Abstract
Objectives
Asymptomatic patients, together with those with mild symptoms of coronavirus disease 2019 (COVID-19), may play an important role in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission. However, the dynamics of virus shedding during the various phases of the clinical course of COVID-19 remains unclear at this stage.
Methods
A total of 18 patients found to be positive for SARS-CoV-2 infection by real-time reverse transcription PCR (RT-PCR) assay and admitted to Chongqing University Central Hospital between 29 January and 5 February 2020 were enrolled into this study. Medical data, pulmonary computed tomographic (CT) scan images and RT-PCR results were periodically collected during the patients' hospital stay. All participants were actively followed up for 2 weeks after discharge.
Results
A total of nine (50%) asymptomatic patients and nine (50%) patients with mild symptoms of COVID-19 were identified at admission. Six patients (66.7%) who were asymptomatic at admission developed subjective symptoms during hospitalization and were recategorized as being presymptomatic. The median duration of virus shedding was 11.5, 28 and 31 days for presymptomatic, asymptomatic and mildly symptomatic patients, separately. Seven patients (38.9%) continued to shed virus after hospital discharge. During the convalescent phase, detectable antibodies to SARS-CoV-2 and RNA were simultaneously observed in five patients (27.8%).
Conclusions
Long-term virus shedding was documented in patients with mild symptoms and in asymptomatic patients. Specific antibody production to SARS-CoV-2 may not guarantee virus clearance after discharge. These observations should be considered when making decisions regarding clinical and public health, and when considering strategies for the prevention and control of SARS-CoV-2 infection.
Keywords: Antibodies, Asymptomatic infections, Convalescence, SARS-CoV-2, Virus shedding
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in a wide variety of clinical manifestations: asymptomatic infection, mildly symptomatic infection, severe respiratory failure—and even death [[1], [2], [3]]. Asymptomatic and mildly symptomatic coronavirus disease 2019 (COVID-19) patients may play an important role in SARS-CoV-2 transmission; however, these particular patients are difficult to identify for essential isolation and quarantine, which consequently further complicates COVID-19 prevention and control.
Knowledge of SARS-CoV-2 virus shedding dynamics is essential for the planning, design and development of appropriate public health strategies for effective control of COVID-19. Two recent studies regarding virus shedding in mild and severe COVID-19 patients during hospitalization showed that SARS-CoV-2 RNA may well be detected in the respiratory tract for up to 21 and 34 days, respectively [4,5]. However, gaps in knowledge remain concerning SARS-CoV-2 shedding dynamics during the convalescent phase in asymptomatic infections and in COVID-19 patients with mild symptoms.
In the present study, we longitudinally assessed 18 asymptomatic and mildly symptomatic patients with SARS-CoV-2 infection during the clinically apparent and convalescent phases of COVID-19 in Chongqing, China, in order to characterize virus shedding dynamics and serologic responses in this population.
Methods
Study design and participants
Eighteen participants, positive for SARS-CoV-2 by real-time reverse transcription PCR (RT-PCR) assay and admitted to Chongqing University Central Hospital between 29 January and 5 February 2020, were enrolled into this study. Medical records for these patients regarding epidemiologic and demographic characteristics, symptom history and relevant exposure data at admission were retrospectively reviewed, along with the results of periodic pulmonary CT scanning and RT-PCR testing during hospitalization. All patients received antiviral treatment in hospital according to guidance provided by the New Coronavirus Pneumonia Prevention and Control Protocol issued by the National Health Commission of China. No patient received steroids. Patients were discharged from hospital if they met the following criteria: (a) body temperature reverted to normal and remained normal for more than 3 days; (b) respiratory symptoms improved significantly; (c) lung CT image showed significant improvement; and (d) RT-PCR of respiratory specimens taken on two consecutive occasions 24 hours apart were negative. After discharge, patients were quarantined and followed up for 2 weeks, with RT-PCR testing performed within the follow-up period. For those with recurrence of clinical symptoms, such as fever, cough and myalgia, RT-PCR testing was expedited so clinical experts would subsequently evaluate whether readmission to hospital would be necessary.
Participants who tested positive for SARS-CoV-2 by RT-PCR assay only, without having any subjective symptoms before admission, were classified as having asymptomatic infections. Such participants were screened from the list of close contacts of confirmed COVID-19 patients. Participants having no subjective symptoms before admission but having developed mild subjective symptoms during hospitalization were reclassified as having presymptomatic infections. Other participants, who had positive results for SARS-CoV-2 RT-PCR together with mild subjective symptoms at admission, such as fever or respiratory symptoms, but without clinical manifestation of severe pneumonia, were classified as having mild cases of COVID-19, in line with recommendations detailed in the 6th edition of the Chinese guidelines for the diagnosis and treatment of COVID-19 in China [6].
This study was reviewed and approved by the medical ethical committee of Chongqing University Central Hospital (approval no. Scientific research 2020(8)).
Laboratory testing
Clinical specimens (throat swabs, nasal swabs, nasopharyngeal swab, sputum and anal swabs) were collected and analysed via RT-PCR assay with primers and probes targeting the N and Orf1b genes of SARS-CoV-2. A nucleic acid extraction kit was used to extract virus nucleic acids from clinical samples via specific nucleic acid extraction instrumentation (Shengxiang Biotechnology, Hunan, China). Nucleic acid amplification and identification were performed with the ABI QuantStudi05 Real-Time PCR Amplifier (Thermo Fisher Scientific, Waltham, MA, USA) using a coronavirus nucleic acid detection kit (Da'an Gene, Guangdong, China). The cycle threshold (C t) value from RT-PCR analysis was used as a proxy measure of virus load; a value of C t < 40 was considered positive.
Plasma samples were collected and tested for total antibody against SARS-CoV-2 with a commercial chemiluminescence microparticle immunoassay (CMIA) (InnoDx Biotech, Xiamen, China), which was based on the double-antigen sandwich method as previously described [7]. In this immunoassay, mammalian cell-expressed SARS-CoV-2 spike receptor binding domain (RBD) proteins were utilized as antigens. The assay was performed with an automatic CMIA Caris200 analyser (UMIC Medical Instrument, Xiamen, China) according to the manufacturers' instructions. Briefly, 50 μL of paramagnetic microparticles coated with recombinant RBD antigen were mixed with 50 μL of plasma and 50 μL of sample dilution buffer; then the mixture was incubated at 37°C for 15 minutes. After the microparticles were washed twice, 100 μL of acridinium ester–conjugated RBD was added, followed by incubation at 37°C for 10 minutes. Then the microparticles were washed four times, and each 100 μL of pre–trigger solution (hydrogen peroxide) and trigger solution (NaOH) was added, and relative light units measured. On the basis of the measured relative light units, the cutoff index for each sample was determined. A cut-off index ≥1 was defined as a positive result.
All kits and instruments we used are commercially available and are approved by the National Medical Products Administration of China.
Statistical analysis
The incubation period was defined as the interval between the earliest date of SARS-CoV-2 exposure and the earliest date of symptom onset. Virus shedding time was defined as the interval between the date of the first positive test of any specimen by RT-PCR and the midpoint date between the dates of the last positive test and subsequent first negative test. For patients with a positive result at their last follow-up, virus shedding time was defined as interval between the date of the first positive test by any specimens and date of the last positive test. For description of continuous variables, means with standard deviation (SD) were used for normally distributed data, and medians with interquartile range (IQR) were used for data that were not normally distributed. The Student t test or the Wilcoxon rank-sum test was used for comparison of continuous variables. Categorical data were described as numbers and percentages, and were compared by the Fisher exact test.
All statistical analyses were conducted by SAS 9.4 software (SAS Institute, Cary, NC).
Results
Epidemiologic and clinical features of patients at admission
A total of nine each asymptomatic and mildly symptomatic SARS-CoV-2 infections were identified at admission. The demographic and clinical details of enrolled participants at admission are presented in Table 1 . The median age of the patients was 46 years old (range, 12-72 years), and six patients (33.3%) were male. There were five clusters of transmission, including four family clusters and one cluster of restaurant staff with untraceable sources or contacts (Fig. 1 ). Fever was present in six (66.7%) of the nine mildly symptomatic patients, and cough was present in seven of them (77.8%). Four asymptomatic patients (44.4%) and four patients with mild symptoms (44.4%) exhibited typical imaging signs of ground-glass opacities or patchy shadows in the lungs on chest CT images, while five patients (27.8%) had neither clinical symptoms nor visible abnormalities on chest CT imaging at admission.
Table 1.
Clinical features of enrolled COVID-19 patients at admission
| Variable | All patients (N = 18) | RT-PCR result after discharge |
p | ||
|---|---|---|---|---|---|
| Negative (n = 11) | Positive (n = 7)a | ||||
| Age (years), median (IQR) | 46 (36, 50) | 46 (36, 50) | 45 (28, 51) | 0.82 | |
| Sex | |||||
| Male | 6 (33.3) | 2 (18.2) | 4 (57.1) | 0.14 | |
| Female | 12 (66.7) | 9 (81.8) | 3 (42.9) | ||
| Exposure | |||||
| Recently visited Wuhan | 2 (11.1) | 2 | 0 | 1.00 | |
| Contact with COVID-19 patients | 15 (83.3) | 9 | 6 | ||
| Unknown | 1 (5.6) | 0 | 1 | ||
| Subjective symptoms | |||||
| No | 9 (50.0) | 6 (75.0) | 3 (50.0) | 1.00 | |
| Yes | 9 (50.0) | 5 (25.0) | 4 (50.0) | ||
| Fever | 6 | 4 | 2 | ||
| Cough | 7 | 4 | 3 | ||
| Headache | 3 | 2 | 1 | ||
| Myalgia | 2 | 1 | 1 | ||
| Fatigue | 2 | 1 | 1 | ||
| Sore throat | 3 | 2 | 1 | ||
| Rhinorrhea | 1 | 1 | 0 | ||
| Nasal congestion | 1 | 0 | 1 | ||
| Abnormalities on chest CT | 8 (44.4) | 5 (62.5) | 3 (50.0) | 1.00 | |
| Vital signs, median (IQR) | |||||
| Temperature (°C) | 36.9 (36.5, 38.0) | 37.3 (36.5, 38) | 36.7 (36.5, 37.8) | 0.71 | |
| O2 saturation (%) | 98 (98, 98) | 98 (98, 98) | 98 (97, 98) | 0.32 | |
| Heart rate (bpm) | 85 (78, 92) | 85 (78, 92) | 85 (79, 92) | 0.43 | |
| Diastolic blood pressure (mm Hg) | 121 (120, 124) | 122 (120, 124) | 120 (112, 124) | 0.46 | |
| CD4+, median (IQR) | 480 (257, 745) | 477.5 (366, 866) | 480 (252, 745) | 0.53 | |
| CD8+, median (IQR) | 316 (305, 403) | 314 (209, 370) | 372 (305, 561) | 0.35 | |
| CD4+/CD8+, mean (SD) | 1.7 (0.8) | 2.0 (0.8) | 1.3 (0.6) | 0.09 | |
| RT-PCR cycle threshold values, mean (SD) | 31.4 (4.7) | 31.6 (4.5) | 31.1 (5.2) | 0.81 | |
Data are presented as n (%) unless otherwise indicated.
COVID-19, coronavirus disease 2019; CT, computed tomography; IQR, interquartile range; RT-PCR, real-time reverse transcription PCR; SD, standard deviation.
Among 7 patients with positive RT-PCR results after discharge, three were readmitted to hospital for recurrence of clinical symptoms.
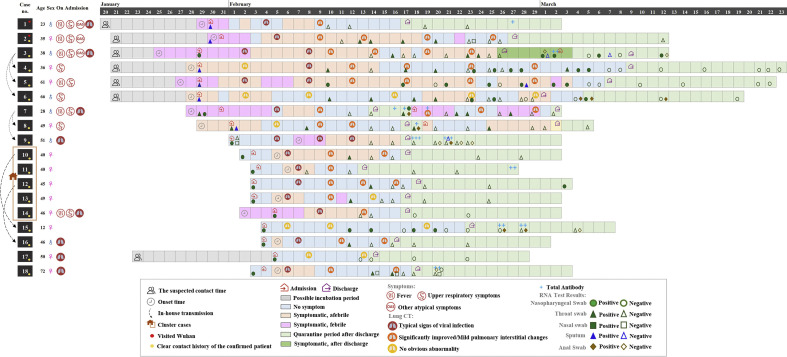
Fig. 1.
Virus shedding dynamics in asymptomatic and mildly symptomatic COVID-19 patients. COVID-19, coronavirus disease 2019. For total antibody, "+" indicates cut-off index between 1 to 10, "++" cut-off index between 10 to 100, "+++" cut-off index 100 to 1000.
SARS-CoV-2 virus shedding dynamics
As shown in Fig. 1, the median incubation period of seven subjects who had definite exposure through close contact with confirmed patients was 7.0 days (IQR 6–9 days, range 4–13 days). Of the nine asymptomatic infections at admission, six subjects (66.7%) developed subjective symptoms during hospitalization; these subjects were recategorized as being presymptomatic. The median interval from hospital admission to symptom onset in presymptomatic subjects was 3 days. The nine subjects who had subjective symptoms before seeking medical advice took, on average, 2.2 days from symptom onset to admission. Most of the patients (16/18, 88.9%) had typical chest CT imaging changes at admission or shortly after admission, and their clinical conditions gradually improved during hospitalization. Two subjects (cases 6 and 15) developed no discernable abnormalities on chest CT imaging during the period between admission and discharge. Up until 9 March 2020, all subjects were discharged from hospital, with a median length of stay of 18.0 days (IQR 15–31 days, range 10–41 days). Among them, three subjects were readmitted to hospital for recurrence of clinical symptoms, with positive RT-PCR results during the 2-weeks follow-up period. None of the subjects in our cohort died of COVID-19.
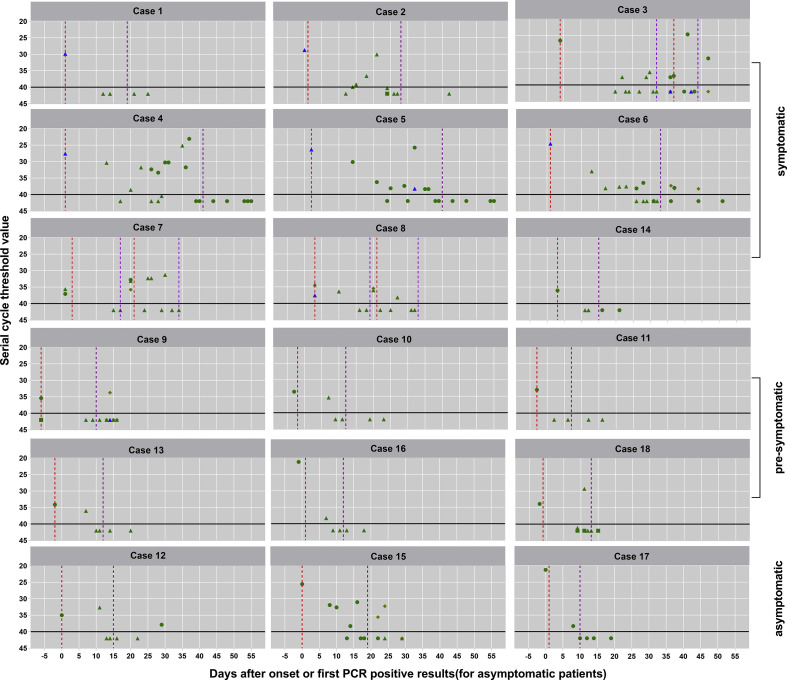
The median (IQR) duration of virus shedding was 22.5 (10–31) days (range, 3–47 days), and ten patients (56%) had virus shedding in the nasopharynx or stool detectable for more than 14 days. The median (IQR) duration of virus shedding in presymptomatic patients, asymptomatic patients and mildly symptomatic patients was 11.5 (10–14), 28 (5–30) and 31 (23.5–38) days, respectively. The sampling site, combined with detailed C t values for each test, are presented in Fig. 2 . Of particular concern, seven patients (cases 3, 6, 7, 8, 9, 12 and 15) continued to shed virus after hospital discharge, with median C t value of the last positive PCR test of 33.7 (IQR 31.9–38.2). An asymptomatic child patient aged 12 years (case 15), surprisingly, had neither clinical symptoms nor noticeable abnormalities on CT imaging, but remained RT-PCR positive for SARS-CoV-2 for 28 days.
Fig. 2.
Serial cycle threshold (Ct) values of each test for each participant. Dotted red line indicates hospital admission; purple dotted line, hospital discharge; green triangle, throat swab; green circle, nasopharyngeal; green square, nasal swab; blue diamond, sputum; orange diamond, anal swab.
The mean CD4+/CD8+ ratio of inpatients whose RT-PCR remained negative after discharge was higher than that of those who subsequently developed positive results after discharge (respectively, 2.0 ± 0.8 vs. 1.3 ± 0.6, p 0.09). No statistically significant differences were found between other clinical indexes in these two groups. This is likely related to our limited sample size.
In addition, the plasma samples of eight patients were also tested for anti–SARS-CoV-2 antibodies after discharge, and all samples had positive results. Among these results, higher titre antibodies were detected in the serum of case 9 (Fig. 1). During the convalescent phase, detection of antibodies to SARS-CoV-2 as well as the presence of SARS-CoV-2 RNA was observed in one (33.3%) asymptomatic patient (case 15), one presymptomatic patient (16.7%, case 9) and three (33.3%) mildly symptomatic patients (cases 3, 7 and 8).
Discussion
In this study, we observed that long-term intermittent virus shedding was occurred in the respiratory tract of some patients with mild symptoms and some asymptomatic patients. During the convalescent phase, detectable antibodies to SARS-CoV-2 and RNA were simultaneously observed in five patients, which suggests that the production of specific antibodies to SARS-CoV-2 may not guarantee virus clearance after discharge.
The median duration of virus shedding in our cohort was 22.5 days (range, 3–47 days), which is longer than that reported in hospital patients in Zhuhai, China (maximum 21 days) [5], and Singapore (median 12 days, maximum 24 days) [8]. This may be because patients in our study were actively followed up during the 14 days of quarantine after hospital discharge. This finding confirms that it may be necessary to monitor RNA and antibody status in discharged COVID-19 patients. The median duration of virus shedding appears to be shorter in presymptomatic patients (11.5 days) than in asymptomatic (28 days) and mildly symptomatic (31 days) patients. Sustained virus shedding in faeces was found after virus clearance from the respiratory tract in an asymptomatic child aged 12. Another asymptomatic patient, a 45-year-old woman, had a positive SARS-CoV-2 RT-PCR result 14 days after hospital discharge subsequent to four consecutive negative RT-PCR results from respiratory tract samples (C t = 37.9). One case of household transmission by asymptomatic patients was observed in this study, with an asymptomatic patient transmitting SARS-CoV-2 to the husband, indicating that asymptomatic cases may be a source of SARS-CoV-2 transmission and infection, likely by means of human-to-human transmission via close contact. Data from a study conducted in Nanjing indicates that transmission from asymptomatic subjects may eventually lead to the development of severe COVID-19 in some patients [9]. RNA combined with antibody testing is necessary for all close contacts during the 14 days of quarantine, as opposed to testing only those with subjective symptoms. However, most asymptomatic patients, together with those with mild symptoms not presenting for active screening for SARS-CoV-2 infection, are inherently difficult to efficiently track and trace for subsequent diagnostic confirmation and placement into quarantine. This actively infectious population represents a tangible and complex epidemiologic challenge to COVID-19 public health control in China, and indeed the rest of the world.
In the convalescent phase, 38.9% of our entire cohort of patients (four symptomatic, two asymptomatic and one presymptomatic) continued to shed virus, with a median C t value of 33.7. One study conducted in Wuhan found that four discharged patients had positive RT-PCR test results 5 to 13 days after discharge [10]. Compared to cases that reverted to PCR positivity in the convalescent phase, CD4+/CD8+ ratios tended to be higher in patients whose PCR results continued to be negative, which may indicate that virus shedding in the convalescent phase may occur in individuals with intrinsically flawed immune function or immune response. Five patients whose PCR results reverted to positive were also found to be positive for SARS-CoV-2 antibodies in the convalescent phase. The presence of adaptive antibodies together with RNA may suggest that production of antibodies may not guarantee SARS-CoV-2 virus clearance. The phenomenon has also been described in patients recovering from Middle East respiratory syndrome [11,12]. It is unknown whether higher specific antibody titres are required for SARS-CoV-2 clearance; this warrants further study.
An analysis of 72 314 cases by the Chinese Center for Disease Control reported that 1.2% of patients (approximately 867 patients) were asymptomatic in that cohort, and approximately 80% of COVID-19 cases (approximately 57.850 patients) had mild to moderate disease [1,2]. The pathogenic transmission potential of the observed large number of asymptomatic patients with COVID-19, as well as the relatively large fraction of patients with mildly symptomatic COVID-19, coupled with the extended period of virus shedding in asymptomatic and symptomatic patients with COVID-19, represents a significant epidemiologic and logistical challenge to local, regional and global public healthcare systems in their diligent ongoing attempts to restrict the transmission of SARS-CoV-2 and limit mortality from severe COVID-19.
Our study had several limitations. Firstly, we only enrolled 18 patients in our study. Larger-scale studies with larger cohorts are warranted in the future in order to validate our findings. Secondly, serial plasma samples were not performed for all patients during their hospital stay, and not all patients consented to providing plasma samples after discharge. Thirdly, contemporary RT-PCR testing is unable to distinguish between viable replicating virus and residual virus RNA after viral lysis and destruction; therefore, not all of our positive PCR results may represent potential virus shedding.
In conclusion, our study observed that long-term virus shedding was present in patients with mild symptoms and in asymptomatic patients, and specific antibody production to SARS-CoV-2 may not guarantee virus clearance after hospital discharge. Specific information regarding virus shedding dynamics and antibody production should be considered by public health authorities as well as clinicians when addressing SARS-CoV-2 infection prevention and control.
Transparency declaration
Supported by grants from Chongqing Young and Middle-aged Medical Professionals Cultivation Program (ZQNYXGDRCGZS2019008), Xiamen Science and Technology Major Project (3502Z2020YJ01) and Chongqing Special Research Project (cstc2020jscx-fyzxX0005). All authors report no conflicts of interest relevant to this article.
Acknowledgements
We thank all the study participants.
Editor: L. Kaiser
References
- 1.Epidemiology Working Group for NCIP Epidemic Response. Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Commission of China New coronavirus pneumonia prevention and control program. 6th ed. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf Available at:
- 7.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Abdely H.M., Midgley C.M., Alkhamis A.M., Abedi G.R., Lu X., Binder A.M., et al. Middle East respiratory syndrome coronavirus infection dynamics and antibody responses among clinically diverse patients, Saudi Arabia. Emerg Infect Dis. 2019;25:753–766. doi: 10.3201/eid2504.181595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]