Abstract
Extracellular matrix metalloproteinase inducer (EMMPRIN), which is also called BASIGIN/CD147, is a cell surface glycoprotein that belongs to the immunoglobulin superfamily and plays a significant role in intercellular recognition in immunology, cellular differentiation and development. Apart from ACE-2, recently EMMPRIN, has been regarded as a target for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) attachment and entry into the host cell. Since one of the routes of entry for the virus is the oral cavity, it becomes imperative to percept oral comorbidities such oral squamous cell carcinoma (OSCC) and oral potentially malignant disorders (OPMDs) in terms of EMMPRIN as a target for SARS-CoV-2. In the present paper, it is proposed that OSCC, by the virtue of upregulation of EMMPRIN expression, increases the susceptibility to coronavirus disease (COVID-19). In turn, COVID-19 in OSCC patients causes exhaustion of EMMPRIN receptor due to binding with ‘S’ receptor leading to a downregulation of related carcinogenesis events. We proposed that in the ACE-2 depleted situation in OSCC, EMMPRIN receptor might get high jacked by the COVID-19 virus for the entry into the host cells. Apart from the anti-monoclonal antibody, it is recommended to explore the use of grape seed and skin containing mouthwash as an adjunct, which could also have anti EMMPRIN effects in patients with OSCC and OPMDs.
Keywords: EMMPRIN, BASIGIN, CD 147, ACE-2, Oral cancer, Oral potentially malignant disorder, SARS-CoV-2, COVID-19
Introduction
Coronavirus disease (COVID-19) pandemic has created a significant global health impact and affected population in developing and developed nations of the world causing significant morbidity and mortality [1]. Angiotensin-Converting Enzyme 2 (ACE-2) on the host cells is the attachment protein for the spike receptor present on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. Intriguingly, ACE-2 expression has been reported at various sites in the oral cavity and is regarded as one of the potential modes of entry for the virus and its infectivity [3]. Moreover, differential expression of ACE-2 expression in various pathologies prompt researcher to draw many speculative conclusion in pathologies such as oral squamous cell carcinoma (OSCC), oral submucous fibrosis (OSMF), periodontitis, etc [4], [5], [6].
Apart from ACE-2, recently extracellular matrix metalloproteinase inducer (EMMPRIN), which is also called BASIGIN/CD147, has been regarded as a target for SARS-CoV-2 attachment and its entry into the host cell [7], [8]. EMMPRIN is a cell surface glycoprotein that belongs to the immunoglobulin superfamily and plays a significant role in intercellular recognition, which is an important event in immunology, cellular differentiation and development [9]. A research study has demonstrated that Meplazumab, an anti-CD147 humanized antibody, was found to prevent the SARS-CoV-2 invasion into the host cell [7]. An affinity constant of 1.85 × 10−7 M was reported on the validation of EMMPRIN and spike (‘S’) protein interaction. The binding of both the proteins was established by co-immunoprecipitation and ELISA technique. Immunoelectron-microscopic studies also confirmed the co-localization of EMMPRIN and ‘S’ protein in infected Vero E6 cell lines thereby confirming the significance of EMMPRIN as a potential COVID-19 receptor [7].
Since one of the routes of entry for SARS-CoV-2 is the oral cavity, it becomes imperative to percept oral comorbidities such as OSCC and OPMDs in terms of EMMPRIN expression as a target for SARS-CoV-2. In the present paper, efforts have been made to propose a hypothesis based on EMMPRIN role in oral carcinogenesis and COVID-19 along with possible ramifications of the complex interaction.
Hypothesis
OSCC, by the virtue of upregulation of EMMPRIN expression (potential and alternative site for ‘S’ receptor), increases the susceptibility to SARS-CoV-2 infection. In turn, COVID-19 in OSCC patients causes exhaustion of EMMPRIN receptor leading to downregulation of related carcinogenesis pathways.
Discussion
EMMPRIN and carcinogenesis
EMMPRIN being a member of the immunoglobulin superfamily has a diversified role in maintaining tissue homeostasis, growth and development and hence is known to express on a variety of tissues [9]. It is highly expressed in a variety of malignant neoplasms and is involved in many carcinogenesis related events that lead to initiation and progression of malignancy [10]. A meta-analysis published in literature found a significant association between EMMPRIN overexpression and adverse tumor outcomes, such as overall survival, disease-specific survival, progression-free survival, metastasis-free survival or recurrence-free survival, irrespective of the model analysis. In addition, CD147/EMMPRIN overexpression predicted a high risk for chemotherapy drugs resistance [11].
Many matrix metalloproteinases molecules such as MMP-1, MMP-3, MMP-9 and membrane-type 1-MMP are activated by EMMPRIN thus promoting tumor cell proliferation, invasions and migration [12]. EMMPRIN also upregulates angiogenesis in the tumor microenvironment by virtue of its potential to stimulate vascular endothelial growth factors in tumor and stromal cells [13]. Metabolic reprogramming in tumor cells is the hallmark of carcinogenesis need for survival. In this regard, EMMPRIN regulates expression and activity of monocarboxylate transporters-1 (MCT-1) and MCT-4, and form complexes on the membrane to transport lactic acid produced by anaerobic glycolysis [14]. EMMPRIN has been found to facilitate the activation of phosphatidylinositol 3-hydroxy kinase and mitogen-activated protein kinase pathways, which are important events in carcinogenesis and mainly responsible for chemoresistance in tumor cells [11]. Moreover, EMMPRIN interact with α3β1, α6β1 integrins to regulate adhesion with extracellular matrix proteins, collagen, laminin or fibronectin and also promote expression of cyclophilin A to induce cancer cell proliferation [15].
Oral cancer, EMMPRIN and COVID-19
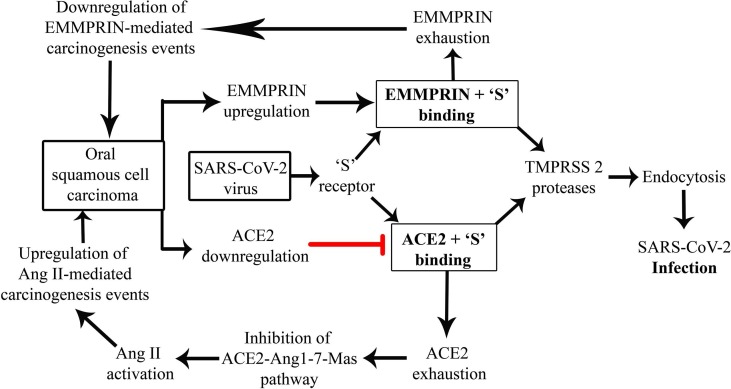
It is noteworthy that the upregulation of EMMPRIN is reported in oral carcinogenesis [16], [17]. Increased expression of EMMPRIN was reported in oral premalignant cells and primary and metastatic cell lines of OSCC [16]. All the aforementioned signaling pathways have been reported to modulate the oral cancer pathogenesis. Thus, by virtue of increased expression and availability of EMMPRIN, OSCC patients could be at high risk for COVID-19. Also, COVID-19 in OSCC patients might exhaust the EMMPRIN due to binding with the 'S' receptor and cause perturbation of related aforementioned carcinogenesis events (Fig. 1 ).
Fig. 1.
Proposed role of EMMPRIN/BASIGIN as a biological modulator of oral cancer and SARS-CoV-2 interaction.
This proposed viewpoint is in contradiction to the previously proposed proposition, which suggested a reduction in ACE-2 receptors in OSCC patients thus, making this condition less susceptible to COVID-19 [18]. It is the other way round, if COVID-19 infection occurs in OSCC patients, there will be a further reduction in the availability of ACE-2. ACE-2 downregulation/exhaustion can lead to inhibition of the ACE2-Ang-1-7-MAS pathway. This situation will increase the concentration of Angiotensin II, which provokes the stromal-tumor paracrine interaction. AT1R mediates the actions of Ang II by receptor phosphorylation and subsequently triggering a chain of intra and extracellular events thus promoting pro-tumoral activities.
It is also quite conceivable that in the ACE-2 depleted situation in OSCC, EMMPRIN receptor might get high jacked by SARS-CoV-2 for the entry into the host cells. If this contention holds true, then it might be applicable for all the situations wherein pre-existing pathology causes depletion in ACE-2 protein. This further increases the complexity of COVID-19 infectivity prediction and disease progression in OSCC patients, which needs serious consideration in the future. Moreover, ACE-2 and EMMPRIN both have a contrasting impact on the disease progression of OSCC and hence, deserve in-depth analysis of both the aspects together in future research.
Oral potentially malignant disorder, EMMPRIN and COVID-19
The prevalence of OPMDs varies between populations and a recent meta-analysis showed a prevalence of 4.47% (95% CI = 2.43–7.08) [19]. Among all the OPMDs, OSMF was the most prevalent one (4.96%; 95% CI = 2.28–8.62) followed by leukoplakia (4.11%; 95% CI = 1.98–6.97) [19]. With increasing consumption of tobacco and betel nut, the prevalence rate of OPMDs is likely to increase in future. Hence, it is expected that OPMD patients might encounter COVID-19 with a higher propensity based on the factors previously elucidated. Since EMMPRIN was detected as high and low glycosylated forms in OPMDs, it could have a serious impact on the spread of COVID-19 [9]. However, in contrast, OSMF might cause exhaustion of ACE2 receptor by underlying fibrosis and thus might decrease the chances of COVID-19 infection [5]. It will be interesting to investigate the expression of EMMPRIN in OSMF patients as a marker of an alternative pathway for COVID-19 infectivity. To better understand the outcome analysis, it is recommended to take cognizance of all OPMDs in the form of detailed history in COVID-19 positive patients.
Future direction
Since both ACE-2 and EMMPRIN are potential targets for COVID-19′s ‘S’ receptor, it is mandatory to consider both the proteins together in any future molecular research. Besides in vitro and in vivo studies, in silico approach to understand at the molecular level, crystal structure of the receptor-binding domain of the spike protein of SARS-CoV-2 in complex with cell receptor ACE2 is reported [20]. However, similar studies on EMMPRIN and SPIKE binding domain of EMMPRIN is warranted that may reveal detained information on binding pocket that may encourage future development of pharmacological inhibitors against EMMPRIN and SPIKE. Prospective cohort studies are recommended on COVID-19 positive OSCC cases to understand the prognostic aspect in comparison with conventional OSCC cases. Quantification of immunohistochemical expression of EMMPRIN and ACE-2 in such patients can be correlated with 5-year survival data to establish them as prognostic biomarkers. In-depth molecular investigations are recommended on primary culture obtained from COVID-19 positive OSCC cases. Molecular expression of EMMPRIN and ACE-2 can be correlated with genomic, proteomic and metabolomics data obtained from the primary cell culture.
EMMPRIN abrogation may serve as a potential strategy for the blockade of SARS-CoV-2. Besides the monoclonal antibody approach, peptide mimetics as metalloproteinase inhibitors, pharmacological inhibitors, and natural compounds from plants, small RNA interference, and CRISPR-Cas9 based system with precision within the oral epithelial tissue may be speculated. Future epidemiological studies are warranted with those patients under the medical for OPMD and severity of COVID-19 infections. In any case, still a speculation that SARS-CoV-2 will find a more convenient way to infect normal human epithelial tissue or perturbed epithelial tissues including downregulation of EMMPRIN or other marker proteins in these tissues. A strong possibility arises that if SARS-CoV-2 is natural, then it will prefer normal epithelial tissues. Therefore, any deviations in the molecular landscape of epithelial cells including EMMPRIN will be an obstruction to the entry of SARS-CoV-2.
Therapeutic implications
EMMPRIN directed antibody called Meplazumab has been suggested as a treatment modality for COVID 19 management [7]. We hypothesize the alternative use of grape seed and skin containing mouthwash as an adjunct, which could also have anti EMMPRIN effect in patients with OSCC and OPMDs. It has been documented that grape seed and grape skin contain resveratrol, which could inhibit EMMPRIN expression in macrophages and monocytes via the inhibition of IκB protein phosphorylation and thus indirectly suppresses the NF-κB pathway [21]. This resveratrol mediated EMMPRIN inhibition could be achieved by the use of mouthwashes containing the grape seed and grape skin extracts in patients with OSCC and OPMDs which could reduce the SARS-CoV-2 entry and its further infection.
Conflict of interest
None declared.
Funding source
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110089.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ahn D.G., Shin H.J., Kim M.H. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madapusi Balaji T., Varadarajan S., Rao U.S.V. Oral cancer and periodontal disease increase the risk of COVID 19? A mechanism mediated through furin and cathepsin overexpression [published online ahead of print, 2020 Jun 1] Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarode S.C., Sarode G.S., Gondivkar S., Gadbail A., Gopalakrishnan D., Patil S. Oral submucous fibrosis and COVID-19: perspective on comorbidity. Oral Oncol. 2020:104811. doi: 10.1016/j.oraloncology.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mhaske S., Yuwanati M., Mhaske A., Desai A., Sarode S.C., Sarode G.S. Perspective on oral exfoliative cytology and COVID-19. Oral Oncol. 2020:104858. doi: 10.1016/j.oraloncology.2020.104858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K., Chen W., Zhou Y.S. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv Preprint. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 8.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nabeshima K., Iwasaki H., Koga K., Hojo H., Suzumiya J., Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56(7):359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 10.Xin X., Zeng X., Gu H., Li M., Tan H., Jin Z., Hua T., Shi R., Wang H. CD147/EMMPRIN overexpression and prognosis in cancer: a systematic review and meta-analysis. Sci Rep. 2016;6(1) doi: 10.1038/srep32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bovenzi C.D., Hamilton J., Tassone P. Prognostic indications of elevated MCT4 and CD147 across cancer types: a meta-analysis. Biomed Res Int. 2015;2015 doi: 10.1155/2015/242437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang P., Chang S., Jiang X. RNA interference targeting CD147 inhibits the proliferation, invasiveness, and metastatic activity of thyroid carcinoma cells by down-regulating glycolysis. Int J Clin Exp Pathol. 2015;8(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro C., Garcia E.A., Morais-Santos F., Moreira M.A.R., Almeida F.M., Jubé L.F., Queiroz G.S., Paula É.C., Andreoli M.A., Villa L.L., Longatto-Filho A., Baltazar F. Reprogramming energy metabolism and inducing angiogenesis: co-expression of monocarboxylate transporters with VEGF family members in cervical adenocarcinomas. BMC Cancer. 2015;15(1) doi: 10.1186/s12885-015-1842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S., Nguyen T.T., Bonanno J.A. CD147 required for corneal endothelial lactate transport. Invest Ophthalmol Vis Sci. 2014;55(7):4673. doi: 10.1167/iovs.14-14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Sun Z., Sun Y. Association of increased ligand cyclophilin A and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology. 2012;60(5):793–803. doi: 10.1111/j.1365-2559.2011.04130.x. [DOI] [PubMed] [Google Scholar]
- 16.Vigneswaran N., Beckers S., Waigel S. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp Mol Pathol. 2006;80(2):147–159. doi: 10.1016/j.yexmp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro L.S., Delgado M.L., Ricardo S. EMMPRIN expression in oral squamous cell carcinomas: correlation with tumor proliferation and patient survival. Biomed Res Int. 2014;2014 doi: 10.1155/2014/905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarode S.C., Sarode G.S., Sengupta N., Kumar Sharma N., Patil S. Biological behavior of oral squamous cell carcinoma in the background of novel corona virus infection [published online ahead of print, 2020 May 7] Oral Oncol. 2020;104781 doi: 10.1016/j.oraloncology.2020.104781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mello F.W., Miguel A.F.P., Dutra K.L. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med. 2018;47(7):633–640. doi: 10.1111/jop.12726. [DOI] [PubMed] [Google Scholar]
- 20.Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 21.Ge H., Zhang J.F., Guo B.S. Resveratrol inhibits macrophage expression of EMMPRIN by activating PPARgamma. Vascul Pharmacol. 2007;46(2):114–121. doi: 10.1016/j.vph.2006.08.412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.