To the Editor:
As coronavirus 2019 (COVID-19) expands globally, its clinical heterogeneity has become increasingly apparent. In addition to varying presentations of respiratory failure and Acute Respiratory Distress Syndrome (ARDS), patients infected with the SARS-CoV-2 virus are also observed to have an increased incidence of thrombotic complications [1]. Autopsy reports performed on patients with severe COVID-19 support these findings, demonstrating intra-alveoloar fibrin deposition, endothelial injury, and wide-spread thrombosis with microangiopathy without obvious hyaline membrane formation or neutrophilic invasion seen commonly in other forms of ARDS [2,3].
Given these concerns, we sought to evaluate evidence of hypercoagulability in a cross-section of critically ill patients with confirmed COVID-19 admitted for hypoxemic respiratory failure to the Intensive Care Unit (ICU) at Walter Reed National Military Medical Center. On a single date, we evaluated the records of five patients residing in the ICU (3 female and 2 male). Four patients were on mechanical ventilation and one patient was on a combination of high-flow nasal oxygen and non-invasive ventilation.
The patients ranged in age from 24 to 80 years-old with length of admission to the ICU from three to 22 days; total ventilator days ranged from one to 18. All patients were obese and had a minimum of two chronic medical conditions. Four patients were placed on mechanical and chemoprophylaxis (enoxaparin 40 mg subcutaneous once daily) at the time of hospital admission, and one patient was on mechanical prophylaxis and therapeutic anticoagulation with unfractionated heparin for atrial fibrillation (verified by serial aPTT). In addition to supportive care, all patients received a 5-day course of azithromycin and hydroxychloroquine.
In light of elevated fibrin degradation product (d-dimer) levels, worsening clinical status, and hypoxemia disproportionate to imaging, all five patients were assessed for venous thromboembolism (VTE). Clinically indicated laboratory studies including thromboelastography (TEG), coagulation panels [prothrombin time, PT; activated partial thromboplastin time, aPTT; international normalized ratio, INR; and fibrinogen], platelet count, d-dimer, and lower extremity Doppler ultrasounds were obtained and analyzed over a twenty-four hour period. Venous blood samples were collected to measure coagulation panels, platelet counts, and d-dimer levels. Lower extremity ultrasounds were performed in accordance with standards of practice for the clinical indication of ruling-out VTE. Radiographic evaluation for pulmonary embolism was not performed due to patient instability for transport.
TEG was performed within 1 h of specimen collection using TEG ® 5000 Thromboelastograph ® Hemostasis Analyzer System (Haemonetics Corporation, 125 Summer Street, Boston, MA 02110). Quality-control checks were performed according to manufacturer instruction.
TEG analyzes clot initiation, kinetics, strength, stability, and breakdown. Various parameters were recorded and used to derive the Coagulation Index (CI), which is calculated as follows:
(CI): 0.1227 (R) + 0.1655 (MA) − 0.0241 (⍺) − 5.0220, where (R) is the time (min) from clot initiation to formation; (K) and the (⍺)-angle denote velocity (minutes) of clot formation; and Maximum Amplitude (MA) is a measure of maximum clot strength [4].
A normal (CI) is −3.0 to +3.0 with higher values concerning for increased risk of thrombosis and lower values concerning for a bleeding diathesis. A typical hypercoagulability profile on TEG would demonstrate decreased (R) and (K) times with increased (⍺)-angle and (MA) [4].
Depending on the severity and stage of the fibrinolytic process, fibrinolysis leads to clot dissolution. Several sets of parameters are computed to quantify the fibrinolytic state and rely on the loss of clot strength over time after the (MA) is reached. LY30 measures percent lysis 30 min after (MA) and is used to diagnose either primary or secondary fibrinolysis. Clot strength (G) along with the (CI) are used to interpret hypocoagulability [low (G) and (CI) values] or hypercoagulability [elevated (G) and (CI) values] [4].
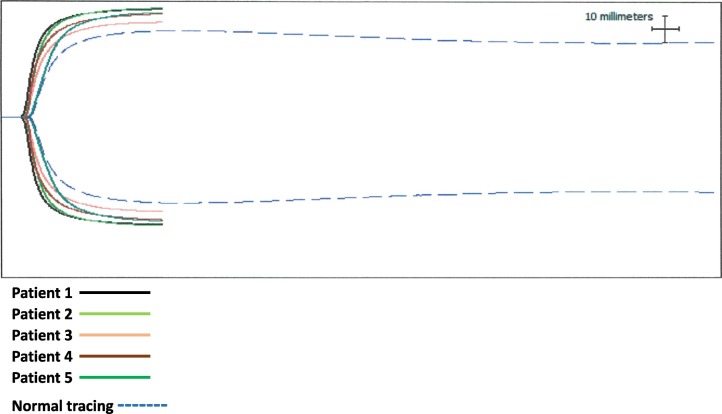
As seen in Table 1 and Fig. 1 , evidence of hypercoagulability was universal among our patients. All five had elevations in D-dimer and fibrinogen levels. Three patients had abnormal (CI) values and demonstrated a typical TEG pattern for hypercoagulability. Two patients were diagnosed with proximal deep vein thrombosis and subsequently treated with therapeutic doses of enoxaparin (1 mg/kg twice-daily subcutaneously). Zero patients had evidence of fibrinolysis as indicated by LY30 levels of 0%. At the time of this submission, all five patients were alive and successfully discharged home on room air.
Table 1.
COVID case series.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (years) | 59 | 52 | 59 | 24 | 80 |
| Gender | Female | Male | Male | Female | Female |
| Weight (kg) | 106 | 102 | 113 | 106 | 75 |
| Body mass index (BMI, kg/m2) | 38 | 33 | 32 | 39 | 30 |
| Comorbidities | |||||
| Hypertension | Yes | Yes | Yes | No | Yes |
| Diabetes | No | No | No | No | No |
| Malignancy | No | No | No | No | Yes |
| Cardiovascular disease | No | No | No | No | Yes |
| Immunosuppression | No | No | No | No | No |
| Laboratory | |||||
| Platelets (K/UL) | 259 | 294 | 224 | 203 | 236 |
| Fibrin d-dimer (μg/ml) | 1.68 | 15.79 | 20 | 12.48 | 3.41 |
| Prothrombin time (s) | 14.7 | 14 | 14.6 | 13.9 | 14.5 |
| Partial thromboplastin time (s) | 31.2 | 27.2 | 27.2 | 31.4 | 72.6 |
| Fibrinogen (mg/dL) | 675 | 612 | 540 | 671 | 792 |
| Thromboelastography | |||||
| R (5–10 min) | 4.1 | 4.6 | 4.6 | 4.8 | 5.5 |
| K (1–3 min | 0.9 | 0.9 | 1.2 | 0.8 | 1.5 |
| Alpha angle (50–72°) | 77.1 | 76.4 | 73.1 | 78.2 | 69.6 |
| Maximum amplitude (MA 50–70 mm) | 79.2 | 80.4 | 69.8 | 76 | 77.1 |
| G (4.5–11 dyn/s) | 19 | 20.5 | 11.5 | 15.8 | 16.9 |
| LY 30 (0–8%) | 0 | 0 | 0 | 0 | 0 |
| Coagulation index (−3 to +3) | 4.7 | 4.5 | 2.8 | 4 | 2.8 |
| Anticoagulation | Enoxaparin 40 mg SQ daily | Enoxaparin 40 mg SQ daily | Enoxaparin 40 mg SQ daily | Enoxaparin 40 mg SQ daily | Therapeutic heparin |
| Venous thromboembolism | None | None | Left popliteal | Right common femoral | None |
Fig. 1.
COVID.
.
Our findings lend support to the growing literature-base showing increased hypercoagulability and thrombotic complications in critically-ill patients with COVID-19. While the exact mechanism(s) responsible for the increased micro- and macro-thrombotic burden in this patient population have yet to be elucidated, prevailing theories center around the following: 1) prothrombotic state secondary to severe immune dysregulation, 2) impairment of the fibrinolytic system, and/or 3) upregulation of ACE2 receptors with direct viral invasion and perivascular inflammation leading to endothelial injury [2,3,5].
Analysis of the TEG results from our small cohort highly suggest dysregulation of the fibrinolytic system as a prominent factor in promoting the hypercoagulable state observed in this patient population. Unlike severe sepsis, which typically demonstrates a consumptive coagulopathy, our patients had relatively normal platelet counts, (near) normal PT/aPTT findings, and elevated fibrinogen levels. Viral-mediated upregulation of fibrinogen production with excessive fibrinogen polymerization would account for significant elevations in D-Dimer levels, offer explanation to the 0% LY30 in our patient population (reflecting minimal to no fibrinolysis), and support autopsy findings of increased fibrin deposition in alveoli, lung interstitium, and microvasculature [2,3,5].
Our study did not evaluate Factor VIII or von Willebrand factor (vWF), but other studies have noted increased levels of each in their severe COVID-19 population [6,7]. These findings would support endothelial injury and subsequent release of vWF as contributors to the increased incidence of thrombotic disease. This is not surprising as endothelial cells express ACE2, which is the primary receptor for SARS-CoV-2. Upregulation of these receptors along with diffuse endothelial injury from direct viral invasion has been confirmed on autopsy findings in patients with severe COVID-19 [2].
Given uncontrolled findings such as ours and the risks of therapeutic anticoagulation, a number of questions remain: Which patients might benefit from empiric anticoagulation? When should it be initiated, at what dose, and for what duration? Fortunately, clinical trials seeking to address such questions are now enrolling patients [8]. In the interim, major medical societies have published recommendations to help guide decisions on anticoagulation regimens. The American Society of Hematology currently recommends standard prophylactic-dose anticoagulation for all patients diagnosed with COVID-19, reserving therapeutic anticoagulation only for those with documented VTE [9] More recent guidelines published by the Anticoagulation Forum acknowledge the increased thrombotic complications observed in patients with severe disease, and recommend considering heightened levels of VTE prophylaxis defined as one of the following: enoxaparin 40 mg subcutaneous twice daily; enoxaparin 0.5 mg/kg subcutaneous twice daily; heparin 7500 units subcutaneous three times daily; or low-intensity heparin infusion [10].
By limiting systemic anticoagulation to only those with evidence of macrothrombotic complications, we may be insufficiently treating the full spectrum of disease. Microthrombi are not detectable with standard laboratory or imaging modalities, and thus our only means to assess severity of disease is through indirect measures. Determining what combination of clinical, laboratory, and imaging findings reflect severe hypercoagulability is challenging. Several studies have shown a positive correlation between D-dimer levels and evidence of VTE, one of which cited values ranging from 1.5μg/mL to 3 μg/mL for use as potential surrogates for the prediction of VTE and initiation of therapeutic anticoagulation [11] Another proposed using a combination of the Sepsis-Induced Coagulopathy (SIC) score and d-dimer levels to guide treatment decisions [12] Applying this information to our patients, we would have initiated therapeutic anticoagulation in all five (100%) patients using a d-dimer value of 1.5μg/mL and four out of five (80%) with a cut-off value of 3.0μg/mL. In contrast, using a combination of the SIC score with the D-dimer level, none (0%) of our patients would have received therapeutic anticoagulation.
There are several limitations to our case series, most notably the small number of patients. It is also not clear how the underlying biologic milieu of COVID-19, the therapies given (to include hydroxychloroquine and azithromycin), and patient heterogeneity impact the relevance of our findings. Laboratory evaluation, TEG, and venous Doppler ultrasound performed at a single point in the hospitalization does not exclude risk for developing VTE and constant vigilance is warranted. Lastly, all of our patients were obese and thus standard-dose enoxaparin (as opposed to 0.5 mg/kg BID) could have been inadequate.
Aberrations in the coagulation cascade are proving to play a significant role in the pathophysiology of severe COVID-19 disease. As we anxiously await the results of ongoing investigations to provide a more specific approach to the prevention, diagnosis and treatment of thrombotic disease in this patient population, we recommend evaluating and treating these patients based on existing evidence-based guidelines.
Author approval
All authors have met requirements for authorship, have read and approved the manuscript, and agree with its submission for peer review and publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial disclosure
The authors have no financial relationships to disclose.
Declaration of competing of interest
The authors have no conflicts of interest to disclose. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Acknowledgements
Dr. Hightower is the first author and conducted the clinical care, investigations, wrote the initial manuscript and completed the final revisions. Dr. Ellis, Nau, Dr. Brown, and Dr. Grasso were intimately involved in the ongoing medical care of these patients. Dr. Clark is the senior author and the lead clinician in charge and revised the manuscript and created Table 1. Dr. Collen participated in patient care, manuscript revision, submission, and is the corresponding author and guarantor of the data contained in this manuscript. Mr. Ellis conducted the TEG assays and created the figure. Dr. Cap, Dr. Chung, Dr. Grasso, Dr. Ellis, Dr. Nau, Dr. Brown, Dr. Prescher, Dr. Roswarski and Dr. Kavanaugh provided expert consultation and assisted in editing and revising the finished manuscript.
References
- 1.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;100(3):1065–1075. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C., Guan Z., Xu Q., Zhao L., Song Y., Wang H. Relation of thromboelastography parameters to conventional coagulation tests used to evaluate the hypercoagulable state of aged fracture patients. Medicine (Baltimore) 2016;95(24):e3934. doi: 10.1097/MD.0000000000003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spyropoulos A. “Full Dose Heparin vs Prophylactic or Intermediate Dose Heparin in High Risk COVID-19 Patients.” ClinicalTrials.gov. Identifier: NCT04401293. 26 May 2020.
- 9.COVID-19 and coagulopathy: frequently asked questions. American Society of Hematology. https://www.hematology.org/covid-19/covid-19-and-coagulopathy (accessed June 6 2020)
- 10.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A., Dager W.E., Deitelzweig S.B., Ellsworth S., Garcia D., Kaatz S., Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]