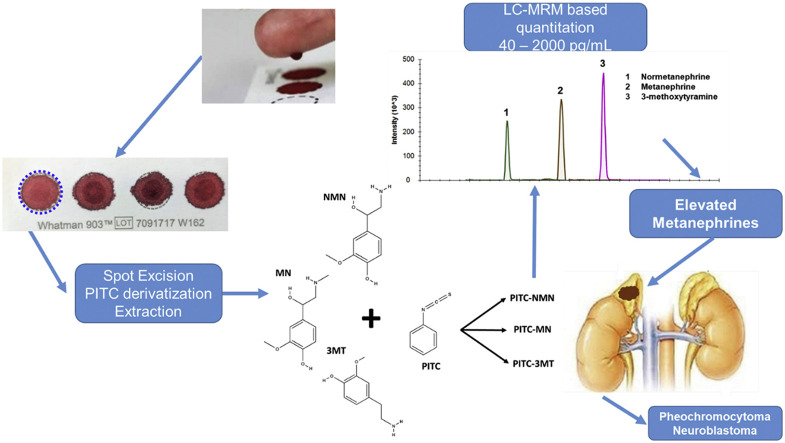
Abstract
The quantitation of metanephrine (MN), normetanephrine (NMN), and 3-methoxytyramine (3-MT) – referred to as metanephrines -- by LC-MS/MS is the gold-standard for screening for pheochromocytoma and paragangliomas (PPGLs), tumours of the adrenal gland and the peripheral nervous system. An assay for metanephrines from dried blood spots (DBSs) would be of high clinical utility as it simplifies sample collection, enables remote sampling, and could increase compliance with the clinical recommendation for supine sampling. Moreover, DBS sampling facilitates the measurement of blood-derived metanephrines in pediatric patients – where DBSs are well-established – in order to diagnose neuroblastomas.
Here, we adapted an established derivatization-based LC-MRM-MS assay for plasma catecholamines, and optimized the sample extraction, LC, and MS parameters to produce a fast, sensitive, and robust method for the measurement of metanephrines from DBSs, including 3-methoxytyramine. The DBS samples were excised, derivatized with phenyl isothiocyanate (PITC) on-spot, extracted, and measured by LC-MRM-MS. To validate assay suitability and performance, we assessed the linearity, precision, accuracy, recovery, and matrix effects of the method, and determined the stability of metanephrines in DBSs under different storage conditions. Assay performance for NMN, MN, and 3-MT was sufficient for quantitation from a single DBS within a linear range from 40 to 2000 pg/mL. MN and NMN were stable in DBSs for 2 weeks, whereas 3-MT was stable for one week regardless of storage temperature. Altogether, this work represents the first quantitative LC-MS/MS method for metanephrines from DBSs and provides a novel opportunity for the diagnosis of PPGLs and neuroblastomas in the future.
Keywords: Dried blood spots (DBSs), Multiple reaction monitoring (MRM), Catecholamines, Metanephrines, Clinical analysis, Pheochromocytoma, Paraganglioma, Neuroblastoma
Graphical abstract
Highlights
-
•
Development and validation of an assay for metanephrines from dried blood spots.
-
•
Method utilizes on-spot derivatization followed by LC-MRM-MS based quantitation.
-
•
Method demonstrated high degree of sensitivity, precision, and accuracy.
-
•
High relevance for the clinical diagnosis of pheochromocytomas and paragangliomas.
1. Introduction
Pheochromocytomas and paragangliomas (PPGLs) are tumours arising from the adrenal medulla or extra-adrenal ganglia which produce excessive amounts of catecholamines, having potentially life-threatening effects on the cardiovascular system. Metanephrine (MN), normetanephrine (NMN), and 3-methoxytyramine (3-MT) are the o-methylated metabolic products of the catecholamines norepinephrine, epinephrine, and dopamine, respectively, and are referred to as metanephrines (MNs). Under proper sampling conditions, LC-MS based determination of plasma MNs is the gold standard for first-line screening of PPGLs [1]. Despite the proven efficacy of methods for determination of MNs from urine or plasma, there exist considerable drawbacks with regard to sampling time, storage, and cost. Urine measurements are made after 24-hour collection, which is both time-consuming and inconvenient for patients. In contrast, pediatric urine samples are typically normalized to creatinine due to large inter- and intra-patient changes in urine concentration, which requires an additional measurement. The current clinical guidelines for the sampling of venous blood for the quantification of plasma MNs recommend rest in a supine position for approximately 30 min [2], which is time consuming and hard to realize under typical hospital test centre conditions. Another important consideration in favour of DBS sample collection for the analysis of metanephrines is that DBS samples may actually be more stable for short term storage than whole blood which is often stored at room temperature for extended periods of time prior to centrifugation (for plasma or serum) and either analysis or storage – which is known to lead to bias in the measurement of these analytes [3,4].
The analysis of a variety of metabolites from DBSs has a number of advantages over the analysis of other commonly analysed biofluids [[5], [6], [7], [8], [9]]. In the context of MNs, minimally invasive DBS collection is a fast sampling technique that would be beneficial to the clinical laboratory as it reduces patient discomfort and inconvenience, improves sample collection throughput, allows remote as well as longitudinal testing and alleviates the need for special types of sample storage [10]. In addition, DBS sampling may improve compliance with the clinical recommendation for supine sampling since blood samples can be spotted remotely, thus reducing the burden on the clinic and potentially limiting both exposure of the patient as well as healthcare workers to pathogens (of particular importance during pandemics like COVID-19). Another promising use of DBSs would be for the screening of blood-derived MNs from pediatric patients, since a comparatively low volume of blood (typically 50 μL) is required. For these reasons, we developed a rapid, robust, sensitive, and cost-effective method for the determination of MNs from DBS. We adapted an approach described by Zheng et al. for the analysis of catecholamines in plasma samples [11] and optimized it for the analysis of MNs from DBS, thereby reducing the analytical run time, utilizing MRM transitions that were more sensitive, and including 3-MT which had not been previously quantitated. To this end, we used on-spot phenyl isothiocyanate (PITC) derivatization, followed by organic extraction, and LC-multiple reaction monitoring-mass spectrometry (LC-MRM) based quantitation.
2. Experimental section
2.1. Chemical standards, reagents, blood collection cards, samples
Certified reference standards for MN, NMN, 3-MT, epinephrine (EN), norepinephrine (NEN), dopamine (DOPA), and deuterium labelled analogs MN-D3, NMN-D3, 3-MT-D4 were purchased from Cerilliant Corporation (Round Rock, TX, USA). Dilutions of chemical standards were prepared in methanol in glass HPLC vials and stored at −20 °C. Acetonitrile, methanol, water, formic acid, and ammonium acetate were LC-MS grade purity or higher for all sample preparation and analysis stages and were purchased from Sigma-Aldrich (St. Louis, MO, USA). PITC and pyridine were HPLC grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA). Whatman 903 snap apart DBS collection cards, Ziploc bags, and sorbent packets were purchased from Sigma Aldrich (St. Louis, MO, USA). Human whole blood from healthy consenting donors of mixed sex and age was purchased from BioIVT (Westbury, NY, USA).
2.2. Dried blood spot preparation
2.2.1. Blood spotting
DBSs for method development and optimization of extraction conditions were prepared by spotting 50 μL of whole blood to the centre of collection spots of Whatman 903 collection cards using a micropipettor. Spotted cards were left to dry for 2 h at room temperature in a biosafety cabinet, then transferred to Ziploc bags with sorbent packets prior to storage or analysis.
2.2.2. Derivatization and DBS extraction
DBSs were excised (whole spot) and transferred to 1.5 mL microcentrifuge tubes. PITC derivatization buffer was prepared immediately before DBS extraction and consisted of 5% isothiocyanate in 1:1:1 ethanol:pyridine:water. Fifty-five μL of PITC derivatization solution was directly pipetted onto the DBS and incubated at room temperature for 10 min. The PITC derivatization solution was then removed by vacuum evaporation using a Labconco Centrivap. The derivatized DBS samples were then extracted with 500 μL of 1:1 methanol:acetonitrile containing 5 mM ammonium acetate using an Eppendorf ThermoMixer C at room temperature with shaking at 2000 rpm for 20 min, followed by sonication for 10 min in an ultrasonic bath. Samples were then spun at 20,000×g for 5 min, and the extract was transferred to new 1.5 mL Eppendorf microcentrifuge tubes. Extracts were vacuum-concentrated at room temperature using a Labconco Centrivap, reconstituted in 20 μL of water, and transferred to glass HPLC vial inserts.
2.3. LC-MS/MS
2.3.1. LC parameters
Chromatographic separation of DBS extracts was conducted using an Agilent Zorbax Eclipse Plus C18 column (2.1 × 150 mm, 1.8 μm 95 Å particle size) and a 10-min binary gradient on a Shimadzu Nexera XR UHPLC system. Mobile phase A was 0.1% aqueous formic acid and B was acetonitrile with 0.1% formic acid. The flow rate was set to 400 μL/min. The gradient began with a brief equilibration phase for 0.5 min at 5% B before increasing from 5 to 40% B over 1 min, then 40% to 50% B over 4 min, then immediately from 50% to 95% B which was held for 2 min before returning to 5% B, which was held for 3 min. The injection volume was 20 μL, the column heater compartment was thermostatted at 50 °C, and the autosampler was refrigerated at 4 °C.
2.3.2. MRM parameters and optimization
Samples were analysed by MRM using a Sciex QTrap 6500+ mass spectrometer operated in positive ion mode. The instrument was controlled using Analyst 1.6. Source parameters were optimized as follows: capillary voltage of 5.5 kV, source temperature of 500 °C, ion source gases 1 and 2 were 60 and 50 respectively, collision gas was set to high, and the curtain gas was set to 25. For MRM experiments, Q1 and Q3 were set to unit resolution. Collision energies, declustering potentials, and cell exit potentials were optimized by direct infusion of PITC-derivatized metanephrine reference standards (including MN, NMN, 3-MT, and their deuterium labelled analogs) using the manual tune interface in Analyst v1.7 (see Table 1 ). For MN, both the [M+H]+ and [M-H2O+H]+ ions were monitored, with the ion corresponding to neutral water loss being used for quantitation, as it demonstrated the greatest sensitivity. The intact precursor ion was used as a ‘pseudo-qualifier’ transition -- a diagnostic neutral-loss form of the precursor ion -- instead of using a fragment ion which is typically used as the qualifier ion for detection of an analyte. Notably, in our hands, using the transitions published by Zheng et al. [11], NMN was undetectable even at high concentrations. To investigate this, we used high-resolution MS/MS on a Sciex TripleTOF 6600 using the same ESI source and chromatographic conditions (Supplementary Fig. 1 ). This revealed that the predominant form of the precursor ion was the ion corresponding to the loss of water (−18 Da) (See Supplementary Figs. 1 and 2 for MS and MS/MS spectra). Therefore, we monitored transitions corresponding to the water loss of the NMN precursor [M –H2O+H]+ (see Table 1). From this acquired MS/MS data, we determined the best candidate ion transitions, which were then further optimized as described above. For the chemical structures of the PITC derivatized analytes, as well as the proposed fragments, see Supplementary Fig. 3.
Table 1.
Optimized MRM transitions for PITC derivatized MNs on a QTrap 6500+. EP = Entrance Potential DP = Declustering potential; CE= Collision energy; CXP= Cell exit potential.
| Analyte | Precursor (m/z) | Product (m/z) | EP | DP | CE | CXP |
|---|---|---|---|---|---|---|
| NMN(-H2O) | 301.1 | 134.1 | 10 | 120 | 35 | 9 |
| NMN(-H2O) | 301.1 | 166.1 | 10 | 50 | 25 | 9 |
| NMN-d3(-H2O) | 304.1 | 137.1 | 10 | 120 | 35 | 9 |
| NMN-d3(-H2O) | 304.1 | 169.1 | 10 | 50 | 25 | 9 |
| MN(-H2O) | 315.1 | 180.1 | 10 | 120 | 28 | 9 |
| MN | 333.1 | 180.1 | 10 | 50 | 25 | 9 |
| MN-d3(-H2O) | 318.1 | 183.1 | 10 | 120 | 28 | 9 |
| MN-d3 | 336.1 | 183.1 | 10 | 50 | 25 | 9 |
| 3-MT | 303.1 | 151.1 | 10 | 120 | 25 | 14 |
| 3-MT | 303.1 | 119.0 | 10 | 120 | 25 | 14 |
| 3-MT-d4 | 307.1 | 155.1 | 10 | 120 | 25 | 14 |
| 3-MT-d4 | 307.1 | 123.0 | 10 | 120 | 25 | 14 |
2.3.3. Data analysis
Acquired MRM data was visualized, analysed, and reported using Skyline daily version 4.2.1 available at https://skyline.ms/ [12]. After importing the data, manually inspecting it for correct peak assignment and integration in Skyline, and comparing the relative peak area ratios of quantifier and qualifier transitions to verify the peak purity, the peak area ratios for single quantifier-ion transitions were calculated, and the assay linearity was assessed using linear regression with 1/x2 regression weighting (the qualifier ion transitions are shown in Table 1).
2.4. Assay validation
2.4.1. Assay linearity and sensitivity
Assay validation was conducted in accordance with the recommendations stipulated in the ICH M10 bioanalytical method validation document published in June 2019 [13]. Assay linearity was assessed using the surrogate analyte approach using reverse calibration curves due to the endogenous presence of MN, NMN, and 3-MT in human blood, and the lack of an appropriate surrogate matrix [13]. Whole blood was fortified with light MN reference standards at a fixed concentration of 2 ng/mL to act as a concentration normalizer. Deuterium-labelled MN, NMN, and 3-MT calibration standards were used to generate a dilution series in quadruplicate over eight different concentration levels as follows: 0, 40, 80, 160, 320, 640, 1280, and 2000 pg/mL. For all three analytes, the linear regression of the relative peak area ratios (heavy/light) against the concentration was adjusted with a weighting of 1/x2. Assay quantitation limits were defined as the lowest point on the curve where the signal to noise (S/N) was greater than 3, and which was also within the linear dynamic range of the calibration curve with a coefficient of variation (% CV) below 20%. To determine accuracy of the calibrators, the concentrations of each level were back-calculated from the linear equation, and the values had to meet an acceptance criteria of <20% deviation from the actual concentration for the LLOQ and <15% for all other calibrator levels.
2.4.2. Assay accuracy and precision
Quality control samples were prepared in the same way as calibrators, but from different stock solutions and using four independent preparations at four concentrations levels: 40 (LLOQ), 120 (low), 750 (middle), and 1500 (high) pg/mL. Intra-assay accuracy representing the accuracy of the linear equation for predicting the actual concentration of unknown samples was determined at each concentration level from four consecutive analytical runs and was calculated as a percentage of the nominal concentration for each replicate. A run was accepted if all of the QC samples were within 15% of the nominal concentration. Precision was calculated as the % CV between all runs of the same concentration level (N = 4 for intra-assay, N = 12 for inter-assay precision), and was deemed to be acceptable if the % CV was <15%. Inter-assay accuracy and precision were assessed over a minimum of three separate time points, separated by at least one day and with a minimum of three replicates each. Samples for inter-day precision were spiked to 500 pg/mL with each analyte, and stored at three separate conditions: room temperature, 4 °C, and −20 °C. These samples were analysed within the context of assessing compound stability, as described in section 2.4.4.
2.4.3. Recovery and matrix effects
To determine analyte recovery and matrix effects, three sets of DBS samples at low, medium, and high concentrations were prepared in independent triplicate replicates as follows: (A) whole blood fortified with internal standard (IS)-MNs (1 ng/mL) pre-DBS extraction; (B) extracted DBS samples which had then been spiked with PITC-derivatized IS-MNs (1 ng/mL); and (C) PITC-derivatized IS-MNs (1 ng/mL) in buffer as an un-extracted control. Recovery of MNs was calculated as the ratio A to B (whole blood spiked pre-extraction/DBS samples spiked post extraction). Matrix induced ion suppression was calculated as the ratio of B to C (DBS samples spiked post-extraction/un-extracted PITC-derivatized IS-MN standards in buffer).
2.4.4. Analyte stability
To determine the stability of MNs in DBS under various storage conditions, DBS samples were prepared and fortified to 500 pg/mL of MN, NMN, and 3-MT and stored in triplicate at either room temperature (RT), 4 °C, or −20 °C for 1 (D2), 5 (D6), 8 (D9), 14 (D15), and 16 days (D17) prior to spot excision, addition of IS (1 ng/mL), derivatization, extraction, and analysis by LC-MRM-MS as described above. The peak areas of the MN reference standards were normalized to IS and plotted as a percentage of the initial time point to generate stability curves.
2.4.5. Carryover
Carryover was assessed in triplicate by measuring matrix double blank samples immediately after injecting the highest calibrator samples. Acceptable carryover required the total area of the blanks to be less than 20% of the LLOQ peak area.
3. Results and discussion
3.1. Method adaptation, optimization of extraction conditions
In order to develop a method for MNs from DBS, we initially used a relatively complex sample preparation protocol entailing whole DBS excision, resolubilization of the DBS in water, molecular weight cut-off filtration (MWCO), and solid phase extraction (Waters HLB). Although this method met our performance criteria (linearity, precision, accuracy, reproducibility, etc.) it suffered from several issues, the first of which was the length, complexity, and cost of the sample preparation itself. This was due to the multiple transfer steps and the lengthy drying times, as well as the dependence on a relatively expensive means of sample clean-up using MWCO filters and SPE cartridges or plates. The second problem was the reliance on reversed-phase chromatography using pentafluorophenyl (PFP) and biphenyl column phases, which, in our hands, showed poor robustness due to repeated issues with deteriorating peak shape and progressive loss of compound retention. Third, despite the relatively complex sample preparation, pronounced matrix effects were still observed with this method (data not shown). To address these issues, we modified a method that had been developed for the analysis of catecholamines in human serum [11], using PITC derivatization, which allowed the separation of the derivatized analytes on a common C18 reversed-phase column. This method does not use SPE or MWCO filtration, thus allowing faster and more cost-effective sample analysis. In order to adapt the original protocol, which employed on-filter derivatization for the analysis of catecholamines from low volumes of serum, our PITC-DBS method involved whole-DBS excision, and transfer to a 1.5 mL microcentrifuge tube, followed by the addition of 50 μL of derivatization solution directly to an excised DBS. The DBS samples were incubated at room temperature for 10 min, followed by extraction of PITC-derivatized MNs using an organic extraction solvent. For organic extraction of PITC-derivatized MNs, we compared methanol and 1:1 methanol:acetonitrile. The methanol:acetonitrile mixture led to improved compound recoveries and improved analytical precision (Supplementary Fig. 4). Extraction in organic solvent not only proved efficient for PITC-MNs (see 3.3), but also effectively precipitated the proteins, allowing the use of a simple vacuum concentration step, reconstitution in water, and the subsequent measurement by LC-MRM.
3.2. Selectivity and chromatographic separation of PITC-derivatized metanephrines
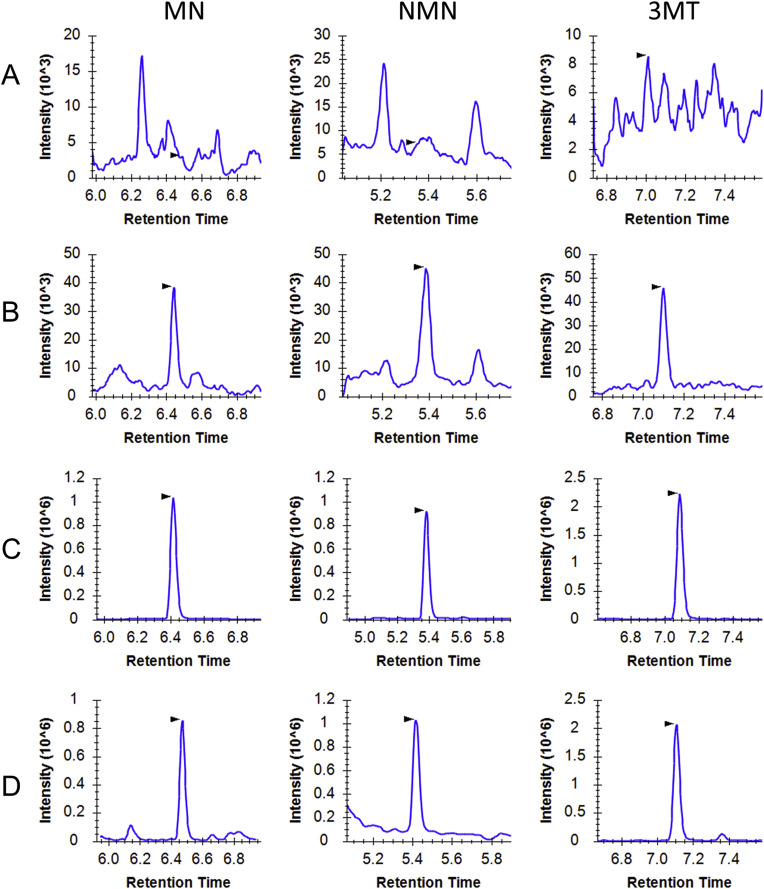
Chromatographic separation of MN, NMN, and 3-MT was achieved using a 10-minute LC gradient (Fig. 1 ). Selectivity was determined by measuring matrix blank samples from at least three separate sources and comparing the responses to those from samples spiked at the LLOQ (Fig. 1A and B). Signals from the matrix double-blanks were less than 20% of the LLOQ, indicating that the monitored MRM transitions were selective. Furthermore selectivity was assessed by analysing DBS samples spiked with a mixture of structurally related catecholamines including epinephrine (EN), norepinephrine (NEN), dopamine (DOPA), as well as MN, NMN, and 3-MT. It should be noted that this method does not allow for the chromatographic resolution of EN and NMN, which does lead to the possibility of interference, since many transitions are shared between the two metabolites. However, the monitored quantifier transition for NMN (endogenous 301 m/z → 134 m/z, IS 304 m/z → 137 m/z) is specific to NMN and thus enables interference-free quantitation of this metabolite.
Fig. 1.
Representative MRM ion chromatograms of stable isotope labelled and light MNs standards. A-C) show MRM chromatograms for PITC-derivatized MN-d3, NMN-d3, and 3-MT-d4, at (A) matrix blank, (B) LLOQ (40 pg/mL), and (C) ULOQ QC (2 ng/mL). (D) MRM chromatograms for a PITC-derivatized DBS sample fortified to 2 ng/mL with MN, NMN, and 3-MT.
3.3. Recovery and matrix effects
Assay recovery and matrix effects are summarized in Table 2 . Recovery was modest for all three metabolites, and matrix effects were observed for all three metabolites, but were highest for NMN and 3-MT (49.5%, and 45.6% respectively). Despite the modest recovery and considerable matrix effects for all three metabolites, the assay achieved low to acceptable CVs between independently-generated triplicates, indicating that the extraction is reproducible.
Table 2.
Recovery and matrix interferences for MNs extracted from DBS samples.
| Compound | Recovery % (n = 9) |
Matrix Effects % (n = 9) |
||
|---|---|---|---|---|
| Average | %CV | Average | %CV | |
| NMN | 57.1% | 7.8% | 49.5% | 15.4% |
| MN | 66.7% | 11.6% | 84.8% | 12.0% |
| 3-MT | 56.8% | 8.5% | 45.6% | 15.1% |
3.4. Assay sensitivity and linearity
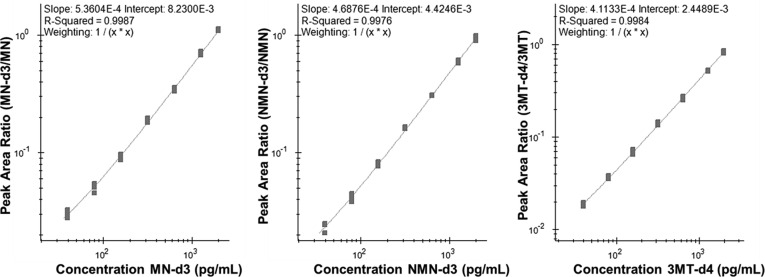
Due to the presence of endogenous MNs in human blood, and the apparent lack of an appropriate surrogate matrix, assay sensitivity and linearity was determined using a surrogate-analyte approach. Reverse calibration curves were generated in whole human blood through dilution of deuterium labelled analogs of each analyte in whole blood prior to volumetric spotting on DBS collection cards. The concentration range of each analyte was selected to span a diagnostically relevant concentration range for all three metabolites based on commonly utilized plasma reference ranges [1,14]. During DBS extraction, samples were spiked with light certified reference standards (fortifying the sample to 1 ng/mL for all three analytes), which were used to normalize the signal for the surrogate analytes. Assay linearity, precision, and accuracy criteria were met for MN (R2 = 0.9987) (Fig. 2 A), NMN (R2 = 0.9976) (Fig. 2B), and 3-MT (Fig. 2C), within the defined concentration range of 40–2000 pg/mL. At all calibration levels, precision was within 15% deviation of the actual concentration with a mean absolute deviation of 6.9%, 7.2%, and 9.5% at the LLOQ for MN, NMN, and 3-MT respectively (Table 3 ). Because the endogenous concentration of 3-MT is typically 5 to 10-fold lower than MN/NMN, and because typical clinical-cutoffs recommended for 3-MT to be used as a diagnostic criterion are below the validated limit of quantitation for our DBS assay (typically below 20 pg/mL) [15,16], further evaluation of the method in a larger cohort would be needed to determine the efficacy of our method of PCC/PPGL screening. Interestingly, the assay may still provide sufficient analytical sensitivity to measure 3-MT in the context of neuroblastoma diagnosis, where it has been demonstrated to serve as a highly sensitive and accurate biomarker [[17], [18], [19], [20]]. In such cases, 3-MT concentrations in plasma have been found to be as high as 255 pg/mL, with typical concentrations of approximately 100 pg/mL, compared to 2–9 pg/mL in healthy controls [17]. As such, this assay may prove useful for neuroblastoma diagnosis.
Fig. 2.
Representative LC-MRM calibration curves for (A) MN, (B) NMN, (C) 3-MT, acquired as reverse calibration curves with increasing IS levels normalized to a constant level of unlabelled reference standards (2 ng mL-1).
Table 3.
Assay linearity, precision, and accuracy data for calibration curves.
| Compound | R2 (n = 4) | Actual Concentration (pg/mL) | Measured Concentration (pg/mL) (n = 4) | Accuracy (n = 4) | % CV (n = 4) |
|---|---|---|---|---|---|
| MN | 0.9987 | 40 | 40.8 | 101.9% | 5.9% |
| 80 | 77.8 | 97.2% | 4.9% | ||
| 160 | 154.3 | 96.5% | 5.1% | ||
| 320 | 329.2 | 102.9% | 4.1% | ||
| 640 | 634.8 | 99.2% | 3.8% | ||
| 1280 | 1283.7 | 100.3% | 3.0% | ||
| 2000 | 2040.8 | 102.0% | 1.5% | ||
| NMN | 0.9976 | 40 | 40.4 | 101.0% | 9.6% |
| 80 | 78.2 | 97.7% | 8.0% | ||
| 160 | 157.7 | 98.6% | 4.4% | ||
| 320 | 333.1 | 104.1% | 1.7% | ||
| 640 | 638.7 | 99.8% | 0.6% | ||
| 1280 | 1261.2 | 98.5% | 3.1% | ||
| 2000 | 2005.1 | 100.3% | 4.5% | ||
| 3-MT | 0.9984 | 40 | 39.3 | 98.3% | 4.9% |
| 80 | 81.7 | 102.1% | 4.4% | ||
| 160 | 159.6 | 99.8% | 6.1% | ||
| 320 | 330.3 | 103.2% | 4.0% | ||
| 640 | 630.5 | 98.5% | 3.7% | ||
| 1280 | 1256.6 | 98.2% | 2.0% | ||
| 2000 | 1989.0 | 99.5% | 3.2% | ||
3.5. Assay precision and accuracy
Assay precision and accuracy were determined by quantifying MNs in independently-prepared QC quadruplicates at the LLOQ, 3 x LLOQ, mid-point of the calibration curve, and at 75% of the highest point of the calibration curve (Table 4 ). Our criteria for analytical validation were that the accuracy must be 100% ± 15% and the CVs must be below 15%. The experimental precisions in % CV were 1.8%–3.4%, 3.9–9.3%, and 1.7–10.4% for MN, NMN, and 3-MT, respectively. Assay accuracies were 93.0–105.5%, 98.2–103.8%, and 97.9–109.4% for MN, NMN, and 3-MT, respectively. All of the QC samples passed our validation criteria, with the exception of one of the four QC samples at the LLOQ of 3-MT, which deviated by more than 15% from the nominal concentration. Since the remaining three QC samples showed acceptable accuracies and low % CVs, we believe that the failure of this single QC-sample is likely attributable to a technical problem during injection. It was, therefore, excluded from the data analysis. Based on these results, all measurements met the validation criteria, and demonstrated excellent reproducibility and accuracy (see Table 5).
Table 4.
Intra-assay performance metrics as determined from quadruplicate replicates of 4 QC concentration levels. The difference between the average measured and actual concentrations was defined as the percent accuracy. CVs were calculated as an average of the 4 replicates.
| Compound | Actual Concentration (pg/mL) | Measured Concentration (pg/mL) (n = 4) | Accuracy (n = 4) | % CV (n = 4) | |
|---|---|---|---|---|---|
| MN | 40 | 37.2 | 93.0% | 3.4% | |
| 120 | 122.2 | 101.8% | 3.2% | ||
| 750 | 790.9 | 105.5% | 1.8% | ||
| 1500 | 1494.3 | 99.6% | 3.2% | ||
| NMN | 40 | 39.9 | 99.8% | 9.3% | |
| 120 | 118.1 | 98.4% | 3.9% | ||
| 750 | 778.1 | 103.8% | 4.8% | ||
| 1500 | 1472.5 | 98.2% | 4.8% | ||
| 3-MT | 40 | 43.7 | 109.4% | 10.4% | |
| 120 | 122.8 | 102.3% | 2.4% | ||
| 750 | 781.8 | 104.3% | 3.6% | ||
| 1500 | 1467.8 | 97.9% | 1.7% | ||
Table 5.
Inter-assay precision and accuracy in relation to storage condition. N = 12 for all replicates except for ∗ where n = 6 because only the D2 and D6 time points were considered to be stable under these storage conditions.
| Compound | Storage Condition | Inter-assay Bias | Inter-assay precision |
|---|---|---|---|
| NMN | RT | 7.6% | 5.3% |
| 4 °C | 4.0% | 4.0% | |
| −20 °C | 6.8% | 6.5% | |
| MN | RT | 7.7% | 12.9% |
| 4 °C | 5.3% | 12.9% | |
| −20 °C | 6.5% | 13.6% | |
| 3-MT | RT | 12.1% | 7.3% |
| 4 °C | 12.0% | 4.0%∗ | |
| −20 °C | 11.3% | 10.8%∗ | |
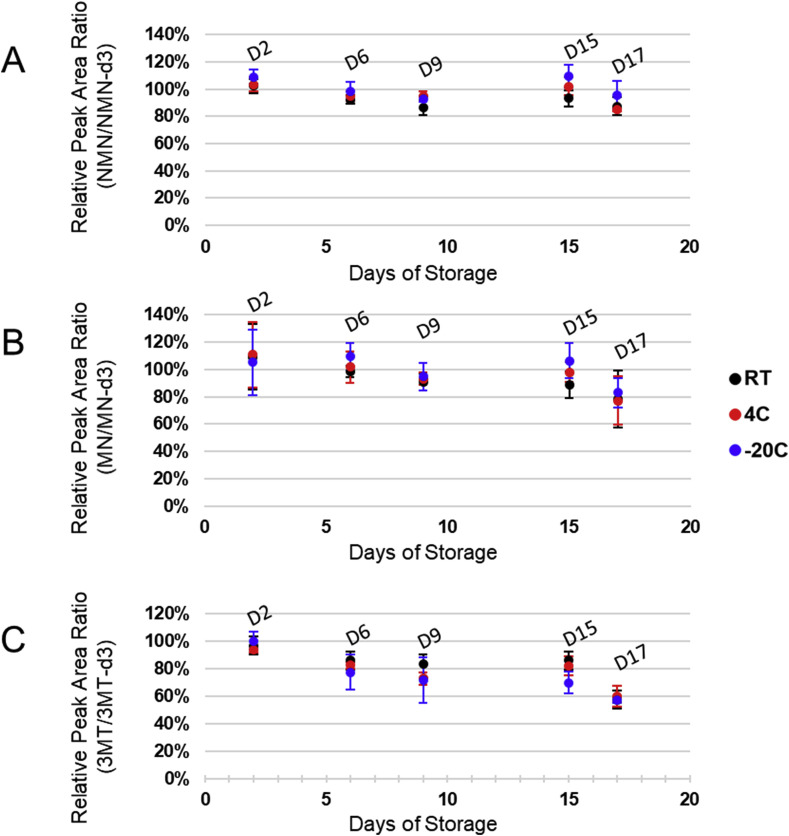
3.6. Effect of storage conditions on analyte stability
In order to assess the stability of MNs from DBS samples under different storage conditions, we fortified whole blood using an equal mixture of MNs reference standards to a final concentration of 500 pg/mL. DBSs were prepared as described above and stored at either room temperature out of direct sunlight, at 4 °C, or frozen at −20 °C. DBS samples were extracted on the following day, and 6, 9, 15, and 17 days after spotting (Fig. 3 ). Inter-day stability for MN and NMN was satisfactory, with the average sample loss being −6.7% and −11.2% for MN and NMN respectively after storage for two weeks at room temperature, with no appreciable sample loss when stored at 4 °C or −20 °C. Although MN and NMN were most stable in samples stored at −20 °C, storage at 4 °C or room temperature also demonstrated acceptable stabilities (average relative intensity of 93.0% and 102.0% for NMN and 88.8% and 98.1% for MN at RT and 4 °C respectively). 3-MT demonstrated lower inter-day stability than MN or NMN demonstrating an average reduction in relative response of −18% after a week in storage. From this data we conclude that MN and NMN are sufficiently stable in DBS to allow for relatively long term storage (2 weeks) at RT and that 3-MT demonstrated comparatively low stability, regardless of the storage condition, thus precluding long-term storage of DBSs for the analysis of this metabolite. In order to assess the stability of all three analytes as PITC conjugates under conditions of storage in the autosampler (10 °C), derivatized reference standards were repeatedly injected every 6 h over a 48-hour period. No signal loss was observed during this time frame (Supplementary Fig. 5).
Fig. 3.
Stability of (A) NMN, (B) MN, and (C) 3-MT in DBS samples stored at room temperature (RT), refrigerator temperature (4 °C), or freezer (−20 °C), out of direct sunlight. All samples were stored with sorbent packets in ZipLoc bags.
3.7. Carryover
Carryover was assessed by injecting blanks immediately after the analysis of the highest calibrator samples (Supplementary Fig. 6). The integrated area ratio of the blank to that of the LLOQ must be below 20% for there to be acceptably low levels of carryover between samples. This analysis was done in triplicate, and, for MN, NMN, and 3-MT, the average ratios of the blank signals to the LLOQ peak areas were 3.9%, 12.2%, and 3.5%, respectively, indicating that our assay satisfied the requirements for validation.
4. Conclusions
In conclusion, we have demonstrated that on-spot PITC-derivatization LC-MRM is a sensitive, robust, and cost-effective method for the quantification of MN and NMN from a single DBS. This work, therefore, provides a novel diagnostic assay for PPGLs, which facilitates the use of supine sampling as recommended. It alleviates patient discomfort at test centres, which can contribute to falsely elevated levels of metanephrines. Furthermore, because the preparation and storage of DBSs could be done remotely and without causing great patient discomfort and inconvenience, this would facilitate repeated follow-up determination of MN concentrations by DBS sampling over a period of several days. Together, this could result in improvements to diagnostic sensitivity and accuracy, thereby reducing the number of false-positives. This method, therefore, might also reduce the need for follow-up urine/plasma metanephrines, CT-scan, and other confirmatory diagnostic procedures, which are costly, time-consuming, and inconvenient for patients. From our established assay, measurement of 3-MT is unlikely to be sensitive enough for application toward the diagnosis of PPGLs from a single DBS due to the intrinsically low concentration of this metabolite, and the relatively modest increase which is of diagnostic utility (<15 pg/mL) [15]. It is possible that by combining multiple DBS samples our validation criteria for sensitivity could be met for this analyte in the context of PPGL diagnosis. In contrast, levels of 3-MT are expected to be greatly elevated in pediatric patients with neuroblastomas. Reported concentrations for these patients are sometimes higher than 1000 pg/mL and usually around 100 pg/mL in plasma [15,17,20]. This new assay would therefore provide a major advantage over other measurements of 3-MT, since DBS sampling is already well established for newborn screening because of the need for sampling of very low amounts of blood with minimal invasiveness.
CRediT authorship contribution statement
Vincent R. Richard: Conceptualization, Investigation, Writing - original draft. René P. Zahedi: Conceptualization, Supervision, Writing - original draft. Shaun Eintracht: Writing - original draft, Writing - review & editing. Christoph H. Borchers: Funding acquisition, Project administration, Resources, Writing - review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CHB is the CSO of MRM Proteomics, Inc. and the CTO of Molecular You. The other authors declare no conflicts of interest.
Acknowledgements
This work was supported by funding to “The Metabolomics Innovation Centre (TMIC)” through the Genomics Technology Platform (GTP) from Genome Canada, Genome Alberta, and Genome British Columbia for operations and technology development (265MET). CHB is grateful for support from the Segal McGill Chair in Molecular Oncology at McGill University (Montreal, Quebec, Canada). CHB is also grateful for support from the Warren Y. Soper Charitable Trust and the Alvin Segal Family Foundation to the Jewish General Hospital (Montreal, Quebec, Canada). The study was also supported by the MegaGrant of the Ministry of Science and Higher Education of the Russian Federation (Agreement with Skolkovo Institute of Science and Technology, No. 075-10-2019-083).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aca.2020.06.020.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Eisenhofer G., Peitzsch M., Kaden D., Langton K., Mangelis A., Pamporaki C., Masjkur J., Geroula A., Kurlbaum M., Deutschbein T., Beuschlein F., Prejbisz A., Bornstein S.R., Lenders J.W.M., Eisenhofer G., Lenders J.W.M., Masjkur J., Kurlbaum M., Beuschlein F., Peitzsch M., Geroula A., Langton K., Prejbisz A., Mangelis A., Deutschbein T., Pamporaki C., Kaden D. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin. Chim. Acta. 2018;490:46–54. doi: 10.1016/j.cca.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Katznelson L., Laws E.R., Melmed S., Molitch M.E., Murad M.H., Utz A., Wass J.A.H. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 3.Willemsen J.J., Sweep C.G.J., Lenders J.W.M., Ross H.A. Stability of plasma free metanephrines during collection and storage as assessed by an optimized HPLC method with electrochemical detection. Clin. Chem. 2003 doi: 10.1373/clinchem.2003.023135. [DOI] [PubMed] [Google Scholar]
- 4.Danese E., Montagnana M., Brentegani C., Lippi G. Short-term stability of free metanephrines in plasma and whole blood. Clin. Chem. Lab. Med. 2019 doi: 10.1515/cclm-2019-0020. [DOI] [PubMed] [Google Scholar]
- 5.Han J., Higgins R., Lim M.D., Atkinson K., Yang J., Lin K., Borchers C.H. Isotope-labeling derivatization with 3-nitrophenylhydrazine for LC/multiple-reaction monitoring-mass-spectrometry-based quantitation of carnitines in dried blood spots. Anal. Chim. Acta. 2018;1037:177–187. doi: 10.1016/j.aca.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Chambers A.G., Percy A.J., Yang J., Borchers C.H. Multiple reaction monitoring enables precise quantification of 97 proteins in dried blood spots. Mol. Cell. Proteomics. 2015;14:3094–3104. doi: 10.1074/mcp.O115.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraf R., Ma J., Jensen B.P., Berry S., Camargo C.A., Grant C.C., Sies C.W. Quantitation of 25-hydroxyvitamin D in dried blood spots by 2D LC-MS/MS without derivatization and correlation with serum in adult and pediatric studies. Clin. Chim. Acta. 2018;481:61–68. doi: 10.1016/j.cca.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Yakkundi S., Millership J., Collier P., Shields M.D., McElnay J. Development and validation of a dried blood spot LC-MS/MS assay to quantify ranitidine in paediatric samples. J. Pharmaceut. Biomed. Anal. 2011;56:1057–1063. doi: 10.1016/j.jpba.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Han J., Higgins R., Lim M.D., Lin K., Yang J., Borchers C.H. Short-term stabilities of 21 amino acids in dried blood spots. Clin. Chem. 2018;64:400–402. doi: 10.1373/clinchem.2017.278457. [DOI] [PubMed] [Google Scholar]
- 10.Freeman J.D., Rosman L.M., Ratcliff J.D., Strickland P.T., Graham D.R., Silbergeld E.K. State of the science in dried blood spots. Clin. Chem. 2018;64:656–679. doi: 10.1373/clinchem.2017.275966. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J., Mandal R., Wishart D.S. A sensitive, high-throughput LC-MS/MS method for measuring catecholamines in low volume serum. Anal. Chim. Acta. 2018;1037:159–167. doi: 10.1016/j.aca.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Henderson C.M., Shulman N.J., MacLean B., MacCoss M.J., Hoofnagle A.N. Skyline performs as well as vendor software in the quantitative analysis of serum 25-hydroxy Vitamin D and Vitamin D binding globulin. Clin. Chem. 2018;64:408–410. doi: 10.1373/clinchem.2017.282293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m10-bioanalytical-method-validation access date: Apr. 10, 2020.
- 14.Eisenhofer G., Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin. Chem. 2014;60:1486–1499. doi: 10.1373/clinchem.2014.224832. [DOI] [PubMed] [Google Scholar]
- 15.Rao D., Peitzsch M., Prejbisz A., Hanus K., Fassnacht M., Beuschlein F., Brugger C., Fliedner S., Langton K., Pamporaki C., Gudziol V., Stell A., Januszewicz A., Timmers H.J.L.M., Lenders J.W.M., Eisenhofer G. Plasma methoxytyramine: clinical utility with metanephrines for diagnosis of pheochromocytoma and paraganglioma. Eur. J. Endocrinol. 2017;177:103–113. doi: 10.1530/EJE-17-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peitzsch M., Prejbisz A., Kroiß M., Beuschlein F., Arlt W., Januszewicz A., Siegert G., Eisenhofer G. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamineproducing metastatic phaeochromocytoma. Ann. Clin. Biochem. 2013;50:147–155. doi: 10.1258/acb.2012.012112. [DOI] [PubMed] [Google Scholar]
- 17.Barco S., Verly I., Corrias M.V.V., Sorrentino S., Conte M., Tripodi G., Tytgat G., van Kuilenburg A., van der Ham M., de Sain-van der Velden M., Garaventa A., Cangemi G. Plasma free metanephrines for diagnosis of neuroblastoma patients. Clin. Biochem. 2019;66 doi: 10.1016/j.clinbiochem.2019.02.012. 0–1. [DOI] [PubMed] [Google Scholar]
- 18.Verly I.R.N., van Kuilenburg A.B.P., Abeling N.G.G.M., Goorden S.M.I., Fiocco M., Vaz F.M., van Noesel M.M., Zwaan C.M., Kaspers G.J.L., Merks J.H.M., Caron H.N., Tytgat G.A.M., 3-Methoxytyramine An independent prognostic biomarker that associates with high-risk disease and poor clinical outcome in neuroblastoma patients. Eur. J. Canc. 2018;90:102–110. doi: 10.1016/J.EJCA.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Lam L., Woollard G.A., Teague L., Davidson J.S. Clinical validation of urine 3-methoxytyramine as a biomarker of neuroblastoma and comparison with other catecholamine-related biomarkers. Ann. Clin. Biochem. 2017;54:264–272. doi: 10.1177/0004563216654723. [DOI] [PubMed] [Google Scholar]
- 20.Peitzsch M., Butch E.R., Lovorn E., Mangelis A., Furman W.L., Santana V.M., Hero B., Berthold F., Shulkin B.L., Huebner A., Eisenhofer G. Biochemical testing for neuroblastoma using plasma free 3-O-methyldopa, 3-methoxytyramine, and normetanephrine. Pediatr. Blood Canc. 2020;67:1–9. doi: 10.1002/pbc.28081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.