 mM EGTA or 5 µM PAO for 30 min. Disruption of cell-cell adhesion was associated with reduced RAP1GTP (Fig. 1A). Removal of EGTA and PAO was correlated with recovery of E-cadherin expression at cell-cell junctions (Fig. 1B) and an increase in RAP1GTP (Fig. 1A). Both EGTA and PAO effectively disrupted cell-cell adhesion as indicated by the removal of E-cadherin (green fluorescent intensity) from cell-cell junctions (Fig. 1B). Similar results were observed with UM-SCC-17B, an independent cell line. Disruption of cell-cell junctions by both EGTA and PAO also reduced cell-matrix adhesion in fibronectin-coated plates in both cell lines (Fig. 1C). Collectively, these data show that disruption of cell-cell adhesion is correlated with inactivation of RAP1 and affects cell adhesion to fibronectin.
Figure 1.

Disruption of cell-cell adhesion inactivates RAP1. (A) UM-SCC-1 (left panels) and UM-SCC-17B (right panels) were serum starved for 3 h and treated with EGTA (5 mM) or phenylarsine oxide (PAO) (1 mM) for 30 min. Cells were lysed, and a RAP1 pulldown assay was performed. (B) UM-SCC-1 and UM-SCC-17B were treated with EGTA (5 mM) or PAO (1 mM) and fixed. Cells were immunofluorescently labeled with E-cadherin antibody (FITC-green) and nuclear DAPI stain (blue). (C) UM-SCC-1 and UM-SCC-17B cells were treated as above and an adhesion assay was performed (SCC1: control vs. EGTA, P = 0.0134; control vs. PAO, P = 0.0018; SCC17B: control vs. EGTA, P < 0.00019; control vs. PAO, P < 0.0001; 1-way analysis of variance). Data are representative of 3 independent experiments with 3 replicates in each experiment. DU, arbitrary densitometry unit of immunoblot signals as measured by ImageJ software (National Institutes of Health). RAP1GTP was normalized to corresponding GAPDH-normalized total RAP1 and expressed as percent of control. *P ≤ 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P ≤ 0.0001.
Active RAP1 Is Required for Cell-Matrix Adhesion
Since reduced cell-fibronectin adhesion was correlated with decreased RAP1GTP (Fig. 1C), we assessed if RAP1GTP enhances cell-matrix adhesion. RAP1 was activated by 8-CPT, which specifically activates Epac, a RAP1-specific GEF (Enserink et al. 2002). Increased RAP1GTP (Fig. 2A) correlated with enhanced cell-matrix adhesion in both cell lines (Fig. 2B). To verify the role of RAP1GTP in cell-matrix adhesion, HNSCC cell lines stably overexpressing RAP1GAP, which inactivates RAP1, were used. Lower RAP1GTP (Fig. 2C) reduced adhesion to fibronectin in comparison with control cells transfected with empty vector (Fig. 2D). As expected, RAP1GTP was not induced by 8-CPT in RAP1GAP-overexpressing cells compared to pcDNA cells (Fig. 2E, G). Consistent with these findings, impaired cell-matrix adhesion of RAP1GAP-overexpressing cells was not rescued by treatment with 8-CPT in contrast to pcDNA cells (Fig. 2F, H). These events were verified in both cell lines, although UM-SCC-17B transfected with empty vector had a more dramatic response to 8-CPT compared to UM-SCC-1 (Fig. 2G). In summary, these data show that RAP1GTP is required for cell-fibronectin adhesion.
Figure 2.
Active RAP1 is required for cell-matrix adhesion. (A) UM-SCC-1 (left panel) and UM-SCC-17B (right panel) cells were serum-starved for 3 h and RAP1 was stimulated with 8-CPT (10 µM) for 30 min. A RAP1 pulldown assay was performed and RAP1GTP was quantified. (B) An adhesion assay was performed (UM-SCC-1, P = 0.0024; UM-SCC-17B, P = 0.0019; t test). (C) Flag-tagged RAP1GAP and pcDNA empty vector was stably transfected in UM-SCC-1 and UM-SCC-17B. Cell lysates were immunoblotted with anti-FLAG antibody (upper panel). A RAP1 pulldown assay was performed and quantified (lower panel). (D) UM-SCC-1 and UM-SCC-17B stably transfected with pcDNA or RAP1GAP were plated at an equal density and an adhesion assay was performed (UM-SCC-1: pcDNA vs. RAP1GAP, P = 0.0022; UM-SCC-17B: pcDNA vs. RAP1GAP, P = 0.0085; t test). (E) UM-SCC-1 and (G) UM-SCC-17B stably transfected with pcDNA or RAP1GAP were stimulated with 8-CPT (10 µM) for the indicated times and a RAP1 pulldown assay was performed. (F) UM-SCC-1 and (H) UM-SCC-17B stably overexpressing pcDNA or RAP1GAP were stimulated with 8-CPT, and adhesion assays were performed (UM-SCC-1-pcDNA: control vs. 8-CPT, P = 0.0072; UM-SCC-17B-pcDNA: control vs. 8-CPT, P = 0.0270; t test). Data are representative of 3 independent experiments with 3 replicates in each experiment. DU, arbitrary densitometry unit: RAP1GTP was normalized to corresponding GAPDH-normalized total RAP1 and expressed as percentage of control. *P ≤ 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P ≤ 0.0001.
Inside-Out Signaling via RAP1 Is Required for α5β1 Integrin-Mediated Cell Adhesion to Fibronectin
Since α5β1 integrin is the receptor for fibronectin, α5β1’s role in mediating the effects of RAP1 on cell-fibronectin adhesion was verified. A functional approach showed that concomitant neutralization of α5 and β1 reduces adhesion to fibronectin of UM-SCC-1 and UM-SCC-17B (Fig. 3A). These results were further confirmed in stably transfected control cell lines, UM-SCC-1 and UM-SCC-17B; however, in cell lines stably overexpressing RAP1GAP, which inactivates RAP1GTP, there was no additional inhibitory effect associated with neutralization of α5 and β1 (Fig. 3B). These results were verified by an orthogonal approach, using siRNA-mediated silencing of either α5 or β1. Initially, we investigated the efficacy of individual siRNAs to α5 or β1 (Fig. 3C). Inhibition of α5 or β1 expression reduced cell-matrix adhesion in both parent HNSCC cell lines (Fig. 3D). Similar results were observed in both control cell lines (Fig. 3E), confirming the relevance of α5β1 for HNSCC adhesion to fibronectin. Since there was no additional inhibitory effect associated with silencing α5 or β1 in cells overexpressing RAP1GAP (Fig. 3E), these results indicate that inside-out signaling via RAP1 is required for α5β1-mediated adhesion to fibronectin.
Figure 3.
Inside-out signaling via RAP1 is required for α5β1 integrin-mediated cell adhesion to fibronectin. (A) UM-SCC-1 and UM-SCC-17B were incubated with antibodies to α5, β1, and α5β1 or control IgG (0.025 mg/mL), and an adhesion assay was performed (P < 0.05, 1-way analysis of variance [ANOVA]). (B) UM-SCC-1 and UM-SCC-17-B stably overexpressing RAP1GAP or pcDNA were incubated with anti-α5β1 (0.025 mg/mL) or control IgG and an adhesion assay was performed (P < 0.05, t test). (C) UM-SCC-1 and UM-SCC-17B were treated with 4 distinct small interfering RNAs (siRNAs) to α5 (left panel) and β1 (right panel) or with nontargeting (NT) siRNA. Signal intensities were quantified and normalized to GAPDH and expressed as a percentage of corresponding NT control. (D) An adhesion assay was performed with UM-SCC-1 and UM-SCC-17B with 2 distinct siRNAs to α5 (siα5: –10 and −11) and β1 (siβ1: −7 and –8), or NT (P < 0.02, 1-way ANOVA). (E) siα5 or siβ1 or NT siRNAs were transfected in UM-SCC-1 (left) and UM-SCC-17B (right), stably overexpressing RAP1GAP or pcDNA, and adhesion assays were performed (P < 0.05). Data are representative of 3 independent experiments with 3 replicates in each experiment (1-way ANOVA). *P ≤ 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P ≤ 0.0001.
RAC1 Is Required for RAP1-Mediated Cell Adhesion to Fibronectin
Since RAC1GTP regulates cell-cell and cell-matrix adhesion and is associated with aggressive tumors, we assessed the role of RAC1 in RAP1-regulated cell-matrix adhesion. 8-CPT induced RAC1 activation in both HNSCC cell lines (Fig. 4A). Conversely, overexpression of RAP1GAP reduced activation of RAC1, further supporting that RAP1GTP is upstream of RAC1GTP (Fig. 4B). Silencing RAC1 (Fig. 4C) reduced cell-fibronectin adhesion in both UM-SCC-1 and UM-SCC-17B parent cell lines (Fig. 4D), verifying the relevance of RAC1 for cell adhesion to fibronectin in HNSCC. In support of RAP1 as an upstream activator of RAC1-mediated cell adhesion, siRAC1 reduced adhesion of both HNSCC cell lines transfected with the empty vector, whereas siRAC1 did not have additional inhibitory effects in both RAP1GAP-overexpressing cell lines (Fig. 4E). Inhibition of α5β1 integrin reduced RAC1 activation in pcDNA-transfected control cells but not in RAP1GAP-overexpressing cells (Fig. 4F).
Figure 4.
RAC1 is required for RAP1-mediated cell adhesion to fibronectin. (A) UM-SCC-1 and UM-SCC-17B were stimulated with 8-pCPT-2′O-Me-cAMP (8-CPT) (10 µM) for the indicated time. A RAC1 pulldown assay was performed; data were normalized to corresponding GAPDH-normalized total RAC1 and expressed as a percentage of control. (B) A RAC1 pulldown assay was performed with UM-SCC-1 and UM-SCC-17B stably overexpressing RAP1GAP or pcDNA. Arrowhead shows RAC1GTP. (C) RAC1 was downregulated in UM-SCC-1 and UM-SCC-17B with 4 different small interfering RNAs (siRNAs) targeting RAC1. Nontargeting (NT) siRNA was used as a control. Lysates were immunoblotted with anti-RAC1 antibody to verify knockdown. GAPDH was used as a loading control. (D) Adhesion assays were performed with siRAC1-14 and siRAC1-15 in UM-SCC-1 and UM-SCC-17B. NT siRNA was used as a control (UM-SCC-1: NT vs. siRAC1-14, P = 0.2916, NT vs. siRAC1-15, P = 0.0377; UM-SCC-17B: NT vs. siRAC1-14, P = 0.0175, NT vs. siRAC1-15, P = 0.0071, 1-way analysis of variance [ANOVA]). (E) Adhesion assays were performed with UM-SCC-1 (top panel) and UM-SCC-17B (bottom panel) stably transfected with pcDNA or RAP1GAP and transiently transfected with siRAC1 (SCC1-pcDNA: NT vs. siRAC1-14, P = 0.0102, NT vs. siRAC1-15, P = 0.0001; SCC17B-pcDNA: NT vs. siRAC1-14, P = 0.2217, NT vs. siRAC1-15, P = 0.1217). Data are representative of 3 independent experiments with 3 replicates in each experiment (1-way ANOVA). (F) α5β1 integrin was neutralized in UM-SCC-1 and UM-SCC-17B stably transfected with pcDNA and RAP1GAP. A RAC1 pulldown assay was performed. Arrowhead shows RAC1GTP. (G) Focal adhesion kinase (FAK) phosphorylation was evaluated in UM-SCC-1 induced by 8-CPT (left panel) and in cells stably transfected with pcDNA or RAP1GAP (right panel). Data are representative of 2 experiments. DU, arbitrary densitometry unit: RAP1GTP or RAC1GTP was normalized to corresponding GAPDH-normalized total RAP1 or total RAC1 and expressed as a percentage of control. *P ≤ 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P ≤ 0.0001.
Since focal adhesion kinase (FAK) activation precedes RAC1 activation (Huveneers and Danen 2009), we investigated whether RAP1-mediated activation of RAC1 occurs via FAK phosphorylation (Fig. 4G and Appendix Fig.). FAK was phosphorylated at tyrosine 397 when RAP1 was activated with 8-CPT (Fig. 4G, left panel). In a complementary approach, in RAP1GAP-overexpressing cells that have endogenously inactive RAP1, phosphorylation of FAK was less than in control cells (Fig. 4G, right panel). Collectively, these data indicate that RAC1 is required for RAP1-mediated adhesion of HNSCC to fibronectin.
RAC1GTP Is Also Required for RAP1-Mediated Migration
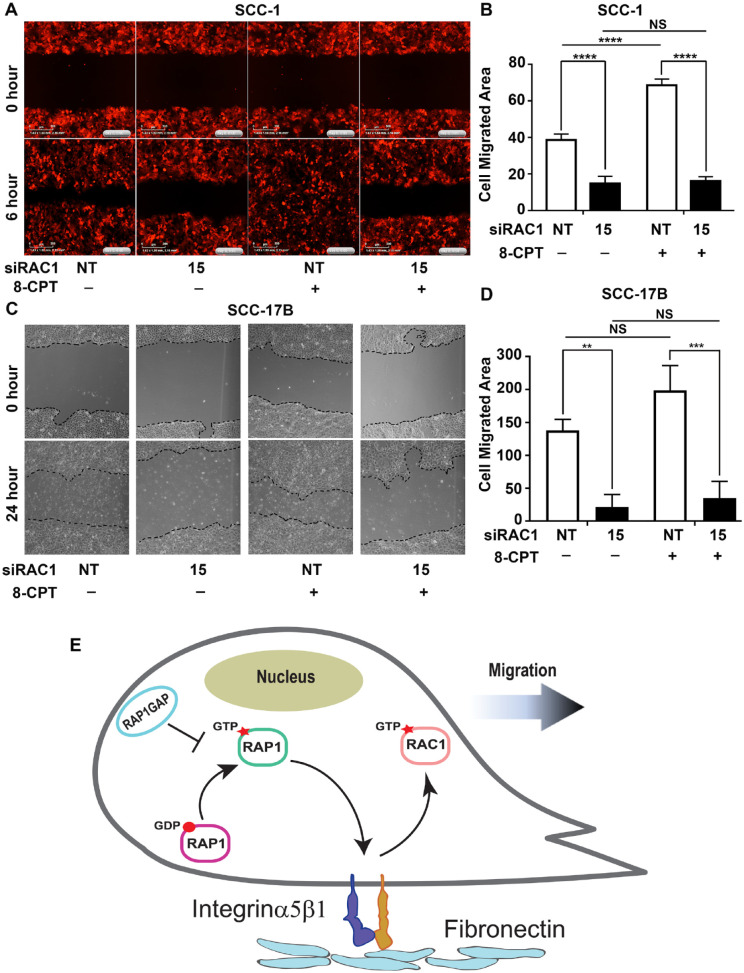
Cell-matrix adhesion affects cell migration, an important phenotype in the spread of HNSCC. Since RAP1GTP and RAC1GTP have a prominent role in adhesion to fibronectin, we subsequently determined the impact of RAP1/RAC1 signaling on HNSCC cell migration. m-Cherry labeled UM-SCC-1 with nontarget or siRAC1 were activated by 8-CPT and a wound-healing assay was recorded in time-lapse microscopy using a live-cell imaging system (Johnston et al. 2015). RAP1GTP significantly increased cell migration (Fig. 5A, third panel, Appendix Video; Fig. 5B). However, this effect was abrogated when RAC1 was downregulated (Fig. 5A, fourth panel, Appendix Video; Fig. 5B). Similar findings were observed in UM-SCC-17B (Fig. 5C, D). These data show that RAP1-induced cell migration is dependent on RAC1, indicating that the Epac/RAP1/RAC1 signaling axis promotes migration (Fig. 5A–D). Together, these results show that RAP1 signaling facilitates cell migration via RAC1.
Figure 5.
Rac1 activation is also required for RAP1-mediated migration of head and neck squamous cell carcinoma cells. (A, B) RAC1 was downregulated in UM-SCC-1 stably transfected with Life-Act-mCherry and stimulated with 8-pCPT-2′O-Me-cAMP (8-CPT) (10 µM). A scratch assay was performed and cell migration was recorded on a live-cell imaging system (a video file is also available in the Appendix). Cell migration was quantified as change in covered area relative to 0 h in 5 representative fields (B; P < 0.02, 1-way analysis of variance [ANOVA]). (C, D) RAC1GTP was downregulated in UM-SCC-17B stimulated with 8-CPT (10 µM). Cell migration was investigated by scratch assay. Cell migration was quantified as change in covered area relative to 0 h in 5 representative fields (P < 0.03, 1-way ANOVA). Each experiment was performed 2 (B) and 3 (C) times and representative results are shown (1-way ANOVA). (E) A schematic diagram shows that RAP1GTP activates RAC1 via inside-out signaling through α5β1 integrin, resulting in cell migration. *P ≤ 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P ≤ 0.0001.
Discussion
Previously, we showed that RAP1 activation in HNSCC is correlated with proliferation and survival (Banerjee et al. 2012). Here, we show that RAP1GTP is correlated with cell-cell adhesion via E-cadherin and is required for cell-fibronectin interaction via α5β1 integrin in HNSCC. Previous studies showed that RAP1GTP is required for initial cell-cell adhesion and for reformation of adherens junctions (Dube et al. 2008) but not for maintenance of adhesion since RAP1GTP decreases after restoring cell-cell contacts (Wittchen et al. 2005). In this study, overexpression of RAP1GAP reduced RAP1GTP and cell-fibronectin adhesion via α5β1 integrin; however, this approach does not allow for the assessment of the relevance of RAP1GTP in established cell-cell contacts. In fact, silencing RAP1 does not affect mature cell-cell adhesion contacts in cell monolayers, indicating that RAP1 is required for formation or reformation of cell-cell junctions but not for maintenance (Dube et al. 2008).
There are 6 major groups of RAP-activating GEFs (C3G, PDZ-GEFs, Epac proteins, RASGRP2 and 3, phospholipase C epsilon, and the atypical RAPGEF DOCK4) (Pannekoek et al. 2009), which respond to different stimuli to activate RAP1 and control its spatiotemporal localization (Kooistra et al. 2007). Epac, C3G, PDZ-GEF, and DOCK4 regulate cell-cell and cell-ECM adhesion (Pannekoek et al. 2009). The role of Epac in mediating RAP1 activation and subsequent tightening of E-cadherin cell-cell junctions has been described primarily in endothelial cells, both in vitro and in vivo (Kooistra et al. 2007). In epithelial cells, both PDZ-GEF and Epac promote maturation of cell-cell adhesion (Dube et al. 2008).
High fibronectin expression in HNSCC is associated with lymph node metastasis (Luksic and Suton 2017) and with worse prognosis in tongue cancer, suggesting that cell-fibronectin adhesion via α5β1 integrin is involved in spread. Inhibition of RAP1GTP reduced cell adhesion to fibronectin, indicating that RAP1 is involved in inside-out α5β1 integrin signaling. RAP1 has also been shown to mediate reelin-induced neuronal adhesion to fibronectin via inside-out signaling to α5β1 (Sekine et al. 2012). The fact that RAP1 activation promotes migration of HNSCC in vitro while promoting both cell-cell and cell-fibronectin adhesion may be related with the regulation of coordinated events involving strengthening of leading-edge focal adhesions, actomyosin contraction, and disassembling of rear-end focal adhesions in an “anchor-pull” mechanism of polarized cells (Ridley et al. 2003).
In HNSCC, RAP1GTP activation modulates cell-cell and cell-ECM adhesion. A conflicting report shows that RAP1 activation via cAMP/Epac inhibits epithelial cell migration independently of cell-cell or cell-ECM adhesion by stabilizing focal cell-ECM adhesions and inhibiting membrane protrusions (Lyle et al. 2008). These contrasting findings may be related to the context in which migration was assessed. We observed that RAP1 activation via cAMP/Epac increased spontaneous migration of human HNSCC, as opposed to the inhibition of HGF- and TGFβ1-induced migration (Lyle et al. 2008). These contrasting findings on RAP1 activation and cell migration may also be related to differences in cell types: human HNSCC versu human lung adenocarcinoma and renal carcinoma and dog epithelial kidney cells. Moreover, genetic and epigenetic backgrounds of different cell types can influence the relative expression of the 2 RAP1 isoforms, and it is possible that contrasting findings are related with relative levels of expression of RAP1A and RAP1B. RAP1A and RAP1B are 95% homologous; however, null mice have very distinct phenotypes: deletion of RAP1B is embryonically lethal (Chrzanowska-Wodnicka et al. 2005), while RAP1A null mice are viable but their hematopoietic cells have impaired adhesion to ICAM1 and fibronectin (Duchniewicz et al. 2006). RAP1A and RAP1B isoforms can have specific effects on the regulation of cell-cell and cell-ECM adhesion. Silencing expression of RAP1B did not affect maturation of cell-cell adhesion but reduced total E-cadherin protein and its localization to the cell surface (Dube et al. 2008). In this study, we did not distinguish between the effects of the 2 RAP1 isoforms. Just as the isoforms of RAP1 may have distinct roles in cell adhesion, it is possible that specific GEFs are preferentially used for the formation or reformation of adherens junctions and for the pattern of cell migration (Pannekoek et al. 2009).
The dynamic interplay between cell-cell and cell-ECM adhesion driving neoplastic cell migration involves crosstalk between E-cadherin and integrin signaling pathways and also the physical interaction between E-cadherin- and integrin-mediated adhesions. Small GTPases can also be signaling intermediates in the crosstalk between E-cadherin and integrin signaling. We show that RAP1 activation is correlated with cell-cell (via E-cadherin) and required for cell-fibronectin (via α5β1 integrin) adhesion of HNSCC cells. It is possible that promotion of fibronectin adhesion via α5β1 by RAP1GTP also modulates the transition from dormant to actively proliferating/migrating cells. Decrease of urokinase plasminogen activator receptor in an oral squamous cell carcinoma cell line reduced adhesion to fibronectin via α5β1 integrin, which induced a dormant state (Aguirre Ghiso et al. 1999). It is tempting to speculate that the crosstalk of RAP1/α5β1 integrin signaling is involved in reversing this effect in HNSCC. Our study showed that inhibition of α5β1 integrin partially inhibited cell adhesion (Fig. 3A, B), suggesting that other fibronectin receptors, such as αVβ3, αVβ5, αVβ6, and αVβ1, and surface proteoglycan, such as syndecans, may be involved (Geiger et al. 2001). Moreover, ligand interaction site in fibronectin varies widely depending on integrin dynamics and other unidentified nonintegrin receptors depending on cell type (Geiger et al. 2001).
Among downstream targets of RAP1, Cdc42 from the Rho family of small GTPases increases the recruitment of E-cadherin to newly formed cell-cell contacts (Hogan et al. 2004). RAC1, another member of the Rho family, has a role in cell migration and integrin signaling/rearrangement of the cytoskeleton. RAC1 may also participate in cell-cell adhesion (Menke and Giehl 2012), and cAMP/Epac-activated RAP1 activates RAC1 (Maillet et al. 2003). In this study, we show that RAC1 in HNSCC has an important role in mediating RAP1-induced cell adhesion and migration. RAC1GTP triggers lamellipodia formation (Ehrlich et al. 2002), which is critical for cell migration (Yasui et al. 2017). RAP1 also activates RAC1 at specific sites of lamellipodia by binding and promoting the localization of the RacGEFs VAV2 and Tiam1 to these sites (Arthur et al. 2004). Interestingly, a constitutively active mutant of RAP1 and 8-CPT/Epac-activated RAP1 binds directly to STEF, a RacGEF, to induce RAC1GTP (Zaldua et al. 2007). Collectively, this information supports various mechanisms for RAP1-induced activation of RAC1 at different subcellular sites. Intriguingly, increased RAC1 activation has been linked to a single-cell or mesenchymal phenotype of cell migration, with reduced cell-cell junctions (Parri and Chiarugi 2010). The specificity of the GEF involved in RAC1 activation may play an important role, as RAC1 activation via Tiam1 inhibits cell migration and invasion (Nola et al. 2011).
Small GTPases influence multiple aspects of cell biology, including proliferation, survival, gene expression, and migration. We show that in HNSCC, Epac-mediated RAP1 activation is required for α5β1 integrin-mediated adhesion to fibronectin and is correlated with cell-cell adhesion. Moreover, we show that the Epac/RAP1 signaling axis activates RAC1, which enhances cell migration. Contrasting evidence on the role of RAP1 in cell migration and invasion suggests a cell-type and/or microenvironment-specific influence. Moreover, the specific GEF activating RAP1 can have a decisive influence on the outcome. Understanding the role of RAP1 and RAC1, as well as of the activating GEF, may lead to the development of more effective approaches for inhibition of neoplastic cell migration without affecting the physiologic role.
Author Contributions
M. Liu, contributed to design, data acquisition, and analysis, critically revised the manuscript; R. Banerjee, contributed to conception, design, data acquisition, and analysis, critically revised the manuscript; C. Rossa Jr., contributed to data analysis, critically revised the manuscript; N.J. D’Silva, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520917058 for RAP1-RAC1 Signaling Has an Important Role in Adhesion and Migration in HNSCC by M. Liu, R. Banerjee, C. Rossa and N.J. D’Silva in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by grants from the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) DE027551 and DE022567 (N.J. D’Silva), University of Michigan–FAPESP 2014/50312-4 (N.J. D’Silva and C. Rossa Jr.), and 2017/14283-5 (C. Rossa Jr.). All data files and uncropped images are available on request.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aguirre Ghiso JA, Kovalski K, Ossowski L. 1999. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 147(1):89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. 2004. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 167(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Russo N, Liu M, Van Tubergen E, D’Silva NJ. 2012. Rap1 and its regulatory proteins: the tumor suppressor, oncogene, tumor suppressor gene axis in head and neck cancer. Small GTPases. 3(3):192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Yen CY, Chen CH, Tsai JH, Tang JY, Chang YT, Kao YH, Wang YY, Yuan SF, Lee SY. 2018. Evaluation of the mRNA expression levels of integrins α3, α5, β1 and β6 as tumor biomarkers of oral squamous cell carcinoma. Oncol Lett. 16(4):4773–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., II 2005. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 115(3):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Owen KA, Ly KT, Park D, Black SG, Wilson JM, Sifri CD, Ravichandran KS, Ernst PB, Casanova JE. 2011. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of gram-negative bacteria. Proc Natl Acad Sci USA. 108(5):2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube N, Kooistra MR, Pannekoek WJ, Vliem MJ, Oorschot V, Klumperman J, Rehmann H, Bos JL. 2008. The RapGEF PDZ-GEF2 is required for maturation of cell-cell junctions. Cell Signal. 20(9):1608–1615. [DOI] [PubMed] [Google Scholar]
- Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. 2006. Rap1a-deficient t and b cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 26(2):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, Nelson WJ. 2002. Spatio-temporal regulation of rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 3(2):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 4(11):901–906. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2(11):793–805. [DOI] [PubMed] [Google Scholar]
- Hamidi H, Ivaska J. 2018. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 18(9):533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. 2004. Rap1 regulates the formation of e-cadherin-based cell-cell contacts. Mol Cell Biol. 24(15):6690–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. 2008. Epac-selective camp analogs: new tools with which to evaluate the signal transduction properties of camp-regulated guanine nucleotide exchange factors. Cell Signal. 20(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Gillette JM, Kang H, Rivera MR, Wandinger-Ness A. 2018. Ovarian tumor microenvironment signaling: convergence on the Rac1 GTPase. Cancers (Basel). 10(10). pii: E358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. 2009. Adhesion signaling—crosstalk between integrins, src and rho. J Cell Sci. 122(Pt 8):1059–1069. [DOI] [PubMed] [Google Scholar]
- Johnston ST, Shah ET, Chopin LK, Sean McElwain DL, Simpson MJ. 2015. Estimating cell diffusivity and cell proliferation rate by interpreting IncuCyte ZOOM™ assay data using the Fisher-Kolmogorov model. BMC Syst Biol. 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AL, Brown NH. 2002. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 295(5558):1285–1288. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Dube N, Bos JL. 2007. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 120(Pt 1):17–22. [DOI] [PubMed] [Google Scholar]
- Luksic I, Suton P. 2017. Predictive markers for delayed lymph node metastases and survival in early-stage oral squamous cell carcinoma. Head Neck. 39(4):694–701. [DOI] [PubMed] [Google Scholar]
- Lyle KS, Raaijmakers JH, Bruinsma W, Bos JL, de Rooij J. 2008. cAMP-induced Epac-Rap activation inhibits epithelial cell migration by modulating focal adhesion and leading edge dynamics. Cell Signal. 20(6):1104–1116. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F. 2003. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 5(7):633–639. [DOI] [PubMed] [Google Scholar]
- Maldonado MDM, Dharmawardhane S. 2018. Targeting Rac and Cdc42 GTPases in cancer. Cancer Res. 78(12):3101–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Giehl K. 2012. Regulation of adherens junctions by rho GTPases and p120-catenin. Arch Biochem Biophys. 524(1):48–55. [DOI] [PubMed] [Google Scholar]
- Mitra RS, Zhang Z, Henson BS, Kurnit DM, Carey TE, D’Silva NJ. 2003. Rap1a and Rap1b Ras-family proteins are prominently expressed in the nucleus of squamous carcinomas: nuclear translocation of GTP-bound active form. Oncogene. 22(40):6243–6256. [DOI] [PubMed] [Google Scholar]
- Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. 2011. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 195(5):855–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. 2009. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 1788(4):790–796. [DOI] [PubMed] [Google Scholar]
- Parri M, Chiarugi P. 2010. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA. 2009. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 69(4):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. 2003. Cell migration: integrating signals from front to back. Science. 302(5651):1704–1709. [DOI] [PubMed] [Google Scholar]
- Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, Hattori M, Kinashi T, Nakajima K. 2012. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron. 76(2):353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. 2010. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 11(4):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA Cancer J Clin. 66(1):7–30. [DOI] [PubMed] [Google Scholar]
- Su YF, Liang CY, Huang CY, Peng CY, Chen CC, Lin MC, Lin RK, Lin WW, Chou MY, Liao PH, et al. 2014. A putative novel protein, DEPDC1B, is overexpressed in oral cancer patients, and enhanced anchorage-independent growth in oral cancer cells that is mediated by Rac1 and ERK. J Biomed Sci. 21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies.J Cell Biol. 137(1):231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. 2005. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 280(12):11675–11682. [DOI] [PubMed] [Google Scholar]
- Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, Reynolds P, Bell DW, Lake R, van den Heuvel S, et al. 2003. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 112(5):673–684. [DOI] [PubMed] [Google Scholar]
- Yasui H, Ohnishi Y, Nakajima M, Nozaki M. 2017. Migration of oral squamous cell carcinoma cells are induced by HGF/c-Met signalling via lamellipodia and filopodia formation. Oncol Rep. 37(6):3674–3680. [DOI] [PubMed] [Google Scholar]
- Zaldua N, Gastineau M, Hoshino M, Lezoualc’h F, Zugaza JL. 2007. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett. 581(30):5814–5818. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Fu X, Yang A. 2017. Identification of key genes and pathways in tongue squamous cell carcinoma using bioinformatics analysis. Med Sci Monit. 23:5924–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520917058 for RAP1-RAC1 Signaling Has an Important Role in Adhesion and Migration in HNSCC by M. Liu, R. Banerjee, C. Rossa and N.J. D’Silva in Journal of Dental Research