Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is a vasculitis characterized by multisystemic manifestations including asthma. Mepolizumab (300 mg/4 weeks) has recently been approved for EGPA. However, real-life data are scarce and report experiences with high doses of mepolizumab intravenously administered (750 mg/4 weeks). The aim of our study was to investigate in a real-life setting whether mepolizumab in EGPA patients at low doses would enable us 1) to control asthma symptoms, 2) to obtain oral corticosteroids (OCS) and/or immunosuppressors tapering and 3) to maintain clinical remission and avoid disease relapses. Mepolizumab (100 mg/4 weeks) was subcutaneously administered for 12 months in 18 EGPA patients with uncontrolled severe asthma. Symptoms, annual asthma exacerbation rates, OCS-sparing effects, lung function and eosinophil activation markers were monitored. The proportion of patients with clinical remission or relapse was also evaluated in month 12. A significant decrease in the annual rate of asthma exacerbations in association with significant changes in asthma control were observed. Specifically, 66.6% of the patients experienced no exacerbations during the mepolizumab treatment. Most patients (77.7%) were able to reduce the daily OCS dose by at least 50%. Four patients also stopped cyclosporine A during the study period. No EGPA relapse was observed and a large majority of the patients achieved clinical remission (94.3%). Clinical benefits were paralleled by reduction in blood eosinophils and serum levels of eosinophil activation markers. Low-dose mepolizumab showed clinically relevant benefits in exacerbation rates, asthma symptoms, OCS and immunosuppressive use in EGPA patients. These effects occurred without any EGPA relapse for extrapulmonary manifestations.
Keywords: Asthma, IL-5, eosinophil, Churg-Strauss syndrome, vasculitis, steroid, antibody
INTRODUCTION
Eosinophilic granulomatosis with polyangiitis (EGPA) is a small-vessel necrotizing vasculitis in which the lung is the most commonly involved organ.1,2 Its treatment remains a challenge for physicians because the current available therapies corticosteroids and immunomodulators are not always able to control symptoms and are often associated with significant morbidity and relapse.3,4 Mepolizumab, approved for the treatment of patients with severe eosinophilic asthma, works by binding to circulating interleukin (IL)-5, thus preventing its function on eosinophils.5,6,7,8,9 The pathogenesis of EGPA is not fully defined; however, a direct pathogenic effect of eosinophil infiltration to different tissues is strongly suggested by releasing cytotoxic granule proteins and lipid mediators.10,11,12 IL-5 may represent a useful target in the treatment of EGPA. The current evidence for the efficacy and safety of mepolizumab in patients with EGPA supports its use in the induction and maintenance of remission of refractory, relapsing or glucocorticoid-dependent EGPA, and has led to mepolizumab approval for EGPA therapy at a dose of 300 mg/4 weeks subcutaneously (s.c.).13,14 However, real-life data are scarce and its optimal use has not yet been fully studied. Specifically, literature data report experiences with very high doses of mepolizumab with a different route of administration (750 mg/4 weeks intravenously [i.v.]).15,16 In this paper, we report our clinical experience in the treatment of EGPA patients with mepolizumab at a dose of 100 s.c.
MATERIALS AND METHODS
Patients
We selected 18 patients (females/males: 12/6) who had been diagnosed as EGPA at least 24 months before (Table 1). EGPA was defined as a history and presence of asthma, a blood eosinophil level of 10%, or an absolute eosinophil count > 1,000 cells/mm3 and the presence of at least 2 of the following features17:
Table 1. EGPA diagnostic disease characteristics of patients.
| Patient type of disease | Asthma/eosinophil (% max value) | Biopsy evidence | PNS | Nonfixed lung infiltrates | CRSwNP | Heart | Kidney | Palpable purpura | Alveolar hemorrhage | ANCA status | Disease duration (yr) | Immuno suppressive therapy since diagnosis | OCS dose (mg/day)* | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + (17) | − | + | + | + | − | − | − | − | + | 20 | CyA | 5 | Arth |
| 2 | + (11) | − | − | + | + | − | − | − | − | + | 4 | MTX | 5 | Arth |
| 3 | + (16) | − | + | + | + | − | − | − | − | − | 29 | No | 5 | - |
| 4 | + (13) | + | − | − | + | − | − | − | − | − | 8 | No | 7.5 | - |
| 5 | + (15) | − | − | + | + | − | − | + | − | − | 15 | CyA/IFX | 5 | - |
| 6 | + (61) | − | + | + | + | − | + | + | − | + | 21 | CyA/CKF/IFX | 15 | - |
| 7 | + (25) | + | − | + | + | − | − | + | − | − | 22 | CyA | 5 | GI/Arth |
| 8 | + (36) | − | + | + | + | − | + | + | − | + | 3 | CyA/AZA/RTX | 5 | - |
| 9 | + (31) | + | − | + | + | − | + | + | − | + | 4 | AZA/CyA | 10 | - |
| 10 | + (17) | − | − | + | + | − | − | − | − | + | 13 | CyA/MTX | 20 | - |
| 11 | + (30) | − | − | + | + | − | − | − | − | − | 12 | CyA | 15 | - |
| 12 | + (12) | + | − | + | + | + | − | + | − | − | 27 | No | 5 | Sierositis |
| 13 | + (13) | − | − | + | + | − | − | + | − | − | 32 | CyA/IFX | 5 | - |
| 14 | + (31) | − | + | − | + | − | − | − | − | + | 9 | OMA | 7.5 | - |
| 15 | + (26) | + | − | + | + | − | − | − | − | − | 26 | CyA/AZA | 5 | - |
| 16 | + (36) | + | − | + | + | + | + | − | − | − | 6 | MTX/IFX | 12.5 | - |
| 17 | + (19) | + | + | + | + | − | − | − | − | + | 6 | MMF | 7.5 | GI |
| 18 | + (12) | + | − | + | + | − | + | − | − | − | 7 | CyA | 7.5 | - |
PNS, peripheral nervous system; CRSwNP, chronic rhinosinusitis with nasal polyps; ANCA, anti-neutrophil cytoplasmic antibodies; OCS, oral corticosteroids; CyA, cyclosporine A; MTX, methotrexate; IFX, infliximab; CKF, cyclophosphamide; AZA, azathioprine; RTX, rituximab; OMA, omalizumab; MMF, mycophenolate mofetil; Arth = arthritis; GI = gastrointestinal.
*At beginning of mepolizumab.
1) Biopsy showing histopathological evidence of eosinophilic vasculitis or perivascular eosinophilic infiltration or eosinophil-rich granulomatous inflammation
2) Pulmonary infiltrates non-fixed
3) Sino-nasal abnormality
4) Neuropathy, mono or poly (motor deficit or nerve conduction abnormality)
5) Cardiomyopathy
6) Glomerulonephritis
7) Palpable purpura and
8) Positive test for anti-neutrophil cytoplasmic antibodies (ANCA) immunofluorescence and/or positive test for myeloperoxidase (MPO) and/or proteinase-3 (PR3) antibodies.
Allergic bronchopulmonary aspergillosis and Strongyloides stercoralis infection were excluded. At enrolment, all patients displayed a refractory asthma state, without clinically active renal, gastrointestinal, skin, central nervous system and/or cardiac disease with a Birmingham Vasculitis Activity Score (BVAS) of 0. All patients were receiving treatment with high doses of inhaled corticosteroids, long-acting beta2 agonists, and a stable dose of prednisone or equivalent (mean ± standard error [SE], 8.1 ± 1.1 mg; range, 5–20 mg/day). Oral corticosteroids (OCS) tapering was started from month 1 post-baseline onwards in patients who displayed improvement in asthma symptom control without BVAS changes, according to the standard of care practice. Before OCS interruption, assessment of hypothalamic-pituitary-adrenal axis integrity was performed, according to standard of care by the adrenocorticotropic hormone stimulation test (Synacthen) associated with early morning cortisol level. Five patients (5/18; 27.7%) were also receiving cyclosporin A (mean ± SE, 181.2 ± 27.7 mg; range, 125–200 mg/day). In Table 2, patients' demographic and clinical characteristics at baseline are summarized. Mepolizumab 100 mg was injected s.c. at 4-week intervals. The clinical effects were analyzed by measuring Asthma Control Test (ACT), Asthma Control Questionnaire-5 (ACQ-5) and Sino-Nasal Outcome Test 22 (SNOT-22) under the supervision of the same physician at each follow-up visit, until 12 months. The exacerbations rate of asthma was also assessed. Lung function was monitored measuring pre-bronchodilator forced expiratory volume in 1 second (FEV1) during the follow-up period in 14 (77.7%) patients; 8 patients were evaluated at baseline, and 6 and 12 months after the start of therapy, while in the remaining 6 patients evaluation was done at baseline and at either 6 (n = 5) or 12 months (n = 1). Blood samples for eosinophil count, serum eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) were monitored.
Table 2. Demographic and clinical characteristics of patients at enrolment.
| Characteristics | Values |
|---|---|
| Total No. | 18 |
| Male/female | 6/12 |
| Age | 57.0 ± 8.7 |
| Disease duration (yr) | 15.7 ± 8.9 |
| ANCA positive status | 4 (22.2) |
| Absolute eosinophil count (/mm3) | 790.0 ± 76.0 |
| ACT | 15.1 ± 0.7 |
| ACQ-5 | 2.3 ± 0.2 |
| SNOT-22 | 44.9 ± 4.5 |
| FEV1 (1) | 2.3 ± 0.2 |
| Annual exacerbations (pre-exposure) | 5.2 ± 0.6 |
| Prednisone dose (mg/day) | 8.1 ± 1.1 |
| Cyclosporine A dose (mg/day) | 181.2 ± 27.7 |
| ECP (mcg/L) | 72.9 ± 12.0 |
| EDN (ng/mL) | 47.0 ± 8.3 |
Values are means ± standard error or number (%).
ANCA, anti-neutrophil cytoplasmic antibodies; ACT, Asthma Control Test; ACQ-5, Asthma Control Questionnaire-5; SNOT-22, sino-nasal outcome test 22; FEV1, forced expiratory volume 1 second; ECP, eosinophil cationic protein; EDN, eosinophil derived neurotoxin.
Laboratory tests
Serum ECP levels were measured using a commercially available fluoroimmunoassay kit (ThermoFisher Scientific AB, Uppsala, Sweden) with a detection limit of less than 2 mcg/L, according to the manufacturer's instructions. EDN levels were measured by an ELISA kit (Elabscience, Houston, TX, USA). ANCA were detected by an indirect immunofluorescence assay (Euroimmun, Lubeck, Germany). Serum MPO and PR3 antibodies were measured by fully automated fluorescent-enzyme immuno-assay technology (ThermoFisher Scientific AB), according to the manufacturer's instructions.
Statistical analysis
Continuous variables were summarized using mean and SE and compared using Student's t-test. All tests were 2-sided and a P value of <0.05 was considered significant.
RESULTS
Mepolizumab consistently improves clinical asthma outcomes in EGPA patients
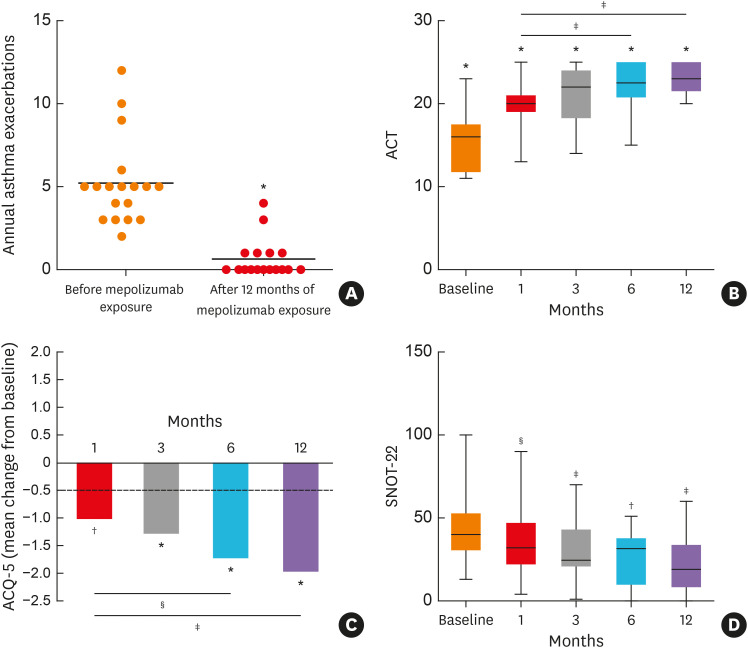
The exacerbation frequency decreased in all patients, and most (n = 12; 66.6%) experienced no exacerbations during mepolizumab treatment. Mepolizumab significantly decreased the annual rate of clinically relevant asthma exacerbations (−87.6%; 5.2 ± 0.6 vs. 0.6 ± 0.3, P < 0.001) (Fig. 1A). A few weeks after the start of therapy, significant changes in both ACT and ACQ-5 scores were observed (Fig. 1B and C). The improvement in asthma control was maintained over time, during the 12-month follow-up period. Overall, considering the last evaluation for each patient, 9/14 (64.2%) showed an improved outcome regarding lung function defined as an increase in FEV1 ≥ 200 mL. Among the remaining patients, we observed FEV1 stability in 4 patients, while in 1 patient a decline was shown, despite the persistence of asthma control. Considering the entire study population, FEV1 improvement was already detectable 6 months after the start of therapy (2.49 ± 0.2 vs. 2.9 ± 0.6; P = not significant) and it remained stable over time (data not shown). Regarding the sinonasal involvement by chronic rhinosinusitis with nasal polyps (CRSwNP), a significant improvement in SNOT-22 was experienced in 66.6% of patients (12/18), starting from the first month of therapy. The improvement was maintained during the 12-month follow-up period with a progressive trend, which showed a reduction in the score over time (Fig. 1D).
Fig. 1. Clinical outcomes of patients suffering from eosinophilic granulomatosis with polyangiitis who received mepolizumab therapy. (A) Reduction in the annual exacerbation rate, evaluated before and after mepolizumab exposure. (B) Changes in ACT and (C) ACQ-5 scores during mepolizumab therapy. (D) SNOT-22 improvement during the study period. Values with symbols refer to comparison with baseline; values with lines and symbols refer to comparison at 1 month of therapy.
ACT, asthma control test; ACQ-5, asthma control questionnaire-5; SNOT-22, Sino-Nasal Outcome Test 22.
*P < .001, †P < 0.001, ‡P < 0.01, §P < 0.05.
Effectiveness of mepolizumab on biomarkers of eosinophilic inflammation
Mean eosinophil counts were consistently reduced (83.9%) from baseline by month 1 (790 ± 88 vs. 127 ± 24; P < 0.001). Reduction in eosinophil counts was maintained throughout the study (91% by month 12, Fig. 2A). A significant reduction in both ECP and EDN was also observed (Fig. 2B).
Fig. 2. Impact of mepolizumab on biomarkers of eosinophilic inflammation. (A) Reduction in blood eosinophils during mepolizumab treatment. (B) Decreased levels of serum ECP (left panel) and EDN (right panel) during therapy. Values with symbols refer to comparisons with baseline; values with lines and symbols refer to comparisons with 1 month of therapy.
ECP, eosinophil cationic protein; EDN, eosinophil derived neurotoxin.
*P < .001, †P < 0.01, ‡P < 0.05.
Mepolizumab effectively serves as corticosteroid- and immunosuppressor-sparing therapy
In month 12, most patients (n = 14; 77.7%) reduced the dose of OCS (3.8 ± 0.6 mg of prednisone/day) (P < 0.001), being able to reduce the daily dose by at least 50%. Three patients (3/18; 16.6%) stopped OCS therapy. No significant difference in eosinophil count at baseline or total immunoglobulin E was observed between patients with or without OCS reduction (data not shown). A proportion of patients (5/18; 27.7%) decreased the OCS dose after the first month of therapy and it progressively increased over time. Notably, in 1 patient, after an initial decrease in OCS doses, it was necessary to return to the initial dose due to a non-satisfactory control of nasal symptoms (Table 3). Among the 5 patients that had been receiving cyclosporine A (CyA), 1 reduced the daily dose (125 mg) and 4 (80%) stopped it after a progressive and controlled reduction.
Table 3. Summary of oral corticosteroid dose reduction in all patients at different times throughout the study.
| 1st month | 3rd month | 6th month | End of the study | |
|---|---|---|---|---|
| Reduction from baseline | 5 (27.7) | 12 (66.6) | 15 (83.3) | 14 (77.7) |
| Mean dose reduction (%) | 9.5 ± 3.8 | 28.1 ± 5.6 | 47.2 ± 6.7 | 58.1 ± 8.7 |
| Reduction of at least 50% | 0 | 5 | 12 | 14 |
| Stop treatment (No./total No.) | 0/18 | 0/18 | 1/18 | 3/18 |
Values are means ± standard error or number (%).
Clinical stability of vasculitis manifestations during mepolizumab treatment
We then asked if the reduction/cessation of OCS and/or CyA could have affected the stability of vasculitis manifestations. At baseline, all patients displayed a BVAS of 0, taking into account that both pulmonary and extra-pulmonary manifestations were persistent for ≥3 months. Most of them (12/18; 66.6%) were in clinical remission (except for asthma and sinonasal symptoms) according to the European League against Rheumatism definition (BVAS = 0 and OCS ≤ 7.5 mg/die). After 12 months of treatment, clinical remission was reached by a large majority of patients (17/18; 94.4%). Only 1 patient, despite the significant improvement of asthma symptoms, did not reach the clinical remission due to the requirement of prednisone ≥ 7.5 mg/day. In addition, none experienced disease relapse (BVAS > 0), active vasculitis and/or active asthma symptoms, or signs with a worsening in the ACQ-5 score, requiring increase in OCS dose, initiation/increased dose of immunosuppressive therapy or hospitalization) during the 12-month period.
DISCUSSION
Our work demonstrated the effectiveness of mepolizumab s.c. at a low dose (100 mg/4 weeks) in EGPA patients with severe asthma and nasal symptoms in a real-life setting. The clinical benefit of mepolizumab allowed a significant OCS- and immunosuppressor-sparing effect. More importantly, the reduction/interruption of OCS and/or CyA was not associated with a relapse of EPGA, but rather an increase in the proportion of patients in clinical remission. The improvement in asthma was already evident after the first administration of the drug and persisted over a period of 12 months of therapy.
Two other open-label studies on mepolizumab in EGPA were conducted15,16; however, both examined a high dose (750 mg/4 weeks i.v.). In both studies, mepolizumab allowed for OCS reduction in most patients, with no relapses during the active treatment phase. The MIRRA trial has recently led to mepolizumab approval for EGPA therapy at a dose of 300 mg s.c./4 weeks. In our patients, we used mepolizumab at a dose of 100 mg due to administrative regulations/issues as mepolizumab is only approved for severe eosinophilic asthma in our country. Our results are in agreement with preliminary data obtained by Thompson et al.18 in a small case series (6 patients) with a short-term course of mepolizumab therapy at low doses. Our promising results should be confirmed in a wider range of cases, to support the non-inferiority of 100 mg vs. the standard dose. In addition, a placebo-controlled study could be useful to strengthen our results.
Our results are in agreement with previous data about OCS-sparing effects, reported in clinical trials performed in patients with severe asthma and EGPA.7,13 Notably, in comparison to this previous data, we observed a more pronounced impact, with a higher proportion of patients that consistently reduced (>50%) the OCS dosage and a proportion of them (over 15%) stopped OCS. Additionally, we showed that mepolizumab allowed the reduction in the immunosuppressor CyA in all patients, and even a complete interruption for most of them. This result is a key point in appreciating the efficacy of mepolizumab, taking into account that most EGPA patients use high doses of OCS in order to control asthma, which exposed them to an increased risk of corticosteroid-related adverse events associated with high direct and indirect costs.4 Furthermore, the stability of extrapulmonary vasculitis manifestations observed in our patients, despite the changes in concomitant systemic treatment, may suggest a potential role for mepolizumab, even at low doses, in the control of the multisystemic EGPA signs and symptoms. However, our data are not sufficient to support the evidence of low-dose mepolizumab effectiveness in EGPA patients with active vasculitis.
One of the features differentiating our real-life study with the registrative trial on EGPA13 is the higher proportion of ANCA-positive patients (4/18) enrolled (22.2% vs. 10%). Interestingly, we observed that the ANCA status does not affect the overall mepolizumab-induced clinical benefit (data not shown).
From a clinical point of view, what we have observed reflects the effects exerted by mepolizumab on the biological parameters that we have analyzed in the follow-up period. In fact, after the first administration, the number of peripheral blood eosinophils and serum marker activation (ECP and EDN) drastically fall and remain stable over time.
In our patients, we observed a clinically significant improvement in FEV1 in about half of the subjects monitored for lung function; it is possible to hypothesize that patients with a long history of the disease may have irreversible damage at the bronchial level partially dependent on eosinophils, therefore could not respond to an anti-IL-5 strategy from a functional point of view.
One of the main clinical features of EGPA is represented by sinonasal involvement that may negatively affect patients' quality of life.19 Although investigating the efficacy of mepolizumab in CRSwNP was not the primary objective of our analysis, we observed a decrease in SNOT-22 scores during treatment. The effect on the rhinosinusal component occurred more gradually than that on asthma symptoms, with a tendency to become progressively more significant as treatment progressed. The different effect of mepolizumab at the bronchial and nasal levels could be a consequence of a different histopathological substrate at the sinus level compared to that present at the bronchial level. Furthermore, a drug's variable capacity to effectively reach therapeutic levels in various anatomical districts cannot be ruled out.
At present, to our knowledge, this is the largest experience in real-life of mepolizumab therapy in EGPA patients, even if our results must be confirmed in a wider range of case series. Further studies on larger populations with prolonged follow-up periods could be useful to better define the mepolizumab dose with the best performance (efficacy/safety) in EGPA patients.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen Y, Guillevin L. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Semin Respir Crit Care Med. 2018;39:471–481. doi: 10.1055/s-0038-1669454. [DOI] [PubMed] [Google Scholar]
- 3.Thompson GE, Specks U. Update on the management of respiratory manifestations of the antineutrophil cytoplasmic antibodies-associated vasculitides. Clin Chest Med. 2019;40:573–582. doi: 10.1016/j.ccm.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Canonica GW, Colombo GL, Bruno GM, Di Matteo S, Martinotti C, Blasi F, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12:100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 6.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 7.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 8.Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143:1742–1751.e7. doi: 10.1016/j.jaci.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 10.Chaigne B, Terrier B, Thieblemont N, Witko-Sarsat V, Mouthon L. Dividing the Janus vasculitis? Pathophysiology of eosinophilic granulomatosis with polyangitis. Autoimmun Rev. 2016;15:139–145. doi: 10.1016/j.autrev.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LY, Gu Q, Lin AH, Khosravi M, Gleich G. Airway hypersensitivity induced by eosinophil granule-derived cationic proteins. Pulm Pharmacol Ther. 2019;57:101804. doi: 10.1016/j.pupt.2019.101804. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinfeld J, Bradford ES, Brown J, Mallett S, Yancey SW, Akuthota P, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2019;143:2170–2177. doi: 10.1016/j.jaci.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–1343. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Moosig F, Gross WL, Herrmann K, Bremer JP, Hellmich B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann Intern Med. 2011;155:341–343. doi: 10.7326/0003-4819-155-5-201109060-00026. [DOI] [PubMed] [Google Scholar]
- 17.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 18.Thompson G, Vasilevski N, Ryan M, Baltic S, Thompson P. Low-dose mepolizumab effectively treats chronic relapsing eosinophilic granulomatosis with polyangiitis. Respirology. 2018;23(Suppl 1):178. [Google Scholar]
- 19.Chung JH, Lee YJ, Kang TW, Kim KR, Jang DP, Kim IY, et al. Altered quality of life and psychological health (SCL-90-R) in patients with chronic rhinosinusitis with nasal polyps. Ann Otol Rhinol Laryngol. 2015;124:663–670. doi: 10.1177/0003489415576181. [DOI] [PubMed] [Google Scholar]