Abstract
Currently, the world is facing its 3rd coronavirus outbreak of the 21st century, which has turned to a pandemic recently. Starting on December 2019, coronavirus disease (COVID-19) was first detected in Wuhan City, Hubei province, China. As of 31st March, 2020, more than 900,000 COVID-19 cases have been reported across the globe involving more than 200 countries. The first case of the United States (US) was confirmed on 20th January, 2020 in a 35-year-old male who had a travel history to Wuhan, China, before returning to the U.S. Since then, the SARS-CoV-2 virus has spread to all the 50 states of US, with more cases being reported every day. New York, New Jersey, Michigan, and California are the worst-hit states. As COVID-19 is growing, every day has been marked by novel developments and updates. We hereby talk about how the U.S. is leading the multiagency effort to fight against this pandemic and the steps that have been taken so far.
Keywords: COVID-19; CoronaVirus, Epidemiology, Pandemic Disease
Introduction
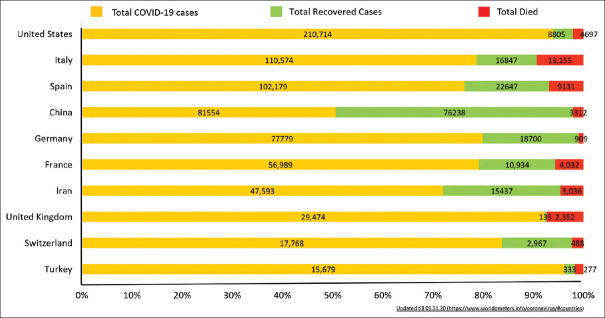
Coronavirus disease (COVID-19) occurs due to the severe acute eespiratory syndrome coronavirus-2 virus (SARS-CoV-2). This virus is a new member of the β coronavirus family, which also has the other offenders of previous coronavirus outbreaks namely, SARS-CoV and MERS-CoV.[1] Initially started as a result of zoonotic spillover, the further spread of the SARS-CoV-2 virus is the result of human to human community transmission, the majority through droplets and direct contact. Despite low mortality (2%–3%) of COVID-19 as compared to the MERS (35%–40%) and SARS outbreak (9%–10%), the total disease burden has outnumbered the previous coronavirus outbreaks by humongous margins [Link for up to date information: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6][Figure 1].
Figure 1.
No of COVID-19 cases in top 10 nations with breakdown of details in confirmed, recovered and died cases
Facts and Figures of COVID-19 Disease Burden in United States
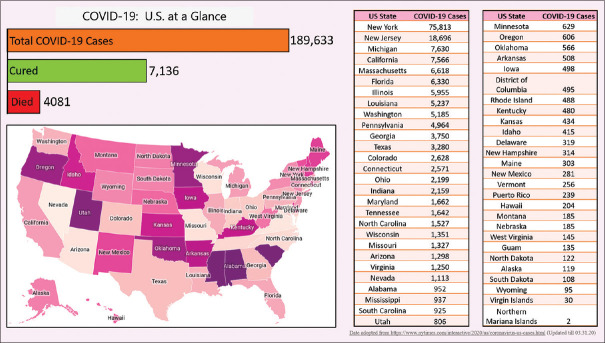
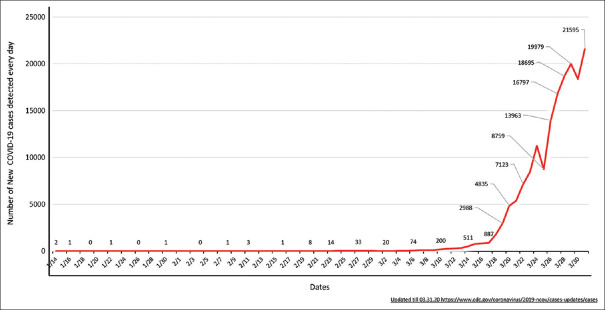
The number of known COVID-19 cases in the United States (US) is rising exponentially [Figure 2]. As of 31st March 2020, there are at least 211,408 confirmed COVID-19 cases with 4,718 deaths, involving every U.S. state, plus Washington, D.C., and the three U.S. territories.[2] For most of the cases, the reason of how the virus was contracted is not clear [Link for upto date information: https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html][Table 1]. The number of new cases took a gigantic surge around the last week of March, and since then it has been up-trending [Link for up to date information: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2019coronavirus-summary][Figure 3].
Figure 2.
United States Map showing detailed breakdown of COVID-19 cases state wise
Table 1.
Breakdown of the possible reasons of how SARS-CoV-2 Virus Was Contracted in U.S.
| (Top 20 reasons) | Cases |
|---|---|
| Travel overseas | 176 |
| Travel within the U.S. | 168 |
| Life Care nursing facility; Kirkland, Wash. | 129 |
| Community in New Rochelle, N.Y. | 119 |
| Gallatin Center for Rehabilitation and Healing; Gallatin, Tenn. | 115 |
| Cook County Jail; Chicago | 113 |
| Biogen conference in Boston | 109 |
| Pleasant View Nursing Home; Mount Airy, Md. | 77 |
| Aboard the U.S.S. Theodore Roosevelt; Guam | 70 |
| Cedar Mountain Post-Acute Care Facility; Yucaipa, Calif. | 57 |
| Travel in Egypt | 52 |
| Careage of Whidbey; Coupeville, Wash. | 44 |
| Diamond Princess cruise ship | 43 |
| Long-term care facility; DuPage County, Ill. | 42 |
| Lambeth House senior living facility; New Orleans | 42 |
| Travel in Italy | 39 |
| First Assembly of God; Greers Ferry, Ark. | 37 |
| Shuksan Healthcare Center; Bellingham, Wash. | 32 |
| Family of Caring nursing home; Montclair, N.J. | 30 |
| St. Joseph’s Senior Nursing Home; Woodbridge, N.J. | 29 |
Data adapted from https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html (Updated till 03.31.20)
Figure 3.
Date wise details of new COVID-19 cases every day from 14th January till 31stMarch 2020.
The first case of the U.S. was confirmed in a 35-year-old male from Snohomish County, Washington on 20th January 2020. He had a travel history to Wuhan, China and had been symptomatic for 4 days with upper respiratory tract symptoms before presenting to the emergency room. Nasopharyngeal and oropharyngeal swabs were collected, which came back positive for SARS-CoV-2 by real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay. Very soon, on 30th January 2020, the first COVID-19 case resulting from a person to person transmission within the U.S. without any travel history was detected in a 54-year-old male from Chicago, Illinois.[3] This tightened the scrutiny nationwide to look for more COVID-19 suspects that led to the more detection and isolation of confirmed cases. As this pandemic is a rapidly evolving scenario, and new data are constantly pouring in, we recommended readers to refer to link provided above to ensure they are up-to-date with latest information or facts. There are many reports of cluster detection throughout the U.S., but the following are examples of different case scenarios reported from three different states, which confirm that COVID-19 is now a nationwide problem.
Nursing home tragedy, Kirkland, Washington
Unfortunately, just like in the other parts of the world, the U.S. is also having more fatalities amongst the older population, individuals with medical comorbidities and friable sick residents of old age homes and nursing facilities. Lifecare Nursing facility in Kirkland, Washington is a classic example of the catastrophe that costed the life of the COVID-19 struck 59 elderly residents. Similar reports are pouring in from many other nursing homes and rehabilitation centers nationwide. Considering the high risk of acquiring the SAR-CoV-2 infection, the Centers for Disease Control and Prevention (CDC) has issued interim guidance with advice to all nursing homes, hospitals, and rehabilitation centers to try to limit all visitors, and non essential personnel barring an end-of-life situation.
Synagogue tragedy, New Rochelle, New York
Another unfortunate incident happened in New Rochelle, New York at a synagogue where there was a mass community transmission of SAR-CoV-2 virus during a local prayer gathering. The incident came into notice after the first case detection on 3rd March 2020. Soon, more than 100 confirmed COVID-19 cases have been reported in individuals who were either directly or indirectly associated with that synagogue. This led to an emergent situation and in order to halt further transmission, a prompt decision was made to create a 1-mile “containment area“ around the synagogue, which was considered as an epicenter of COVID-19 outbreak in New Rochelle. Currently, New York has the greatest number of COVID-19 cases than any other states combined together.
Cruise ships tragedy (Diamond Princess, and Grand Princess)
Cruise ships have always been known for outbreaks related to various infectious diseases in the past. This fear came to reality during this pandemic as well. The first incident was related with the Diamond Princess cruise ship which was docked at Yokohama port, Japan with more than 3,700 people on board for the concerns of COVID-19 outbreak. Japan government ordered for a quarantine of the cruise ship and its occupants at port itself with an immediate effect from 5th February 2020 for 2 weeks. On 17th February 2020, the U.S. finally evacuated its 300 Americans from the cruise ship. Since then, similar incidents of COVID-19 positive cases have been reported in other cruise ships as well for instance- Caribbean Princess and California Princess ship.
Role of Centers for Disease Control and Prevention (CDC)
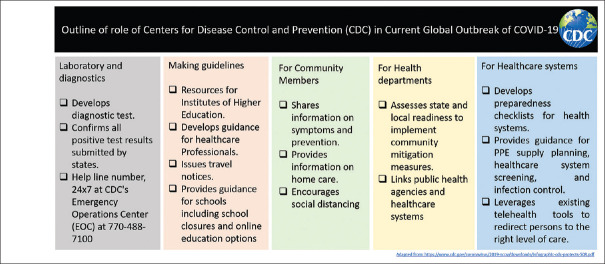
COVID-19 pandemic is a rapidly evolving situation. CDC is the U.S.'s health protection agency, committed to save lives and protect its people from health threats.[4] CDC is constantly monitoring COVID-19 pandemic and providing interim guidance to the public health authorities, hospitals, schools, aviation, and homeland security department and other partners that come in the U.S. jurisdictions [Figure 4]. The CDC's Epidemic Intelligence Service (EIS) officials have so far done a commendable job in their attempt to identify the community transmission, i.e. the source of the COVID-19 cases clusters and rapidly implementing the adequate control measures. On 12th March 2020, CDC recommended considering postponing or canceling any mass gathering to contain community transmission and discouraged handshakes and high fives. Similarly, the CDC recommended to implement social distancing and avoid gatherings of more than 10 at public places.
Figure 4.
Detailed outline of the role of Centers for Disease Control and Prevention (CDC)
COVID-19 Pandemic: A National Emergency Situation
Considering the worsening situation, the U.S. government recently declared COVID-19 pandemic as a national emergency on 13th March 2020. The government also signed an $8.3 billion emergency spending bill on 6th March 2020 to be used in various sectors. As of now, the proposal aims to direct the funds (approximate numbers) towards the research and development of vaccines ($3 billion); in public health funding ($2.2 billion); to aid coronavirus struck countries overseas ($1.25 billion); towards medical supplies, health-care preparedness ($1 billion). Numerically, this is more than the amount released during the previous epidemics [$5.4 billion during the Ebola outbreak (2014) and $7 billion during the H1N1 outbreak (2009)]. The following are the other major decisions and actions being taken by the U.S. authorities in order to tackle the COVID-19 pandemic and its consequences [Figure 5]. As the numbers are rising every day and economic slowdown has already hit rock-bottom, the U.S. administration is also considering releasing another stimulus package, which could worth anywhere from $850 billion to more than $1 trillion in order to battle the economic crisis.
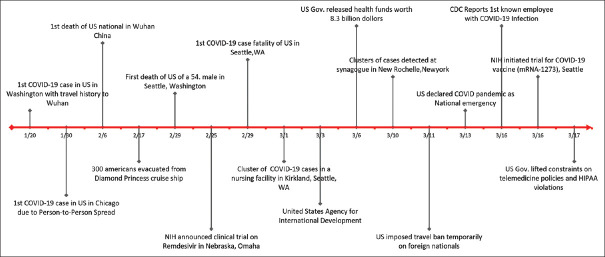
Figure 5.
Timeline of Major events in United States since COVID-19 Pandemic (Jan- March 2020)
Recognizing the power of Telemedicine during COVID-19 crisis
In the U.S., telemedicine services have always played an important component in health care. From making an appointment to discussing the lab results with health care providers via the patient portal, telemedicine has always been considered as a backbone of the U.S. health care system. Now with COVID-19 pandemic, its importance has been felt more than ever. Many hospitals acknowledged concerns over the enormous volumes of patients trying to consult doctors about their symptoms. Considering this unprecedented tele query loads, many state's individual medical societies approached to the U.S.'s Board of Registration in Medicine and requested greater flexibility and clarification regarding the health insurance coverage and Health Insurance Portability and Accountability Act (HIPAA) regulations and policies related to telemedicine. The matter was immediately addressed and the U.S. administration on 17thMarch 2020 announced to loosen the regulations and lifted the constraints on telemedicine policies. As per the statement, the Medicare payments will be exempted for telehealth services (valid from 6thMarch 2020). Also, patients will be able to assess their doctors and consult them remotely through teleconferencing (phone, video conferences) at no extra cost. During this period of the COVID-19 crisis, physicians are now waived off the penalty for violating the HIPAA act, for using communications technologies, like FaceTime or Skype for discussing health issues with the patients.
Embarking on the clinical trials: Search for the COVID-19 vaccine and target antiviral therapy
NIH funded research on new antiviral agent, Remdesivir at Nebraska, Omaha
Remdesivir is an antiviral agent that has shown better activity than the antiretroviral agents like lopinavir and ritonavir. Unfortunately, remdesivir is not commercially available for clinical use and hence there is not much literature regarding its side effect profile and in vivo interactions with other drugs. Moving a step ahead, the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) has initiated a clinical trial to test remdesivir for its clinical effectiveness at the University of Nebraska Medical Center (UNMC) in Omaha (NCT04280705)[Table 2].[5]
Table 2.
Description of the Clinical trials currently actively running related to SARS-CoV-2 and COVID-19 Disease
| NCT No. | STATUS | STUDY TITLE | STUDY DESIGN | COUNTRY |
|---|---|---|---|---|
| 04308668 | New, not yet recruiting | To test if post-exposure prophylaxis with hydroxychloroquine can prevent progression development of symptomatic COVID19 disease after known exposure to the SARS-CoV2 virus. |
Study Type: Interventional Estimated Enrollment:1500 participants Allocation: Randomized Intervention Model: Parallel Assignment Estimated Study Start Date: April 2020 Estimated Study Completion Date: May 2021 |
University of Minnesota Minneapolis, Minnesota, |
| 04283461 | Active, Recruiting | To study the Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection | Study Type: Interventional Estimated Enrollment: 45 participants Allocation: Non-Randomized Intervention Model: Sequential Assignment Actual Study Start Date: March 3, 2020 Estimated Study Completion Date: June 1, 2021 |
Kaiser Permanente Washington Health Research Institute - Vaccines and Infectious Diseases Seattle, Washington |
| 04280705 | Active, Recruiting | To study the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Adults |
Study Type: Interventional Estimated Enrollment:394 participants Allocation: Randomized Intervention Model: Parallel Assignment Actual Study Start Date: February 21, 2020 Estimated Study Completion Date: April 2023 |
Multicentric study, 50 sites globally, 17 centers in US |
| 04292899 | Active, Recruiting | To Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants with Severe Coronavirus Disease (COVID-19) | Study Type: Interventional Estimated Enrollment:400 participants Allocation: Randomized Intervention Model: Parallel Assignment Actual Study Start Date: March 6, 2020 Estimated Study Completion Date: May 2020 |
Multicentric study, US Centers: -Providence Regional Medical Center Everett Everett, Washington, -Swedish Center for Comprehensive Care Seattle, Washington, |
| NCT04329923 | New, not yet recruiting | To Study Prevention And Treatment of COVID-19 with Hydroxychloroquine | Study Type: Interventional Estimated Enrollment: 400 participants Allocation:Randomized Intervention Model:Parallel Assignment Estimated Study Start Date: April 6, 2020 Estimated Study Completion Date: December 1, 2021 |
University of Pennsylvania Philadelphia, Pennsylvania, United States |
| NCT04315298 | Recruiting | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | Study Type: Interventional Estimated Enrollment:400 participants Allocation:Randomized Intervention Model:Parallel Assignment Actual Study Start Date: March 16, 2020 Estimated Study Completion Date:March 16, 2021 |
-Multicentric Study -54 Centers across U.S. |
| NCT04328467 | New, not yet recruiting | To determine if pre-exposure prophylaxis with hydroxychloroquine is effective for the prevention of COVID-19 disease. | Study Type: Interventional Estimated Enrollment:3500 participants Allocation:Randomized Intervention Model: Parallel Assignment Estimated Study Start Date: April 1, 2020 Estimated Study Completion Date: August 2020 |
University of Minnesota Minneapolis, Minnesota, United States |
| NCT04325672 | New, not yet recruiting | Convalescent Plasma to Limit Coronavirus Associated Complications: An Open Label, Phase 2A Study of High-Titer Anti-SARS-CoV-2 Plasma in Hospitalized Patients With COVID-19 |
Study Type: Interventional Estimated Enrollment: 20 participants Intervention Model:Single Group Assignment Estimated Study Start Date: April 1, 2020 Estimated Study Completion Date: December 31, 2022 |
Mayo Clinic in Rochester Rochester, Minnesota, United States |
| NCT04326452 | Recruiting | Treating COVID-19 With a Bidirectional Oxygenation Valve | Study Type: Interventional Estimated Enrollment :15 participants Intervention Model:Single Group Assignment Estimated Study Start Date: March 27, 2020 Estimated Study Completion Date: June 1, 2020 |
-TMC HealthCare Tucson, Arizona, -Stanford University Stanford, California, -Emory Saint Joseph’s Hospital Atlanta, Georgia, |
| NCT04311697 | New, not yet recruiting | Intravenous Aviptadil for COVID-19 Associated Acute Respiratory Distress (COVID-AIV) | Study Type: Interventional Estimated Enrollment: 120 participants Allocation: Randomized Intervention Model:Parallel Assignment Estimated Study Start Date: April 2020 Estimated Study Completion Date: September 2020 |
-Research Center New York, New York, United States -Rambam Health Care Campus Haifa, Israel |
| NCT04329832 | New, not yet recruiting | Hydroxychloroquine vs. Azithromycin for Hospitalized Patients with Suspected or Confirmed COVID-19 (HAHPS) | Study Type: Interventional Estimated Enrollment:300 participants Allocation:Randomized Intervention Model:Parallel Assignment Estimated Study Start Date: April 1, 2020 Estimated Study Completion Date: December 31, 2021 |
-Intermountain Medical Center Murray, Utah, United States -University of Utah Salt Lake City, Utah, United States |
(Adopted from https://clinicaltrials.gov/ct2/results?cond=coronavirus.)
1st COVID-19 vaccine trial (mRNA-1273) at Seattle, Washington
Currently, there is no approved vaccine to prevent SARS-CoV-2 infection. On 16th March 2020, NIAID launched a Phase 1 clinical trial (NCT04283461) to evaluate an investigational vaccine devised against COVID-19. It has begun at the Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. This is an open-label trial that aims to enroll 45 healthy adult volunteers ages 18 to 55 years over the next 6 weeks. Dr. Lisa A. Jackson, M.D., a senior investigator at KPWHRI is leading the trial. The investigational vaccine is called mRNA-1273 and has been built by the collaborative effort of NIAID scientists and biotechnology company Moderna, Inc., based in Cambridge, Massachusetts.[6] The 45 participants will be grouped into 3 sets of 15 people in order to test different doses of the vaccine. Group 1, 2, and 3 will receive a dose of 25 micrograms (mcg), 100 mcg, and 250 mcg respectively in 2 sessions, 28 days apart. In total there will be 11 in-person and 4 phone visits over a 14-month period to capture the results.
Convalescent sera as an option to contain COVID-19: A Johns Hopkins initiative, Baltimore
Scientists realize that there is still a long way to go before an anti-COVID-19 vaccine becomes a reality. Passive antibodies have successfully been used in patients exposed to hepatitis B and rabies viruses. Using this as a platform to work, Johns Hopkins immunologist, Dr. Arturo Casadevall proposed to use convalescent sera from COVID-19 recovered patients to treat individuals with early symptoms.[7] This basically involves identifying recovered COVID-19 individuals with high titers of neutralizing antibodies. Serum from such individuals can be administered to high-risk populations like[1] vulnerable individuals with comorbidities,[2] health care providers, and[3] individuals with exposure to confirmed cases. The possible risks involved with such therapy are immunological reactions like serum sickness, transfusion-related acute lung injury, and so on. The Johns Hopkins research team has released the funds to set up an operating facility at their center in Baltimore within the next few weeks. On similar lines, on 4thMarch 2020, Japan's one of the largest drug makers, Takeda Pharmaceuticals initiated a new program (TAK-888) to develop an anti-SARS-CoV-2 polyclonal hyperimmune globulin.
Emergency use authorization of antimalarial drug for use in COVID-19 by FDA
Anti malarial drug, hydroxyl chloroquine (HCQ) has shown some anti-SARS-CoV activity in vitro studies. Following this, Gautret et al. found the combination of HCQ and azithromycin to be more effective than HCQ alone or standard care in their study group on COVID-19 patients in an open labelled study.[8] Considering the limited options, the Food and Drug Administration (FDA) on 28th March gave authorization for the emergency use of chloroquine phosphate and hydroxyl chloroquine sulfate for the treatment of COVID-19 patients when clinical trials are not available, or participation is not feasible. Many trials are already underway to confirm the efficacy of HCQ for COVID-19[Link for upto date information: https://clinicaltrials.gov/. (NCT04329923, NCT04329832, NCT04318444)].
Securing the borders: COVID-19 related International travel restrictions
With the growing threat of COVID-19 pandemic, just like many other countries, on 11th March 2020 the U.S.'s Homeland Security suspended the entry of foreign nationals from Iran, China, and most of the European countries. Before as well, in January and February, a similar travel restriction was imposed on individuals who had been in China and Iran.
Overcoming the limited COVID-19 testing and insurance hurdle
Currently, in the U.S., CDC is providing the COVID-19 test kits. CDC, in turn, procures these kits from the International Reagent Resource (IRR), a designated organization that acquires, authenticates, and produces these reagents. As of 16th March 2020, in the U.S., approximately 25,162 specimens have already been tested. But still, a large gap between the demand and supply of COVID-19 testing kits have been felt. Considering the need of the hour, the FDA has already granted an emergency clearance to many biotechnical companies to commercialize their COVID-19 testing kits into the market for use by various tertiary and public community health centers. FDA aims for 1.4 million test kits to be accessible by next week, and approximately 5 million within a month. The US Congress also passed a bill on 13th March 2020, which ensures a free access to COVID-19 testing, paid extension of sick leave for vulnerable, and provision of free food aid to the needy.
Tacking the economic downturn
Worldwide, global markets are fluctuating due to the unstable economy and there is a fear of a recession even worse than 09/11. With mounting economic toll on Wall Street, New York, the US Treasury Secretary, Mr. Steven Mnuchin, in his press briefing said that the administration would consider for an $850 billion stimulus proposal to shield the economic hit on business. Discussion is going on as well for a payroll tax cut for this year. The administration is also considering a $50 billion economic relief package towards the airline industry. All these measures are being taken to ensure that the economy and trade do not suffer a huge loss and could survive in this time of crisis.
Predicting the Trajectory of COVID-19: US Preparedness and Strategy
Different countries have responded to the current pandemic with different strategies based on their previous experiences (example SARS pandemic, 2002-03), economic situation, health care infrastructure, and preparedness beforehand. Talking in the terms of numbers, apart from China, the other three countries who have successfully contained the further spread of COVID-19 are Hong Kong, Singapore, and South Korea. In contrast, European countries like France, Germany, and Spain, which were affected late, now all have more than 30–100 times more cases than the earlier mentioned countries. “Singapore Model“ has shown that if acted early then the mortality can be minimized. Disease Outbreak Response System Condition (DORSCON) is a color-coded framework that Singapore follows when an infectious outbreak occurs, potential of the health hazard to its citizens. The framework has set general guidelines on what must be done in order to avoid and minimize the impact of infections. Countries like South Korea and China had to follow the “suppression model,“ which is basically doing a lockdown of the heavily inflicted cites thereby halting all daily activities, strict quarantine to homes for everyone unless medically needed. In contrast, by acting ahead of curve, Hong Kong and Singapore followed their suspected contacts, tested them liberally with strict quarantine if required, and sent strong and regular communication about social distancing and public awareness.
The U.S. has already entered into Stage III of the COVID-19 pandemic. We assume that the sky-high numbers of new COVID-19 cases being diagnosed daily across all the states of the U.S. is both due to the increased testing as well as community transmission. Till now U.S. administration is urging the public to keep following the social etiquettes, social distancing, and to stay in home as much as possible. Unlike countries like China, India, and Italy, the U.S. so far has not taken any stringent actions like a complete lockdown across all the states. With so many COVID-19 related deaths every day, the U.S is just behind Italy and Spain in terms of death toll. Hence, there is an ongoing debate of switching mode to suppression strategy from the current mitigation strategy. Few experts are vouching for a national-level lockdown order, with uniform measures across the whole country. Next few weeks are anticipated to be crucial for the U.S., as the scarcity of medical devices, lack of critical care beds, falling sick of health care professionals, and shortage of personal protective equipments have already plagued the health care infrastructure of many states like New York, New Jersey, etc., CDC, WHO, FDA and U.S administration are keeping an eye on the volatile COVID-19 situation and we hope to see some strict actions in near future to contain this COVID-19 pandemic.
Conclusion
COVID-19 pandemic has caused enormous damage to the health, economy, trade, and finance sectors of the U.S. The current situation on COVID-19 is concerning and remains very fluid. The U.S. has always been a front runner when it comes to developing new medicines, vaccines, and diagnostics. Hopefully, the current ongoing trials on antiviral agents and vaccines will give us a new ray of hope soon. We appreciate the unwavering dedication of health care professionals and scientists towards their patients and the community during this health crisis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
None
References
- 1.Sahu KK, Mishra AK, Lal A. Comprehensive update on current outbreak of novel coronavirus infection (2019-nCoV)? Ann Transl Med. 2020;8:393. doi: 10.21037/atm.2020.02.92. doi: 10.21037/atm. 2020.02.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html .
- 3.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. Washington State 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-inus.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019ncov%2Fcases-in-us.html .
- 5. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins .
- 6. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins .
- 7.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138003. pii: 138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. 10594doi: 101016/jijantimicag 2020105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]