Graphical abstract
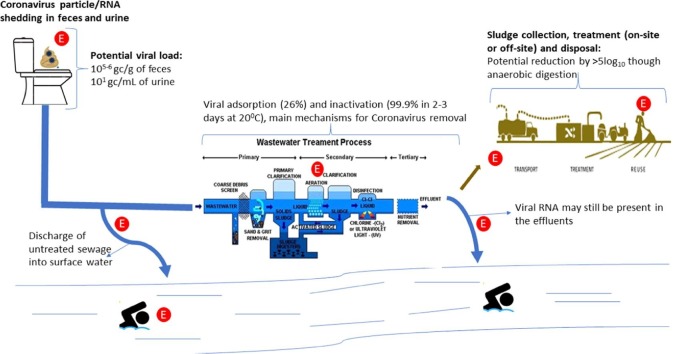
A graphical representation of the source and fate of Coronavirus in wastewater, showing the potential viral load in feces and urine and their survival removal by during wastewater/sludge treatment. The potential exposure points are represented by the letter ‘E’ in a red background. These exposure points include; the home setting due to faulty plumbing, general public exposure to untreated sewage due to inadequate sanitation and burst sewer pipes. Exposure for WWTW workers from aerosols and direct contact due to sludge collection and treatment. The final exposure scenario is for the general public due to the potential presence of infectious viral particles in treated sewage.
Keywords: Human coronavirus, Severe Acute Respiratory Syndrome (SARS), Middle Eastern Respiratory Syndrome (MERS), COVID-19, Wastewater, Sewage
Abstract
The last 17 years have seen three major outbreaks caused by coronaviruses, with the latest outbreak, COVID-19, declared a pandemic by the World Health Organization. The frequency of these outbreaks, their mortality and associated disruption to normal life calls for concerted efforts to understand their occurrence and fate in different environments. There is an increased interest in the occurrence of coronaviruses in wastewater from the perspective of wastewater-based epidemiology. However, there is no comprehensive review of the knowledge on coronavirus occurrence, fate and potential transmission in wastewater.
This paper, provides a review of the literature on the occurrence of coronaviruses in wastewater treatment processes. We discuss the presence of viral RNA in feces as a result of diarrhoea caused by gastrointestinal infections. We also reviewed the literature on the presence, survival and potential removal of coronaviruses in common wastewater treatment processes. The detection of infectious viral particles in feces of patients raises questions on the potential risks of infection for people exposed to untreated sewage/wastewater. We, therefore, highlighted the potential risk of infection with coronaviruses for workers in wastewater treatment plants and the public that may be exposed through faulty plumbing or burst sewer networks.
The mortalities and morbidities associated with the current COVID-19 pandemic warrants a much more focused research on the role of environments, such as wastewater and surface water, in disease transmission. The current wealth of knowledge on coronaviruses in wastewater based on the reviewed literature is scant and therefore calls for further studies.
1. Background
Coronaviridae (coronavirus) is a family of positive-sense single-stranded RNA viruses, responsible for several common-cold-like and severe respiratory infections (Yeo et al., 2020, Qu et al., 2020, Casanova et al., 2009). This family has over 30 viruses and has the largest reported genome of all RNA viruses of 30 Kb (Amirian, 2020, Woo et al., 2005, Marra et al., 2003). Coronaviruses are subdivided into four groups; Alphacoronavirus (Alpha-CoV), Betacoronavirus (Beta-CoV), Gammacoronavirus (Gamma-CoV), and Deltacoronavirus (Delta-CoV) (Qu et al., 2020). To date there are six known coronaviruses that have caused infections in humans, these are HCoV-229E, HCoV-OC43, HCoV-NL-63, HCoV-HUK-1, SARS-CoV, and MERS-CoV, mainly belonging to the Beta-CoV group (Hemida, 2019). In the past 17 years, there have been three major outbreaks caused by human coronaviruses. This include the severe acute respiratory syndrome coronavirus (SARS-CoV) that emerged in 2003 in China and affected 26 countries (Hemida, 2019, Lau and Chan, 2015, Peiris et al., 2003a). The second outbreak of human coronaviruses caused by the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) occurred in 2012 (Hemida, 2019, Zaki et al., 2012), affecting 27 countries wth over 2400 cases as at the end of 2019 (WHO, 2020a). More recently in 2019, a novel coronavirus, called the SARS-CoV-2 emerged in Wuhan, China (Liu et al., 2020, Zhu et al., 2020). As at 4th July 2020 the official data from the World Health Organization (WHO) indicates that over 10.9 million people globally have the COVID-19, the disease caused by SARS-CoV-2, (WHO, 2020c).
The short time between the emergence of new coronaviruses has been attributed to the poor proofreading capability of RNA polymerases (Hofer, 2013). Additionally, the simultaneous occurrence of different coronaviruses in the same environment may contribute to the emergence of new strains of this virus through recombination (Hemida, 2019, Su et al., 2016).
This group of viruses have the potential to grow in epithelial cells and usually causes respiratory infection, hence the names Severe Acute Respiratory Syndrome Coronavirus or Middle East Respiratory Syndrome Coronavirus (Liu et al., 2020, Casanova et al., 2009). Clinical symptoms include, diarrhea, nausea, fever, cough and myalgia, although some may be asymptomatic (Liu et al., 2020, Guan et al., 2020, Chan et al., 2020). In the SARS-CoV outbreak of 2003, 16–73% of the patients had diarrhoea, which was reported during the first week of the illness (Yeo et al., 2020). During the current outbreak of COVID-19 evidence suggests that 2–35% of the patients have gastrointestinal (GI) symptoms, such as diarrhoea, abdominal pain, and vomiting, although it is less frequent compared to respiratory symptoms (Yeo et al., 2020, Wang et al., 2020a). This has led to the detection of the viral RNA in feces and sewage (Amirian, 2020, Bowser, 2020, Pan et al., 2020, Xie et al., 2020). Some studies have reported infectious virions of coronavirus in feces (Zhang et al., 2020a, Wang et al., 2020b, Xiao et al., 2020). Information on the persistence of coronaviruses in wastewater is therefore important, especially to understand the potential risks for exposed populations.
This review, therefore, presents the current state of knowledge on coronaviruses in wastewater processes. We reviewed studies that report on coronaviruses in feces and urine, that may result in the occurrence of these in wastewater. Additionally, we address the fate of these viruses in wastewater; which looks at their survival and removal during wastewater treatment. Since infective virions have been found in feces, we also looked at the potential of infections for workers/operators of wastewater treatment plants and the general public.
2. Methodology
The review was prepared based on literature search in the following databases for publications up to 2nd May 2020; Pub Med; Web of Science, ScienceDirect, google scholar and ResearchGate. The keywords and word strings used for the search were; Coronavirus OR Coronaviridae OR Severe Acute Respiratory Syndrome OR SARS OR SARS-CoV OR SARS-CoV-2 OR COVID-19 OR Middle Eastern Respiratory Syndrome OR MERS OR MERS-CoV AND Wastewater OR Sewage OR Feces OR Urine OR Gastrointestinal infections. All results of the search were screened manually for relevant information and their references searched for additional publications that may be relevant. Due to the urgency of the current COVID-19 pandemic, there are several manuscripts in the pre-print form with a wealth of information relevant for the review, therefore both peer-reviewed and pre-print articles were reviewed. Articles reporting on coronavirus infections that do not present information on the detection of the virus in feces, urine, wastewater, and sewage were only considered if they gave information on the fate of these viruses in wastewater.
3. Source of coronaviruses in wastewater
Coronaviruses may be introduced into wastewater (domestic and hospital) through several sources, such as handwashing, sputum and vomit (Han et al., 2020a, Sung et al., 2016, Haagmans et al., 2014). Additionally, there are reports of viral shedding in urine of individuals infected with SARS-CoV (Xu et al., 2005), MERS (Drosten et al., 2013) and SARS-CoV-2 (Nomoto et al., 2020). However, the main route that has been reported extensively is via the shedding of the viral RNA in feces of infected individuals (Chen et al., 2020b; Ling et al., 2020, Xiao et al., 2020, Zhang et al., 2020b). This section, therefore, focuses on the GI infections and reports of viral RNA in feces.
3.1. Gastrointestinal infections and detection of viral RNA in feces
Gastrointestinal infections (GI) symptoms, such as abdominal discomfort, diarrhea, GI bleeding, nausea and vomiting, have been observed in patients, indicating GI tract infection (Yang et al., 2020, Wang et al., 2020a). Studies have shown that the MERS-CoV infects and replicates in human primary intestinal epithelial cells, through the dipeptidyl peptidase receptor (van Doremalen et al., 2014, Wang et al., 2013). In-vivo studies showed inflammation and epithelial degeneration in the small intestine before the development of pneumonia and brain infection associated with MERS-CoV (Zhou et al., 2017). These findings suggest that in some MERS-CoV, pulmonary infections may be secondary to intestinal infections.
Evidence suggests that the SARS-CoV and SARS-CoV-2 GI infections in humans are mediated through the angiotensin-converting enzyme 2 (ACE2) cell receptor (Yeo et al., 2020, Wan et al., 2020, Bertram et al., 2012, Chan et al., 2004b). The ACE2 enzyme is mainly found attached to the cell membranes of cells in the lungs, arteries, heart, kidney, and intestines (Hamming et al., 2004). The binding affinity of the ACE2 receptors has been observed as the most important factor for the infectivity of the virus (Yeo et al., 2020, Holshue et al., 2020). Structural analysis indicates that SARS-CoV-2 uses the human ACE2 receptor more efficiently than the SARS-CoV (Yeo et al.,2020). This may be another reason for the faster spread of the SARS-CoV-2. Early reports from Wuhan showed that abdominal pain (an indication of GI infections) was reported more frequently in patients admitted into intensive care, than individuals not requiring intensive care (Yang et al., 2020, Wang et al., 2020a, Chen et al., 2020a, Jin et al., 2020). These reports also showed that in about 10% of the patients, diarrhoea and nausea symptoms occurred 1–2 days before the development of fever and respiratory symptoms (Wang et al., 2020a). This again supports the hypothesis that in some patients, GI infections may occur before the respiratory symptoms. It is estimated that in the first SARS outbreak, between 20 and 25% of the patients had diarrhoea (Hui et al., 2003), a sign of GI infections. Other publications have reported higher diarrhoea incidence among infected people, for instance 20.3%-38.4% (Leung et al., 2003) and 73% (Peiris et al., 2003a, Peiris et al., 2003b) of patients are reported to have watery dirahhoea. In the current pandemic (COVID-19), reports on frequency of GI infections varies. For instance, Huang et al. (2020b) reported 3% diarrhoeal frequency. Wang et al. (2020a) reported GI infections like diarrhea (10.1%), nausea (10.1%), vomit (3.6%), abdominal pain (2.2%). However, official data from the WHO reports that between 2 and 27% of COVID-19 patients have diarrhoea (WHO, 2020b).
The detection of coronavirus in the feces of infected persons is therefore not surprising, based on the GI infections reported. Corman et al. (2016) reported MERS-CoV RNA in 14.6% of stool samples from patients. There are emerging reports of SARS-CoV-2 RNA in stool from patients in the current COVID-19 pandemic (Amirian, 2020, CDC-Centers for Disease Control & Prevention, 2020a). Table 1 presents reports of coronavirus RNA in stool samples from different geographical locations. However, it is unclear how long the shedding continues, a few studies have suggested that RNA can be found in the stool from day 1–25 days after onset of the GI illness (Amirian, 2020, Ling et al., 2020, The COVID-19 Investigation Team, 2020, Xiao et al., 2020, Zhang et al., 2020b). In another study, SARS-CoV RNA was detected in stool samples from the fifth day, with a peak in viral titer at the 11th day and lasted till the 30th day (Amirian, 2020, Bell, 2003). It is also unclear if there is any association between the detection of the viral RNA in stool and the severity or pattern of symptomatology of the disease (Amirian, 2020). However, it is assumed that both symptomatic and asymptomatic people could spread the virus through their excreta/feces (Núñez-Delgado, 2020). In a study conducted in Wuhan, it was demonstrated that in about 10% of COVID-19 patients, viral RNA (some infectious) were still found in the feces even after no viral RNA was found in samples from the respiratory tract (Xiao et al., 2020).
Table 1.
Reports of coronaviruses in fecal material.
| Virus | Sample | Detection methods | Location | Reference |
|---|---|---|---|---|
| SARS-CoV | Feces and Urine | Cell monolayer culture and confirmed with RT-PCR | Hong Kong | Chan et al., 2004a |
| Feces and Urine | Cell monolayer culture and confirmed with electron microscopy and RT-PCR | Beijing, China | Xu et al., 2005 | |
| Feces | Cell culture and semi-nested RT-PCR | Beijing, China | Wang et al., 2005a | |
| Feces and Urine | RT-PCR | Hong Kong | Cheng et al., 2004 | |
| Feces and Urine | RT-PCR | Hong Kong | Hung et al., 2004 | |
| Feces | RT-PCR | Hong Kong | Peiris et al., 2003b | |
| Feces and Urine | RT-PCR | China | Zheng et al., 2004 | |
| MERS-CoV | Urine | RT-PCR | France | Poissy et al., 2014 |
| Feces and Urine | RT-PCR and sequencing | Germany (patient from Abu Dhabi) | Drosten et al., 2013 | |
| Feces and Urine | RT-PCR and cell culturing | Riyadh, Saudi Arabia | Corman et al., 2016 | |
| SARS-CoV-2 | Feces | RT-PCR | Hubei, Shandong and Beijing, China | Wang et al., 2020b |
| Feces | RT-PCR | Jinhua, China | Zhang et al., 2020b | |
| Anal swabs | full genome sequencing, Cell culture (vero cells) and electron microscopy | Wuhan, China | Zhang et al., 2020a | |
| Feces | RT-PCR | Shanghai and Qingdao, China | Cai et al., 2020 | |
| Feces | viral nucleocapsid staining | Zhuhai, China | Xiao et al., 2020 | |
| Feces | RT-PCR | Zhoushan, China | Tang et al., 2020 | |
| Feces | RT-PCR and sequencing | Singapore | Young et al., 2020a | |
| Feces | RT-PCR | AZ, CA, IL, MA, WA, and WI, United States | The COVID-19 Investigation Team, 2020 | |
| Feces | RT-PCR | Shanghai, China | Ling et al., 2020 | |
| Feces | RT-PCR | Guangdong, China | Chan et al., 2020 | |
| Feces | RT-PCR | Kallang, Singapore | Kam et al., 2020 | |
| Feces | RT-PCR | Heilongjiang, China | Zhang et al., 2020b | |
| Feces | RT-PCR | China | Xie et al., 2020 | |
| Feces | RT-PCR | Macau | Lo et al., 2020 | |
| Feces | RT-PCR | China | Wu et al., 2020c | |
| Feces | RT-PCR | Wuhan, China | Chen et al., 2020b | |
| Feces | RT-PCR | China | Rampelli et al., 2020 | |
| Feces | RT-PCR | Shandong Province, China | Xing et al., 2020 | |
| Feces | RT-PCR | Tianjin, China | Zhang et al., 2020c | |
| Feces | RT-PCR | Korea | Park et al., 2020b |
Detection of the virus in fecal samples has been mainly through RT-PCR, as shown in Table 1. This approach is the gold standard accepted globally for the detection of viral RNA in several types of clinical samples, however a stool sample positive for the virus may only have the RNA but not the infective viable virus. A few methods have used cell cultures (Chan et al., 2004a, Xu et al., 2005, Corman et al., 2016), electron microscopy (Xu et al., 2005, Zhang et al., 2020a) and viral nucleocapsid staining (Xiao et al., 2020) for the detection of these viruses in feces. These approaches have shown that some fecal samples may contain viable viruses (Chan et al., 2004a, Xu et al., 2005, Zhang et al., 2020a), which raises concern for transmission of infections through exposure to feces. The early onset of GI symptoms during infections with coronaviruses could therefore serve as an early warning system, based on early detection of the viral RNA in feces. Furthermore, continuous shedding of the viral RNA in feces after no respiratory symptoms are observed could potentially cause fecal-oral transmission from ‘recovered’ patients.
3.2. Occurence of coronaviruses in wastewater
Majority of studies on the occurence of viruses in wastewater have focused on nonenveloped enteric viruses, like adenoviruses, polio viruses, enteroviruses, noroviruses and rotaviruses (Ye et al., 2016, Fumian et al., 2010, Katayama et al., 2008). This is mainly because these are transmitted primarily through the fecal-oral route (Ye et al., 2016). However, the presence of enveloped viruses like coronaviruses in wastewater could differ greatly due to differences in their survival and partitioning behaviour in water (Ye et al., 2016, Arbely et al., 2006).
Since the cluster of SARS cases in an apartment block in Hong Kong, traced to droplets containing coronavirus from the wastewater system (WHO, 2003), there has been an increased interest in the detection of coronaviruses in wastewater. Initial reports of SARS-CoV RNA in wastewater came from studies conducted at the Xiao Tang Shan Hospital and 309th Hospital of PLA, the designated hospitals to receive SARS patients in Beijing during the 2003 outbreak (Wang et al., 2005c). Another reason for increased interest in the occurrence of coronaviruses in wastewater is wastewater-based epidemiology (WBE). This concept aims to use sewage/untreated wastewater analysis as an early warning system for disease outbreak (Xagoraraki & O’Brien, 2020), since viral RNA can be detected in feces, and subsequently wastewater, weeks before the onset of illness. A few studies have reported the detection of coronavirus in untreated wastewater/sewage (Table 2 ). These studies have focused mainly on the detection of these viruses without quantification, therefore it challenging to compare the concentration of the viral titer between studies. The available information shows an increase in these studies during the current pandemic, this could be attributed to the WBE concept,the need for further information on the occurrence of these viruses in wastewater and the avaibility of advanced molecular techniques for viral load quantification. In Paris, Wurtzer et al. (2020) detected the presence of the SARS-CoV-2 viral RNA even in the treated wastewater. However, the presence of the viral RNA does not indicate that these viral particles are intact and infectious. Addtionally, in the report the wastewater treatment processes used was not stated.
Table 2.
Reports of coronaviruses in wastewater or sewage.
| Virus | Sample | Detection method | Location | Reference |
|---|---|---|---|---|
| SARS-CoV | sewage | RT-qPCR | China | Wang et al., 2005c |
| SARS-CoV-2 | wastewater | RT-qPCR | USA | Wu et al.,2020a |
| SARS-CoV-2 | sewage | RT-qPCR | Netherlands | Medema et al., 2020 |
| SARS-CoV-2 | Wastewater | RT-qPCR | Australia | Ahmed et al., 2020a |
| SARS-CoV-2 | Wastewater (treated and untreated) | RT-qPCR | France | Wurtzer et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Netherlands | Lodder & de Roda Husman,2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Spain | Randazzo et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | USA | Nemudryi et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | USA | Green et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Israel | Or et al., 2020 |
| SARS-CoV-2 | primary and secondary wastewater and sludge | RT-qPCR | Spain | Balboa et al., 2020 |
| SARS-CoV-2 | Primary sludge | RT-qPCR | USA | Peccia et al., 2020 |
| SARS-CoV-2 | wastewater | nested RT-PCR and real-time qPCR | Italy | La Rosa et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Japan | Haramoto et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Pakistan | Sharif et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | Japan | Hata, et al., 2020 |
| SARS-CoV-2 | wastewater | RT-qPCR | India | Kumar et al., 2020 |
| SARS-CoV-2 | Wastewater and rivers | RT-qPCR and whole genome sequencing | Italy | Rimoldi et al., 2020 |
| SARS-CoV-2 | Waste Activated sludge | RT-qPCR | Turkey | Kocamemi et al., 2020 |
The main factor influencing the occurrence of coronaviruses in wastewater will be the concentration of viral RNA shed per gram of feces of an infected person. In general, the concentration of enteric viral particles per gram of feces during diarrhoea has been reported to be 1010-1012 (Haas et al., 2014). For SARS viral loads of 106.1 gc/g of feces and 101.3 gc/mL of urine have been reported (Hung et al., 2004). Reports of viral load in stool of persons infected with SARS-CoV-2 has varied. For instance, 1.7 × 106–4.1 × 107 gc/mL have been reported by Han et al. (2020b), in contrast to 6.3 × 106 –1.26 × 108 gc/g of stool reported by Lescure et al. (2020). In anal swabs viral loads of 105 gc/swab for SARS-CoV-2 has been reported (Woelfel et al., 2020). This shows that the viral load of coronaviruses in feces may be lower than that of enteric viruses. However, additional studies are required to understand how frequently coronaviruses are shed in feces and urine of infected individuals. This information will not only give an idea to the concentrations expected in wastewater but may also provide vital information in understanding the potential of fecal transmission. Information on the shedding frequency will also aid in correlating the viral load in the wastewater with infection rate in the community.
In addition, the per capita water use could affect the concentration of viruses detected in wastewater. Peak times (like mornings and evenings) are associated with higher domestic water usage (Gerba et al., 2017, Almeida et al., 1999), this could result in dilution, therefore, reducing the concentrations of viral load at these times. The survival of these coronaviruses in the environment could be another major factor influencing their occurrence in wastewater. The current belief is that coronaviruses can survive for only a few days in the environment (Kampf et al., 2020), however, some studies paint a different picture, this is discussed in detail in Section 4.1.
3.2.1. Methods for the detection and quantification of coronavirus RNA in wastewater
Detection of coronavirus RNA in wastewater has been mainly through molecular techniques involving PCR based methods such as reverse transcription-polymerase chain reaction (RT-PCR) and digital PCR. This is achieved through the amplification of parts of the viral genome, like the genes coding for either the nucleocapsid (CDC, 2020b), and viral envelop (Corman et al., 2020). Molecular detection of the viral RNA involves three major steps. These include;
-
•
Viral concentration/enrichment: Due to the potential low concentration of viral titer in wastewater, several options have been used to concentrate the viral particles for analysis. These include; direct analysis of unfiltered wastewater/sewage samples after precipitation with polyethylene glycol (PEG) (Wu et al., 2020a, Wu et al., 2020b). Viral concentration/enrichment through filtration using 0.2um filters (Wu et al., 2020a, Wu et al., 2020b), ultrafilters (Medema et al., 2020, Wurtzer et al., 2020, Ahmed et al., 2020a, Ahmed et al., 2020b) and ultracentrifugation (Green et al., 2020, Ahmed et al., 2020b) have also been reported. Direct RNA extraction from electronegative membranes (0.45-μm) is another method that can be used (Ahmed et al., 2020a).
-
•
RNA extraction: RNA extraction has generally been performed using commercial kits from a variety of supplies. The most common RNA extraction kits used are RNeasy PowerMicrobiome Kit (Medema et al., 2020, Ahmed et al., 2020a, Ahmed et al., 2020b), Biomerieux Nuclisens kit (Medema et al., 2020), PowerFecal Pro kit (Wurtzer et al., 2020) and RNeasy PowerWater Kit and RNeasy (Ahmed et al., 2020a). In addition to these RNA extraction kits that have been used in the detection of coronavirus RNA in wastewater, the Centers for Disease Control and Prevention of US has published a list of RNA extraction kits that can be used for SARS-CoV-2 (CDC, 2020b).
Amplification of viral RNA: Amplification of viral RNA extracted from wastewater has been performed with a set of five primers/probes. These primers and probes target different parts of the viral particle as shown in Table 3 . Varying results have been reported using these primer/probe sets targeting different parts of the viral genome. For instance, Medema et al. (2020) observed positive amplification from all study sites (6) using the N1 primer, as compared to the N3 and E primers that were positive in 5 and 4 study sites respectively. However, in contrast Rimoldi et al. (2020) reported a high frequency of positive amplification targeting the ORF1ab gene, compared with the N and E genes that were only positive in three of the positive wastewater samples. These results therefore indicate inconclusive results in relation to the best primer/probe set for amplification of the viral RNA in wastewater. This could be attributed to primer/probe sensitivity, PCR inhibitors in the wastewater sampels from different regions/sites and potential stability of the virus and viral genome in these different areas.
Table 3.
Primers/Probes used for the amplification of coronavirus RNA in wastewater.
| Target gene | Primer/Probe | Sequence | Reference |
|---|---|---|---|
| Nucleocapsid (N) | 2019-nCoV_N1-F | 5′-GACCCCAAAATCAGCGAAAT-3′ | Wu et al., 2020a, Medema et al., 2020, Ahmed et al., 2020a |
| 2019-nCoV_N1-R | 5′-TCTGGTTACTGCCAGTTGAATCTG-3′ | ||
| 2019-nCoV_N1-P | 5′-FAM-ACCCCGCATTACGTTTGGTGGACC-ZEN/Iowa Black-3′ | ||
| Nucleocapsid (N) | 2019-nCoV_N2-F | 5′-TTACAAACATTGGCCGCAAA-3′ | Wu et al., 2020a, Medema et al., 2020 |
| 2019-nCoV_N2-R | 5′-GCGCGACATTCCGAAGAA-3′ | ||
| 2019-nCoV_N2-P | 5′-FAM-ACAATTTGCCCCCAGCGCTTCAG- ZEN/Iowa Black-3′ | ||
| Nucleocapsid (N) | 2019-nCoV_N3-F | 5′-GGGAGCCTTGAATACACCAAAA-3′ | Wu et al., 2020a, Medema et al., 2020 |
| 2019-nCoV_N3-R | 5′-TGTAGCACGATTGCAGCATTG-3′ | ||
| 2019-nCoV_N3-P | 5′-FAM-AYCACATTGGCACCCGCAATCCTG- ZEN/Iowa Black-3′ | ||
| Envelope(E) | E_Sarbeco_F | 5′-ACAGGTACGTTAATAGTTAATAGCGT-3′ | Medema et al., 2020;Wurtzer et al., 2020 |
| E_Sarbeco_R | 5′-ATATTGCAGCAGTACGCACACA-3′ | ||
| E_Sarbeco_P1 | 5′-FAM-ACACTAGCCATCCTTACTGCGCTTCG-ZEN/Iowa Black-3′ | ||
| Cor-p-F2(+) | 5′-CTAACATGCTTAGGATAATGG-3′ | Wang et al., 2005c | |
| Cor-p-F3 (+) | 5′-GCCTCTCTTGTTCTTGCTCGC-3′; | ||
| Cor-p-R1 (−) | 5′-CAGGTAAGCGTAAAACTCATC-3′ | ||
| ORF1ab | 5′-CCCTGTGGGTTTTACACTTAA-3′ | Arora et al., 2020, Kumar et al., 2020, Rimoldi et al., 2020 | |
| 5′-ACGATTGTGCATCAGCTGA-3′ | |||
| 5′-FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′ |
Other molecular methods have been used for the detection of coronavirus RNA in clinical samples, such as pharyngeal swabs, these include reverse transcription (RT) loop-mediated isothermal amplification (LAMP) (Park et al., 2020a, Lamb et al., 2020, Yu et al., 2020, Huang et al., 2020a, Shirato et al., 2018, Li et al., 2015, Shirato et al., 2014). This method has shown great potential in detecting these viruses in clinical samples, producing results in less than an hour, in some instances within 11 min (Thai et al., 2004). Another molecular technique used for coronavirus detection in clinical and wastewater samples is the digital droplet PCR. This has shown to be have an improved lower limit of detection, more sensitive and accurate compared to RT-PCR for environmental samples (Lu et al., 2020, Dong et al., 2020, Zhou et al., 2020). The use of dPCR may therefore aid in reducing false negatives and positives, especially in samples with low viral titer, like wastewater (Zhou et al., 2020).
The molecular detection and quantification of viral RNA in wastewater using these molecular techniques has shown the potential for the use of wastewater analysis to determine the infection incidence with the population. This could be achieved by performing a mass balance on the viral titer in wastewater per day and relating to the RNA copies shed per gram of stool of infected individual in a day (Ahmed et al., 2020a). For instance, Wurtzer et al. (2020) reported a correlation between viral load in untreated wastewater with COVID-19 infections in the population served by the wastewater treatment plant in Paris, France. Ahmed et al. (2020a) also used this approach in estimating infection within the catchment of a wastewater treatment plant, with the estimates showing reasonable agreement with clinical observations in Queensland, Australia. In addition to the estimation of infected people through the accurate detection and quantification of viral load, this approach could also act as an early warning system. This is possible due to the early shedding (1–2 days) of viral RNA in the stool of a proportion of infected individuals before the onset of pulmonary symptoms (Wang et al., 2020a). For instance, Medema et al. (2020) detected SARS-CoV-2 viral RNA in wastewater taken almost a week (6 days) before the first case of COVID-19 was reported in the city of Amersfoort in the Netherlands.
4. Fate of coronaviruses in wastewater
Human enveloped viruses, like coronaviruses, are presumed to undergo rapid inactivation in the water environment (Kampf et al., 2020, Ye et al., 2016). However, several reports of these viruses in feces and wastewater, as discussed in the sections above, indicate these may be able to survive longer than presumed. The fate of coronaviruses in wastewater may be mediated by two processes; their ability to survive in the extreme wastewater environment and its removal during different stages of wastewater treatment. In this section, we review the current knowledge surrounding coronavirus survival in wastewater and discuss their possible removal by different wastewater treatment processes.
4.1. Survival of coronaviruses in wastewater
There is currently evidence to suggest that SARS-CoV and MERS-CoV are viable under different environmental conditions. Gundy et al. (2009) reported that it will take 2–3 days for a 99.9% reduction of coronavirus in wastewater, this agrees with data from Wang et al (2005b) at 20 °C. In unpasteurized wastewater Ye et al. (2016), observed that it takes 13(±1) hours for 90% inactivation. In contrast, Casanova et al. (2009), reported that it could take up to a week for coronaviruses in wastewater to reduce by 99%. This data was generated using the mouse hepatitis virus (MHV) and transmissible gastroenteritis virus (TGEV) as surrogates for coronaviruses. The experiments were also performed using pasteurized water, which could have eliminated the possible predation action from other microbes in the wastewater. Additionally, the longer survival of the surrogates, MHV and TGEV, as compared to the human coronaviruses, SARS-CoV and MERS-CoV, could be attributed to the subtle difference between these different viruses. It can be concluded, based on the data available, that it could take a maximum of 3 days for 99.9% or a 3 Log reduction of coronaviruses in wastewater at 20 °C. This could, however, be affected greatly by several factors discussed in Section 4.1.1.
Infectivity of coronaviruses in wastewater could also be affected. The study by Casanova et al. (2009), indicated that it takes 7–9 days for a 99% reduction in infectious viral titer at 25 °C. Additionally, SARS-CoV seeded into sewage remained infectious for 2 days at 20 °C (Yeo et al., 2020, Wang et al., 2005b). Reports on the occurrence of SARS-CoV-2 in wastewater is mainly based on nucleic acid (RNA) detection. Rimoldi et al. (2020) reported no viable SARS-CoV-2 in wastewater based on cell cultures. They estimated that it took 6–8 h from excretion in feces to the wastewater sampling point, therefore the virus may have been inactivated within that period. However, based on data from other coronaviruses (as discussed above) and occurrence of viable SARS-CoV-2 viral particles in feces (Chan et al., 2004a, Xu et al., 2005, Zhang et al., 2020a), untreated wastewater may contain some viable and infective human coronaviruses.
4.1.1. Factors affecting coronavirus survival in wastewater
Survival of coronaviruses and other viruses, in wastewater could be influenced by several factors. These factors can either be intrinsic or external, based on the wastewater or environmental conditions mentioned below;
-
•
Viral structure: Enveloped viruses, like coronavirus, have been found to have shorter survival periods compared to nonenveloped viruses and are consistent with the survival of tailed phages (Gundy et al., 2009). The shorter survival time for enveloped viruses may be due to the action of proteolytic enzymes and detergents on the external lipid envelope of the virus (Aquino de Carvalho et al., 2017, Ye et al., 2016). Additionally, single stranded RNA is extremely fragile and may be degraded rapidly by RNases abundant in nature (Brisebois et al., 2018). Therefore being a single-stranded RNA virus, coronaviruses may be easily degraded in the wastewater. However, there is the need for further research to validate these assumptions on the survival of coronaviruses, especially, SARS-CoV-2.
-
•
Wastewater characteristics/composition: Comparison of the inactivation or survival of coronaviruses suggests that the characteristics/composition of the wastewater may play a major role in their survival. Casanova et al. (2009), reported that in reagent grade water it will take 44 days for 4 log10 (99.99%) reduction of TGEV and 35 days for MHV at 25 °C. In pasteurized wastewater, it will take 19 days for TGEV and 14 days for MHV to achieve the same inactivation at the same conditions (Casanova et al., 2009). The faster inactivation of coronaviruses in wastewater could be attributed to the presence of chemicals with antiviral activity (Sobsey & Meschke, 2003), proteolytic enzymes produced by bacteria (Casanova et al., 2009), protozoan and metazoan predation in the wastewater (Ye et al., 2016). Additionally, survival studies on viruses in wastewater have found that the high molecular weight of dissolved matter, which is common wastewater, may influence their survival (Noble & Fuhrman, 1997).
-
•
Temperature: Just like many other microorganisms’ temperature has been found to have a greater influence on the survival of coronaviruses in wastewater. Using SARS-CoV seeded into sewage, it was observed that the viruses remain infectious for 14 days at 4 °C, but for only 2 days at 20 °C (Wang et al., 2005c). Studies on the survival of TGEV and MHV also showed that at 25 °C it took 19 days for TGEV and 14 days to reduce by 4 log10 in wastewater. However, at 4 °C for the same level of reduction, it will take 98 days for TGEV and 139 days for MHV (Casanova et al., 2009). These findings show that in temperate or colder regions coronaviruses may survive longer in wastewater as compared to tropical regions. The decrease in persistence of the virus with increasing temperature could be attributed to the denaturation of proteins and nucleic acids, as well as increase in the activity of extracellular enzymes (Aquino de Carvalho et al., 2017).
-
•
pH: There is a lack of information on the impact of pH on the survival of coronaviruses. However, based on the stability of MHV and TGEV we can deduce the impact of wastewater pH on coronaviruses survival. A pH range of 5–7.4 at 37 °C and 3–10 at 4 °C is considered to be the stable range for MHV (Casanova et al., 2009, Daniel and Talbot, 1987). For TGEV, the stable pH range is 5–7 at 37 °C and 5–8 at 4 °C. Acidic pH has been shown to result in reversible acid denaturation of RNA, through protonation of GC base pairs and consequent formation of Hoogsteen base pairing (Mariani, et al., 2018). In addition to the impact on viral stability, pH influences viral survival through the impact on adsorption on particles in the wastewater. An increase in viral adsorption is observed with decreasing pH (Schaub et al., 2017).
4.2. Removal of coronavirus during wastewater treatment
Conventional wastewater treatment processes are mainly designed for the removal of organic matter and suspended solids (Droste and Gehr, 2018, Qasim, 2017). Some degree of pathogen removal is however achieved in the process, but this is mainly effective for bacteria than viruses (Dias et al., 2018, Diston et al., 2012, Sinton et al., 2002, Grabow, 2001).
Several studies have reported the removal efficiency of enteric viruses during wastewater treatment; however, only one study has reported coronavirus removal during wastewater treatment. Wurtzer et al. (2020) detected SARS-CoV-2 RNA in treated and untreated wastewater from Paris, concentrations in the treated wastewater were found to be 100 times lower than viral load in the untreated wastewater. However, this study did not report the type of wastewater treatment processes employed in these treatments, nor the viability or otherwise of these viral particles. The viral RNA detected in the treated wastewater could also be pieces of the viral particle, therefore this information is inconclusive in helping to understand the possible removal of coronaviruses during wastewater treatment. Viral adsorption and inactivation have been given as the two main reasons for reduction in water (Bibby et al., 2015). This has been observed for Φ6 bacteriophage, which is also an enveloped RNA virus (Bibby et al., 2015).
Information from the general removal of viruses and coronavirus surrogates could be used, cautiously, to give additional information on the possible removal of these viruses. For instance, Sidhu et al. (2018) observed that activated sludge treatment processes (ASP) under sub-tropical conditions achieved above 3 log10 removal of enteric viruses. ASP is a common wastewater treatment process used extensively across the globe (Kitajima et al., 2014, Simmons and Xagoraraki, 2011, Nordgren et al., 2009). This treatment process includes primary settling, biological degradation and secondary clarification (Sidhu et al., 2018, Keegan et al., 2013). At equilibrium in wastewater, Ye et al. (2016) demonstrated that MHV (used as a surrogate for human coronaviruses) adsorbs to the wastewater solids more rapidly. They estimated that 26% of the MHV will be adsorbed to the wastewater solids at equilibrium, with 99% equilibrium occurring at 0.4–2.9 h. Therefore, it can be postulated that during ASP processes the highest removal of coronaviruses may occur at the primary settling stage. It has been reported that microbial inactivation increases with increasing hydraulic retention time (HRT), till a saturation is reached (García, et al., 2003). For instance, in a wastewater pond system, Verbyla and Mihelcic, (2015) reported an average of 1 log10 reduction of viruses for every 14.5–20.9 days of retention. Therefore, in addition to adsorption to solids, a longer HRT may also be critical in inactivating coronaviruses in wastewater. The adsorption of coronaviruses to the solids therefore means a high concentration may be expected in the sludge. Anaerobic digestion of sludge, which is a common sludge treatment process, has proven to result in reduction of pathogenic microorganisms. Sassi et al. (2018) demonstrated that mesophilic anaerobic digestion could achieve above 5.9 log10 reduction of the Φ6 bacteriophage. This could be attributed to protein and nucleic acid denaturation at higher temperatures. We can there conclude that coronavirus adsorbed on the wastewater solids could be effectively removed (almost by 6 Log10 units) during anaerobic digestion.
Viral removal between 2 and 3 log10 has been reported for different types of viruses during membrane bioreactor (MBR) treatment (Prado et al., 2019, Miura et al., 2018, Purnell et al., 2016). Other studies have reported Log removal of viral particles greater than 4 (Chaudhry et al., 2015, Simmons et al., 2011, Kuo et al., 2010). The main mechanism in the MBR processes responsible for viral, and other pathogen removals, is retention or size exclusion. Chaudhry et al. (2015) reported that retention by a 0.04 µm membrane accounts for over 50% removal of different viruses and phages. An additional 1.0 Log10 of these viruses were attached to mixed liquor suspended solids which facilitates the retention. The most commonly used membrane technologies in wastewater treatment are the microfiltration (0.1–0.2 μm) and ultrafiltration (0.005 ≈ 10 μm). There are reports of microfiltration membranes of bigger pore sizes (0.2–0.4) in use (Nqombolo et al., 2018). With an average viral particle diameter of 120 nm (0.12 μm) and envelop diameter of 80 nm (0.08 μm) (Neuman & Buchmeier, 2016) coronaviruses, the best membrane technology for their removal will be ultrafiltration. The adsorption of coronaviruses to solids in wastewater may enhance their removal.
Tertiary wastewater treatment processes such chlorination and UV treatment may also result in further removal of remaining coronaviruses in the wastewater. Wang et al. (2005b) reported that SARS-CoV can be inactivated completely by 20 mg/L chlorine in 1 min. They observed that Chlorine dioxide was less effective for the inactivation of SARS-CoV as compared with free chlorine, similar results have been reported for other viruses (Young et al., 2020b, Wati et al., 2019, Cromeans et al., 2019, Lim et al., 2010). Chlorine has been reported to inactivate viruses through the cleavage of the capsid protein backbone of viruses, therefore inhibiting viral genome injection into host cells (Wigginton et al., 2012, Wigginton and Kohn, 2012, Page et al., 2010).
Several studies have also reported the inactivation of coronaviruses using UV irradiation (Shirbandi et al., 2020, Kim and Jang, 2018, Casanova et al., 2009, Wang et al., 2005b). Pinon and Vialette (2018) reported that enveloped viruses, like coronaviruses are more sensitive to UV than non-enveloped viruses. The main mechanism through which UV inactivates coronaviruses could be through the generation of pyrimidine dimers which damages the nucleic acid (Smith & Denison, 2013).
It must be noted that in addition to the specific wastewater treatment processes that may result in the removal or inactivation of coronaviruses, factors affecting their survival in wastewater (Section 4.1.1) may also contribute significantly.
5. Risk of infection with coronaviruses found in wastewater
The occurrence of infective viral particles of coronaviruses in wastewater may pose health concerns for people who come into contact with the wastewater. Live SARS-CoV-2 has been isolated from stools of patients (Zhang et al., 2020a, Wang et al., 2020b, Xiao et al., 2020), in one instance 15 days after onset of the disease (Zhang et al., 2020a). With the observation that it may take 2 days for a 99% reduction in infectivity (Section 4.1), it is safe to assume that some of the viral particles may still be infectious. The present belief is that SARS-CoV-2 has a low infectious dose (Lee & Hsueh, 2020), therefore the viral loads in the wastewater could still pose a great risk. The available information on the viral survival implies that the populations at greatest risks are people exposed to the raw sewage. This could be workers at wastewater treatment plants (WWTPs) and the general public who may be directly exposed to the sewage via faulty plumbing or sewer networks. Despite these fears till date there is no evidence for the transmission of COVID-19 due to exposure to wastewater (WHO, 2020b).
5.1. Potential risks of infection for wastewater operators/workers
There is evidence to suggest that GI infections may occur first in a subset of coronavirus infections (Section 3.1), which means that workers at WWTPs may be exposed to these pathogens days before an outbreak is reported. Exposure within the WWTPs could be through two major routes; aerosol inhalation or direct contact with infectious viral particles.
5.1.1. Inhalation of aerosols containing the infectious viral particles
Inhalation of aerosols or droplets contaminated with infectious viral particles has been reported as the main route through which coronaviruses are transmitted in WWTPs (Lee, 2020, Ge et al., 2020, Tellier et al., 2019, Adhikari et al., 2019). However, no study has considered the risks posed to workers in WWTPs, therefore there is a lack of information on the possible infections from this exposure. Aerosolization of Ebola virus (also an RNA enveloped virus) surrogates have been reported in wastewater systems (Lin and Marr, 2017), which shows the potential of detecting coronaviruses in aerosols from wastewater. Several factors may influence the infection of WWTP workers through inhalation of aerosols contaminated with coronaviruses;
-
A.
Emission rate: This refers to the amount of the infectious viral particles released per unit of time. This is a function of the availability of the virus and its aerosolization rate (Van Leuken et al., 2016, Shao, 2009, Viana et al., 2008). Currently, there is no information on the emission rate for coronaviruses in aerosols during wastewater treatment.
-
B.
Meteorological factors: Wind speed, wind direction, turbulence and deposition are all factors that will greatly influence the transmission of pathogens, including viruses, through aerosols (Van Leuken et al., 2016). These factors influence, the height of the aerosols generated, and the distance covered. Higher wind speed could also result in the exposure of populations living close to the WWTPs to the coronavirus.
-
C.
Viral inactivation: This is expressed as a function of time and meteorological factors, such as temperature and humidity (Zhao et al., 2014). At 25 °C and relative humidity (RH) of 79%, over 60% of coronaviruses in aerosols/droplets have been found to remain infectious for up to 60 min, however at much warmer temperature, of 38 °C and 24% RH, only 4.7% remained infectious (Pyankov et al., 2018). Therefore, it is safe to assume that within an hour aerosol contaminated with coronaviruses will still contain some number of infectious particles that may result in infections.
-
D.
Amount of infectious viral particle inhaled: The breathing rate, lung volume and particle size are important factors here ((Després et al., 2012, Wilkinson et al., 2012, Rostami, 2009). Men have larger nasal cavities, and longer, narrower and higher nasal floors than females of the same body size (García-Martínez et al., 2016, Bastir et al., 2011). This may result in inhalation of higher number of infectious viral particles by male than female workers of WWTPs resulting in higher risks of coronavirus infection.
-
E.
Host health response: The final and most important factor to consider in assessing the possibility of infection for WWTP workers is the host response to the inhaled dose. It is currently known that most critical cases of infection with coronaviruses are seen in people with underlying conditions like diabetes mellitus, chronic lung disease, and cardiovascular disease (Wu et al., 2020b, Bonow et al., 2020, Garg, 2020). However, healthy individuals are also infected upon exposure, therefore all workers within the WWTPs irrespective of their health status may be at risk of infection especially when surface aeration is used as compared to diffused aeration.
5.1.2. Direct contact with the infectious viral particle deposited on formites
Beyond direct inhalation of aerosols in the WWTPs, direct contact with the infectious viral particles deposited on contact surfaces may also result in infections. The potential for aerosols contaminated with infectious viral particles been deposited on contact surfaces/formites is high within the WWTPs. The detection of coronaviruses in environmental samples, especially in nosocomial infections (Xiao et al., 2017, Booth et al., 2005) and evidence that intranasal instillation could cause infections (Xiao et al., 2017) shows that direct contact with the viral particles on formites may be a major route of transmission. The findings that handwashing reduces the risks of infections with these viruses further supports the role of direct hand contact in the transmission (Xiao et al., 2017, Lau et al., 2004, Teleman et al., 2004). Analysis of studies on the survival of coronaviruses on surfaces, showed that these infectious viral particles can survive up to nine days at room temperature (Kampf et al., 2020). However, they can be easily inactivated within a minute using 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite. In addition to viral particles deposited on formites/contact surfaces within the WWTP, direct exposure to the viral particles may occur during removal and transportation of primary and secondary sludge. Some WWTPs may not have facilities for onsite sludge treatment, which requires the collection and transportation of the sludge for treatment off-site. Occurrence of human coronaviruses been reported in sludge both treated (Bibby et al., 2011, Bibby and Peccia, 2013) and untreated sludge (Bibby and Peccia, 2013). This could therefore be another significant exposure route for workers of the WWTPs. For instance, Westrell et al. (2004) reported a high risk of viral infection for workers during sludge dewatering. Therefore, without proper hygienic practices and use of PPE, WWTP workers could be exposed to infectious coronavirus particles deposited on surfaces and in sludge.
5.2. Potential risks for the general public
The case of SARS infections from the apartment block in Hong Kong, highlighted the role of the wastewater system or sewer networks in the spread of infections (WHO, 2003). This was spread through droplets from SARS-CoV contaminated sewage from a faulty plumbing. It therefore shows that exposure to the sewage containing infectious coronaviruses may lead to the spread of the virus. In addition to faulty plumbing within residential facilities, burst sewer networks discharging untreated wastewater into the community may be another route of transmission. Faults in sewer networks, either structural or hydraulic, may take a long time before repairs (Khan et al., 2009), which could increase the risks. Areas with inadequate sanitation, which means no sewer networks, will have higher risks of exposure to the coronavirus in the sewage. Exposure to the untreated sewage in these circumstances may pose higher risks than the risks exposed to the workers at the WWTPs. This is because this exposure may occur minutes or hours after excretion of the viral particles where infectivity may still be high. Furthermore, the discharge of treated wastewater directly into surface water bodies could potentially lead to exposure for the general public. This is especially critical in areas where WWTPs are not working effectively resulting in occurrence of pathogens in the final effluent. Occurrence of coronaviruses in surface water has been reported in Saudi Arabia (Blanco et al., 2019) and Kazakstan (Alexyuk et al., 2017). Rimoldi et al (2020) also detected SARS-CoV-2 RNA in surface water which they attributed this occurrence to discharge of non-collected domestice wastes or urban runoff from domestic effluents. However, no viable viral particle was founf based on cell cultures, which indicates the potential absence of risks of infections from surface water.
6. Scope for further reseach
Further work is required to fully assess the possible risks from coronaviruses in wastewater. Future studies in this regard could focus on;
-
1.
The shedding frequency of coronavirus RNA in feces and urine: This is necessary in understanding the viral load in the wastewater per an infected person or population.
-
2.
Optimization of methods for the detection and quantification of coronaviruses in wastewater: There is the need for more research on the effective recovery of coronavirus from wastewater and optimization of the RNA extraction methods. Additionally, the different primers used for the amplification of the viral particles could introduce uncertainties into the results due to difference in stability of the various parts of the viral particle. Therefore, there is the need for research to understand how stable the different viral particles are in wastewater.
-
3.
Survival of coronavirus in wastewater under field conditions: Although a few studies have looked at the survival of these viruses in wastewater, these have been laboratory scale studies either using viral surrogates or pasteurized wastewater. The survival rates could therefore be different under field conditions.
-
4.
Efficiency of different conventional wastewater treatment processes in removing or inactivating coronaviruses. Only one study so far has analysed treated wastewater for coronaviruses. The lack of interest could be attributed to the earlier belief that these viruses may not occur, and even if they do will be in low viral loads, in wastewater. However, the increasing evidence that this might not be the case, calls for studies on how conventional wastewater treatment processes may either remove or inactivate coronaviruses.
-
5.
To fully understand the risks posed to WWTP workers, a full risk assessment is necessary. This should consider; emission quantification, atmospheric dispersion, dose estimation and probability of infection using dose response models.
-
6.
Survival in sludge and receiving waters: Due to the potential risks of infection from exposure to coronavirus in sewage sludge and the receiving environment (rivers and lakes), there is the need to ascertain the survival of the virus in these environments.
7. Conclusion
Coronavirus infections are a serious threat to health systems globally. The frequency of outbreaks with these viruses calls for concerted efforts to understand their occurrence and survival in different environments and how that may contribute towards an increase in infections. The current knowledge on the occurrence of coronaviruses in wastewater is scant, this makes it difficult to fully understand their behaviour in this environment. The few reports of viral RNA belonging to these viruses in wastewater means this could potentially expose larger numbers of people to these infections.
The main conclusions that can be drawn from this review are;
-
1.
Coronavirus RNA are shed in feces leading to their occurrence in wastewater. This could assist in early detection of outbreaks as well the use of wastewater-based epidemiology for estimation of infection levels in populations.
-
2.
The viruses can survive for few hours to days in wastewater, remaining infectious in the process. Therefore, exposing the general public and wastewater treatment plant workers to possible risks of infections.
-
3.
The survival of coronaviruses in wastewater is influenced by several factors, such as viral structure, temperature, wastewater composition/characteristics and pH.
-
4.
Additionally, conventional wastewater treatment processes can potentially inactivate or remove these viruses. However, the viral RNA may still be found in the treated wastewater.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We are grateful to the South African Research Chair Initiative (SARChI) of the Department of Science and Technology and the National Research Foundation of South Africa for funding this research. We also acknowledge the support from our institution, the Durban University of Technology and specifically the Institute for Water and Wastewater Technology.
Author contributions
All authors: Conceptualization; I. D. Amoah: Data curation, Writing- Original draft preparation, F. Bux and S. Kumari: Supervision; All authors: Writing- Reviewing and Editing.
Handling Editor: Frederic Coulon
References
- Adhikari U., Chabrelie A., Weir M., Boehnke K., McKenzie E., Ikner L., Wang M., Wang Q., Young K., Haas C.N., Rose J. A Case Study Evaluating the Risk of Infection from Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) in a Hospital Setting Through Bioaerosols. Risk Anal. 2019;39(12):2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. https://linkinghub.elsevier.com/retrieve/pii/S0048969720322816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. https://linkinghub.elsevier.com/retrieve/pii/S004896972033480X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexyuk M.S., Turmagambetova A.S., Alexyuk P.G., Bogoyavlenskiy A.P., Berezin V.E. Comparative study of viromes from freshwater samples of the Ile-Balkhash region of Kazakhstan captured through metagenomic analysis. VirusDisease. 2017;28(1):18–25. doi: 10.1007/s13337-016-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M.C., Butler D., Friedler E. At-source domestic wastewater quality. Urban water. 1999;1(1):49–55. [Google Scholar]
- Amirian, E.S., 2020. Potential Fecal Transmission of SARS-CoV-2: Current Evidence and Implications for Public Health. [DOI] [PMC free article] [PubMed]
- Aquino de Carvalho N., Stachler E.N., Cimabue N., Bibby K. Evaluation of Phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017;51(15):8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- Arbely E., Granot Z., Kass I., Orly J., Arkin I.T. A trimerizing GxxxG motif is uniquely inserted in the severe acute respiratory syndrome (SARS) coronavirus spike protein transmembrane domain. Biochemistry. 2006;45(38):11349–11356. doi: 10.1021/bi060953v. [DOI] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. medRxiv; 2020. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater-based epidemiology (WBE) tracking tool in India. [DOI] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. MedRxiv; 2020. The fate of SARS-COV-2 in WWTPs points out the sludge line as a suitable spot for monitoring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastir M., Godoy P., Rosas A. Common features of sexual dimorphism in the cranial airways of different human populations. Am. J. Phys. Anthropol. 2011;146(3):414–422. doi: 10.1002/ajpa.21596. [DOI] [PubMed] [Google Scholar]
- Bell D.M. World Health Organization Working Group on prevention of international and community transmission of SARS. Public Health Interv. SARS Spread. 2003:1900–1906. [Google Scholar]
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pöhlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Fischer R.J., Casson L.W., Stachler E., Haas C.N., Munster V.J. Persistence of Ebola virus in sterilized wastewater. Environ. Sci. Technol. Lett. 2015;2(9):245–249. doi: 10.1021/acs.estlett.5b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Viau E., Peccia J. Viral metagenome analysis to guide human pathogen monitoring in environmental samples. Lett. Appl. Microbiol. 2011;52(4):386–392. doi: 10.1111/j.1472-765X.2011.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco A., Abid I., Al-Otaibi N., Pérez-Rodríguez F.J., Fuentes C., Guix S., Pintó R.M., Bosch A. Glass Wool Concentration Optimization for the Detection of Enveloped and Non-enveloped Waterborne Viruses. Food Environ. Virol. 2019;11(2):184–192. doi: 10.1007/s12560-019-09378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (covid-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser, A.D., 2020. Coronavirus May Cause Environmental Contamination Through Fecal Shedding. Medscape Medical News. Accessed on 20th April, 2020 at https://www.medscape.com/viewarticle/926390.
- Brisebois E., Veillette M., Dion-Dupont V., Lavoie J., Corbeil J., Culley A., Duchaine C. Human viral pathogens are pervasive in wastewater treatment center aerosols. J. Environ. Sci. 2018;67:45–53. doi: 10.1016/j.jes.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Xu J., Lin D., Xu L., Qu Z., Zhang Y., Zhang H., Jia R., Wang X., Ge Y., Xia A. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC-Centers for Disease Control & Prevention, 2020. Checklist for Healthcare Facilities: Strategies for Optimizing the Supply of N95 Respirators during the COVID-19 Response. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/hcp/checklist-n95-strategy.html.
- CDC-Centers for Disease Control & Prevention, 2020. CDC 2019-Novel Coronavirus (2019-nCoV). Real-Time RT-PCR Diagnostic Panel. Available at https://www.fda.gov/media/134922/download (accessed on the 1th of May, 2020).
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Poon L.L., Cheng V.C.C., Guan Y., Hung I.F.N., Kong J., Yam L.Y., Seto W.H., Yuen K.Y., Peiris J.S.M. Detection of SARS coronavirus in patients with suspected SARS. Emerg. Infect. Dis. 2004;10(2):294. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K., To K.F., Lo A.W., Cheung J.L., Chu I., Au F.W., Tong J.H., Tam J.S., Sung J.J., Ng H.K. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 2004;74(1):1–7. doi: 10.1002/jmv.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry R.M., Nelson K.L., Drewes J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49(5):2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., Muth D., Sieberg A., Meyer B., Assiri A.M., Binger T. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromeans T., Jothikumar N., Lee J., Collins N., Burns C.C., Hill V.R., Vinjé J. A new solid matrix for preservation of viral nucleic acid from clinical specimens at ambient temperature. J. Virol. Methods. 2019;274 doi: 10.1016/j.jviromet.2019.113732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Talbot P.J. Physico-chemical properties of murine hepatitis virus, strain A 59. Brief report. Arch. Virol. 1987;96:241–248. doi: 10.1007/BF01320963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després V.R., Alex Huffman J., Burrows S.M., Hoose C., Safatov A.S., Buryak G., et al. Primary biological aerosol particles in the atmosphere: a review. Tellus. 2012;B64 [Google Scholar]
- Dias E., Ebdon J., Taylor H. The application of bacteriophages as novel indicators of viral pathogens in wastewater treatment systems. Water Res. 2018;129:172–179. doi: 10.1016/j.watres.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Diston D., Ebdon J.E., Taylor H.D. The effect of UV-C radiation (254 nm) on candidate microbial source tracking phages infecting a human-specific strain of Bacteroides fragilis (GB-124) J. Water Health. 2012;10(2):262–270. doi: 10.2166/wh.2012.173. [DOI] [PubMed] [Google Scholar]
- Dong L., Wang X., Wang S., Du M., Niu C., Yang J., Li L., Zhang G., Fu B., Gao Y., Wang J. Interlaboratory assessment of droplet digital PCR for quantification of BRAF V600E mutation using a novel DNA reference material. Talanta. 2020;207:120293. doi: 10.1016/j.talanta.2019.120293. [DOI] [PubMed] [Google Scholar]
- Droste R.L., Gehr R.L. John Wiley & Sons; 2018. Theory and practice of water and wastewater treatment. [Google Scholar]
- Drosten C., Seilmaier M., Corman V.M., Hartmann W., Scheible G., Sack S., Guggemos W., Kallies R., Muth D., Junglen S., Müller M.A. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet. Infect. Dis. 2013;13(9):745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumian T.M., Leite J.P.G., Castello A.A., Gaggero A., de Caillou M.S.L., Miagostovich M.P. Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. J. Virol. Methods. 2010;170(1–2):42–46. doi: 10.1016/j.jviromet.2010.08.017. [DOI] [PubMed] [Google Scholar]
- García-Martínez D., Torres-Tamayo N., Torres-Sanchez I., García-Río F., Bastir M. Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am. J. Phys. Anthropol. 2016;161(3):467–477. doi: 10.1002/ajpa.23051. [DOI] [PubMed] [Google Scholar]
- García J., Vivar J., Aromir M., Mujeriego R. Role of hydraulic retention time and granular medium in microbial removal in tertiary treatment reed beds. Water Res. 2003;37(11):2645–2653. doi: 10.1016/S0043-1354(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Garg, S., 2020. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR. Morbidity and Mortality Weekly Report, 69. [DOI] [PMC free article] [PubMed]
- Ge Z.Y., Yang L.M., Xia J.J., Fu X.H., Zhang Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ.-Sci. B. 2020:1–8. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Betancourt W.Q., Kitajima M. How much reduction of virus is needed for recycled water: A continuous changing need for assessment? Water Res. 2017;108:25–31. doi: 10.1016/j.watres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow W.O.K. Bacteriophages: update on application as models for viruses in water. Water Sa. 2001;27(2):251–268. [Google Scholar]
- Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B., Zeng T., Larsen D.A. medRxiv; 2020. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S., Du B. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10. [Google Scholar]
- Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A., Ghobashy H. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet. Infect. Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C.H., Rose J.B., Gerba C.P. In: Quantitative Microbial Risk Assessment. Haas C., editor. John Wiley & Sons Inc; Hoboken, NJ: 2014. Microbial agents and transmission; pp. 15–62. [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. J. Pathol. Soc. Great Britain Ireland. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Luo Q., Mo F., Long L., Zheng W. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.S., Seong M.W., Heo E.Y., Park J.H., Kim N., Shin S., Cho S.I., Park S.S., Choi E.H. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. medRxiv; 2020. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R., Hara-Yamamura H., Meuchi Y. medRxiv; 2020. Detection of SARS-CoV-2 in wastewater in Japan by multiple molecular assays-implication for wastewater-based epidemiology (WBE) [Google Scholar]
- Hemida M.G. Middle East respiratory syndrome coronavirus and the one health concept. PeerJ. 2019;7 doi: 10.7717/peerj.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer U. Viral evolution: variation in the gut virome. Nat. Rev. Microbiol. 2013;11(9):596. doi: 10.1038/nrmicro3092. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.E., Lim B., Hsu C.C., Xiong D., Wu W., Yu Y., Jia H., Wang Y., Zeng Y., Ji M., Chang H. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020 doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S.C., Wong P.C., Wang C. SARS: clinical features and diagnosis. Respirology. 2003;8:S20–S24. doi: 10.1046/j.1440-1843.2003.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.N., Cheng V.C.C., Wu A.K.L., Tang B.S.F., Chan K.H., Chu C.M., Wong M.M.L., Hui W.T., Poon L.L.M., Tse D.M.W., Chan K.S. Viral loads in clinical specimens and SARS manifestations. Emerg. Infect. Dis. 2004;10(9):1550. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K.Q., Yung C.F., Cui L., Tzer Pin Lin R., Mak T.M., Maiwald M., Li J., Chong C.Y., Nadua K., Tan N.W.H., Thoon K.C. A Well Infant with Coronavirus Disease 2019 with High Viral Load. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42(6–7):1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Keegan A.R., Robinson B., Monis P., Biebrick M., Liston C. Validation of activated sludge plant performance for virus and protozoan reduction. J. Water Reuse Desal. 2013;3(2):140–147. [Google Scholar]
- Khan, Z., Zayed, T., Moselhi, O., 2009. Stochastic Analysis of Factors Affecting Sewer Network Operational Condition. In: ICPTT 2009: Advances and Experiences with Pipelines and Trenchless Technology for Water, Sewer, Gas, and Oil Applications, pp. 389–400.
- Kim J., Jang J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci. Technol. 2018;52(5):557–566. [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes—identification of potential viral indicators. Sci. Total Environ. 2014;488:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. medRxiv; 2020. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges. [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. medRxiv; 2020. The first proof of the capability of wastewater surveillance for COVID-19 in India through the detection of the genetic material of SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo D.H.W., Simmons F.J., Blair S., Hart E., Rose J.B., Xagoraraki I. Assessment of human adenovirus removal in a full-scale membrane bioreactor treating municipal wastewater. Water Res. 2010;44(5):1520–1530. doi: 10.1016/j.watres.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Lamb, L.E., Bartolone, S.N., Ward, E., Chancellor, M.B., 2020. Rapid Detection of Novel Coronavirus (COVID19) by Reverse Transcription-Loop-Mediated Isothermal Amplification. Available at SSRN 3539654. [DOI] [PMC free article] [PubMed]
- La Rosa Giuseppina, Iaconelli Marcello, Mancini Pamela, Bonanno Ferraro Giusy, Veneri Carolina, Bonadonna Lucia, Lucentini Luca, Suffredini Elisabetta. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. https://linkinghub.elsevier.com/retrieve/pii/S0048969720331727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Chan J.F. Coronaviruses: emerging and re-emerging pathogens in humans and animals. Virol. J. 2015;12:209. doi: 10.1186/s12985-015-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.T., Tsui H., Lau M., Yang X. SARS transmission, risk factors, and prevention in Hong Kong. Emerg. Infect. Dis. 2004;10(4):587. doi: 10.3201/eid1004.030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Ping-Ing, Hsueh Po-Ren. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J. Microbiol. Immunol. Infect. 2020;53(3):365–367. doi: 10.1016/j.jmii.2020.02.001. https://linkinghub.elsevier.com/retrieve/pii/S1684118220300116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B.U., 2020. Minimum Size of Respiratory Droplets Containing SARS-CoV-2 and Aerosol Transmission Possibility.
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Nie K., Zhang D., Li X., Wang Y., Tan W., Ma X. Detection of middle east respiratory syndrome coronavirus by reverse-transcription loop-mediated isothermal amplification. Bing du xue bao= Chin. J. Virol. 2015;31(3):269–275. [PubMed] [Google Scholar]
- Lu R., Wang J., Li M., Wang Y., Dong J., Cai W. medRxiv; 2020. SARS-CoV-2 detection using digital PCR for COVID-19 diagnosis, treatment monitoring and criteria for discharge. [Google Scholar]
- Lim M.Y., Kim J.M., Ko G. Disinfection kinetics of murine norovirus using chlorine and chlorine dioxide. Water Res. 2010;44(10):3243–3251. doi: 10.1016/j.watres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Lin K., Marr L.C. Aerosolization of Ebola virus surrogates in wastewater systems. Environ. Sci. Technol. 2017;51(5):2669–2675. doi: 10.1021/acs.est.6b04846. [DOI] [PubMed] [Google Scholar]
- Ling Y., Xu S., Lin Y., Tian D., Zhu Z., Dai F., Wu F., Song Z., Huang W., Chen J., Hu B. The persistence and clearance of viral RNA in 2019 novel coronavirus disease survivors. Chin. Med. J. (Engl.) 2020 doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Ma Q., Han H., Su H., Liu F., Wu K., Wang W., Zhu C. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin. Chem. Lab. Med. (CCLM) 2020;1 doi: 10.1515/cclm-2020-0220. [DOI] [PubMed] [Google Scholar]
- Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X., Tian Y., Sin N.N. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16(10):1698. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A., Bonfio C., Johnson C.M., Sutherland J.D. pH-Driven RNA strand separation under prebiotically plausible conditions. Biochemistry. 2018;57(45):6382–6386. doi: 10.1021/acs.biochem.8b01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. medRxiv; 2020. Presence of SARS-Coronavirus-2 in sewage. [DOI] [PubMed] [Google Scholar]
- Miura T., Schaeffer J., Le Saux J.C., Le Mehaute P., Le Guyader F.S. Virus type-specific removal in a full-scale membrane bioreactor treatment process. Food Environ. Virol. 2018;10(2):176–186. doi: 10.1007/s12560-017-9330-4. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. medRxiv; 2020. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman, B.W., Buchmeier, M.J., 2016. Supramolecular architecture of the coronavirus particle. In: Advances in virus research, vol. 96. Academic Press, pp. 1–27. [DOI] [PMC free article] [PubMed]
- Noble R.T., Fuhrman J.A. Virus decay and its causes in coastal waters. Appl. Environ. Microbiol. 1997;63(1):77–83. doi: 10.1128/aem.63.1.77-83.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto H., Ishikane M., Katagiri D., Kinoshita N., Nagashima M., Sadamasu K., Yoshimura K., Ohmagari N. Cautious handling of urine from moderate to severe COVID-19 patients. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren J., Matussek A., Mattsson A., Svensson L., Lindgren P.E. Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res. 2009;43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Nqombolo A., Mpupa A., Moutloali R.M., Nomngongo P.N. Wastewater treatment using membrane technology. Wastew. Water Qual. 2018:29. [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020:138647. doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or I.B., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H. medRxiv; 2020. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.A., Shisler J.L., Mariñas B.J. Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl. Environ. Microbiol. 2010;76(9):2946–2954. doi: 10.1128/AEM.02267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.S., Ku K., Baek S.H., Kim S.J., Kim S.I., Kim B.T., Maeng J.S. Development of Reverse Transcription Loop-mediated Isothermal Amplification (RT-LAMP) Assays Targeting SARS-CoV-2. J. Mol. Diagn. 2020 doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., Lee C.W., Park D.I., Woo H.Y., Cheong H.S., Shin H.C., Ahn K., Kwon M.J., Joo E.J. Detection of SARS-CoV-2 in Fecal Samples from Patients with Asymptomatic and Mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Weinberger D.M. medRxiv; 2020. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2018;61(5):214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Poissy J., Goffard A., Parmentier-Decrucq E., Favory R., Kauv M., Kipnis E., Mathieu D., Guery B., The M.E.R.S. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J. Clin. Virol. 2014;61(2):275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., de Castro Bruni A., Barbosa M.R.F., Garcia S.C., Moreno L.Z., Sato M.I.Z. Noroviruses in raw sewage, secondary effluents and reclaimed water produced by sand-anthracite filters and membrane bioreactor/reverse osmosis system. Sci. Total Environ. 2019;646:427–437. doi: 10.1016/j.scitotenv.2018.07.301. [DOI] [PubMed] [Google Scholar]
- Purnell S., Ebdon J., Buck A., Tupper M., Taylor H. Removal of phages and viral pathogens in a full-scale MBR: implications for wastewater reuse and potable water. Water Res. 2016;100:20–27. doi: 10.1016/j.watres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim S.R. Routledge; 2017. Wastewater treatment plants: planning, design, and operation. [Google Scholar]
- Qu, G., Li, X., Hu, L., Jiang, G., 2020. An Imperative Need for Research on the Role of Environmental Factors in Transmission of Novel Coronavirus (COVID-19). [DOI] [PubMed]
- Rampelli, S., Biagi, E., Turroni, S., Candela, M., 2020. Retrospective Search for SARS-CoV-2 in Human Faecal Metagenomes. Available at SSRN 3557962.
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C. medRxiv; 2020. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A.A. Computational modeling of aerosol deposition in respiratory tract: a review. Inhalation Toxicol. 2009;21(4):262–290. doi: 10.1080/08958370802448987. [DOI] [PubMed] [Google Scholar]
- Sassi H.P., Ikner L.A., Abd-Elmaksoud S., Gerba C.P., Pepper I.L. Comparative survival of viruses during thermophilic and mesophilic anaerobic digestion. Sci. Total Environ. 2018;615:15–19. doi: 10.1016/j.scitotenv.2017.09.205. [DOI] [PubMed] [Google Scholar]
- Schaub, S.A., Sorber, C.A., Taylor, G.W., Lefler, E., Kott, Y., Malina, J.F., Ranganathan, K.R., Moore, B.E.D., Sagik, B.P., Nupen, E.M., Bateman, B.W., McKenny, N.C., Gerba, C.P., Sobsey, M.D., Wallis, C., 2017, Virus survival in water and wastewater systems. In: Tex Univ, Cent for Res in Water Resour, Symp Ser, no. 7.
- Shao, Y., 2009. Physics and Modelling of Wind Erosion-Front Matter. Physics and Modelling of Wind Erosion. Springer, Germany. http://dx. doi. org/10.1007/978-1-4020-8895-7iexv.
- Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., Ahmad J., Angez M., Alam M.M., Rehman L., Mujtaba G. medRxiv; 2020. Detection of SARS-Coronavirus-2 in wastewater, using the existing environmental surveillance network: An epidemiological gateway to an early warning for COVID-19 in communities. [Google Scholar]
- Shirato K., Semba S., El-Kafrawy S.A., Hassan A.M., Tolah A.M., Takayama I., Kageyama T., Notomi T., Kamitani W., Matsuyama S., Azhar E.I. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J. Virol. Methods. 2018;258:41–48. doi: 10.1016/j.jviromet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Yano T., Senba S., Akachi S., Kobayashi T., Nishinaka T., Notomi T., Matsuyama S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2014;11(1):139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbandi, Kiarash, Barghandan, Sara, Mobinfar, Omid, Rahim, Fakher, Inactivation of Coronavirus with Ultraviolet Irradiation: What? How? Why? (April 8, 2020). Available at SSRN: https://ssrn.com/abstract=3571418 or 10.2139/ssrn.3571418. [DOI]
- Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Simmons F.J., Kuo D.H.W., Xagoraraki I. Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45(9):2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Sinton L.W., Hall C.H., Lynch P.A., Davies-Colley R.J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 2002;68(3):1122–1131. doi: 10.1128/AEM.68.3.1122-1131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.C., Denison M.R. Coronaviruses as DNA wannabes: a new model for the regulation of RNA virus replication fidelity. PLoS Pathog. 2013;9(12) doi: 10.1371/journal.ppat.1003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M.D., Meschke J.S. Report for the World Health Organization; Geneva, Switzerland: 2003. Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin; p. 70. [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Yong D., Ki C.S., Kim J.S., Seong M.W., Lee H., Kim M.N. Comparative evaluation of three homogenization methods for isolating Middle East respiratory syndrome coronavirus nucleic acids from sputum samples for real-time reverse transcription PCR. Ann. Lab. Med. 2016;36(5):457–462. doi: 10.3343/alm.2016.36.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Early Release-Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child China. Emerg Infect Dis. 2020;26(6) doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The COVID-19 Investigation Team, 2020. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Preprint. Retrieved from https://www.medrxiv.org/content/10.1101/2020.03.09.20032896v1. [DOI] [PMC free article] [PubMed]
- Teleman M.D., Boudville I.C., Heng B.H., Zhu D., Leo Y.S. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol. Infect. 2004;132(5):797–803. doi: 10.1017/s0950268804002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai H.T.C., Le M.Q., Vuong C.D., Parida M., Minekawa H., Notomi T., Hasebe F., Morita K. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004;42(5):1956–1961. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Miazgowicz K.L., Milne-Price S., Bushmaker T., Robertson S., Scott D., Kinne J., McLellan J.S., Zhu J., Munster V.J. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J. Virol. 2014;88(16):9220–9232. doi: 10.1128/JVI.00676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuken J.P.G., Swart A.N., Havelaar A.H., Van Pul A., Van der Hoek W., Heederik D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock–A review to inform risk assessment studies. Microbial. Risk Anal. 2016;1:19–39. doi: 10.1016/j.mran.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Viana M., Kuhlbusch T.A.J., Querol X., Alastuey A., Harrison R.M., Hopke P.K., Winiwarter W., Vallius M., Szidat S., Prevot A.S.H., Hueglin C., Bloemen H., Wahlin P., Vecchi R., Miranda A.I., Kasper-Giebl A., Maenhaut W., Hitzenberger R. Source apportionment of particulate matter in Europe: a review of methods and results. Aerosol. Sci. 2008;39:827–849. [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Wu X.M., Xiao W.J. Excretion and detection of SARS coronavirus and its nucleic acid from digestive system. World J. Gastroenterol. WJG. 2005;11(28):4390. doi: 10.3748/wjg.v11.i28.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., Song N., Jin M., Xiao W., Zhu X. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People's Liberation Army. Water Sci. Technol. 2005;52(8):213–221. [PubMed] [Google Scholar]
- Wati S., Robinson B.S., Mieog J., Blackbeard J., Keegan A.R. Chlorine inactivation of coxsackievirus B5 in recycled water destined for non-potable reuse. J. Water Health. 2019;17(1):124–136. doi: 10.2166/wh.2018.393. [DOI] [PubMed] [Google Scholar]
- Westrell T., Schönning C., Stenström T.A., Ashbolt N.J. QMRA (quantitative microbial risk assessment) and HACCP (hazard analysis and critical control points) for management of pathogens in wastewater and sewage sludge treatment and reuse. Water Sci. Technol. 2004;50(2):23–30. [PubMed] [Google Scholar]
- WHO-World Health Organization, 2020. Middle East respiratory syndrome coronavirus (MERS-CoV). Available at https://www.who.int/emergencies/mers-cov/en/ (accessed on the 30th of April, 2020).
- WHO-World Health Organization, 2020. Water, sanitation, hygiene and waste management for COVID-19: technical brief, 03 March 2020 (No. WHO/2019-NcOV/IPC_WASH/2020.1). World Health Organization.
- WHO-World Health Organization, 2020. Coronavirus disease (COVID-19) Situation Report – 166. Available at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200704-covid-19-sitrep-166.pdf?sfvrsn=6247972_2 (accessed on 4th July, 2020).
- WHO-World Health Organization Regional Office for the Western Pacific. Environmental health team reports on Amoy Gardens. 2003. Available at http://www.info.gov.hk/info/ap/who-amoye.pdf.
- Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr. Opin. Virol. 2012;2(1):84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton K.R., Pecson B.M., Sigstam T., Bosshard F., Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46(21):12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.M., Koumoutsaris S., Mitchell E.A., Bey I. Modelling the effect of size on the aerial dispersal of microorganisms. J. Biogeogr. 2012;39(1):89–97. [Google Scholar]
- Woelfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A., Niemeyer D., Vollmar P., Rothe C., Hoelscher M., Bleicker T. medRxiv; 2020. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C. medRxiv; 2020. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Moulin L. medRxiv; 2020. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. [Google Scholar]
- Xagoraraki I., O’Brien E. Women in Water Quality. Springer; Cham: 2020. Wastewater-Based Epidemiology for Early Detection of Viral Outbreaks; pp. 75–97. [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Li Y., Wong T.W., Hui D.S. Role of fomites in SARS transmission during the largest hospital outbreak in Hong Kong. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0181558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Jiang L., Huang G., Pu H., Gong B., Lin H., Ma S., Chen X., Long B., Si G., Yu H. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Ni W., Wu Q., Li W., Li G., Tong J., Song X., Xing Q. medRxiv; 2020. Prolonged presence of SARS-CoV-2 in feces of pediatric patients during the convalescent phase. [Google Scholar]
- Xu D., Zhang Z., Jin L., Chu F., Mao Y., Wang H., Liu M., Wang M., Zhang L., Gao G.F., Wang F.S. Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(3):165–171. doi: 10.1007/s10096-005-1299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Torrey J., Bachmann V., Kohn T. Relationship Between Inactivation and Genome Damage of Human Enteroviruses Upon Treatment by UV 254, Free Chlorine, and Ozone. Food Environ. Virol. 2020;12(1):20–27. doi: 10.1007/s12560-019-09411-2. [DOI] [PubMed] [Google Scholar]
- Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V., Chen W.H., Yin X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., Song Y., Zhen W., Zijian F., Wu G., Xu J. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92(6):680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Cui X., Zhao X., Wang J., Zheng J., Zheng G., Guo W., Cai C., He S., Xu Y. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A.J., De Jong M.C., Groot Koerkamp P.W. Airborne microorganisms from livestock production systems and their relation to dust. Crit. Rev. Environ. Sci. Technol. 2014;44(10):1071–1128. doi: 10.1080/10643389.2012.746064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Dongping X., Fusheng W. Dynamic detection of RNA of SARS-associated coronavirus in blood and excreta of SARS patients. Med. J. Chin. People's Liberat. Army. 2004;5:16. [Google Scholar]
- Zhou J., Li C., Zhao G., Chu H., Wang D., Yan H.H.N., Poon V.K.M., Wen L., Wong B.H.Y., Zhao X., Chiu M.C. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3(11):p.eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J.B., Kong, W.H., Wang, S., Long, Y.B., Dong, L.H., He, Z.Y., Liu, M.Q., 2020. Potential transmission risk of SARS-CoV-2 through medical wastewater in COVID-19 outbreak cities.
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]