Abstract
Background
Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor is the cornerstone for prevention ischemic events in patients with acute coronary syndromes (ACS) and undergoing percutaneous coronary intervention. However, the optimal antiplatelet strategy for ACS patients with both high bleeding and high ischemic risks is unclear.
Study design
The OPT-BIRISK trial is a multicenter, double-blinded, placebo-controlled randomized study designed to test the superiority of extended antiplatelet therapy with clopidogrel monotherapy compared with aspirin and clopidogrel for reduction of bleeding events in ACS patients with both high bleeding and high ischemic risks (“bi-risk”). A total of 7,700 patients who completed 9- to 12-month dual antiplatelet therapy after new-generation drug-eluting stent implantation for the treatment of ACS will be randomized to receive clopidogrel monotherapy or aspirin plus clopidogrel for 9 months followed by aspirin monotherapy for 3 months. The primary end point is Bleeding Academic Research Consortium type 2, 3, or 5 bleedings at 9 months after randomization. The key secondary end point is major adverse cardiac and cerebral events at 9 months after randomization, defined as a composite of all-cause death, myocardial infarction, stroke, or coronary artery revascularization.
Conclusions
OPT-BIRISK is the first large-scale randomized trial aimed to explore the optimal antiplatelet strategy for bi-risk ACS patients after percutaneous coronary intervention in current clinical practice. The results will add evidence regarding de-escalation antiplatelet therapy for patients at special risk.
Background
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the cornerstone to prevent stent thrombosis and atherothrombotic events in patients with acute coronary syndromes (ACS) and/or undergoing percutaneous coronary intervention (PCI).1., 2., 3. Although DAPT reduces thrombotic events, this comes at the expense of increased bleeding, underscoring the importance of careful assessment of risks and benefits in the decision-making process of choice and duration of antiplatelet therapy. A number of predictors have been associated with the risk of ischemic and bleeding complications, allowing for the development of scoring systems.4., 5., 6., 7., 8. However, some predictors have been associated with high risk of both bleeding as well as ischemic events, such as elderly, diabetes mellitus, renal dysfunction, anemia, and prior stroke.9
Defining the optimal antiplatelet treatment regimen for patients at high risk for both ischemic and bleeding complications, hereby called bi-risk, is a clinical challenge. Current practice guidelines recommend DAPT for at least 12 months after an episode of ACS event, followed by aspirin monotherapy indefinitely.1 , 2 For patients at high risk of ischemic events, DAPT beyond 1 year is reasonable, but it is based on the prerequisite that patients are not at high risk of bleeding. Several pivotal randomized trials have demonstrated that extended DAPT beyond 1 year resulted in significantly reduced major cardiac and cerebral ischemic events compared to aspirin alone but was associated with higher bleeding risk even among patients who did not previously experience ischemic or bleeding events during the first year or 1 to 3 years.10., 11., 12. Therefore, extended DAPT beyond 1 year might not be suitable for bi-risk patients due to their high bleeding risk.
In recent years, a novel approach of dropping aspirin after a period of DAPT has emerged as a strategy aimed to reduce bleeding while preserving efficacy.13 DAPT followed by chronic P2Y12 inhibitor monotherapy has now been tested in a number of randomized trials.14., 15., 16., 17. Compared with aspirin monotherapy, P2Y12 inhibitor monotherapy has the potential to maintain anti-ischemic efficacy and prevent bleeding, especially gastrointestinal, which is mainly caused by aspirin and which accounts for most (up to 61.7% of post-PCI bleedings) bleeding complications.18 Therefore, extended antiplatelet therapy with P2Y12 inhibitor monotherapy is an attractive option for bi-risk patients. To test this hypothesis, we designed the Optimal antiPlatelet Therapy for high Bleeding and Ischemic RISK patients (OPT-BIRISK) trial to determine if extended clopidogrel monotherapy will be superior to DAPT with clopidogrel and aspirin following completion of 9 to 12 months of DAPT for ACS patients who received drug-eluting stent (DES) implantation and possess both high bleeding and high ischemic characteristics.
Methods
Study design
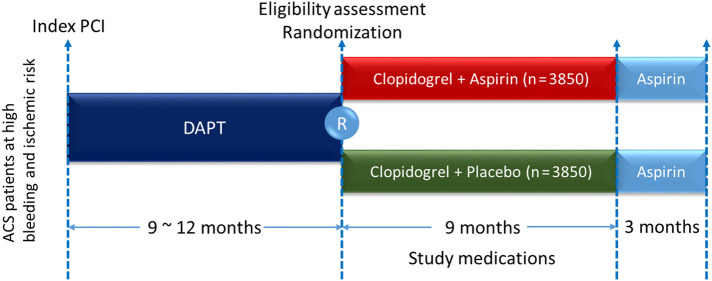
OPT-BIRISK, formerly named East Asian Outcome (EA OUTCOME) trial, is a multicenter, prospective, double-blinded, active-controlled randomized trial designed to examine the superiority of clopidogrel monotherapy for 9 months over DAPT with aspirin and clopidogrel on reducing bleeding in ACS patients with both high ischemic and bleeding risk who have completed 9 to 12 months of DAPT following PCI using new-generation DES. The study design is shown in Figure 1 . This study was approved by the institutional ethics committee of the General Hospital of Northern Theater Command and conducted in accordance with the Declaration of Helsinki. The study is registered at Clinicaltrials.gov (Identifier: NCT 03431142).
Figure 1.
Study flow chart.
Study population
The study will enroll 7,700 patients who completed 9 to 12 months of DAPT after new-generation DES implantation for the treatment of ACS and are free from major adverse clinical events during the prior 6 months. Specific study inclusion and exclusion criteria are shown in Table I . Bleeding and ischemic risks will be evaluated according to criteria listed in Table II based upon the assessment at the time of index PCI. Each risk criterion of bleeding or ischemia was previously reported in literatures or was an individual component of established risk scores. Eligibility assessment will be performed between 9 and 12 months after index PCI. Patients eligible for enrollment and willing to participate in the study will be called back to clinic, and an in-person interview will be performed. A written informed consent will be signed before screening. Eligibility assessment using medical records free from consent was approved by the ethics committee at General Hospital of Shenyang Northern Theater Command.
Table I.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • ACS patients undergoing PCI and received at least 1 new-generation DES⁎ • Completed 9-12 m of DAPT (either with clopidogrel or ticagrelor on top of aspirin) after index PCI procedure • 18-85 y • Meet criteria of both high bleeding and ischemic risk† - Patients <65 y old must meet at least 1 of clinical criteria of high bleeding risk and at least 1 of clinical criteria of high ischemic risk - Patients 65-75 y old must meet 1 of clinical criteria of either high bleeding risk or high ischemic risk - Patients ≥75 y old • Written informed consent |
• Any interruption, abruption, or discontinuation on DAPT during the recent 6 m because of adverse events (hemorrhagic or ischemic) or other reasons • Planned surgery within 90 d • Planned coronary revascularization (surgical or percutaneous) within 90 d • Dialysis-dependent renal failure • Moderate to severe liver function dysfunction (ALT or AST >2 times upper limit of normal) • Life expectancy <1 y • Unable or unwilling to provide informed consent • Women of child-bearing potential • Platelet count <100,000/mm3 • Subjects on warfarin or other anticoagulant therapy |
ACS, acute coronary syndromes; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention.
New-generation DESs refer to any DES with thin strut/Co-Cr alloy platform, or with biodegradable polymer/polymer free, or with more biocompatible drugs other than paclitaxel or sirolimus.
Criteria of high bleeding and ischemic risk; see Table II.
Table II.
Clinical criteria of high bleeding and ischemic risks
| Bleeding risk | Ischemic risk |
|---|---|
| • Female gender • Iron deficiency anemia • Stroke (hemorrhagic or ischemic) history • Diabetes mellitus treated with medications (oral hypoglycemic therapy or subcutaneous insulin) • Chronic kidney disease defined as an estimated glomerular filtration rate< 60 mL/min per 1.73 m2 or creatinine clearance <60 mL/min |
• Multivessel coronary artery disease • Target lesion requiring total stent length >30 mm • Thrombotic target lesion • Bifurcation lesions with Medina 0, 1, 1 or 1, 1, 1 classification requiring at least 2 stents • Left main (≥50%) or proximal LAD (≥70%) lesion • Calcified target lesion(s) requiring atherectomy • Troponin-positive ACS • Established vascular disease defined as previous MI, ischemic stroke, diagnosed PAD, or CAD/PAD revascularization • Repeat MI, coronary revascularization, stent thrombosis, stroke within prior 9 m before index PCI • Diabetes mellitus treated with medications (oral hypoglycemic therapy or subcutaneous insulin) • Chronic kidney disease defined as an estimated glomerular filtration rate <60 mL/min per 1.73 m2 or creatinine clearance <60 mL/min |
LAD, left anterior descending; PAD, peripheral artery disease; CAD, coronary artery disease; MI, myocardial infarction.
Randomization, study treatment, and follow-up
Patients eligible for randomization will be randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo in a 1:1 ratio for 9 months followed by open-label aspirin monotherapy for 3 months. Allocation to treatment will be acquired by an interactive Web response system incorporated within an electronic data capture system (Taimei Tech, Shanghai, China). After randomization, all patients will receive open-label, on-marketing packaged clopidogrel (Plavix, Sanofi Co Ltd, Paris, France) at a dose of 75 mg/d. Repackaged aspirin (Bayer Aspirin; Bayer Pharmacy, Berlin, Germany) at a dose of 100 mg/d or matching placebo (Boji Pharmacy, Guangzhou, China) will be given to patients allocated in the DAPT group and clopidogrel-alone group, respectively. Study medications will not be discontinued unless patients are under unnecessary risk judged by investigators or uncompliant for any reason. Other cardiovascular medications will be at physicians' discretion.
Clinical follow-up will be scheduled at 3, 6, 9, and 12 months via telephone or clinic visit after randomization. Throughout the study period, patients will be monitored for the occurrence of the following clinical events: death, myocardial infarction, stroke, any revascularization, and any bleeding. Adverse events will be evaluated by the independent clinical event adjudication committee. Every event will be monitored until it is adequately resolved or explained.
Study end points
The primary end point is clinically relevant bleeding at 9 months after randomization, which is defined as Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding. The definition of BARC bleeding types was published elsewhere. In brief, BARC types 2, 3, and 5 bleeds are defined as clinically overt hemorrhage requiring medical attention (type 2); requiring transfusion or surgical correction or associated with a hemoglobin drop of at least 3 g/dL (type 3); or fatal events (type 5).19
The key secondary end points are as follows: (1) major adverse cardiac and cerebral events (MACCE) at 9 months, defined as a composite of all-cause death, myocardial infarction, stroke, or coronary artery revascularization; (2) each individual components of MACCE; (3) all bleeds, including BARC type 1, 2, 3, or 5 bleedings; and (4) definite/probable stent thrombosis. All deaths are considered cardiac related unless noncardiac causes were clearly identified. Myocardial infarction is defined according to the third universal definition of myocardial infarction.20 Stroke is defined as an acute event of nonhemorrhagic cerebrovascular origin causing focal or global neurologic dysfunction lasting >24 hours, which is confirmed by both clinical and radiographic means. Coronary artery revascularization is defined as any PCI or bypass surgery of lesion(s) in the main epicardial coronary artery or branches. Stent thrombosis is defined as the Academic Research Consortium definitions.21
Sample size and statistical analysis
We assume BARC type 2, 3, or 5 bleedings to occur in 6.0% of patients during the 9-month study period in the DAPT group.10 To detect a 25% reduction in primary end point events, with 2-tailed α of .05 and power of 80%, 3,470 patients for each group are needed to draw a conclusion that clopidogrel alone is superior to DAPT. Considering a loss of follow-up rate of 10%, the final sample size is 7,700 patients, that is, 3,850 patients in each group.
For key secondary end point testing, we assume MACCE occurred in 8.0% of patients in the DAPT group, with a noninferior margin of 1.6% (20% difference), 1-sided α of .025, 7,700 patients will provide 73% power to conclude that clopidogrel is noninferior to DAPT in anti-ischemic efficacy.
All analyses will be performed according to the intention-to-treat principle based on the number of patients available for each analysis. The data will be presented as means ± SD, medians with 25th and 75th percentiles, or counts or percentages. Baseline clinical and angiographic characteristics as well as procedural variables will be checked for statistically significant differences with Student t test (continuous data) or contingency table analysis (categorical data). Time-to-event variables will be estimated using Kaplan-Meier method and compared with log-rank test. Last observation carried forward will be used in patient loss of follow-up but has clinical outcomes by at least 1 follow-up visit. Prespecified analysis will consist of comparisons between the 2 study groups with regard to the primary and secondary end points. The analysis will also involve prespecified subsets of interest: age; sex; diabetes mellitus; renal dysfunction; heart failure; anemia; prior systemic ischemic events; troponin-positive ACS; complex PCI (including PCI for left main disease, chronic total occlusion, small vessel disease, diffused lesion, bifurcation, or severe calcification); complete revascularization; DES type; prior bleeding events; and risk stratification by GRACE (The Global Registry of Acute Coronary Events) score, CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) score, and DAPT (Dual Antiplatelet Therapy) score.
No interim analysis will be performed for this study. An independent data safety monitoring board will monitor study data periodically during the trial and determine reporting and stopping rules in the data safety monitoring board charter.
Study organization
Details of the study organization are shown in the Appendix. This trial is a physician-initiated study and is supported by research fund from Chinese National key R & D project (contract ID: 2016YFC1301303, 2016YFC1301300) and an investigator-initiated grant from Sanofi (Paris, France). The sponsor is not involved in the study design; study processes, including site selection and management; data collection and analysis; or decision to submit for publication. The authors are responsible for all the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Discussion
OPT-BIRISK is the first large-scale, placebo-controlled randomized trial focused on an important patient cohort composed of ACS patients at high risk for both ischemic and bleeding complications. Defining the optimal antiplatelet therapy for such patients is challenging. Although prior studies have been conducted on defining duration and choice of antiplatelet therapy in patients at either high bleeding or high ischemic risk, to date, there has not been any study focusing on bi-risk patients. In this trial, we will test the hypothesis that, for bi-risk ACS patients, extended antiplatelet therapy beyond 9-12 months with sustained clopidogrel monotherapy is superior to sustained DAPT in reducing bleeding events and is noninferior to sustained DAPT on anti-ischemic efficacy. This trial will provide new insights and evidence on optimal antiplatelet therapy for a high-risk patient cohort which is frequently encountered in real-world practice (about 60% according to our unpublished data).
The importance of aggressive antithrombotic therapies has been emphasized since the introduction of coronary stents more than 2 decades ago and was reinforced because of the fear of stent thrombosis after first -generation DES implantation and concerns surrounding antiplatelet drug resistance.22., 23., 24. However, over the most recent years, improvements in stent design leading to better safety profiles and improved operator skills have led to a dramatic reduction in thrombotic complications leading to a change in perception on the duration and potency of antiplatelet agents needed.25., 26., 27. Moreover, significantly improved compliance to guidelines-recommended secondary prevention measures,28 such as cholesterol lowering, and antihypertensive and hypoglycemic treatment, resulted in significant decrease on ischemic risks. In parallel, there has been growing appreciation for the prognostic implications associated with bleeding, including increased risk of all-cause and cardiac mortalities.29., 30., 31., 32. Even nuisance or minor bleeding can result in unexpected discontinuation of antiplatelet treatment and consequently increase ischemic risk.33 , 34 Accordingly, the primary end point of OPT-BIRISK is clinically relevant bleeding.
De-escalation of antiplatelet potency has emerged as strategy to reduce bleeding and a topic of ongoing investigation.23 , 35 This includes reduced antiplatelet agent dose, short DAPT duration, switching to a less potent agent, and even early switching to monoantiplatelet agent.36 , 37 Traditionally, chronic aspirin monotherapy is the standard antiplatelet strategy recommended by current practice guidelines after discontinuation of DAPT. This approach has never been challenged for about 2 decades because of its established anti-ischemic effectiveness and very low cost. However, aspirin alone or in adjunct to a P2Y12 inhibitor is the most important cause of antiplatelet-related bleeding, especially gastrointestinal bleedings.38 Therefore, it is reasonable to consider P2Y12 inhibitor monotherapy instead of aspirin-based DAPT for patients who finished standard DAPT duration but still at high ischemic risk to reduce bleeding risk without compromise in anti-ischemic effects. Recently published GLOBAL LEADERS/GLASSY, STOPDAPT 2, SMART-CHOICE, and TWILIGHT are all trials intent to test the superiority of P2Y12 inhibitor monotherapy over DAPT in selected patients.14., 15., 16., 17. , 39 GLOBAL LEADERS failed to demonstrate the superiority of sustained ticagrelor monotherapy compared with standard DAPT; 1 major reason might be the unselected study populations in which more than half had stable coronary artery disease.14 The open-label STOPDAPT 2 study focused on patients treated with Co-Cr EES, a device with low risk of stent thrombosis, and found that 1-month DAPT followed by sustained clopidogrel monotherapy was superior to standard 12-month DAPT with significantly improved net clinical benefits.15 The open-label SMART-CHOICE study demonstrated that P2Y12 inhibitor monotherapy after mandatory 3-month DAPT was noninferior to prolonged DAPT on ischemic risks and was better on reducing bleeding risks in a broad spectrum of PCI patients.16 Different from the above studies, TWILIGHT and OPT-BIRISK are both double-blinded trials focused on high-risk patients, and the high-risk criteria of these 2 trials partly overlap. TWILIGHT has demonstrated that, among high-risk patients who underwent PCI and completed 3-month DAPT, ticagrelor alone is better than ticagrelor plus aspirin in reducing bleeding events with no high risk of major ischemic events.17 Compared with TWILIGHT, OPT-BIRISK is looking only into ACS patients and is more conscious to the concept of bi-risk, that is, high bleeding combined with high ischemia, so more specific inclusion criteria were formulated. Considering that all patients are at high bleeding risk, clopidogrel and not a potent P2Y12 inhibitor (ie, ticagrelor) was used as the experimental intervention. Our single-center real-world data analysis has showed that the anti-ischemic benefits of ticagrelor over clopidogrel were only demonstrated in low–bleeding risk patients.40 Therefore, clopidogrel alone may represent a more appropriate treatment option for a bi-risk patient population as enrolled in the present study.41
Another important distinguishing feature of the OPT-BIRISK trial is the duration of DAPT before switching to P2Y12 inhibitor monotherapy. DAPT duration was 1 month in GLOBAL LEADERS and STOPDAPT 2, 3 months in SMART CHOICE and TWILIGHT, and 9-12 months in the present study.14., 15., 16., 17. The rationale for considering 9-12 months of DAPT in the OPT-BIRISK trial was due to the following considerations: First, for high-risk ACS patients, residual ischemic risks exist through the first 1-3 years, and 9-12 months of DAPT was reasonable according to current guidelines, especially for patients without bleeding events. Second, this study will answer the question of how to treat bi-risk ACS patients after completion of 9 to 12 months DAPT; that is, treatment decision will be made at 9 to 12 months after index PCI, and the target population will be those that survived free from major events. In most trials, the decision making of antiplatelet regimen was performed at time of the index PCI regardless the patient's clinical status at follow-up. Therefore, at 9-12 months after PCI, the following antiplatelet strategy needs to be answered. Third, according to our experience, DAPT is usually stopped at 9-12 months after PCI for most ACS patients.42 Therefore, enrollment of patients at 9 to 12 months was consistent to clinical practice and will be of benefit to enhance patient's and physician's compliance to the trial.
Study medication will last for 9 months in the present trial, which is shorter than other above-mentioned trials. Considering that all patients in the present study have at least 1 criterion of high bleeding risk and overall intervention/observation will be completed at 21-24 months after index PCI, it is reasonable to design a study with 9-month prolonged study medications.
Current status
The first patient was enrolled in OPT-BIRISK trial on Feburary 12, 2018. At present, 103 sites have been activated with a total enrollment of 6,404 patients. After reviewing raw data of the first 5500 patients, we found a significant unbalance between fulfilled ischemic and bleeding criteria (Supplementary Table I and Supplementary Figure 1). A guidance was issued to encourage enrolling patients with more high bleeding risk criteria. Data about ischemic/bleeding criteria will be periodically reviewed until end of patient recruitment to ensure balance between ischemic and bleeding risk profiles. Because of the COVID-19 pandemic and the amended enrollment strategy, the enrollment is expected to continue until April 31, 2021, that is, 3 months later than originally planned. The last patient visit is expected to occur in April 2022.
Disclosure
Dr DJ Angiolillo has received payment as an individual for (a) consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and (b) participation in review activities from CeloNova and St. Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2020.07.005.
Appendix. Supplementary data
Supplementary material
References
- 1.Valgimigli M., Bueno H., Byrne R.A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 2.Levine G.N., Bates E.R., Bittl J.A., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 3.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915–2931. [DOI] [PubMed]
- 4.Yeh R.W., Secemsky E.A., Kereiakes D.J., et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. doi: 10.1001/jama.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa F., van Klaveren D., James S., et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 6.Baber U., Mehran R., Giustino G., et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: Risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. doi: 10.1016/j.jacc.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 7.Han Y., Chen J., Qiu M., et al. Predicting long-term ischemic events using routine clinical parameters in patients with coronary artery disease: the OPT-CAD risk score. Cardiovasc Ther. 2018;36(5) doi: 10.1111/1755-5922.12441. [DOI] [PubMed] [Google Scholar]
- 8.Capodanno D., Angiolillo D.J. Tailoring duration of DAPT with risk scores. Lancet. 2017;389(10073):987–989. doi: 10.1016/S0140-6736(17)30591-3. [DOI] [PubMed] [Google Scholar]
- 9.Tantry U.S., Bonello L., Aradi D., et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013 Dec 17;62(24):2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 10.Mauri L., Kereiakes D.J., Yeh R.W., et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaca M.P., Bhatt D.L., Cohen M., et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 12.Verdoia M., Kedhi E., Ceccon C., et al. Duration of dual antiplatelet therapy and outcome in patients with acute coronary syndrome undergoing percutaneous revascularization: a meta-analysis of 11 randomized trials. Int J Cardiol. 2018;264:30–38. doi: 10.1016/j.ijcard.2018.02.095. [DOI] [PubMed] [Google Scholar]
- 13.Capodanno D., Mehran R., Valgimigli M., et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15(8):480–496. doi: 10.1038/s41569-018-0049-1. [DOI] [PubMed] [Google Scholar]
- 14.Vranckx P., Valgimigli M., Jüni P., et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940–949. doi: 10.1016/S0140-6736(18)31858-0. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H., Domei T., Morimoto T., et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321(24):2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn J.Y., Song Y.B., Oh J.H., et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321(24):2428–2437. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehran R., Baber U., Sharma S.K., et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019 Nov 21;381(21):2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 18.Généreux P., Giustino G., Witzenbichler B., et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036–1045. doi: 10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- 19.Mehran R., Rao S.V., Bhatt D.L., et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J.S., Jaffe A.S., et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 22.Angiolillo D.J., Fernandez-Ortiz A., Bernardo E., et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49(14):1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Sibbing D., Aradi D., Alexopoulos D., et al. Updated expert consensus statement on platelet function and genetic testing for guiding p2y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(16):1521–1537. doi: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Aradi D., Kirtane A., Bonello L., et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36(27):1762–1771. doi: 10.1093/eurheartj/ehv104. [DOI] [PubMed] [Google Scholar]
- 25.Philip F., Agarwal S., Bunte M.C., et al. Stent thrombosis with second-generation drug-eluting stents compared with bare-metal stents: network meta-analysis of primary percutaneous coronary intervention trials in ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2014;7(1):49–61. doi: 10.1161/CIRCINTERVENTIONS.113.000412. [DOI] [PubMed] [Google Scholar]
- 26.Colmenarez H., Fernández C., Escaned J. Impact of technological developments in drug-eluting stents on patient-focused outcomes: a pooled direct and indirect comparison of randomised trials comparing first- and second-generation drug-eluting stents. EuroIntervention. 2014;10(8):942–952. doi: 10.4244/EIJV10I8A161. [DOI] [PubMed] [Google Scholar]
- 27.Moon J.Y., Franchi F., Rollini F., et al. Evolution of coronary stent technology and implications for duration of dual antiplatelet therapy. Prog Cardiovasc Dis. 2018;60(4–5):478–490. doi: 10.1016/j.pcad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Eccleston D., Horrigan M., Rafter T. Improving guideline compliance in australia with a national percutaneous coronary intervention outcomes registry. Heart Lung Circ. 2017;26(12):1303–1309. doi: 10.1016/j.hlc.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Eikelboom J.W., Mehta S.R., Anand S.S., et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 30.Vaduganathan M., Harrington R.A., Stone G.W., et al. Short- and long-term mortality following bleeding events in patients undergoing percutaneous coronary intervention: insights from four validated bleeding scales in the CHAMPION trials. EuroIntervention. 2018;13(15):e1841–e1849. doi: 10.4244/EIJ-D-17-00723. [DOI] [PubMed] [Google Scholar]
- 31.Urban P., Mehran R., Colleran R., et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140(3):240–261. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmerini T., Bacchi Reggiani L., Della Riva D., et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J Am Coll Cardiol. 2017;69(16):2011–2022. doi: 10.1016/j.jacc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Roy P., Bonello L., Torguson R., et al. Impact of "nuisance" bleeding on clopidogrel compliance in patients undergoing intracoronary drug-eluting stent implantation. Am J Cardiol. 2008;102(12):1614–1617. doi: 10.1016/j.amjcard.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Dor I., Torguson R., Scheinowitz M., et al. Incidence, correlates, and clinical impact of nuisance bleeding after antiplatelet therapy for patients with drug-eluting stents. Am Heart J. 2010;159(5):871–875. doi: 10.1016/j.ahj.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Angiolillo D.J., Rollini F., Storey R.F., et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. [DOI] [PubMed] [Google Scholar]
- 36.Han Y.L. De-escalation of anti-platelet therapy in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a narrative review. Chin Med J (Engl) 2019;132(2):197–210. doi: 10.1097/CM9.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo C., Li M., Lv Y.H., et al. De-escalation versus standard dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Platelets. 2019:1–11. doi: 10.1080/09537104.2019.1574969. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt D.L., Scheiman J., Abraham N.S., et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502–1517. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi S., Franzone A., Piccolo R., et al. Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported randomised controlled trial in patients with coronary artery disease: the GLOBAL LEADERS Adjudication Sub-StudY (GLASSY) BMJ Open. 2019;9(3) doi: 10.1136/bmjopen-2018-026053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H.Y., Li Y., Xu X.M., et al. Impact of baseline bleeding risk on efficacy and safety of ticagrelor versus clopidogrel in chinese patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin Med J (Engl) 2018;131(17):2017–2024. doi: 10.4103/0366-6999.239306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park T.K., Song Y.B., Ahn J., et al. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(1) doi: 10.1161/CIRCINTERVENTIONS.115.002816. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Li Y., Qiu M., et al. Impact of dual antiplatelet therapy duration on 1-year clinical outcomes in diabetic patients with acute coronary syndrome undergoing percutaneous coronary intervention: Insights from the real-world OPT-CAD study. Catheter Cardiovasc Interv. 2020;95(Suppl. 1):579–586. doi: 10.1002/ccd.28653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material