Abstract
Purpose
It is difficult to assess airway obstruction using spirometry in adult asthmatic patients with preserved lung function. Impulse oscillometry (IOS) can detect not only airway resistance but also reactance. Therefore, IOS may be useful in assessing pulmonary function in such patients. We investigated the applicability of IOS for asthma patients with preserved lung function.
Methods
Between 2015 and 2018, 1,248 adult asthmatic patients suspected of having asthma who visited the Allergy and Asthma Center of Severance Hospital underwent both spirometry and IOS. Consequently, 784 patients had asthma, 111 had chronic obstructive lung disease (COPD) or asthma-COPD overlap, and 7 had parenchymal lung disease. The remaining 346 patients had chronic cough without underlying lung or airway disease. Among the 784 asthmatic patients, 191 with decreased lung function (predicted forced expiratory volume in 1 second [FEV1] < 80%) were excluded. Propensity score matching was performed to adjust baseline characteristics between 346 non-asthmatic and 593 asthmatic patients with preserved lung function. Subsequently, we compared the spirometry and IOS parameters between the 329 asthmatic and 329 non-asthmatic patients.
Results
Multiple logistic regression analysis showed that the area of reactance (AX) was associated with asthma with preserved lung function. In receiver operating characteristic (ROC) curve analysis, the area under the curve (AUC) of AX (AUC = 0.6823) for asthma was not significantly different from that of FEV1 (AUC = 0.6758). However, the AUC of a combination of AX and FEV1 (AUC = 0.7437) for asthma was significantly higher than that of FEV1 alone. The cutoff value of AX was 0.51 kPa/L in univariate ROC analysis.
Conclusions
AX is associated with adult asthma with preserved lung function. Performing spirometry together with IOS is more beneficial than performing spirometry alone for diagnosing asthma in adult patients with preserved lung function.
Keywords: Asthma, oscillometry, spirometry, lung function, airway
INTRODUCTION
Many asthmatic patients have preserved lung function (predicted forced expiratory volume in 1 second [FEV1] ≥ 80%),1,2 despite having asthma symptoms.3 Therefore, although spirometry is the most commonly used procedure to assess pulmonary function,4,5,6,7 it is limited in assessing asthma in patients with normal airway flow. In these patients, the bronchial challenge test may be helpful in confirming a diagnosis of asthma.8 However, it may be difficult to detect asthma using this challenge test alone.9,10 Therefore, the diagnosis of asthma is confirmed by considering various factors such as patient history, physical examination, the presence of reversible airway obstruction and airway hyperresponsiveness, patient response to treatment, and results of additional tests (blood and sputum analysis, fractional exhaled nitric oxide levels, etc.).8 However, it is still difficult to diagnose asthma in some patients with preserved lung function, and there is an unmet need for additional diagnostic tools.
Unlike spirometry which measures the volume displacement of air through forceful expiratory and inspiratory maneuvers, impulse oscillometry (IOS) measures changes in the pressure and flow of the airway, which are then mapped to mechanical resistances and reactances.11 Therefore, it has been suggested that IOS can detect peripheral airway impairment at an early stage and reflects the elasticity of the peripheral airway.11,12 However, due to the excellent accessibility of spirometry, IOS is mainly used in children who face difficulties in undergoing spirometry and is not commonly used for adults.13 Therefore, there have been limited studies on the applicability of IOS in adult asthmatic patients with preserved lung function. Considering the limitation of spirometry in patients with preserved lung function, performing IOS in such patients may be beneficial, and the forced expiration process during spirometry is difficult to perform in adults, especially patients with fatigue, comorbid medical conditions, sensory deficits, coordination impairments, or cognitive impairments.14,15,16 In contrast, because IOS is an effort-independent modality,16 its parameters are measured during the normal breathing process. Its parameters can be easily measured in most patients.
The Allergy and Asthma Center of Severance Hospital has performed spirometry and IOS in adults suspected to have asthma. In this study, we aimed to investigate the applicability of IOS in the diagnosis of adult asthmatic patients with preserved lung function. We analyzed adult patients who underwent spirometry and IOS from 2015 to 2018.
MATERIALS AND METHODS
Patients
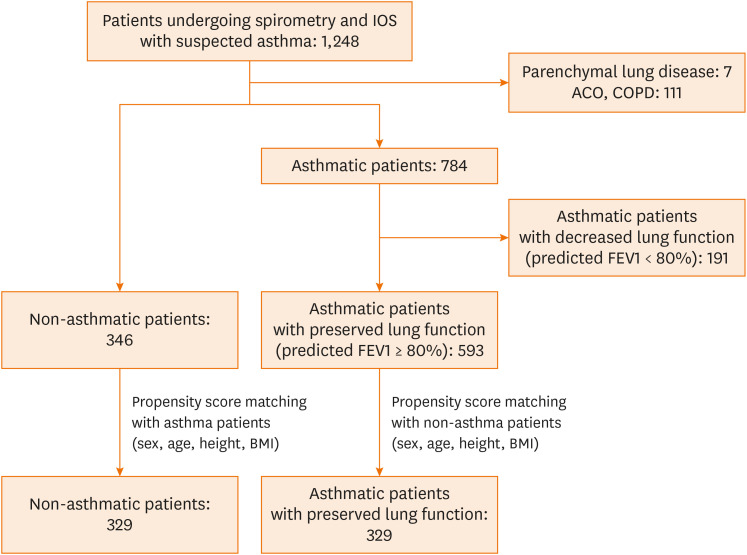
From 2015 to 2018, 1,248 adult patients suspected of having asthma who underwent both spirometry and IOS were enrolled in this study (Fig. 1) Consequently, 784 patients had asthma, 111 had chronic obstructive lung disease (COPD) or asthma-COPD overlap, 7 had parenchymal lung disease, and 346 had chronic cough without underlying lung or airway disease. Among the asthmatic patients, 191 with decreased lung function (predicted FEV1 < 80%) were excluded. After adjusting baseline characteristics such as sex, age, weight, and body mass index (BMI), propensity score matching was performed with the remaining 593 asthmatic patients with preserved FEV1 (predicted FEV1 ≥ 80%) and 346 non-asthmatic patients. We then compared and analyzed spirometry and IOS parameters in 329 asthmatic patients with preserved lung function with those in 329 non-asthmatic patients. This study was approved by the hospital's medical ethics committee (4-2019-0530). The need for informed consent was exempted.
Fig. 1.
Patients investigated in this study.
Diagnosis of patients
The medical records of 1,248 patients were retrospectively reviewed. Patients were diagnosed with asthma based on their clinical histories involving variable respiratory symptoms, their responses to inhaled corticosteroids, presence of elevated sputum eosinophil levels, and results of the bronchial challenge test.17 Patients who had tuberculosis or interstitial lung disease according to chest radiography or computed tomography were diagnosed with parenchymal lung disease. Patients who had persistent peripheral airway obstruction (FEV1/forced vital capacity [FVC] ratio < 70%) after bronchodilator inhalation were diagnosed with COPD or asthma-COPD overlap. Patients who had chronic cough lasting for more than 2 months with preserved lung function and without asthma evidence or parenchymal lung disease as mentioned above were diagnosed as non-asthmatics.
Propensity score matching
A propensity score is the probability that a unit with certain characteristics will be assigned to the asthma group (as opposed to the non-asthma group).18 These scores can be used to reduce or eliminate selection bias in observational studies by balancing covariates (the characteristics of participants) between the asthma and non-asthma groups. When the covariates are balanced, it becomes much easier to match participants with multiple characteristics. This is one of the best alternatives to reduce bias in sample selection when structural randomization is not possible. Therefore, a propensity score matching method was used to match in a 1:1 ratio in order to reduce bias. First, we performed a univariate analysis between the asthma and non-asthma groups, confirmed differences in age, sex, height, and BMI, and used these as matching covariates. After matching, analysis was performed with reduced bias.
IOS
IOS parameters were collected before conventional spirometry parameters using the MasterLab IOS System (Erich Jaeger, Würzburg, Germany). Calibration was performed using a single volume of air (3 L) at different flow rates and a reference resistance device (0.2 kPa/L/s). The patients wore a nose clip and a manufacturer-provided, oval, hard plastic mouthpiece to prevent the expired air from escaping. They were also requested to support their cheeks with their hands to decrease shunt compliance. Artifacts caused by coughing, breath holding, swallowing, and vocalization were not included. A single, experienced, respiratory technician made all the IOS measurements. The parameters evaluated were resonant frequency (Fres), resistance at 5 Hz (R5), resistance at 20 Hz (R20), difference between the resistance at 5 and 20 Hz (R5–R20), reactance at 5 Hz (X5), area of reactance (AX), and impedance at 5 Hz (Z5) as calculated by .
Spirometry
In all patients, spirometric measurements were taken 5 minutes after IOS measurements. The maximal expiratory flow volume measurements were obtained using a pneumotachometer system equipped with a Lilly head (MasterScreen system; Erich Jaeger). International criteria were used to determine the spirometric flow-volume curve.19 The patients used a nose clip and were instructed in the standard forced expiratory maneuver. Each data set consisted of results associated with at least 3 reproducible attempts, with no more than 8 attempts made per patient. The best result from the 3 attempts was chosen for final data analysis. The measurement of lung function was repeated 20 minutes after albuterol administration. Albuterol was administered in 3 puffs of 100 μg through a pressurized metered dose inhaler.
Bronchial challenge test
Methacholine was diluted in an isotonic sodium chloride solution and was administered using a handheld nebulizer (Devilbis 646; Devilbis Health Care, Somerset, UK) that was connected to a Rosental dosimeter (Devilbis Health Care). Each patient took 5 full inhalations from functional residual capacity to total lung capacity without a period of breath holding after each full inspiration. The initial concentration of methacholine that was administered was 0.075 mg/mL, and a dose-response curve was constructed by administering a serial doubling of this concentration of methacholine until a concentration of 25 mg/mL was reached. The provocation concentration that caused a decrease of 20% (PC20) in FEV1 was calculated by a linear interpolation between the last 2 points on the dose-response curve. If the PC20 value was ≤16 mg/mL, the airway was considered positive for hyperresponsiveness. The asthma challenge test was performed 2 weeks after measuring IOS and spirometry parameters.
Induced sputum analysis
Sputum was induced by the inhalation of 3% saline via a nebulizer (ULTRA-NEB 2000; Devilbis Health Care). We mixed 1 mL of sputum with an equal volume of 10% sputolysin (0.1% dithiothreitol in phosphate-buffered saline; Calbiochem-Novabiochem, San Diego, CA, USA) and mildly homogenized it by vortexing for 1 minute. After incubation in a shaking water bath for 20 minutes, the cells were centrifuged at 1,500 rpm for 3 minutes. The resulting cell pellets were resuspended with saline and were cytocentrifuged at 1,000 rpm for 3 minutes onto a slide using the Cytospin 3 (Shandon; Cheshire, United Kingdom). After Wright and Giemsa staining, up to 200 inflammatory cells were counted and eosinophil percentages were calculated. If the eosinophil percentage was ≥ 3%, the sputum was considered eosinophil-positive. The sputum analysis was performed following spirometry and IOS on the same day.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and was compared using an independent 2 sample t test. Categorical variables are expressed as numbers and percentages and were compared using a χ2 test or Fisher's exact test. To adjust differences in baseline characteristics between the non-asthmatic and asthmatic patients, propensity score matching (1:1) was performed. Age, sex, height, and BMI were used as covariates for matching. Multiple logistic regression was performed to identify factors associated with the composite outcome. The areas under the curves (AUCs) and their 95% confidence intervals (CIs) were calculated using the nonparametric method suggested by DeLong et al.19 The cutoff values were determined by using Youden's index.20 Statistical significance was assessed by P values < 0.05, and 95% CIs were constructed. Statistical software (SAS 9.4, SAS Institute; Cary, NC, USA) was used for statistical analyses.
RESULTS
Demographic data
FVC (predicted %), FEV1 (L), FEV1 (predicted %), and FEV1/FVC (%) were lower in asthmatic patients than in non-asthmatic patients; the bronchodilator (BD) response (L) and BD response (%) were higher in asthmatic patients than in non-asthmatic patients (Supplementary Table S1). There were more atopic patients among asthmatic patients than among non-asthmatic patients. A sputum eosinophil percentage of ≥ 3% was observed in 51.1% of the asthmatic patients, and 30.7% of the asthmatic patients had positive results in the asthma challenge test. AX, Fres, R5, R20, and R5–R20 values were higher and X5 values were lower in asthmatic patients than in non-asthmatic patients. Due to differences in baseline characteristics, such as sex, age, height, and BMI, between non-asthmatic and asthmatic patients, which could affect IOS parameters, propensity score matching was performed using these factors. The significant difference in spirometry and IOS parameter values remained after propensity score matching (Table 1).
Table 1. Demographic data of non-asthmatic and asthmatic patients with preserved lung function after propensity score matching.
| Characteristics | Non-asthmatic patients | Asthmatic patients | P value |
|---|---|---|---|
| Patient number | 329 | 329 | |
| Sex (male:female) | 148:181 | 146:183 | 0.8754 |
| Age (yr) | 51.1 ± 15.1 | 50.6 ± 17.1 | 0.7026 |
| Height (cm) | 163.5 ± 8.7 | 163.3 ± 8.3 | 0.7208 |
| Body weight (kg) | 63.0 ± 11.7 | 62.9 ± 11.2 | 0.8503 |
| BMI (kg/m2) | 23.5 ± 3.5 | 23.5 ± 3.1 | 0.8503 |
| FVC (predicted %) | 105.65 ± 13.63 | 100.87 ± 12.21 | < 0.0001 |
| FEV1 (L) | 2.99 ± 0.77 | 2.79 ± 0.78 | 0.0008 |
| FEV1 (predicted %) | 104.55 ± 14.02 | 97.04 ± 12.08 | < 0.0001 |
| BD response (L) | 0.079 ± 0.080 | 0.131 ± 0.130 | < 0.0001 |
| BD response (%) | 2.67 ± 2.59 | 4.73 ± 4.38 | < 0.0001 |
| FEV1/FVC (%) | 82.4 ± 6.4 | 80.2 ± 7.3 | < 0.0001 |
| AX (kPa/L) | 0.341 ± 0.190 | 0.712 ± 0.789 | < 0.0001 |
| Fres (Hz) | 13.121 ± 2.692 | 15.313 ± 4.182 | < 0.0001 |
| R5 (kPa/L/s) | 0.304 ± 0.077 | 0.370 ± 0.129 | < 0.0001 |
| R20 (kPa/L/s) | 0.231 ± 0.065 | 0.256 ± 0.071 | < 0.0001 |
| R5–R20 (kPa/L/s) | 0.073 ± 0.041 | 0.114 ± 0.077 | < 0.0001 |
| X5 (kPa/L/s) | −0.097 ± 0.035 | −0.128 ± 0.082 | < 0.0001 |
| Z5 (kPa/L/s) | 0.320 ± 0.079 | 0.394 ± 0.146 | < 0.0001 |
Propensity score matching (1:1) was performed between the non-asthma and asthma patients. Age, sex, height, and BMI were used as covariates for matching. Continuous variables are expressed as mean ± standard deviation and were compared using an independent 2-sample t test. Categorical variables are expressed as numbers and percentages, and were compared using a χ2 test or Fisher's exact test.
BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; BD, bronchodilator; AX, area of reactance; Fres, resonant frequency; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, difference between the resistance at 5 Hz and 20 Hz; X5, reactance at 5 Hz; Z5, impedance at 5 Hz.
Multiple logistic regression and receiver operating characteristic (ROC) analyses
Multiple logistic regression analysis was performed with a model containing spirometry parameters, a model containing IOS parameters, and a model containing all parameters of both tests (Table 2). In the model containing all parameters of both tests, FEV1 (predicted %, P < 0.0001), AX (P < 0.0001), and Fres (P = 0.0198) were significantly associated with asthma. In the spirometry parameter model, FEV1 (L, P = 0.0289) and FEV1 (predicted %, P < 0.0001) were significantly associated with asthma. In the IOS parameter model, AX was significantly associated with asthma (P < 0.0001). Multiple logistic regression analyses of selected parameter models were performed to compare AUC values of significant parameters. In the selected parameter models, FEV1 (L, P = 0.0112), FEV1 (predicted %, P < 0.0001), and AX (P < 0.0001) were significantly associated with asthma.
Table 2. Multiple logistic regression analyses of spirometry and IOS parameters for asthma in adults with preserved lung function.
| Parameters | All parameters model of both tests | Selected parameters model of both tests | Spirometry parameters model | Selected spirometry parameters model | IOS parameters model | Selected IOS parameters model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | |
| FEV1 (L) | 0.2703 | 0.1646 | −0.3478 | 0.0289 | −0.2784 | 0.0112 | ||||||
| FEV1 (predicted %) | −0.0402 | < 0.0001 | −0.0459 | < 0.0001 | −0.0382 | < 0.0001 | −0.0453 | < 0.0001 | ||||
| BD response (L) | 1.1672 | 0.7393 | 2.7819 | 0.3689 | ||||||||
| BD response (%) | 0.1260 | 0.2439 | 0.0597 | 0.5163 | ||||||||
| FEV1/FVC (%) | −0.00157 | 0.9183 | −0.00071 | 0.9591 | ||||||||
| AX (kPa/L) | 5.8004 | < 0.0001 | 3.2436 | < 0.0001 | 5.4102 | < 0.0001 | 2.6891 | < 0.0001 | ||||
| Fres (Hz) | −0.1369 | 0.0198 | −0.0722 | 0.1423 | −0.0920 | 0.0812 | ||||||
| R5 (kPa/L/s) | −4.8246 | 0.2368 | 31.7721 | 0.4577 | ||||||||
| R5–R20 (kPa/L/s) | 28.3540 | 0.5301 | −3.1906 | 0.4251 | ||||||||
| X5 (kPa/L/s) | 2.4889 | 0.8725 | 2.0470 | 0.8883 | ||||||||
| Z5 (kPa/L/s) | −27.7076 | 0.5561 | −33.2542 | 0.4566 | ||||||||
Multiple logistic regression analyses were performed with several models. Statistically significant variables in multiple logistic regression analysis were chosen as selected parameters.
IOS, impulse oscillometry; FEV1, forced expiratory volume one second; BD, bronchodilator; AX, area of reactance; Fres, resonant frequency; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, difference between the resistance at 5 Hz and 20 Hz; X5, reactance at 5 Hz; Z5, impedance at 5 Hz.
ROC curves were plotted based on predictive scoring equations from the results of the multiple logistic regression analyses. The AUC of all parameter models of both tests was 0.7891, that of the selected parameter model of both tests (FEV1 [predicted %], FEV1 [L] and AX) was 0.7437, that of the selected spirometry parameter model (FEV1 [predicted %] and FEV1 [L]) was 0.6758, and that of the selected IOS parameter model (AX) was 0.6823 (Fig. 2). No significant difference was observed in the AUC value between the selected spirometry parameters and selected IOS parameter models (P = 0.8028). However, there was a significant difference in the AUC value between all parameter and selected spirometry parameter models (P < 0.0001), and between the selected parameters of both tests and selected spirometry parameter model (P < 0.0001).
Fig. 2.
ROC curve analysis of spirometry and IOS parameters for predicting asthma in adults with preserved lung function. ROC curves were plotted based on predictive scoring equations from multiple logistic regression analyses. The AUC values were calculated on the basis of the multiple logistic regression models. All parameter models of both tests included FEV1 (L), FEV1 (%), BD response (L), BD response (%), FEV1/FVC (%), AX (kPa/L), Fres (Hz), R5 (kPa/L/s), R5–R20 (kPa/L/s), X5 (kPa/L/s), and Z5 (kPa/L/s). The selected parameter models of both tests included FEV1 (%) and AX (kPa/L). The selected spirometry parameter model included FEV1 (L) and FEV1 (%). The selected IOS parameter model included AX (kPa/L). AUC values of each model were compared by DeLong's method.
ROC, receiver operating characteristic; AUC, area under the curve; FEV1, forced expiratory volume in 1 second; BD, bronchodilator; AX, area of reactance; Fres, resonant frequency; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, difference between the resistance at 5 Hz and 20 Hz; X5, reactance at 5 Hz; Z5, impedance at 5 Hz.
Scatter plot and cutoff values
Scatter plot of FEV1 (predicted %) and AX are shown in Fig. 3. There was no linear correlation between the 2 parameters in patients with preserved lung function. Non-asthmatic and asthmatic patients overlapped in most FEV1 ranges. However, many asthmatic patients showed high AX, regardless of the FEV1 value. Cutoff values were calculated using univariate ROC analysis (Table 3). The cutoff value of AX was 0.51 kPA/L, and FEV1 (predicted %) was 94%. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of AX were higher than that of FEV1 (predicted %). Scatter plot and cutoff values of all parameters are shown in Supplementary Table S2 and Supplementary Fig. S1.
Fig. 3.
Scatter-plot of FEV1 and AX. There was no linear correlation between the 2 parameters.
FEV1, forced expiratory volume in 1 second; AX, area of reactance.
Table 3. Cutoff values for asthma in adults with preserved lung function.
| Parameters | Cut-off value | AUC | YJ | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| FEV1 (%) | 94.0112 | 0.6692 | 0.27356 | 46.5046 | 80.8511 | 70.8333 | 60.1810 |
| AX (kPa/L) | 0.51000 | 0.6868 | 0.32313 | 49.3865 | 82.9268 | 74.1935 | 62.2426 |
Univariate receiver operating characteristic curve analysis was performed using these parameters, and the cutoff values were then determined using Youden's index.
AUC, area under the curve; YJ, Youden's J; FEV1, forced expiratory volume in 1 second; AX, area of reactance; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In this study, we demonstrated the applicability of IOS in diagnosing asthma in adult patients with preserved lung function. AX was significantly associated with asthma in these patients, and AX showed a similar AUC value as FEV1 when compared using ROC curves. Furthermore, the model of AX and FEV1 had a significantly higher AUC value for asthma than that of FEV1. In addition, AX had higher sensitivity, specificity, PPV, and NPV for asthma than FEV1 in these patients. Considering these results, performing spirometry and IOS together can help clinician diagnose adult asthma.
Compared with non-asthmatic airways, the inflammation of asthmatic airways results in an altered airway composition.21 Moreover, airway remodeling can also occur in asthmatic airways.22 Generally, these changes are evident in patients with severe asthma.23,24 However, this can also occur in asthmatic patients with preserved lung function. Subepithelial fibrosis has been observed in these patients.25,26,27 Such differences in cell composition and physical layers of the airway shift the structural and mechanical resonances of the airway and lead to different frequency response coefficients associated with resonance, which eventually translates to different impedance values.28 When the airway is excited due to the IOS examination, such changes in the frequency domain clearly manifest as differences in IOS outcomes. Therefore, IOS can detect airway changes that do not affect airway flow. As a result, IOS can be useful for asthma diagnosis, especially in patients whose spirometric results show normal lung function.
The multiple logistic regression analysis of all parameters showed that among IOS parameters, AX and Fres were associated with asthma. However, the estimate of −0.1369 for Fres indicated that the risk of asthma was reduced when Fres increased. It is known that Fres is higher in asthmatic patients than in healthy subjects,29 and Fres had a higher mean value in asthmatic patients than in non-asthmatic patients in this study. This paradoxical result is associated with a higher standard deviation of the Fres value in asthmatic patients than in non-asthmatic patients. This affected the results of multiple logistic regression following propensity score matching. Therefore, we considered that AX was the only valid parameter in this analysis. An accurate interpretation of this result requires further analysis with a larger sample size.
AX is the area under the curve of reactance between 5 Hz and Fres30 and reflects the elastic properties of the lung.11 In this study, although we enrolled asthmatic patients who had preserved lung function in spirometry, the patients might have had asthmatic inflammation in their airways. Therefore, this inflammation could have affected airway elasticity, resulting in an abnormal AX. In contrast, multiple logistic regression analysis showed that the R5–R20, which reflects peripheral airway resistance,31 was not associated with asthma in these patients. Relatively small changes in the airway of asthmatic patients who had normal airway flow might not affect the R5 change in a clinically meaningful way. Therefore, we suggest that among IOS parameters, AX is the most useful parameter for the diagnosis of asthma in adult patients who have preserved lung function in spirometry.
An important advantage of IOS is that it can be measured during normal breathing. IOS examines the airway via frequency excitations. IOS superimposes multiple sound waves with different frequency components on a patient's normal tidal breathing.11 The multiple frequency components propagate into various parts of the lung. Frequency components are reflected back at different rates due to the complex physical structure and nonuniform frequency response of the lung. Pressure changes and flows can be determined by comparing incident waves and reflected waves at various frequencies, and corresponding mechanical resistance and reactance (impedance) can be calculated at each frequency. The resistances and impedances at different frequencies map to different regions in the lung. Because tidal breathing is at much lower frequency components (< 1 Hz) compared with relevant frequency components (5–25 Hz) for IOS, a simple low-frequency filter implemented during IOS accurately processes signals, and consequently, data extraction can be completed. Therefore, IOS can be performed during normal tidal breathing with a remarkably low volume of air being displaced. Furthermore, this is a relatively short procedure. As a result, performing spirometry following IOS measurements dose not burden patients.
This study has some limitations. First, we did not consider the effects of medications; some patients were already using inhaled corticosteroid (ICS) when undergoing spirometry and IOS. The AUCs of spirometry and IOS parameters might be undervalued. However, subgroup analysis showed that the mean values of parameters of naive ICS users did not significantly differ from those of continuous ICS users. Therefore, effects of medications might not have significantly affected the AUCs of parameters. Secondly, asthma is not the only condition that can show abnormal IOS values. Airway diseases, such as bronchitis, emphysema, bronchiectasis, COPD, and interstitial lung disease, can also show abnormal IOS values.32,33,34 Therefore, it is always necessary to discriminate among these diseases that may affect IOS values. Thirdly, although there are many reference values associated with adult IOS,13,35,36,37,38 there are no clear reference values for adult asthma. Because IOS values were reported to depend on age, sex, height, body weight, BMI, and race,13,39 it is difficult to establish clear reference values. The cutoff values for IOS parameters suggested in this study did not consider multiple interactions among the parameters. Therefore, further research is needed to determine appropriate reference values that can aid in the diagnosis of adult asthma. Despite these limitations, the results of this study are clinically meaningful because we demonstrated that IOS is helpful in diagnosing asthma in adult patients with preserved lung function.
In conclusion, AX is associated with asthma with preserved lung function. Performing spirometry and IOS together is more clinically useful for diagnosing these asthmatic patients than spirometry only.
ACKNOWLEDGMENTS
JHL has responsibility for the content of the manuscript, including the data and analysis.
Footnotes
Presentation: A preliminary version of the data presented in this study was presented as poster presentation at the World Allergy Congress (WAC) 2019 Conference, December 12-14, 2019, Lyon, France (poster No. 95).
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Demographic data of non-asthmatic and asthmatic patients with preserved lung function
Cutoff values of each parameter for asthma in adults with preserved lung function
Scatter plot of spirometry and IOS parameters. IOS parameters were not correlated with spirometry parameters.
References
- 1.Sears MR. Lung function decline in asthma. Eur Respir J. 2007;30:411–413. doi: 10.1183/09031936.00080007. [DOI] [PubMed] [Google Scholar]
- 2.Siroux V, Boudier A, Dolgopoloff M, Chanoine S, Bousquet J, Gormand F, et al. Forced midexpiratory flow between 25% and 75% of forced vital capacity is associated with long-term persistence of asthma and poor asthma outcomes. J Allergy Clin Immunol. 2016;137:1709–1716.e6. doi: 10.1016/j.jaci.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Rytilä P, Metso T, Heikkinen K, Saarelainen P, Helenius IJ, Haahtela T. Airway inflammation in patients with symptoms suggesting asthma but with normal lung function. Eur Respir J. 2000;16:824–830. doi: 10.1183/09031936.00.16582400. [DOI] [PubMed] [Google Scholar]
- 4.Morris JF. Spirometry in the evaluation of pulmonary function. West J Med. 1976;125:110–118. [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 6.Enright PL, Lebowitz MD, Cockroft DW. Physiologic measures: pulmonary function tests. Asthma outcome. Am J Respir Crit Care Med. 1994;149:S9–18. doi: 10.1164/ajrccm/149.2_Pt_2.S9. [DOI] [PubMed] [Google Scholar]
- 7.Douglas G, Higgins B, Barnes N, Boyter A, Burge S, Cates C, et al. British guideline on the management of asthma: a national clinical guideline. Thorax. 2008;63:1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 8.McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. Diagnosis and management of asthma in adults: a review. JAMA. 2017;318:279–290. doi: 10.1001/jama.2017.8372. [DOI] [PubMed] [Google Scholar]
- 9.Sumino K, Sugar EA, Irvin CG, Kaminsky DA, Shade D, Wei CY, et al. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol. 2012;130:69–75.e6. doi: 10.1016/j.jaci.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Nair P. Methacholine test and the diagnosis of asthma. J Allergy Clin Immunol. 2012;130:556. doi: 10.1016/j.jaci.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Desiraju K, Agrawal A. Impulse oscillometry: the state-of-art for lung function testing. Lung India. 2016;33:410–416. doi: 10.4103/0970-2113.184875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galant SP, Komarow HD, Shin HW, Siddiqui S, Lipworth BJ. The case for impulse oscillometry in the management of asthma in children and adults. Ann Allergy Asthma Immunol. 2017;118:664–671. doi: 10.1016/j.anai.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz H, Flexeder C, Behr J, Heier M, Holle R, Huber RM, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One. 2013;8:e63366. doi: 10.1371/journal.pone.0063366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow L, Coggon D, Osmond C, Holgate ST. A population survey of respiratory symptoms in the elderly. Eur Respir J. 1991;4:267–272. [PubMed] [Google Scholar]
- 15.Chotirmall SH, Watts M, Branagan P, Donegan CF, Moore A, McElvaney NG. Diagnosis and management of asthma in older adults. J Am Geriatr Soc. 2009;57:901–909. doi: 10.1111/j.1532-5415.2009.02216.x. [DOI] [PubMed] [Google Scholar]
- 16.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146:841–847. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 17.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention revised 2019. place unknown: Global Initiative for Asthma; 2019. [cited 2019 Sep 4]. Available from: http://www.ginasthma.org. [Google Scholar]
- 18.Parsons LS. Performing a 1: N case-control match on propensity score; Proceedings of the 29th Annual SAS Users Group International Conference; Montreal, Canada; 2014 May 9-12. San Francisco (CA): SAS Institute; 2004. [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117:496–506. doi: 10.1016/j.jaci.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, et al. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am Rev Respir Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- 27.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 28.Brashier B, Salvi S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe (Sheff) 2015;11:57–65. doi: 10.1183/20734735.020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavalcanti JV, Lopes AJ, Jansen JM, Melo PL. Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respir Med. 2006;100:2207–2219. doi: 10.1016/j.rmed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Komarow HD, Myles IA, Uzzaman A, Metcalfe DD. Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol. 2011;106:191–199. doi: 10.1016/j.anai.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y, Aledia AS, Galant SP, George SC. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol. 2013;131:718–723. doi: 10.1016/j.jaci.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Naglaa BA, Kamal E. Role of IOS in evaluation of patients with interstitial lung diseases. Egypt J Chest Dis Tuberc. 2016;65:791–795. [Google Scholar]
- 33.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Impulse oscillometry in adults with bronchiectasis. Ann Am Thorac Soc. 2015;12:657–665. doi: 10.1513/AnnalsATS.201406-280OC. [DOI] [PubMed] [Google Scholar]
- 34.Frantz S, Nihlén U, Dencker M, Engström G, Löfdahl CG, Wollmer P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med. 2012;106:1116–1123. doi: 10.1016/j.rmed.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Vogel J, Smidt U. Impulse oscillometry: analysis of lung mechanics in general practice and the clinic, epidemiological and experimental research. Frankfurt: PMI-Verlagsgruppe; 1994. [Google Scholar]
- 36.Newbury W, Crockett A, Newbury J. A pilot study to evaluate Australian predictive equations for the impulse oscillometry system. Respirology. 2008;13:1070–1075. doi: 10.1111/j.1440-1843.2008.01375.x. [DOI] [PubMed] [Google Scholar]
- 37.Guo YF, Herrmann F, Michel JP, Janssens JP. Normal values for respiratory resistance using forced oscillation in subjects>65 years old. Eur Respir J. 2005;26:602–608. doi: 10.1183/09031936.05.00010405. [DOI] [PubMed] [Google Scholar]
- 38.Shiota S, Katoh M, Fujii M, Aoki S, Matsuoka R, Fukuchi Y. Predictive equations and the reliability of the impulse oscillatory system in Japanese adult subjects. Respirology. 2005;10:310–315. doi: 10.1111/j.1440-1843.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012;129:671–678. doi: 10.1016/j.jaci.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic data of non-asthmatic and asthmatic patients with preserved lung function
Cutoff values of each parameter for asthma in adults with preserved lung function
Scatter plot of spirometry and IOS parameters. IOS parameters were not correlated with spirometry parameters.