Abstract
The emergence of SARS-CoV-2 has driven a global research effort to identify medical countermeasures at an unprecedented pace. In this issue of Cell, Cao et al. identify thousands of SARS-CoV-2 neutralizing antibodies from convalescent donors. The authors improve our understanding of immunity against the coronavirus spike glycoprotein and detail novel pathways to rapidly identify and characterize protective monoclonal antibodies.
The emergence of SARS-CoV-2 has driven a global research effort to identify medical countermeasures at an unprecedented pace. In this issue of Cell, Cao et al. identify thousands of SARS-CoV-2 neutralizing antibodies from convalescent donors. The authors improve our understanding of immunity against the coronavirus spike glycoprotein and detail novel pathways to rapidly identify and characterize protective monoclonal antibodies.
Main Text
In late 2019 in Hubei province, China, a novel coronavirus, SARS-CoV-2, was shown to cause a highly pathogenic respiratory disease in humans. The publication of the viral genome sequence in January 2020 spurred a global effort to rapidly develop prophylactic and therapeutic countermeasures for this new virus. SARS-CoV-2, a betacoronavirus most closely related, among human coronaviruses, to SARS-CoV, mediates entry into airway epithelial cells through binding of its surface Spike (S) glycoprotein to the angiotensin I converting enzyme 2 (ACE2) receptor (Walls et al., 2020; Wrapp et al., 2020). Immunogens that elicit antibodies against S have been the basis of most vaccine candidates. Monoclonal and polyclonal antibody products that are planned or under assessment in clinical trials also target S, with initial studies showing encouraging results (Salazar et al., 2020; Shi et al., 2020).
While polyclonal convalescent plasma has played an important role in treating infectious diseases in the past, there has been increased momentum in recent years to develop monoclonal antibodies as mainstays of managing viral infections, most notably for treating respiratory syncytial virus and Ebola virus. In the last decade, exciting technological advances have been made in the isolation, characterization, and development of monoclonal antibodies. Several methods in particular have demonstrated great promise: Bcl-6 based B cell immortalization (Kwakkenbos et al., 2016), single-cell heavy-light chain paired BCR sequence amplification, and high-throughput single-cell RNA and variable-diversity-joining (VDJ) gene sequencing combining reverse transcription polymerase chain reaction (RT-PCR), 10X Chromium, and microfluidics platforms to facilitate recovery of unprecedented clonotypic and phenotypic information in a single experiment. These state-of-the-art techniques, alone or in combination with antigen-specific flow cytometric approaches, are advancing the rapid and efficient recovery of neutralizing monoclonal antibodies.
Given the urgency of the current pandemic, rapid identification of potent monoclonal antibodies necessitates a multifaceted search strategy (Cao et al., 2020). Xie and colleagues undertook three interconnected strategies with varying levels of success. The authors first isolated B cells from twelve convalescent individuals and carried out 10X Chromium 5′ mRNA and VDJ sequencing. Using a defined selection criteria of immunoglobulin G1 (IgG1) isotype utilization, memory B cell phenotype, and clonal expansion, a set of antibodies (BD1-175) was assessed for SARS-CoV-2 binding and neutralization. Only two antibodies targeted epitopes in the receptor binding domain (RBD), with a lone antibody, BD-23, demonstrating SARS-CoV-2 neutralization.
Next, in order to enrich for B cells targeting the S glycoprotein, a rapid antigen probe-based B cell pull-down was performed using recombinant RBD or S prior to single-cell RNA-VDJ sequencing. As enrichment reduced the overall B cell numbers recovered, an impressive 60 convalescent donors could be analyzed in 6 different batches, thus allowing more than 8,000 IgG1+ antigen-binding clonotypes to be rapidly identified. From these clonotypes, an expanded set of criteria was applied to identify lead antibodies, excluding exhausted or naïve B cells and selecting for clones with evidence of somatic hypermutation. From this, more than 200 additional antibodies (BD176–425) were assessed, and 14 SARS-CoV-2 potent neutralizing antibodies with ng/mL potency were identified. Seven of these antibodies had pseudovirus neutralization half maximal inhibitory concentration (IC50) titers below 50 ng/mL; the most potent monoclonal antibody (mAb) BD-368-2 had an IC50 of 1.2 ng/mL.
Recent large-scale characterization of influenza-reactive antibodies demonstrated that signature sequences can be used to computationally identify potent neutralizing antibodies (Joyce et al., 2016). Utilizing the complementarity-determining region (CDR) H3 sequences from the SARS-CoV neutralizing antibodies m396 and 80R, Xie and colleagues computationally panned the B cell clonotypes to identify a set of antibodies (BD492–515) with the signature SARS-CoV sequence. This computational method of antibody identification demonstrated a surprisingly high efficiency, with 7 of 12 selected antibodies displaying potent SARS-CoV-2 neutralization.
Antibody BD-23—identified from the first discovery strategy—was structurally characterized by electron microscopy in complex with the S glycoprotein trimer. The antibody binding epitope displayed a set of unique properties in comparison with previously described SARS-CoV-2 neutralizing antibodies. A single BD23-Fab bound to the S trimer with the antibody recognition site overlapping the ACE2 receptor binding site. Unexpectedly, BD23 contacted the RBD oriented in the “down” conformation and utilized only heavy-chain contact residues to do so. The reliance on heavy-chain-only antigen binding is reminiscent of antibodies against other viruses such as influenza, where stereotypic B cell recognition is observed in multiple individuals and provides opportunities for targeted design of vaccine immunogens (Joyce et al., 2016).
The most potent antibody identified in this work, BD-368-2, was assessed for both therapeutic and prophylactic efficacy in a SARS-CoV-2 infection—human ACE2 transgenic mouse model. In both experiments, mice were protected from infection-associated weight loss, although the overall degree of pathology observed was limited. Nevertheless, SARS-CoV-2 was undetectable in the lungs of the mice following prophylactic treatment, while after therapeutic treatment (2 h after infection), viral titers were reduced 3–4 log compared to controls. These results are highly encouraging, and BD-368-2 is currently under assessment in a human clinical trial to prevent or treat disease.
The achievement of isolating, sequencing, and characterizing thousands of clonotypes from tens of donors is a major advance. In this and other recent studies on S-targeting antibodies, neutralizing activity has been largely confined to epitopes within the RBD. This contrasts with prior studies of MERS-CoV (Wang et al., 2018) and SARS-CoV where numerous neutralizing epitopes outside the RBD were also identified. However, an alternative neutralizing epitope in the N-terminal domain (Chi et al., 2020) has recently been identified, and it provides additional pathways for vaccine development and combination antibody therapies. From the SARS-CoV-2 studies, the low frequency of potent neutralizing RBD- and non-RBD-specific monoclonal antibodies should be noted. The comparative scarcity of RBD-specific B and T cell responses (Juno et al., 2020), combined with the relatively low titers of serum neutralization seen in convalescent patients, may suggest that neutralizing epitopes are inefficiently targeted during infection, highlighting the need for vaccine, antibody, or small molecule interventions.
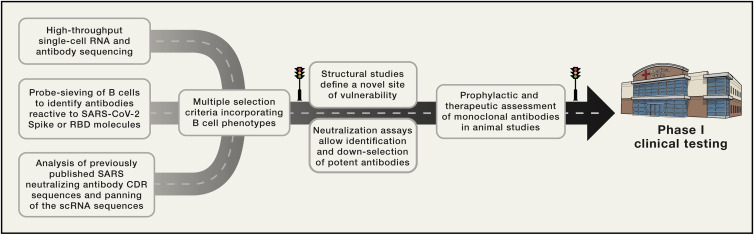
Overall, the work of Xie and colleagues brings together recent technological advancements in concert with significant new understandings of B cell biology. The implications are a rapid process to identify and evaluate monoclonal antibodies: moving from convalescent human samples to animal-protection and efficacy testing in two weeks (Figure 1 ). The pathway mapped out will guide future rapid response efforts.
Figure 1.
Multifaceted Blueprint for Monoclonal Antibody Discovery and Development
Rapid response to identify a protective monoclonal antibody is enabled by a combination of technological advances, matched with B cell selection criteria. High-throughput sequencing, B cell selection, and signature sequence panning identified thousands of SARS-CoV-2 reactive monoclonal antibodies, including a subset with ng/ul neutralization and protective capability, enabling rapid advancement to a phase I clinical trial.
Acknowledgments
This work was supported by funding from the Defense Health Agency, USA, and through a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, USA, and the US Department of Defense. A.K.W. is a NHMRC fellow and is funded by NHMRC project grant GNT1162760, Australia. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
References
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182:1–12. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Meng H., Zhang Z., Fan P., Dong Y., Yang Y. A Neutralizing Human Antibody Binds to the N-terminal Domain of the Spike Protein of SARS-CoV-2. Science. 2020:1–12. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M.G., Wheatley A.K., Thomas P.V., Chuang G.Y., Soto C., Bailer R.T., Druz A., Georgiev I.S., Gillespie R.A., Kanekiyo M., NISC Comparative Sequencing Program Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell. 2016;166:609–623. doi: 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno J.A., Tan H.-X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L. Immunogenic profile of SARS-CoV-2 spike in individuals recovered from COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.17.20104869. [DOI] [Google Scholar]
- Kwakkenbos M.J., van Helden P.M., Beaumont T., Spits H. Stable long-term cultures of self-renewing B cells and their applications. Immunol. Rev. 2016;270:65–77. doi: 10.1111/imr.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., Eubank T., Bernard D.W., Eagar T.N., Long S.W. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am. J. Pathol. 2020 doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi W., Chappell J.D., Joyce M.G., Zhang Y., Kanekiyo M., Becker M.M., van Doremalen N., Fischer R., Wang N. Importance of Neutralizing Monoclonal Antibodies Targeting Multiple Antigenic Sites on the Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein To Avoid Neutralization Escape. J. Virol. 2018;92:e02002-17. doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]