Abstract
Objective:
Osteoporosis is the most common type of bone disorder characterized by low bone mineral density (BMD). It is a multifactorial disease and caused by the interaction of environmental and genetic factors. It has been reported that mutations in the vitamin D receptor (VDR) gene highly affect the metabolism of minerals, which reduces bone density. Therefore, this study aimed to determine the association of VDR gene polymorphisms TaqI (rs731236) and ApaI (rs7975232) with osteoporosis risk in the Saudi population.
Methods:
This case–control study involved 73 individuals with osteoporosis and 73 healthy controls in Jeddah, KSA. DNA extracted from peripheral blood was used to determine the genotypes and allele frequencies of VDR variants by polymerase chain reaction-restriction fragment length polymorphisms. Osteoporosis was confirmed by measuring BMD using dual-energy X-ray absorptiometry. The results were interpreted using the Hardy–Weinberg equilibrium assumption with P < 0.05 considered as significant.
Results:
A significant increase in the genotype frequencies of the ApaI (Aa) and (aa) was observed among osteoporotic patients compared to controls (P = 0.002 and P < 0.0001, respectively). Only the homozygous (tt) genotype of TaqI was significantly higher in those with osteoporosis than in the controls (P = 0.001). The minor “a” allele of ApaI and the “t” allele of TaqI were significantly more common in the patients as compared to controls (P < 0.0001 and P = 0.01, respectively).
Conclusion:
VDR polymorphisms ApaI and TaqI were found to be significantly determinant risk factors for osteoporosis progression in the Saudi population.
Keywords: ApaI Vitamin D receptor variant, osteoporosis, TaqI vitamin D receptor variant
Introduction
Osteoporosis and its related complications are major global problems as the disease is a substantial cause of morbidity and mortality, affecting about 200 million women worldwide.[1] In the 1990s, it was discovered that the problem of osteoporosis in Saudi Arabia was more severe than in the rest of the world, with a reported prevalenceaz between 23% and 31%.[2,3] Considering this, osteoporosis imposes a heavy burden on patients and families as well as the health-care system.[4] Due to the strong association between the disease and bone mineral density (BMD), the scanning of BMD using dual-energy X-ray absorptiometry (DEXA) has become the most commonly used diagnostic technique.[5] Osteoporosis is a multifactorial disease characterized by reduced BMD (<2.5 standard deviations below young adults), with a consequent increase in bone fragility and susceptibility to fracture.[6,7] In fact, the prevalence of osteoporosis varies among different countries and even among different regions and ethnic groups of a single country.[8] Several genes have been studied to reveal their contributions to osteoporosis risk, among them is vitamin D receptor (VDR) gene.[9]
Vitamin D and its active metabolites participate in the processes of bone tissue mineralization, maintaining calcium homeostasis, and bone remodeling which is mediated through its receptor, VDR. The VDR receptor is expressed on the cell surfaces of the intestine, thyroid, and kidney and has a key role in calcium homeostasis.[10] The human VDR gene is localized on the short arm of chromosome 12 and consists of 9 exons that encode a 427 amino acid protein.[11] In the VDR gene, several common polymorphic sequence variations have been reported. VDR genotypes were found to be associated with a wide variety of bone diseases as well as multiple sclerosis, osteoporosis, vitamin D-dependent rickets type II, and other complex maladies.[12,13] However, its association with BMD is controversial and has not been established in different ethnic populations. Polymorphisms in the gene can occur in its coding or noncoding parts and lead to changes in the protein sequence; these can also affect the degree of gene expression.[14] These include single nucleotide polymorphisms (SNPs) that can be identified with the appropriate restriction endonucleases such as ApaI and TaqI.[15] The VDR ApaI (rs7975232) polymorphism is located in intron 8 and it has been suggested to affect mRNA stability.[14] Whereas, the VDR TaqI (rs731236) polymorphism is located in exon 9 and has been shown to influence the biological function of vitamin D.[16]
Mutations in functional regions of the VDR gene affect the metabolism of minerals – especially calcium – and therefore bone density. In recent years, multiple studies have been performed to investigate the correlation between VDR gene variants and osteoporosis risk, suggesting the presence of ethnic differences in the genetic association with osteoporosis.[17,18] A series of characterized VDR gene polymorphisms, including FokI, ApaI, TaqI, and BsmI, have been extensively studied with regard to their association with the disease, but contradictory results have been offered.[19-21] The correlation remains unclear and requires further in-depth studies.
Vitamin D deficiency is common in the Middle East and in Saudi Arabia in particular.[22] According to the National Osteoporosis Foundation, the International Osteoporosis Foundation, and the American Geriatric Society, it has been suggested that the adequate vitamin D level in the blood is 30 ng/ml.[23,24] Therefore, to expose individuals with an increased risk of osteoporosis, it is important to identify gene variants, which are responsible for low BMD. This will suggest a personalized clinical approach to prevent or at least delay the development of this disease. From this point of view, this study was conducted to evaluate the genetic association of selected polymorphic variants within the VDR gene, particularly (ApaI and TaqI), among those with osteoporosis in the Saudi population.
Materials and Methods
Study design
This case–control study was approved by the National Committee for Medical and Bioethics of Health Affairs in Jeddah, Ministry of Health, Saudi Arabia (Approval Number A00406). The study included 146 Saudi participants equally divided into two groups: 73 individuals had osteoporosis (patients) and 73 did not (controls). All participants were at least 40 years old and lived in Jeddah, Saudi Arabia, during the 10-month study period (March 2017–January 2018). The 73 osteoporotic patients were on a regular follow-up schedule in the Orthopedic Department at King Fahd General Hospital in Jeddah, Saudi Arabia.
Participants were selected based on the results of a two-step process: (1) Saudi patients underwent a DEXA scan (Discovery-W fan-beam densitometer, Hologic Inc., Bedford, MA, USA) in the radiology center of King Fahad General Hospital and (2) those having a confirmed diagnostic BMD T-score of <–2.5 were included in the study. The 73 healthy participants were selected based on the results of their bone scans and confirmation that they were free of osteoporosis with T-score values > -1. Study participants who were pregnant, lactating, and/or had any type of organ dysfunction or terminal illness were excluded from the study. Informed consent was obtained from all subjects who agreed to provide their personal information after they were educated to the purpose and procedures of the study. A survey questionnaire was used to collect information regarding age, sex, height, weight, smoking habits, coffee consumption, previous bone fractures, asthma, tumors, renal problems, and family history of osteoporosis. Anthropometric measurements were included: body mass index (BMI) was calculated as body weight (kg) divided by square of body height (m2). The most prominent standard BMI categories, as defined by the WHO, are as follows: Underweight ≤18.5, normal weight = 18.5–24.9, overweight = 25–29.9, and obesity ≥30.
Collection of blood samples and extraction of gDNA samples
Blood samples for gDNA extraction were collected in EDTA tubes from all participants. The gDNA was extracted from whole blood samples using a GeneJET Whole Blood Genomic DNA Purification Mini Kit (Catalog number K0782, Thermo Fisher Scientific, USA) following the manufacturer’s instructions. The quality of each extracted DNA sample was assessed by measuring the absorbance at two wavelengths (260 and 280) on a DeNovix DS-11 spectrophotometer at the Cancer and Mutagenesis Unit in King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. All DNA samples were kept at a temperature of −20°C for future experiments.
Genotyping of VDR gene polymorphisms
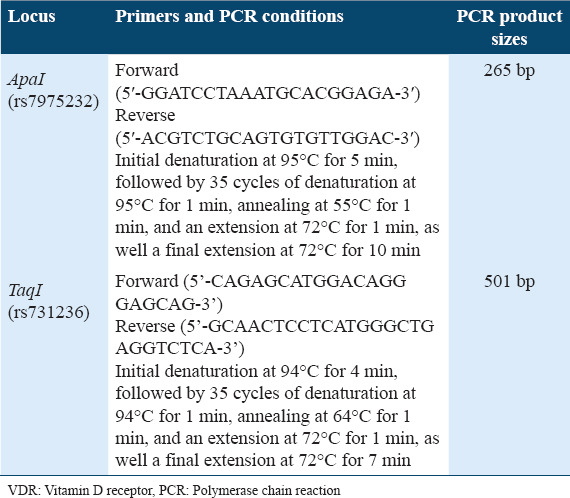
The different genotypes of the VDR gene (ApaI and FokI) were determined using a (polymerase chain reaction-restriction fragment length polymorphism [PCR-RFLP]) technique. PCR was performed to amplify the regions of interest in the VDR gene. To perform PCR in a 25 ml reaction, 12.5 ml Thermo Scientific™ DreamTaq Green PCR Master Mix (2X) (catalog number K1081, Thermo Fisher Scientific, California, USA), 1 ml of each forward and reverse primer, 8.5 ml of nuclease-free water (catalog number 203601, Affymetrix, Santa Clara, CA, USA), and 2 ml genomic DNA (100 ng/ml) were added into PCR tubes. Then, all tubes were centrifuged at 5000 rpm for 5 min before being placed in a PCR thermocycler. The PCR primers and thermocycler reactions [Table 1] were previously published without any modifications.[25,26]
Table 1.
PCR primers and conditions used for VDR polymorphisms amplification
To confirm the different genotypes of the ApaI and TaqI variants in the VDR gene, RFLP was performed on all PCR products using restriction enzymes. To perform RFLP, a 30 ml mixture was prepared as follows: 10 ml of a PCR product, 17 ml of RNase free water, 2 ml of 10x FastDigest Green buffer, and 1 ml of a specific FastDigest enzyme (Thermo Fisher Scientific, Waltham, MA, USA) (ApaI [catalog number FD1414] or TaqI [catalog number FD0674]) were mixed in an Eppendorf tube and incubated at 37°C for 20 min without an inactivation step. Digestion products were visualized on 2% agarose gels. These were resolved on a 1.5% agarose gel containing 0.5 mg/ml ethidium bromide using a gel electrophoresis system (Peqlab, Germany) at 60 V for 20 to 30 min. The gel was visualized under UV light using a transilluminator system (Cleaver Scientific Ltd., UK) at the Central Lab in King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia.
The measurement of serum levels of vitamin D and calcium
Serum samples for measuring biochemical parameters of calcium and vitamin D were obtained from all subjects; plain red vacutainer tubes were used. Then, they were stored at −20°C until the time of analysis. The 25-hydroxyvitamin D [25(OH)D] was the preferred metabolite that has been used to assess serum vitamin D levels.[27] The level of serum 25(OH)D was determined automatically as described previously using a Roche Elecsys modular analytics (Cobas e411) to perform an electrochemiluminescence immunoassay (Roche Diagnostics, GmbH, Mannheim, Germany).[28] Serum calcium was measured on an Olympus AU 400 chemistry autoanalyzer using a photometric color (Arsenazo method) test. Both analyses were performed in the Biochemistry laboratory at King Fahad General Hospital, Jeddah, KSA.
Statistical analysis
In this study, statistical analyses were performed using IBM SPSS statistics version 20.0. A non-parametric Mann–Whitney test was used to compare one parameter between the two groups. Univariate regression analysis was applied to assess the relationship between each single variable with osteoporosis. Allele frequencies were calculated as the number of occurrences of the test allele in the population divided by the total number of alleles. The odds ratio (OR), 95% confidence intervals, and P values of genotype distributions and allele frequencies were determined using a Chi-square test following the Hardy–Weinberg equilibrium assumption. All P < 0.05 were considered as statistically significant.
Results
Determination of participants physical and biochemical characteristics
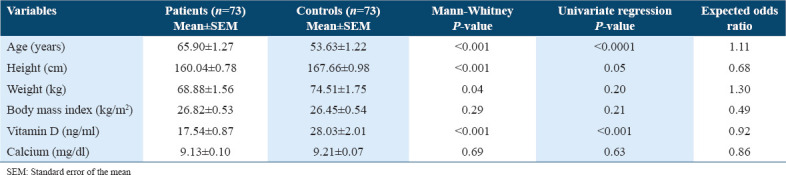
The baseline characteristics of osteoporosis patients and controls are shown in Table 2. Physical characteristics such as age, weight, height, BMI, and biochemical parameters such as vitamin D and calcium levels were presented as mean ± standard error of the mean. The mean age of the patients (65.90 ± 1.27 years) was significantly (P < 0.001) higher than the mean age of controls (53.63 ± 1.22 years). The means of heights and weights were significantly (P < 0.001 and P < 0.05, respectively) lower in osteoporotic patients (160.04 ± 0.78 cm and 68.88 ± 1.56 kg) as compared to healthy controls (167.66 ± 0.98 cm and 74.51 ± 1.75 kg). On the other hand, no significant difference (P = 0.3) was seen in BMI among the two study groups (patients’ BMI mean 26.82 ± 0.53 kg/m2 as compared to 26.45 ± 0.54 kg/m2 in controls). When univariate regression analysis was performed to assess the OR and degree of significance between each variable with the risk of osteoporosis, results showed that only age and vitamin D levels were significantly different between patients and controls. However, these differences had no significant association with osteoporosis risk, as shown by the calculated ORs (the expected OR for age was 1.11 and for vitamin D, it was 0.922). In this study, vitamin D and calcium levels were also estimated in the serum of the participants [Table 2]. The comparison by a Mann–Whitney test showed that the control group had significantly higher levels of vitamin D (28.03 ± 2.01 ng/ml) than those in the patient’s group (17.54 ± 0.87 ng/ml; P < 0.001). In contrast, serum calcium levels were not significantly different between the two groups (P > 0.05).
Table 2.
Physical and biochemical characteristics of patients with osteoporosis and those in the control group
Genotype and allele frequencies distribution of the VDR gene (ApaI) variant
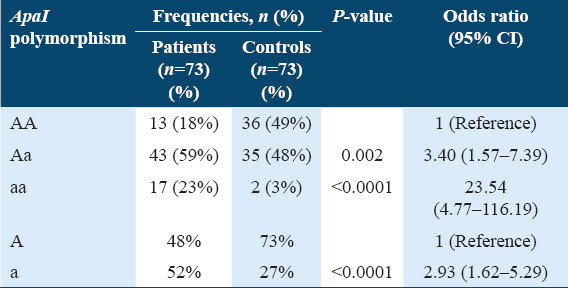
The genotype and allele frequencies of the ApaI variant for the osteoporotic group and the control group are presented in Table 3. The genotypic frequencies of the osteoporotic group were 18% (n = 13) normal (AA); 59% (n = 43) heterozygous (Aa); and 23% (n = 17) homozygous (aa). The allelic frequency of “A” versus “a” was 48% versus 52% in the patients’ group. Genotype distribution for the osteoporosis group was within the Hardy–Weinberg equilibrium (χ2 = 2.59, degree of freedom [DF] =1, 0.1 < P < 0.2). In controls, the results showed 49% (n = 36) normal (AA); 48% (n = 35) heterozygous (Aa); and 3% (n = 2) homozygous (aa). The frequency of “A” allele was 73% and the frequency of “a” allele was 27%. Genotype distribution for the control group was within the Hardy–Weinberg equilibrium (χ2 = 3.6, DF = 1, 0.05 < P < 0.1). A statistically significant increase was found in heterozygous (Aa) and homozygous (aa) genotypes in the ApaI polymorphism among osteoporotic patients as compared to controls (P = 0.002 and P < 0.0001, respectively). Moreover, the minor “a” allele was found to be more common in patients than in healthy controls (P < 0.0001). Comparing the results of the OR showed that this SNP had a higher significant risk for osteoporosis development between osteoporotic patients and healthy controls [Table 3].
Table 3.
Frequency distribution of genotypes and alleles of ApaI among members of the osteoporosis group and healthy controls
Genotype and allele frequencies distribution of the VDR gene (TaqI) variant
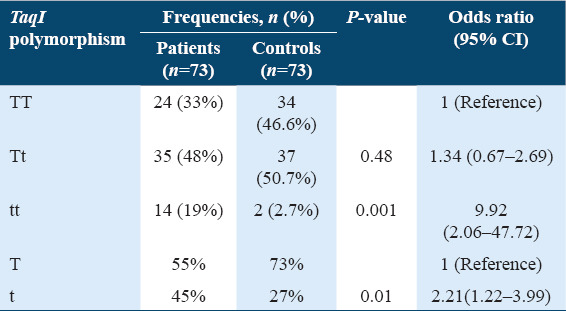
Table 4 shows the frequency distribution of genotypes and alleles of the TaqI polymorphism among osteoporotic patients and controls. The genotypic frequencies of the TaqI variant in the osteoporotic group showed 33% (n = 24) normal (TT); 48% (n = 35) heterozygous (Tt); and 19% (n = 14) homozygous (tt). The genotype distribution for the osteoporosis group was within the Hardy–Weinberg equilibrium (χ2 = 0.3, DF = 1, 0.2 < P < 0.975). The analysis showed that the “T” allele frequency was 55% and the “t” allele frequency was 45% in the osteoporotic patient’s group. On the other hand, in the control group, this variant was distributed as 46.6% (n = 34) normal (TT); 50.7% (n = 37) heterozygous (Tt); and 2.7% (n = 2) homozygous (tt). The frequency of the “T” allele was 73% and the “t” allele was 27% in the control group. The genotype distribution for the control group was out of the Hardy–Weinberg equilibrium (χ2 = 4.99, DF = 1, 0.025 < P < 0.05). Comparing the results of the ORs showed that this SNP had a significant risk for osteoporosis development between osteoporotic patients and healthy controls, especially carriers of the homozygous (tt) genotype which was significantly higher in the osteoporosis patients group than in the control group (P = 0.001). Moreover, a significant increase was seen in the “t” allele in the osteoporosis group as compared to controls (P = 0.01).
Table 4.
Frequency distribution of genotypes and alleles of TaqI among members of the osteoporosis group and healthy controls
Discussion
Bone fragility and fractures in osteoporotic patients have attracted particular attention in Saudi Arabia. The etiology of osteoporosis involves the interaction of genetic and non-genetic factors. Although the genetic influence on the development of osteoporosis is well established, the number of genes involved, their chromosomal locations, the magnitude of their effects, and the way they may interact with each other and with other risk factors are not well defined.[29] Since BMD is under strong genetic control and polymorphisms in the VDR gene locus could be associated with the bone mass, and that the VDR genotyping could be used to assess predisposition to osteoporosis, many studies have been carried out to explain the exact role of VDR alleles and BMD.[30] The relationship between VDR variants (ApaI and TaqI) and bone disorders has been investigated in numerous studies, which have produced disparate results based on the geographical and ethnic factors.[31,32] In this study, the association of ApaI and TaqI polymorphisms of the VDR gene with osteoporosis risk was investigated. A significant increase was found in the genotype frequencies of the ApaI (Aa) and (aa) genotypes among osteoporotic patients as compared to controls. On the other hand, the homozygous (tt) genotype of TaqI was found to be significantly higher in the patients than in the controls. Regarding the allelic levels, the minor “a” allele of ApaI and the “t” allele of TaqI were significantly more common in the patients than in the controls. Moreover, the serum level of total vitamin D was found to be lower in osteoporotic patients compared to controls and no significant difference in calcium serum level between the two groups.
In contrast to our results, Seremak-Mrozikiewicz et al. found that the TaqI polymorphism was associated with BMD in Polish postmenopausal osteoporotic women, especially patients carrying the normal (TT) genotype, who had lower BMD than (Tt) and (tt) genotype patients.[33] In 2003, Zhang et al. reported a significant relationship between the (AA) genotype of ApaI and the incidence of osteoporosis in Chinese menopausal women.[34] In 2009, Dundar et al. studied the genotype distribution of ApaI in Turkish menopausal women and showed that individuals with the (aa) genotype had a lower bone density and higher serum calcium level than those with the (AA) genotype.[35] On the other hand, in 2004, in a similar study involving Turkish menopausal women with osteoporosis, the authors found that individuals with (TT, Tt, and tt) genotypes in the TaqI variant had the highest bone density and the lowest serum calcitonin levels.[36] In Greek menopausal women with osteoporosis, the genotype frequencies of TaqI had a nonsignificant relationship with BMD, osteoporosis, or bone fractures, while they did have a significant relationship with calcium absorption.[37] Similar results were obtained from Tunisian osteoporotic menopausal women in whom it was found that the (Aa) genotype of the ApaI genotype was considered a protective factor against osteoporosis while the (aa) genotype was considered an osteopenic factor related to bone fractures.[38] In Belarusian osteoporotic menopausal women, both ApaI and TaqI polymorphisms were found to be influencing factors of osteoporosis.[39] In spite of the contrasting results of the previously mentioned studies, in a meta-analysis review by Shen et al. on 14 studies consisting of 6500 osteoporotic women, no significant relationship was found among the named polymorphisms and the incidence of bone fracture.[40]
Conclusion
This study adds more knowledge to the genetic variants role of the VDR gene polymorphism (ApaI and TaqI) in the Saudi population. The major strength result of the study includes the high impact of VDR polymorphisms’ distribution on osteoporotic patients and healthy individuals. The analysis revealed that ApaI (Aa) and (aa) genotypes, as well as the TaqI (tt) genotype, are significantly associated with the risk of osteoporosis in the Saudi population. Therefore, the VDR gene polymorphisms (ApaI and TaqI) can be considered as influencing factors of osteoporosis in Saudi Arabia. This could support our understanding of the relationship between VDR polymorphisms and osteoporosis in the Saudi population. Furthermore, it can explain the effect of ethnicity on disease susceptibility when the patients of different populations will be compared to those of the Saudi population. However, the study has a number of limitations, most importantly, the small number of sample size, which might affect the statistical correlations of some comparisons.
Ethics Approval and Consent to Participate
The study was approved by the National Committee for Medical and Bioethics of Health Affairs in Jeddah, Ministry of Health, Saudi Arabia (Approval Number A00406). All participants signed written informed consent before they were involved in the study.
Availability of Data and Material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing Interests
All authors declare no conflicts of interest.
Funding Statement
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. (KEP-PhD-47-130-38).
Authors’ Contributions
Abeer A. Banjabi conducted experiments and write manuscripts; Ayat B. Al-Ghafari performed statistical analyses; Taha A. Kumosani revised the manuscript; Kurunthachalam Kannan designed the project; and Salah M. Fallatah provided participants’ samples and medical information. All authors revised the manuscript and read and approved the final version of the manuscript.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. (KEP-PhD-47-130-38). The authors, therefore, acknowledge with thanks to DSR technical and financial support.
References
- 1.Melton LJ., 3rd How many women have osteoporosis now? J Bone Miner Res. 1995;10:175–7. doi: 10.1002/jbmr.5650100202. [DOI] [PubMed] [Google Scholar]
- 2.Al-Habdan IM, Sadat-Ali M, Al-Muhanna FA, Al-Elq AH, Al-Mulhim AA. Bone mass measurement using quantitative ultrasound in healthy Saudi women A cross-sectional screening. Saudi Med J. 2009;30:1426–31. [PubMed] [Google Scholar]
- 3.Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos Int. 2005;16:43–55. doi: 10.1007/s00198-004-1639-9. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Ralston SH, Fraser J. Diagnosis and management of osteoporosis. Practitioner. 2015;259:15–9. [PubMed] [Google Scholar]
- 6.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis:Synopsis of a WHO report WHO study group. Osteoporos Int. 1994;4:368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 7.Swann JI. Osteoporosis:The fragile bone disease. Br J Healthc Assist. 2012;6:59–62. [Google Scholar]
- 8.Wilkin TJ. Changing perceptions in osteoporosis. BMJ. 1999;318:862–4. doi: 10.1136/bmj.318.7187.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. A vitamin D receptor gene polymorphism in the translation initiation codon:Effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–21. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- 10.Ahn J, Albanes D, Berndt SI, Peters U, Chatterjee N, Freedman ND, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis. 2009;30:769–76. doi: 10.1093/carcin/bgp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto KI, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, et al. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol. 1997;11:1165–79. doi: 10.1210/mend.11.8.9951. [DOI] [PubMed] [Google Scholar]
- 12.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 13.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371:1–12. doi: 10.1016/j.cca.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Marozik PM, Tamulaitiene M, Rudenka E, Alekna V, Mosse I, Rudenka A, et al. Association of vitamin D receptor gene variation with osteoporosis risk in Belarusian and Lithuanian postmenopausal women. Front Endocrinol (Lausanne) 2018;9:305. doi: 10.3389/fendo.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, Van Meurs JBJ, D'Alesio A, Jhamai M, Zhao H, Rivadeneira F, et al. Promoter and 3'-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture:The Rotterdam study. Am J Hum Genet. 2005;77:807–23. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan EL, Brown MA. Genetic studies of osteoporosis-the end of the beginning. Arthiritis Res Ther. 2008;10:214. doi: 10.1186/ar2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naito M, Miyaki K, Naito T, Zhang L, Hoshi K, Hara A, et al. Association between Vitamin D receptor gene haplotypes and chronic periodontitis among Japanese men. Int J Med Sci. 2007;4:216–22. doi: 10.7150/ijms.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbasi M, Hasani S, Sheikholeslami H, Alizadeh SA, Rashvand Z, Yazdi Z, et al. Association between vitamin D receptor Apa1 and Taq1 genes polymorphism and osteoporosis in postmenopausal women. J Qazvin Univ Med Sci. 2012;16:4–10. [Google Scholar]
- 20.Eisman JA. Vitamin D receptor gene alleles and osteoporosis:An affirmative view. J Bone Miner Res. 1995;10:1289–93. doi: 10.1002/jbmr.5650100903. [DOI] [PubMed] [Google Scholar]
- 21.Gennari L, Merlotti D, De Paola V, Calabrò A, Becherini L, Martini G, et al. Estrogen receptor gene polymorphisms and the genetics of osteoporosis:A HuGE review. Am J Epidemiol. 2005;161:307–20. doi: 10.1093/aje/kwi055. [DOI] [PubMed] [Google Scholar]
- 22.Al-Daghri NM, Al-Saleh Y, Aljohani N, Sulimani R, Al-Othman AM, Alfawaz H, et al. Vitamin D status correction in Saudi Arabia:An experts'consensus under the auspices of the European society for clinical and economic aspects of osteoporosis, osteoarthritis, and musculoskeletal diseases (ESCEO) Arch Osteoporos. 2017;12:1. doi: 10.1007/s11657-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Geriatrics Society Workgroup on vitamin D Supplementation for Older Adults Recommendations abstracted from the American geriatrics society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147–52. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 24.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, et al. IOF position statement:vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–4. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 25.Hajj A, Chedid R, Chouery E, Megarbané A, Gannagé-Yared MH. Relationship between vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics. 2016;17:1675–86. doi: 10.2217/pgs-2016-0045. [DOI] [PubMed] [Google Scholar]
- 26.Rizk MM, Zakaria NH, Elshazely WG. Study of vitamin D receptor (VDR) gene polymorphisms among Egyptian cohort patients with different stages of colorectal cancer. J Cancer Ther. 2014;5:253–63. [Google Scholar]
- 27.Al-Daghri NM, Yakout S, Bukhari I, Khattak MN, Al-Saleh Y, Aljohani N, et al. Parathyroid hormone in relation to various vitamin D metabolites in adult females. Medicine (Baltimore) 2017;96:e8071. doi: 10.1097/MD.0000000000008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Ajlan A, Krishnaswamy S, Alokail MS, Aljohani NJ, Al-Serehi A, Sheshah E, et al. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Pregnancy Childbirth. 2015;15:314. doi: 10.1186/s12884-015-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vupputuri MR, Goswami R, Gupta N, Ray D, Tandon N, Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and Vitamin D receptor gene polymorphisms in Asian Indians. Am J Clin Nutr. 2006;83:1411–9. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]
- 30.Douroudis K, Tarassi K, Ioannidis G, Giannakopoulos F, Moutsatsou P, Thalassinos N, et al. Association of vitamin D receptor gene polymorphisms with bone mineral density in postmenopausal women of Hellenic origin. Maturitas. 2003;45:191–7. doi: 10.1016/s0378-5122(03)00148-8. [DOI] [PubMed] [Google Scholar]
- 31.Zintzaras E, Rodopoulou P, Koukoulis GN. BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D receptor (VDR) gene and the risk of osteoporosis:A meta-analysis. Dis Markers. 2006;22:317–26. doi: 10.1155/2006/921694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zmuda JM, Cauley JA, Danielson ME, Wolf RL, Ferrell RE. Vitamin D receptor gene polymorphisms, bone turnover, and rates of bone loss in older African-American women. J Bone Miner Res. 1997;12:1446–52. doi: 10.1359/jbmr.1997.12.9.1446. [DOI] [PubMed] [Google Scholar]
- 33.Seremak-Mrozikiewicz A, Drews K, Mrozikiewicz PM, Bartkowiak-Wieczorek J, Marcinkowska M, Wawrzyniak A, et al. Correlation of vitamin D receptor gene (VDR) polymorphism with osteoporotic changes in Polish postmenopausal women. Neuro Endocrinol Lett. 2009;30:540–6. [PubMed] [Google Scholar]
- 34.Zhang YY, Long JR, Liu PY, Liu YJ, Shen H, Zhao LJ, et al. Estrogen receptor alpha and vitamin D receptor gene polymorphisms and bone mineral density:Association study of healthy pre-and postmenopausal Chinese women. Biochem Biophys Res Commun. 2003;308:777–83. doi: 10.1016/s0006-291x(03)01479-7. [DOI] [PubMed] [Google Scholar]
- 35.Dundar U, Solak M, Kavuncu V, Ozdemir M, Cakir T, Yildiz H, et al. Evidence of association of vitamin D receptor Apa I gene polymorphism with bone mineral density in postmenopausal women with osteoporosis. Clin Rheumatol. 2009;28:1187–91. doi: 10.1007/s10067-009-1220-1. [DOI] [PubMed] [Google Scholar]
- 36.Duman BS, Tanakol R, Erensoy N, Oztürk M, Yilmazer S. Vitamin D receptor alleles, bone mineral density and turnover in postmenopausal osteoporotic and healthy women. Med Princ Pract. 2004;13:260–6. doi: 10.1159/000079524. [DOI] [PubMed] [Google Scholar]
- 37.Stathopoulou MG, Dedoussis GV, Trovas G, Theodoraki EV, Katsalira A, Dontas IA, et al. The role of vitamin D receptor gene polymorphisms in the bone mineral density of Greek postmenopausal women with low calcium intake. J Nutr Biochem. 2011;22:752–7. doi: 10.1016/j.jnutbio.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Sassi R, Sahli H, Souissi C, Sellami S, Ben Ammar El Gaaied A. Polymorphisms in VDR gene in Tunisian postmenopausal women are associated with osteopenia phenotype. Climacteric. 2015;18:624–30. doi: 10.3109/13697137.2015.1007123. [DOI] [PubMed] [Google Scholar]
- 39.Marozik P, Mosse I, Alekna V, Rudenko E, Tamulaitienė M, Ramanau H, et al. Association between polymorphisms of VDR, COL1A1, and LCT genes and bone mineral density in Belarusian women with severe postmenopausal osteoporosis. Medicina (Kaunas) 2013;49:177–84. [PubMed] [Google Scholar]
- 40.Shen H, Xie J, Lu H. Vitamin D receptor gene and risk of fracture in postmenopausal women:A meta-analysis. Climacteric. 2014;17:319–24. doi: 10.3109/13697137.2013.856401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available and will be provided by the corresponding author on a reasonable request.


