Abstract
Background
A multisystem inflammatory syndrome in children (MIS-C) is associated with coronavirus disease 2019. The New York State Department of Health (NYSDOH) established active, statewide surveillance to describe hospitalized patients with the syndrome.
Methods
Hospitals in New York State reported cases of Kawasaki’s disease, toxic shock syndrome, myocarditis, and potential MIS-C in hospitalized patients younger than 21 years of age and sent medical records to the NYSDOH. We carried out descriptive analyses that summarized the clinical presentation, complications, and outcomes of patients who met the NYSDOH case definition for MIS-C between March 1 and May 10, 2020.
Results
As of May 10, 2020, a total of 191 potential cases were reported to the NYSDOH. Of 95 patients with confirmed MIS-C (laboratory-confirmed acute or recent severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection) and 4 with suspected MIS-C (met clinical and epidemiologic criteria), 53 (54%) were male; 31 of 78 (40%) were black, and 31 of 85 (36%) were Hispanic. A total of 31 patients (31%) were 0 to 5 years of age, 42 (42%) were 6 to 12 years of age, and 26 (26%) were 13 to 20 years of age. All presented with subjective fever or chills; 97% had tachycardia, 80% had gastrointestinal symptoms, 60% had rash, 56% had conjunctival injection, and 27% had mucosal changes. Elevated levels of C-reactive protein, d-dimer, and troponin were found in 100%, 91%, and 71% of the patients, respectively; 62% received vasopressor support, 53% had evidence of myocarditis, 80% were admitted to an intensive care unit, and 2 died. The median length of hospital stay was 6 days.
Conclusions
The emergence of multisystem inflammatory syndrome in children in New York State coincided with widespread SARS-CoV-2 transmission; this hyperinflammatory syndrome with dermatologic, mucocutaneous, and gastrointestinal manifestations was associated with cardiac dysfunction.
Since December 2019, the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), has resulted in high morbidity and mortality worldwide. New York State emerged as an epicenter of the outbreak in the United States, with more than 348,000 cases of laboratory-confirmed SARS-CoV-2 infection as of May 10, 2020.1-3 Globally and in New York State, initial reports indicated that children typically have mild or no Covid-19 symptoms and have lower rates of hospitalization and death than adults.3-7 However, in early May, the United Kingdom and several European countries reported the occurrence of a hyperinflammatory process in children that had features similar to atypical Kawasaki’s disease, Kawasaki’s disease shock syndrome, and toxic shock syndrome, possibly related to SARS-CoV-2 infection.8-12 On May 13, 2020, the New York State Department of Health (NYSDOH) released an interim case definition,13 and on May 14, 2020, the Centers for Disease Control and Prevention (CDC) released a Health Alert Network advisory about multisystem inflammatory syndrome in children (MIS-C) associated with Covid-19.14
Between January 1 and May 10, 2020, the NYSDOH received reports of 15,515 cases of SARS-CoV-2 infection detected by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay among children and adolescents younger than 21 years of age; 816 patients (5%) were hospitalized with acute Covid-19, and 14 (0.1%) died.3 After the initial reports of suspected cases of MIS-C in early May, the NYSDOH established active, statewide surveillance to rapidly ascertain cases of possible MIS-C from hospitals through daily required reporting.
We describe the demographic characteristics, presenting symptoms, clinical course, laboratory findings, therapy received, and outcomes among children and adolescents meeting the NYSDOH case definition of MIS-C. We also examine the temporal relationship between the Covid-19 outbreak in New York State and reported cases of MIS-C.
Methods
Data Reporting
Reporting and investigation of reportable communicable diseases, including SARS-CoV-2 (and its complications, such as MIS-C), are mandated under New York State Public Health Law.15 Data were collected as part of a public health response; human research protection review at the CDC determined that institutional-review-board review was not required.
Data Sources
On May 5, 2020, the NYSDOH required 106 hospitals in New York State that provide pediatric medical or surgical care to report potential cases of MIS-C among persons younger than 21 years of age admitted since March 1, 2020, through the NYSDOH Health Emergency Response Data System. Hospitals were asked to identify and submit medical records for patients who received a diagnosis of Kawasaki’s disease, toxic shock syndrome, or myocarditis or who were suspected to have MIS-C. The NYSDOH Electronic Clinical Laboratory Reporting System (ECLRS) was used to collect data on the results of RT-PCR or serologic tests for SARS-CoV-2 from reported patients.
Case Definition
On May 13, 2020, the NYSDOH established an interim case definition of MIS-C (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Confirmed cases were defined by the presence of both clinical and laboratory criteria. Laboratory evidence consisted of elevated levels of two or more inflammatory markers and virologic evidence (any positive molecular test for SARS-CoV-2) or serologic evidence within 10 days after admission (reactivity for IgG or IgM antibodies against SARS-CoV-2). For this evaluation, patients without supportive virologic laboratory evidence of SARS-CoV-2 were categorized as having a suspected case if they met clinical criteria and had exposure to a person with Covid-19 in the 6 weeks before hospitalization.
Standardized Study Definitions
We defined values for tachycardia, tachypnea, hypotension, lymphopenia, neutropenia, and elevated levels of pro–brain natriuretic peptide (proBNP) (all during the first 24 hours of admission) on the basis of age standards.16-18 Myocarditis was determined by discharge diagnosis or the code in the International Classification of Diseases, 10th Revision. Clinical myocarditis was defined as cardiac dysfunction on echocardiography with an elevated troponin level; if troponin levels were not tested, clinical myocarditis was defined as elevated levels of proBNP or brain natriuretic peptide and cardiac dysfunction (defined as any ventricular dysfunction or hypokinesia or decreased contractility or ejection fraction) or arrhythmia on electrocardiography. Coronary-artery aneurysm was reported on the basis of echocardiographic findings and z scores (Boston criteria, when available).
Study Sample
Using a modified version of the CDC Covid-19 standardized case-report form, a trained team of physicians, nurses, and epidemiologists abstracted demographic information, clinical characteristics, laboratory values, hospital course, treatment, and outcomes from possible cases among patients admitted from March 1 through May 10, 2020. Presenting signs and symptoms were classified as constitutional (fever, subjective fever, or chills), cardiovascular (chest pain), gastrointestinal (abdominal pain, nausea, vomiting, or diarrhea), dermatologic (rash or swelling of fingers, hands, toes, or feet), mucocutaneous (conjunctival injection or mucosal changes), neurologic (headache, altered mental status, or confusion), lymphadenopathy, musculoskeletal (myalgias or arthralgias), upper respiratory (congestion or sore throat), or lower respiratory (cough, shortness of breath, or wheezing).
Statistical Analysis
Medians and interquartile ranges for continuous variables and frequencies and proportions for categorical variables were calculated with the use of SAS software, version 9.4 (SAS Institute). Patient age was grouped into three categories: less than 6 years, 6 to 12 years, and 13 to 20 years. The NYSDOH ECLRS laboratory data were used to calculate the incidence of laboratory-confirmed Covid-19 and to generate an epidemiologic curve of laboratory-confirmed cases of Covid-19 among pediatric and adolescent patients according to the date of specimen collection, for comparison with the date of hospital admission among patients with MIS-C.
Authors at the NYSDOH designed and implemented the surveillance system and evaluation. All the authors participated in the data-collection process, analyzed the data, wrote the manuscript, made the decision to submit the manuscript for publication, and vouch for the completeness and accuracy of the data. Data were collected as part of public health reporting of communicable disease, and the confidentiality of the data were maintained by the NYSDOH.
Results
Study Population and SARS-CoV-2 Testing
From March 1 through May 10, 2020, there were 191 reports submitted to the NYSDOH regarding admitted patients younger than 21 years of age. A total of 189 medical records were received, and 161 were abstracted (Fig. S2). Of these 161 patients, 99 met the NYSDOH interim case definition of MIS-C (for comparison of included and excluded patients, see Table S1); 95 patients (96%) were classified as having a confirmed case and 4 (4%) as having a suspected case. Of the 99 patients, 29 (29%) met clinical criteria with one or more of the following: hypotension or shock, severe cardiac illness, or other severe end-organ illness; 6 (6%) met clinical criteria with two or more of the following: rash, conjunctivitis, mucocutaneous signs, or gastrointestinal symptoms; and 64 (65%) met both types of clinical criteria. Between March 1 to May 10, of the 95 patients with confirmed cases, 94 (99%) were tested for SARS-CoV-2 infection with an RT-PCR assay and 77 (81%) were tested for the presence of SARS-CoV-2 antibodies with a serologic assay; 19 (20%) had evidence of SARS-CoV-2 infection from an RT-PCR assay alone, 45 (47%) had evidence of SARS-CoV-2 infection from a serologic assay alone (1 of whom was never tested for SARS-CoV-2 infection with an RT-PCR assay), and 31 (33%) had evidence of SARS-CoV-2 from both RT-PCR and serologic assays. Of the 77 patients tested for the presence of IgG, 76 (99%) were reactive; 3 patients were reactive for IgA, 3 were reactive for IgM, and 21 were nonreactive for IgM. There were no patients with IgM or IgA reactivity alone. Of the 76 patients with serologic evidence of SARS-CoV-2 infection, 40 (53%) had reactive IgG before or on the first day of admission.
All four patients with suspected cases had negative molecular testing; none underwent serologic testing. Two (50%) had Covid-19–like illness in the 6 weeks before MIS-C symptom onset. Because the characteristics of the patients with confirmed cases and of the patients with suspected cases were similar (data not shown), the two groups were combined for further analyses.
Characteristics of the Patients
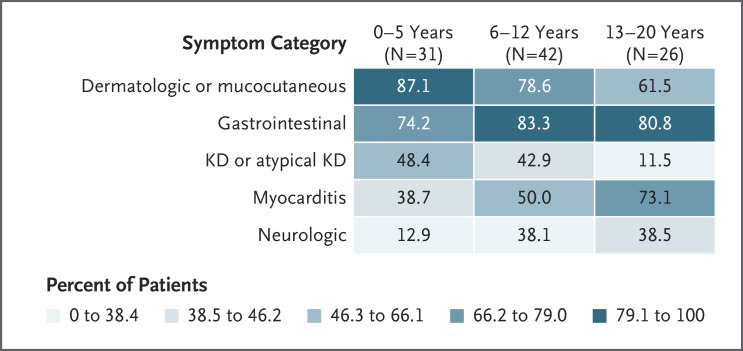
Of the 99 patients with MIS-C, 53 (54%) were male. A total of 31 patients (31%) were 0 to 5 years of age, 42 (42%) were 6 to 12 years of age, and 26 (26%) were 13 to 20 years of age (Table 1). Of 78 patients with data on race, 29 (37%) were white, 31 (40%) were black, 4 (5%) were Asian, and 14 (18%) were of other races; of 85 patients with data on ethnic group, 31 (36%) were Hispanic (Table 2). Of the 36 patients with a preexisting condition, 29 had obesity. All the patients had fever or chills at admission. Other common presenting symptoms were gastrointestinal (80%), dermatologic (62%), mucocutaneous (61%), and lower respiratory (40%). A total of 60 patients (61%) had gastrointestinal and either dermatologic or mucocutaneous symptoms. Figure 1 shows symptom categories according to age group. Neurologic symptoms, predominantly headache, were present in 13% of the patients 0 to 5 years of age and 38% of those 13 to 20 years of age. A total of 48% of the patients 0 to 5 years of age and 43% of those 6 to 12 years of age presented with Kawasaki’s disease or atypical Kawasaki’s disease, whereas 12% of those 13 to 20 years of age had such a presentation.
Table 1. Patients at Hospital Admission with Confirmed or Suspected Multisystem Inflammatory Syndrome in Children (MIS-C).*.
| Characteristic | Overall (N=99) |
Confirmed MIS-C (N=95) |
Suspected MIS-C (N=4) |
|---|---|---|---|
| number (percent) | |||
| Male sex | 53 (54) | 51 (54) | 2 (50) |
| Age | |||
| 0–5 yr | 31 (31) | 27 (28) | 4 (100) |
| 6–12 yr | 42 (42) | 42 (44) | 0 |
| 13–20 yr | 26 (26) | 26 (27) | 0 |
Percentages may not total 100 because of rounding.
Table 2. Demographic and Clinical Characteristics of the Patients at Hospital Admission, According to Age Group.*.
| Characteristic | Overall (N=99) |
0–5 Years (N=31) |
6–12 Years (N=42) |
13–20 Years (N=26) |
|---|---|---|---|---|
| Positivity for SARS-CoV-2 — no./total no. (%) | ||||
| On RT-PCR assay | 50/98 (51) | 15/31 (48) | 19/41 (46) | 16/26 (62) |
| On serologic assay for IgG antibodies | 76/77 (99) | 24/25 (96) | 33/33 (100) | 19/19 (100) |
| Male sex — no. (%) | 53 (54) | 20 (65) | 18 (43) | 15 (58) |
| Race — no./total no. (%)† | ||||
| White | 29/78 (37) | 10/27 (37) | 13/34 (38) | 6/17 (35) |
| Black | 31/78 (40) | 13/27 (48) | 12/34 (35) | 6/17 (35) |
| Asian | 4/78 (5) | 0/27 | 2/34 (6) | 2/17 (12) |
| Other‡ | 14/78 (18) | 4/27 (15) | 7/34 (21) | 3/17 (18) |
| Ethnic group — no./total no. (%)† | ||||
| Hispanic | 31/85 (36) | 12/28 (43) | 11/35 (31) | 8/22 (36) |
| Not Hispanic | 54/85 (64) | 16/28 (57) | 24/35 (69) | 14/22 (64) |
| Coexisting conditions — no. (%)§ | ||||
| Any | 36 (36) | 10 (32) | 14 (33) | 12 (46) |
| Chronic lung disease¶ | 14 (14) | 2 (6) | 5 (12) | 7 (27) |
| Obesity | 29 (29) | 10 (32) | 11 (26) | 8 (31) |
| Symptoms at admission — no. (%) | ||||
| Constitutional: fever or chills | 99 (100) | 31 (100) | 42 (100) | 26 (100) |
| Cardiovascular: chest pain | 11 (11) | 1 (3) | 3 (7) | 7 (27) |
| Any gastrointestinal | 79 (80) | 23 (74) | 35 (83) | 21 (81) |
| Abdominal pain | 60 (61) | 18 (58) | 29 (69) | 13 (50) |
| Nausea or vomiting | 57 (58) | 16 (52) | 25 (60) | 16 (62) |
| Diarrhea | 49 (49) | 13 (42) | 23 (55) | 13 (50) |
| Any dermatologic | 61 (62) | 24 (77) | 25 (60) | 12 (46) |
| Rash | 59 (60) | 23 (74) | 25 (60) | 11 (42) |
| Swollen hands or feet | 9 (9) | 6 (19) | 1 (2) | 2 (8) |
| Any gastrointestinal and any dermatologic | 48 (48) | 17 (55) | 21 (50) | 10 (38) |
| Any mucocutaneous | 60 (61) | 22 (71) | 25 (60) | 13 (50) |
| Conjunctivitis | 55 (56) | 21 (68) | 23 (55) | 11 (42) |
| Mucosal changes | 27 (27) | 15 (48) | 8 (19) | 4 (15) |
| Any gastrointestinal and any mucocutaneous | 48 (48) | 16 (52) | 21 (50) | 11 (42) |
| Any neurologic | 30 (30) | 4 (13) | 16 (38) | 10 (38) |
| Headache | 29 (29) | 4 (13) | 15 (36) | 10 (38) |
| Altered mental status or confusion | 2 (2) | 0 | 1 (2) | 1 (4) |
| Lymphadenopathy | 6 (6) | 4 (13) | 2 (5) | 0 |
| Any musculoskeletal | 20 (20) | 2 (6) | 9 (21) | 9 (35) |
| Muscle aches or myalgias | 17 (17) | 1 (3) | 8 (19) | 8 (31) |
| Joint pain | 4 (4) | 1 (3) | 1 (2) | 2 (8) |
| Upper respiratory | 27 (27) | 12 (39) | 9 (21) | 6 (23) |
| Congestion | 13 (13) | 8 (26) | 2 (5) | 3 (12) |
| Sore throat | 16 (16) | 5 (16) | 8 (19) | 3 (12) |
| Lower respiratory | 40 (40) | 13 (42) | 14 (33) | 13 (50) |
| Cough | 31 (31) | 11 (35) | 11 (26) | 9 (35) |
| Shortness of breath | 19 (19) | 5 (16) | 6 (14) | 8 (31) |
| Wheezing | 1 (1) | 1 (3) | 0 | 0 |
IQR denotes interquartile range, RT-PCR reverse transcriptase–polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Race and ethnic group were determined through medical-record review.
Other race included American Indian or Alaska Native or multiracial or other racial-ethnic group.
Among patients with confirmed MIS-C, 1 patient each had congenital heart disease, psoriatic arthritis, and adult-onset Still’s disease during methotrexate treatment.
A total of 12 patients had asthma or reactive airway disease, 1 had oxygen dependence, and 1 was a former 25-week-premature infant.
Figure 1. Syndrome Clusters According to Age Group among Patients with Multisystem Inflammatory Syndrome in Children (MIS-C).
Color ranges were determined at quintiles of the observed percentages. Dermatologic or mucocutaneous included the following symptoms: rash, conjunctivitis, swollen hands or feet, and mucosal changes. Gastrointestinal included the following symptoms: abdominal pain, nausea or vomiting, and diarrhea. Kawasaki’s disease (KD) or atypical KD was determined by discharge diagnosis or code in the International Classification of Diseases, 10th Revision (ICD-10). Myocarditis was determined by discharge diagnosis or ICD-10 code. Clinical myocarditis was defined as cardiac dysfunction on echocardiography with an elevated troponin level; if the troponin value was missing, clinical myocarditis was defined as an elevated level of pro–brain natriuretic peptide or brain natriuretic peptide and cardiac dysfunction or arrhythmia on electrocardiography in the context of an inflammatory process. Neurologic included the following symptoms: headache, altered mental status, and confusion.
One neonate, who was classified as having a suspected case and whose mother had asymptomatic SARS-CoV-2 infection at delivery, presented with fever and left breast cellulitis between 14 and 28 days of age. Laboratory assessment showed increasing troponin levels (increasing from 43 ng per liter to 51 ng per liter in 10 hours); cardiac two-dimensional ultrasonography showed good ventricular function and unremarkable coronary arteries. Two molecular tests for SARS-CoV-2 were negative. The discharge diagnoses were cellulitis, myocarditis, and shock. One adolescent who was pregnant (between 23 and 26 weeks of gestation), classified as having a confirmed case, was admitted with fever, headache, and chest pain. She had hypotension, and laboratory assessment showed elevated levels of troponin and other inflammatory markers. The discharge diagnoses were acute respiratory distress syndrome, perimyocarditis, and pneumonia.
On admission, 63% of the patients had fever of 38.0°C (100.4°F) or higher, 97% had tachycardia, 78% had tachypnea, and 32% had hypotension (Table 3). The median temperature on admission was 38.3°C, and the median oxygen saturation was 98%. Median and interquartile ranges for systolic and diastolic blood-pressure measurements at admission, according to age, are presented in Table S2. On admission, among patients with suspected or confirmed MIS-C, the median white-cell count was 10,400 per microliter, and 59 of 89 (66%) had lymphopenia; 74 of 82 (90%) had elevated proBNP levels, 63 of 89 (71%) had elevated troponin levels, 98 of 98 had elevated C-reactive protein levels, and 86 of 94 (91%) had elevated d-dimer levels (Table 3). Additional clinical and laboratory findings on admission, according to age group, are provided in Table 2 and Table 3.
Table 3. Vital Signs and Laboratory Values of the Patients at Hospital Admission, According to Age Group.*.
| Characteristic | Overall (N=99) |
0–5 Years (N=31) |
6–12 Years (N=42) |
13–20 Years (N=26) |
|---|---|---|---|---|
| Vital signs | ||||
| Median heart rate (IQR) — beats/min | 133 (120–148) | 145 (130–158) | 138 (123–153) | 121 (116–130) |
| Tachycardia — no. (%)† | 96 (97) | 28 (90) | 42 (100) | 26 (100) |
| Median respiratory rate (IQR) — breaths/min | 27 (23–36) | 31 (24–42) | 26 (23–36) | 26 (20–33) |
| Tachypnea — no. (%)‡ | 77 (78) | 21 (68) | 34 (81) | 22 (85) |
| Hypotension — no. (%)§ | 32 (32) | 3 (10) | 17 (40) | 12 (46) |
| Median temperature (IQR) — °C | 38.3 (37.5–39.3) | 38.3 (37.3–39.4) | 38.4 (37.5–39.3) | 38.3 (37.4–39.3) |
| Temperature ≥38.0°C — no. (%)¶ | 62 (63) | 19 (61) | 27 (64) | 16 (62) |
| Median oxygen saturation (IQR) — % | 98 (97–100) | 98 (96–99) | 99 (97–100) | 98 (95–99) |
| Oxygen saturation <92% — no. (%) | 4 (4) | 3 (10) | 0 | 1 (4) |
| Laboratory values‖ | ||||
| Median white-cell count (IQR) — ×10−3/μl | 10.4 (6.7–14.5) | 11.2 (7.4–14.8) | 9.1 (6.4–13.7) | 10.9 (8.0–14.0) |
| Median lymphocyte level (IQR) — % | 10.0 (5.0–16.0) | 17.9 (11.7–23.0) | 8.5 (4.0–12.0) | 6.0 (4.0–11.3) |
| Lymphopenia — no./total no. (%)** | 59/89 (66) | 11/27 (41) | 29/38 (76) | 19/24 (79) |
| Median neutrophil level (IQR) — % | 82.0 (76.0–89.0) | 75.0 (65.5–79.2) | 86.0 (80.6–91.0) | 87.0 (81.0–90.0) |
| Neutropenia — no./total no. (%)†† | 0/89 | 0/28 | 0/38 | 0/23 |
| Median platelet count (IQR) — ×10−9/liter | 155 (105–233) | 186 (107–297) | 125 (100–200) | 162 (130–223) |
| Platelet count <80×10−9/liter — no./total no. (%) | 10/95 (11) | 2/29 (7) | 7/40 (18) | 1/26 (4) |
| Elevated proBNP level — no./total no. (%)‡‡ | 74/82 (90) | 22/23 (96) | 34/34 (100) | 18/25 (72) |
| Elevated troponin level — no./total no. (%) | 63/89 (71) | 15/26 (58) | 27/38 (71) | 21/25 (84) |
| Median procalcitonin level (IQR) — ng/dl | 6.2 (2.2–19.7) | 6.1 (2.2–15.8) | 11.3 (4.4–24.8) | 2.7 (0.4–18.5) |
| Elevated procalcitonin level — no./total no. (%)§§ | 60/65 (92) | 19/20 (95) | 29/29 (100) | 12/16 (75) |
| C-reactive protein level | ||||
| Median (IQR) — mg/dl | 21.9 (15.0–30.0) | 20.6 (11.3–29.0) | 21.0 (16.0–28.7) | 28.3 (18.5–36.4) |
| Distribution — no./total no. (%) | ||||
| ≥3.0 mg/dl | 98/98 (100) | 31/31 (100) | 41/41 (100) | 26/26 (100) |
| >5.0 mg/dl | 97/98 (99) | 31/31 (100) | 41/41 (100) | 25/26 (96) |
| >10.0 mg/dl | 85/98 (87) | 24/31 (77) | 38/41 (93) | 23/26 (88) |
| >20.0 mg/dl | 59/98 (60) | 17/31 (55) | 23/41 (56) | 19/26 (73) |
| Median fibrinogen level (IQR) — mg/dl | 624 (506–764) | 551 (386–689) | 596 (496–719) | 766 (620–836) |
| Fibrinogen level >400 mg/dl — no./total no. (%) | 57/66 (86) | 13/18 (72) | 27/31 (87) | 17/17 (100) |
| Median d-dimer level (IQR) — mg/liter | 2.4 (1.2–3.7) | 2.7 (1.7–4.4) | 2.5 (1.3–3.6) | 1.8 (0.8–3.3) |
| d-dimer level >0.55 mg/liter — no./total no. (%) | 86/94 (91) | 26/28 (93) | 38/40 (95) | 22/26 (85) |
| Median ferritin level (IQR) — ng/ml¶¶ | 522 (305–820) | 412 (185–698) | 613 (305–932) | 540 (365–880) |
| Ferritin level >300 ng/ml — no./total no. (%) | 62/83 (75) | 16/23 (70) | 27/36 (75) | 19/24 (79) |
| Median albumin level (IQR) — g/dl | 3.1 (2.5–3.6) | 3.2 (2.5–3.6) | 3.1 (2.5–3.5) | 3.1 (2.6–3.8) |
| Albumin level ≤3.0 g/dl — no./total no. (%) | 45/94 (48) | 13/29 (45) | 20/41 (49) | 12/24 (50) |
| Median LDH level (IQR) — U/liter | 313 (267–380) | 328 (273–426) | 288 (256–342) | 325 (283–465) |
| LDH level ≥500 U/liter — no./total no. (%) | 6/69 (9) | 2/19 (11) | 2/31 (6) | 2/19 (11) |
| Median interleukin-6 level (IQR) — pg/ml | 116.3 (37.0–315.0) | 80.0 (7.7–301.4) | 215.0 (47.0–315.0) | 111.0 (63.7–286.0) |
| Interleukin-6 level ≥5.0 pg/ml — no./total no. (%) | 33/34 (97) | 6/7 (86) | 18/18 (100) | 9/9 (100) |
| Median ESR (IQR) — mm/hr | 61.5 (43.0–77.5) | 57.5 (46.0–72.0) | 55.5 (38.0–75.5) | 65.0 (59.0–88.0) |
| ESR ≥40 mm/hr — no./total no. (%) | 40/52 (77) | 15/18 (83) | 14/20 (70) | 11/14 (79) |
ESR denotes erythrocyte sedimentation rate, LDH lactate dehydrogenase, and proBNP pro–brain natriuretic peptide.
An elevated heart rate was coded as more than 160 beats per minute for an age of 0 to younger than 3 months, more than 150 for 3 to younger than 6 months, more than 130 for 6 to younger than 12 months, more than 125 for 1 to younger than 3 years, more than 115 for 3 to younger than 6 years, and more than 100 for older than 6 12 years.16
An elevated respiratory rate was coded as more than 60 breaths per minute for an age of 0 to younger than 3 months, more than 45 for 3 to younger than 6 months, more than 40 for 6 to younger than 12 months, more than 30 for 1 to younger than 3 years, more than 25 for 3 to younger than 6 years, more than 22 for 6 to younger than 12 years, and more than 18 for older than 12 years.16
Hypotension was defined as a systolic blood pressure of less than 60 mm Hg for an age of younger than 1 month, less than 70 mm Hg for 1 month to younger than 1 year, [<70+(2×the age in years)] mm Hg for 1 to 10 years, and less than 90 mm Hg for older than 10 years.16
Patients were considered to have fever if there was either subjective or objective fever.
The normal ranges for selected variables are as follows: fibrinogen level, 200 to 400 mg per deciliter; d-dimer level, 0.20 to 0.55 mg per liter; albumin level, 3.4 to 5.4 g per deciliter; and erythrocyte sedimentation rate (ESR), 0 to 10 mm per hour.
Lymphopenia was defined as a lymphocyte level of less than 2.5% for an age of younger than 1 month, less than 4.0% for 1 to younger than 12 months, less than 3.0% for 1 to younger than 2 years, less than 2.0% for 2 to younger than 4 years, less than 1.5% for 4 to younger than 10 years, less than 1.2% for 10 to younger than 16 years, and less than 1.0% for 16 years or older.16
Neutropenia was classified as a neutrophil level of less than 1.0% for an age of younger than 6 months, less than 1.5% for 6 months to younger than 10 years, and less than 1.8% for 10 years or older.16
An elevated proBNP level (excluding children <1 month of age) was classified as more than 1121 pg per milliliter for an age of 1 month to younger than 1 year, more than 675 pg per milliliter for 1 to younger than 2 years, more than 391 pg per milliliter for 2 to younger than 14 years, and more than 363 pg per milliliter for 14 years or older.17
An elevated procalcitonin level was classified as more than 0.5 ng per deciliter.18
The ferritin level was measured for children older than 2 months of age.
Antecedent Illness and Viral and Bacterial Testing on Admission
In the 6 weeks before admission, of the 99 patients with MIS-C, 24 (24%) had a Covid-19–compatible illness a median of 21 days (interquartile range, 10 to 31) before hospitalization, 38 (38%) had exposure to a person with confirmed Covid-19, and 22 (22%) had direct contact with a person who had a clinically compatible Covid-19–like illness. Testing for respiratory viruses, including influenza A and B, and for respiratory syncytial virus was performed in 57 patients (58%). Of the 57 patients tested for respiratory viruses, 2 had evidence of viral infection: coronavirus 229e and SARS-CoV-2 were detected in 1 patient, and adenovirus, a nontyped coronavirus, and SARS-CoV-2 were detected in 1 patient. Bacterial cultures were reported for 77 patients (78%); none showed evidence of a bacterial blood infection. A total of 71 patients (72%) received empiric systemic antibacterial therapy.
Clinical Course, Treatment, and Outcomes
Overall, 79 patients (80%) were admitted to an intensive care unit (ICU) (median time from admission to ICU entry, <1 day; interquartile range, 0 to 1), and 10 (10%) received mechanical ventilation. The median time from symptom onset to hospital admission was 4 days (interquartile range, 3 to 6) (Table 4). A total of 69 patients (70%) received intravenous immune globulin (IVIG), 63 (64%) received systemic glucocorticoids, and 61 (62%) received vasopressor support; 48 (48%) received both systemic glucocorticoids and IVIG. During hospitalization, at least one echocardiogram was obtained for 93 patients (94%); 51 (52%) had some degree of ventricular dysfunction, 32 (32%) had pericardial effusion, and 9 (9%) had a documented coronary-artery aneurysm. The z scores were reported for 7 of the 9 patients with coronary-artery aneurysms, with 4 (57%) having a score of 2.5 to less than 5. Of 60 patients with examinations for troponin and proBNP levels, an electrocardiogram, and an echocardiogram, 59 had evidence of cardiac abnormalities. Of 90 patients who underwent computed tomography (CT) or radiography of the chest, 35 (39%) had at least one opacity noted. Of 44 patients who underwent CT of the abdomen, ultrasonography of the abdomen, or both, 34 (77%) had abnormal findings; 4 (9%) had hepatomegaly, splenomegaly, or hepatosplenomegaly, 8 (18%) had mesenteric adenopathy, 16 (36%) had ascites, pleural effusions, or pelvic fluid, and 17 (39%) had inflammation or enlargement of the appendix (in 2 patients) or the gallbladder (in 5), enteritis or enterocolitis (in 3), bowel-wall thickening (in 7), or fluid-filled bowel loops (in 4).
Table 4. Clinical Course and Outcomes, According to Age Group.*.
| Variable | Overall (N=99) |
0–5 Years (N=31) |
6–12 Years (N=42) |
13–20 Years (N=26) |
|---|---|---|---|---|
| Median time from symptom onset to hospital admission (IQR) — days | 4 (3–6) | 4 (3–6) | 5 (4–5) | 4 (3–6) |
| ICU admission — no. (%) | 79 (80) | 19 (61) | 38 (90) | 22 (85) |
| Median time to ICU entry (IQR) — days | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–1) |
| Median length of stay (IQR) — days | ||||
| Overall | 6.0 (4.0–9.0) | 6.0 (3.0–8.0) | 6.0 (4.0–10.0) | 6.5 (6.0–10.0) |
| Among those discharged | 6.0 (4.0–8.0) | 5.0 (3.0–7.0) | 4.0 (4.0–8.0) | 6.0 (5.0–10.0) |
| Therapy — no. (%) | ||||
| BiPAP or CPAP† | 7 (7) | 1 (3) | 3 (7) | 3 (12) |
| High-flow nasal cannula† | 16 (16) | 1 (3) | 10 (24) | 5 (19) |
| Mechanical ventilation† | 10 (10) | 3 (10) | 3 (7) | 4 (15) |
| ECMO | 4 (4) | 1 (3) | 2 (5) | 1 (4) |
| Vasopressor support | 61 (62) | 15 (48) | 29 (69) | 17 (65) |
| Systemic glucocorticoids | 63 (64) | 16 (52) | 30 (71) | 17 (65) |
| IVIG | 69 (70) | 26 (84) | 30 (71) | 13 (50) |
| Systemic glucocorticoids and IVIG | 48 (48) | 15 (48) | 25 (60) | 8 (31) |
| Diagnoses — no. (%)‡ | ||||
| Kawasaki’s disease or atypical Kawasaki’s disease | 36 (36) | 15 (48) | 18 (43) | 3 (12) |
| Myocarditis | 52 (53) | 12 (39) | 21 (50) | 19 (73) |
| Shock | 10 (10) | 4 (13) | 5 (12) | 1 (4) |
| Coronary-artery aneurysm | 9 (9) | 4 (13) | 4 (10) | 1 (4) |
| Acute kidney injury | 10 (10) | 3 (10) | 4 (10) | 3 (12) |
| Death — no. (%) | 2 (2) | 1 (3) | 1 (2) | 0 |
BiPAP denotes bilevel positive airway pressure, CPAP continuous positive airway pressure, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, and IVIG intravenous immune globulin.
Therapies were not mutually exclusive; 2 patients received both BiPAP or CPAP and mechanical ventilation, and 3 patients received both high-flow nasal cannula and mechanical ventilation.
One patient received diagnoses of stroke and seizures.
A total of 36 patients (36%) received a diagnosis of Kawasaki’s disease or atypical (or incomplete) Kawasaki’s disease; 7 of the 9 patients with coronary-artery aneurysms also received a diagnosis of Kawasaki’s disease. A total of 36 patients (36%) received a diagnosis of myocarditis, and an additional 16 (16%) had clinical myocarditis. As of May 15, a total of 76 patients (77%) had been discharged and 21 (21%) were still hospitalized; 2 (2%) died in the hospital. The median length of stay was 6 days (interquartile range, 4 to 9) overall, 6 days (interquartile range, 4 to 8) among patients who were discharged, and 7 days (interquartile range, 3 to 11) among those who died.
Death occurred in two children 0 to 12 years of age. Both were admitted with abdominal pain and fever, had tachycardia and hypotension on presentation, and during the course of their hospitalization received vasopressor support and underwent intubation; one received extracorporeal membrane oxygenation. Neither received IVIG, systemic glucocorticoids, or immunomodulators. The contributing cause of death for both children included complications of a possibly inflammatory, coagulopathic, or neurologic process.
Epidemic Curve
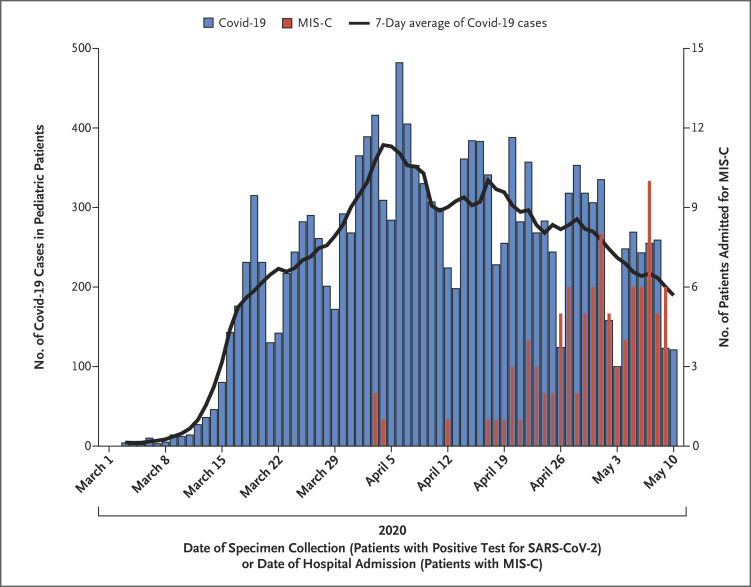
Cases of laboratory-confirmed SARS-CoV-2 infection among persons younger than 21 years of age in New York State, according to date of specimen collection, and cases of confirmed and suspected MIS-C in our study, according to date of admission, are shown in Figure 2. The peak in the number of MIS-C cases followed the peak in the number of cases of laboratory-confirmed SARS-CoV-2 infection by 31 days. From March 1 through May 10, 2020, the incidence of laboratory-confirmed SARS-CoV-2 infection was 322 per 100,000 persons younger than 21 years of age, and the incidence of MIS-C was 2 per 100,000 persons younger than 21 years of age.
Figure 2. Pediatric Cases of Coronavirus Disease 2019 (Covid-19) and of MIS-C.
All data are for patients younger than 21 years of age in New York State from March through May, 2020. Covid-19 was defined by a positive test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Discussion
Our study involving hospitalized pediatric patients with MIS-C in New York State describes a febrile hyperinflammatory syndrome with dermatologic, mucocutaneous, and gastrointestinal manifestations associated with cardiac dysfunction; this syndrome is currently being seen worldwide. Although 32% of the patients had hypotension at admission, 62% (48% of those 0 to 5 years of age) received vasopressor support and 80% were admitted to an ICU. Notable findings were the high prevalence of cardiac dysfunction or depression, coagulopathy, and gastrointestinal symptoms, accompanied by mild respiratory symptoms and occasional indications for supplemental oxygen, in contrast with most cases of acute Covid-19 among hospitalized children.4,19 Patients were commonly treated with IVIG, glucocorticoids, and vasopressors. This constellation suggests an inflammatory vasculopathy, with some similarities to Kawasaki’s disease and toxic shock syndrome. Our findings are consistent with those of other studies to date, which have been limited to case reports, short reports, and small case series.10,11,20-24
As in a study from Italy, MIS-C cases in New York State followed the peak of the Covid-19 epidemic in the state, which supports a temporal and geographic association between Covid-19 and MIS-C.10 Of the patients tested with a serologic assay, nearly all had reactivity, and of the 76 who were tested with both RT-PCR and serologic assays for SARS-CoV-2, 44 (58%) had only serologic evidence of current or recent SARS-CoV-2 infection, which supports a laboratory-based association. Of the 4 patients with suspected cases, none of whom had laboratory evidence of SARS-CoV-2 infection on admission, 2 (50%) had had a Covid-19–like illness in the 6 weeks before MIS-C symptom onset. These findings support that MIS-C is probably a postinfectious, inflammatory process related to Covid-19.
We found variations in presenting symptoms and manifestations according to age. The prevalence of dermatologic symptoms was highest among children 0 to 5 years of age, and the prevalence of myocarditis (diagnoses and clinical) was highest among the adolescents. The prevalence of gastrointestinal symptoms was high in all age groups. Nearly half of children 0 to 5 years of age with MIS-C but only 12% of the adolescents 13 to 20 years of age had a discharge diagnosis of Kawasaki’s disease or atypical Kawasaki’s disease. Children 0 to 12 years of age with MIS-C were more likely to present with Kawasaki’s disease–like symptoms such as conjunctival injection, rash, and oral mucosal changes than adolescents with MIS-C; however, children 0 to 12 years of age in our study presenting with Kawasaki’s disease–like symptoms were older, were more likely to present with hypotension, and were more commonly admitted to the ICU than children with Kawasaki’s disease described in previous reports.25 Although Kawasaki’s disease–like symptoms were less common among adolescents than among younger children with MIS-C, they did still occur, and rare cases of Kawasaki’s disease among adults have been reported.26 Further research could explore whether a post–SARS-CoV-2 inflammatory syndrome exists in adults.
Although classic Kawasaki’s disease disproportionately affects Asian children, MIS-C that is associated with Covid-19 appears to occur among children of all racial and ethnic backgrounds.24 Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS CoV-2 infection among black and Hispanic communities.6,27 In comparison, among persons younger than 21 years of age, an estimated 25% and 32% of people living in the New York Metropolitan Region in 2018 were black and Hispanic, respectively.28
Limitations of our study include presumed underestimation, possibly due to mild cases of MIS-C that did not involve hospitalization, and possible underreporting due to lack of recognition of an emerging syndrome. Furthermore, clinicians may not have ordered a full panel of inflammatory markers, possibly excluding some patients. Children with severe acute Covid-19 who had a concurrent inflammatory syndrome29 complicating their hospitalization may have met the case definition, which would dilute our understanding of MIS-C. In addition, the lack of serologic tests early in the outbreak, limited availability of molecular or serologic testing, and clinician testing practices in children may have led to underrecognition. This may have been particularly true for those excluded because they did not meet the virologic or epidemiologic criteria; the clinical and laboratory characteristics of these patients were more similar to the characteristics of patients with MIS-C than they were to the characteristics of patients excluded for other reasons. However, others not meeting the virologic or epidemiologic criteria may represent patients with baseline Kawasaki’s disease, particularly in geographic areas without ongoing community-based transmission of SARS-CoV-2. The sensitivity or specificity of the case definition for confirmed MIS-C may be further affected by the varying performance of both PCR and serologic tests according to manufacturer and setting. We could not independently verify the diagnosis of Kawasaki’s disease, atypical Kawasaki’s disease, or toxic shock syndrome.
Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options.10,11
Because children often present with mild symptoms of Covid-19 and are less frequently tested than adults,4,7 the incidence of MIS-C among children infected with SARS-CoV-2 is unclear. It is crucial to establish surveillance for MIS-C cases,14 particularly in communities with higher levels of SARS-CoV-2 transmission.
Acknowledgments
We thank our colleagues at the New York State Department of Health for their contributions (K. Alshaer, M. Anand, B. Backenson, K. Buettner, K. Engesser, F. Gesten, D. Gowie, C. Gulotta, E. Heslin, M. Kacica, J. Kirkwood, C. Kus, C. Marbot, T. Morck, A. Osinaga, M. Oxtoby, P. Paiva, P. Pennell Huth, K. Purcell, E. Rausch-Phung, A. Robbins, J. Skidmore, C. Tice, J. White, and Y. Zheng); the Westchester County Department of Health for their public health efforts (S. Amler, D. Hewlett, and A. Huang); and clinicians in New York State for their care of patients with MIS-C and expertise to inform development of a New York State case definition (S. Nolan, L. Saiman, and H. Ushay).
Supplementary Appendix
Disclosure Forms
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article was published on June 29, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Rosenberg ES, Dufort EM, Blog DS, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State — March 2020. Clin Infect Dis 2020. May 8 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Coronavirus Resource Center. COVID-19 United States Cases 2020 (https://coronavirus.jhu.edu/us-map).
- 3.New York State Department of Health. NYSDOH COVID-19 tracker. 2020. (https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Map?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n).
- 4.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020;145(6):e20200702-e20200702. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus Disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC COVID-19 Response Team. Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020;10:537-540. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control. Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children. May 15, 2020. (https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment).
- 10.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. 2020. (https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf).
- 13.New York State Department of Health. Health advisory: pediatric multi-system inflammatory syndrome temporally associated with COVID-19 interim case definition in New York State. May 13, 2020. (https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf).
- 14.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). May 14, 2020 (https://emergency.cdc.gov/han/2020/han00432.asp).
- 15.New York State Department of Health. Communicable disease reporting requirements. 2020. (https://health.ny.gov/forms/instructions/doh-389_instructions.pdf).
- 16.Kahl L, Hughes H. The Harriet Lane handbook. 21st ed. Philadelphia: Elsevier, 2018. [Google Scholar]
- 17.Nir A, Lindinger A, Rauh M, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009;30:3-8. [DOI] [PubMed] [Google Scholar]
- 18.Children’s Minnesota. Chemistry: procalcitonin. 2019. (https://www.childrensmn.org/references/lab/chemistry/procalcitonin.pdf).
- 19.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020. May 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020. May 17 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 21.Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr 2020. June 14 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology 2020. June 4 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020. June 8 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020. June 8 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez SR, Friedman K, Seewald R, Anderson MS, Willis L, Glodé MP. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics 2008;122(4):e786-e790. [DOI] [PubMed] [Google Scholar]
- 26.Ueda Y, Kenzaka T, Noda A, Yamamoto Y, Matsumura M. Adult-onset Kawasaki disease (mucocutaneous lymph node syndrome) and concurrent Coxsackievirus A4 infection: a case report. Int Med Case Rep J 2015;8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Vintage 2018 bridged-race population estimates (https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm).
- 29.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.