Abstract
There are a number of implications of climate change in regard to human health. Among these, the role of rising carbon dioxide (CO2) and temperature in aeroallergen exposure and associated changes in the start, duration and intensity of the pollen season, and associated consequences in aeroallergens and allergic disease are a primary concern. This review is intended to provide a synopsis of CO2 and climate factors associated with likely changes in aeroallergen biology (indoor and outdoor), including changes in the demography of flowering plants, pollen seasonality, aeroallergen production, and potential biotic and abiotic interactions. These factors, in turn, are compared to clinical trials that have linked aeroallergens to allergic disease and associated health impacts. Finally, suggestions to address unmet needs and critical knowledge gaps are offered. Such recommendations are not meant to be inclusive, but to serve as a spur for the additional research and resources that will be necessary to acquire a better understanding of climate change, CO2, aeroallergens and associated allergic diseases. Such resources will be critical to derive time-relevant scientific and policy solutions that will minimize public health consequences in a changing climate.
Keywords: Allergens, rhinitis, allergy; climate change; pollen; carbon dioxide
INTRODUCTION
Pollen and fungal spore production and dissemination are widely recognized as having a major impact on the occurrence and the severity of allergic responses in human systems. Allergic diseases, including asthma, allergic rhinitis (AR), pollinosis and atopic dermatitis, are increasingly acknowledged as among the most prevalent and important non-communicable diseases globally.1,2 The World Health Organization has estimated that over 400 million people globally suffer from AR, and another 300 million from asthma.3 Both AR and asthma have previously been linked; with approximately 40% of AR patients having asthma, and over 80% of asthmatics who have AR.4 A recent meta-analysis of 29 eligible studies has indicated that AR is significantly associated with the occurrence of asthma.5 In addition, AR is an associated risk factor for other co-morbidities such as sinusitis, otitis media, nasal polyps and sleep apnea, which, in turn, can exacerbate AR.4
The global economic costs associated with asthma are significant, but estimates depend on the health impact factors considered. Direct costs can include expenditures related to hospitalization, diagnostic tests and medical treatments including asthma medication. Indirect costs include impact on employment, productivity losses and social costs. Overall, estimates have considerably depended on the disease subset with a more detailed assessment indicating approximately 25 billion USD for AR, 13.5 billion for acute bronchitis and 94.5 billion for asthma morbidity.6
Aeroallergen production from plants or fungi represents complex interactions between biology and environment. Hence, any worldwide environmental change will have a significant impact on aeroallergen production and subsequent changes in allergic disease. The goal of the current review is to elucidate potential climate and/or CO2 induced changes in indoor and outdoor aeroallergen production; provide a biological context for their potential interaction with allergic disease, compare that potential to existing clinical studies; and to provide guidance for future directions and unmet needs.
CLIMATE CHANGE
Energy intensification is a means to provide sufficient food and fuel to keep pace with an expanding population. At present, the basis for this necessary energy production is the burning of fossil fuels. Oxidation of carbon sources (fossil fuels) results in increased atmospheric levels of carbon dioxide. Estimates of total carbon emissions from all human activities, including agriculture and land use, are expected to be about 43 billion tons for 2019.7
As carbon dioxide absorbs infra-red radiation (heat), increasing atmospheric concentrations will result in enhanced surface temperatures. Since 1970, average surface air temperature has increased by approximately 1.6°F (up to 1.0°C).8 The term “global warming” is generally understood in the context of an average increase in surface temperature; yet in reality, differential increases in surface temperatures are occurring. For example, air temperatures in Alaska and artic regions are increasing at a much faster rate.8 In general, these differential temperature increases are occurring because of the relative concentration of water vapor, another greenhouse gas. For example, in the tropics, where water vapor (humidity) is high, rising levels of CO2 are not expected to result in as great an increase in surface temperature relative to regions where the air is dry (i.e., less water vapor). Hence, observed changes in surface temperature are greater at the poles and desert areas, where water vapor is low. Overall, however, in addition to warming, other weather phenomenon is being observed and projected, including spatial and temporal changes in precipitation, flooding and severe weather events.
CLIMATE-INDUCED CHANGES IN AEROALLERGEN PRODUCTION AND EXPOSURE
Demography
Temperature and moisture critically influence species population and demographics. Paleo-ecological data have shown that distribution shifts were a common response among plants during previous episodes of climate change, with corresponding shifts in community composition and ecosystem function.9,10 As such, similar shifts are expected in response to current climatic change.
While such shifts are of interest from an environmental perspective, they will also result in changes in the distribution and frequency of allergen-producing plant species and subsequent consequences for aeroallergen production. For example, warming in Scandinavia has resulted in greater competitiveness of European beech (Fagus sylvatica) over Norway spruce (Picea abies), the latter having decline in population due to drought and insect herbivores.11 Another important plant-based pollen source, common ragweed (Ambrosia artemisiifolia), has significantly expanded its range in Europe due to climate change.12 Rising levels of CO2 and warmer temperatures may also affect the demography of Parthenium weed, a noxious weed species in Asia, Africa and Australia (pollen and plant parts can result in various allergies, including contact dermatitis, hay fever, asthma and bronchitis).13
Similarly, it appears likely that climate change-related factors could be associated with a changing profile of indoor allergens. It is known, for example, that dust mites are very sensitive to relative humidity; in regions that become warmer and wetter with climatic change, dust mite populations could reflect tropical dust mite proliferation, and greater egg and allergen proliferation.14 As the sea level rises, those who live near coastal regions may run a potential risk for greater water exposure and wet housing conditions, including risk of greater indoor mold contamination and spore exposure.15 A meta-analysis of 33 epidemiological studies indicated increases between 30%-50% in adverse respiratory health outcomes for occupants because of dampness and mold exposure.16
Seasonality
Among plant species, annual production of aeroallergens follows a temporal order of tree pollen in the spring, followed by weed and grass pollen in the summer and ragweed in the fall. In absolute pollen production terms, the values follow a bimodal distribution with the largest pollen amounts generated in the spring, a decline during the summer and an uptick in the fall. Fungal species, including Alternaria, Cladosporium and Aspergillus, are associated in some studies with higher prevalence of asthma hospital admissions.17 Fungi, in general, require high humidity, moisture and warmer temperatures. Consequently, anthropogenic climate changes, specifically warmer temperatures, more frost-free days and potential increases in precipitation (e.g., humidity) are almost certain to have significant impacts on fungal life cycles, including, potentially, seasonal sporulation.18
For many global locations birch (Betula) pollen represents the first aeroallergen encountered in the spring. Long-term (30-year) analysis of birch pollen seasons throughout Europe indicated earlier start dates, with estimates that these start dates will advance by up to 6 days over 10 years.19 Additional research regarding the onset and duration of allergenic plant pollen indicates that seasonal changes for spring-flowering birch, oak and grass will shift northward, consistent with observed changes in plant hardiness zones (https://nca2014.globalchange.gov/report/appendices/climate-science-supplement/graphics/shifts-plant-hardiness-zones).20 The greater extent of warming with increasing latitude is also a factor in changing autumn allergies associated with ragweed. A synthesis of ragweed pollen season data along a 2,200 km north-south transect demonstrated that the duration of the ragweed pollen season has been increasing (up to 27 days at latitudes above 44°N) since 1995.21 These latitudinal changes, in turn, were associated with a delay in the first autumn frost and a lengthening of the frost-free period.21
For fungal or arthropod allergen production, there are rational links to climatic change that could influence seasonality; however, unlike plant aeroallergens, clear indications of seasonal changes are lacking. Such temporal disparity may reflect indoor environment, greater physical heterogeneity or social economic status.14 One of the few studies to examine spore production seasonality focused on Cladosporium in France and showed a downward trend in the southernmost locations and an upward trend in other locations. However, such trends appeared, independent of temperature increases that occurred continuously during the study period.22
Aeroallergen production
Annual compilation of airborne aeroallergen concentration, or annual pollen index (API), can differ relative to potential pollen/spore production and release.23 However, changes in API determined from pollen/spore monitoring is an appropriate quantitative measure of pollen season intensity or pollen load.24 As such, long-term changes in API are indicative of sources, but also of potential health consequences.
Temporal changes in API are of obvious interest in documenting anthropogenic climate change influences, with an especial focus on current and projected changes in temperature and/or CO2. Findings from specific pollen monitoring sites include those of Damialis et al.25 who showed a doubling of atmospheric pollen concentration per decade for 12 out of 16 species in Thessaloniki, Greece, suggesting that the factor most responsible for these changes was increasing air temperature. Clot26 analyzed temporal changes in 25 plant taxa from 1979 to 1999, but observed increasing trends for only 4, Alnus, Ambrosia, Artemisia and Taxus/Cupressaceae. Other researchers have noted no temporal increase in pollen for specific sites.27,28 Outdoor experiments where projected CO2 levels were applied are scarce, but Ladeau and Clark29 noted significantly greater pollen cone abundance of loblolly pine (Pinus taeda) and greater pollen production at ambient +200 ppm CO2. Studies with paper birch (Betula papyrifera), a more allergenic species at similar elevated CO2 concentrations, demonstrated large increases in the number of trees producing male flowers relative to ambient CO2 conditions, although pollen per se was not measured.30 Research with sawtooth oak trees (Quercus acutissima) at projected elevated CO2 concentrations of 560 and 720 ppm indicated substantial and significant increases in pollen production per tree as well as increases in allergenic protein concentration.31
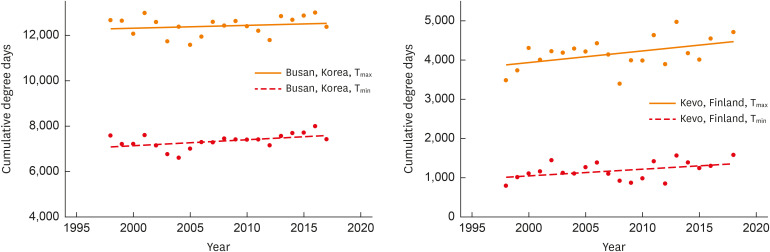
At present, much of the data regarding climate and API has been derived from particular monitoring stations. The first inclusive analysis of temporal metrics for pollen counts was published by Ziello et al.32 for over 1,000 pollen time series over 97 stations across Europe. They showed that 59% of the series increased in API, with 14% being statistically significant; and if significant, there was a significant correlation between API and local temperatures. This comprehensive work was followed by Zhang et al,20,33 who demonstrated that API increased by 46% from 1994-2000 to 2001-2010, with the increase associated with changes in growing degree days, frost-free days and precipitation. In addition, they showed that different Intergovernmental Panel on Climate Change scenarios is likely to increase cumulative and daily pollen counts to 2,100. These data are consistent with recent findings of Ziska et al.34 who analyzed long-term (decadal) pollen data for 17 locations around the northern hemisphere and were able to demonstrate that temperature extremes (Tmin and Tmax) may already be contributing to extended seasonal duration and increased pollen load for multiple aeroallergenic pollen taxa (Table). For this study, changes in latitude were associated with concomitant changes in temperature extremes, suggesting that more northern locations could potentially see a greater increase in overall pollen load (Figure).
Table. Temporal changes in seasonal cumulative pollen load (average percentage change per year) for 17 northern hemisphere locations for the number of years specified.
| Location | Years | Latitude (°N) | Season cumulative pollen | |
|---|---|---|---|---|
| Value (% yr−1) | P value | |||
| Brussels, Belgium | 35 | 50.85 | 2.8 | < 0.001‡ |
| Saskatoon, Canada | 23 | 52.13 | 1.7 | 0.060 |
| Winnipeg, Canada | 23 | 49.90 | 3.7 | 0.002† |
| Kevo, Finland | 38 | 69.45 | 3.1 | 0.050* |
| Turku, Finland | 42 | 60.45 | 10.4 | < 0.001‡ |
| Amiens, France | 29 | 49.85 | 3.8 | < 0.001‡ |
| Thessaloniki, Greece | 27 | 40.64 | 11.2 | < 0.001‡ |
| Reykjavik, Iceland | 25 | 64.12 | 7.4 | < 0.001‡ |
| Legnano, Italy | 21 | 45.60 | −0.3 | 0.744 |
| Busan, Korea | 20 | 35.18 | −1.0 | 0.231 |
| Seoul, Korea | 20 | 37.57 | −1.3 | 0.303 |
| Krakow, Poland | 20 | 50.06 | 9.0 | < 0.001† |
| Moscow, Russia | 23 | 55.76 | 7.7 | 0.010† |
| Geneva, Switzerland | 26 | 46.20 | 2.3 | 0.001‡ |
| Fairbanks, USA | 18 | 64.84 | 12.2 | 0.077 |
| Minneapolis, USA | 23 | 44.98 | 11.5 | < 0.001‡ |
| Papillion, USA | 25 | 41.15 | 3.1 | < 0.001‡ |
Significance was determined by first-order regression.
*P value between 0.05 and 0.01; †P value between 0.01 and 0.001; ‡P value < 0.001 (adapted from Ziska et al.34).
Figure. Changes in cumulative degrees above the freezing point (32°F) for Tmax and Tmin for 2 locations differing in latitude, Busan, Korea, and Kevo, Finland. Changes in annual pollen load, API, were not significant for Busan, Korea, but increased by 1.6% per year for Kevo, Finland. Data are adapted from Ziska et al.34.
Anticipated weather extremes (e.g., flooding and storms) or rising temperatures could also favor an increase in spore counts.22,35 After major flood events, however, a number of studies have observed a weak association between mold exposure and respiratory symptoms.14 This may be contingent of epidemiological factors related to social economic status; i.e., those who have the resources to fix their homes quickly may have less mold exposure.36 Damialis et al.37 examined a number of fungal species with high allergenic potential at differing temperatures (recent and projected) and divergent nutrient availability. They conclude that while all fungal species grew faster at higher temperatures, most species produced fewer spores as temperature increased. The exception was Cladosporium cladosporioides, which increased its spore production at projected 2,100 temperatures.37 The other study by Corden and Millington27 demonstrated that Alternaria spore concentration increased during 1970-1998 in Derby, UK, with the increase likely associated with temperature increases and harvesting periods. Overall, however, long-term evaluations of spore concentrations in the context of anticipated climate events are rare.
Interactions
Although aeroallergen production can be directly related to climatic factors (e.g., temperature) it is highly likely that exposure and health consequences will be exacerbated, or mitigated, by biological or abiotic (i.e., chemical/physical) interactions.
At the biological level, it is likely that both CO2 and temperature will change concomitantly. Yet, the consequences of such simultaneous changes on floral phenology and pollen production are difficult to assess, in part because of methodological challenges in maintaining CO2 and temperature treatments in an outdoor setting. This challenge has been partially overcome by using urbanization—which is characterized by higher CO2 values and warmer temperatures as a proxy for future climate change. For example, Roetzer et al.38 analyzed data for 4 spring-flowering shrubs and trees, and noted that species flowered 2-4 days earlier in urban relative to rural areas. In an urban-rural transect for Baltimore, Maryland, Ziska et al.39 observe that ragweed grew faster, flowered earlier and produced significantly more pollen per plant relative to the rural site.39 However, analysis of such transects is complicated by micro-environmental perturbations and does not allow separation of CO2 from temperature effects.
Another biological interaction is the symbiotic relationship between fungi and plants. As increasing CO2 can significantly affect plant photosynthesis and growth, can this in turn alter fungal sporulation of known hosts? For timothy grass (Phleum pretense) inoculated with Alternaria alternata and grown at recent and projected CO2 concentrations (300, 400, 500 and 600 ppm), Alternata produced nearly 3 times the number of spores and more than twice the total antigenic protein per plant at 600 ppm relative to 300 ppm CO2.40 While additional information is needed, CO2 induced increases in plant growth, combined with warmer temperatures and longer growing seasons could potentially lengthen exposure times and/or spore aeroallergen amounts.
A feature of climatic change is an increase in extreme weather events. Such events, in turn, are likely to have significant effects in regard to aeroallergen exposure. For example, dust storms or increasing wind has obvious implications in spore and pollen dispersal. In contrast, there may be a negative correlation between high precipitation events or drought on pollen transport and exposure.41
Among extreme events is the relationship between thunderstorms and asthma incidence. For example, on November 21, 2016, a severe thunderstorm event in Melbourne Australia resulted in 9,900 patients presented to hospitals with asthma attacks, with 9 deaths linked to the episode. At present, models suggest that future warmer climates may result in less thunderstorms overall, but an increase in the intensity of the thunderstorms that do occur, with projections of an increase in lightning by 10% for every 1 degree increase in global warming.42
Other man-made environmental perturbations, particularly air pollution, can be exacerbated by climate change, e.g., higher temperature can speed up the chemical reactions that lead to tropospheric ozone and secondhand particle formation43,44 However, air pollution, in turn, can have significant interactions in regard to aeroallergen exposure and health consequences.45 For example there is substantial evidence that diesel exhaust particular matter (DEP) can act synergistically with allergens to enhance allergic disease.46 Allergenic proteins and glycoproteins within the pollen grain can be released upon contact with water.47 These smaller size allergens can bind to DEP in the atmosphere and penetrate into the lower airways, resulting in simultaneous co-exposure to DEP and aeroallergens48,49 There is also substantial information regarding the association between living near high-traffic areas and diesel exhaust exposure as it relates to increased risk and severity of asthma and allergic disease.50,51,52
CLINICAL CONSEQUENCES
There are then a number of factors associated with anthropogenic climate change that may alter aeroallergen production and human exposure on a global basis. Physicians who treat allergic airway diseases are reporting an increase in symptomology that they attribute to climate change.53 The World Allergy Organization has suggested that climate change will alter seasonality and the intensity of pollen season while exacerbating the synergistic effects of pollutants on asthma.54 As such, are there clinical data that link climate change and aeroallergen production to human health impacts?
At present, clinical data are scarce, but of obvious interest. Data for spring peaks in tree pollen were associated with over-the-counter allergy medication sales and emergency department visits for asthma attacks, especially among children.55 In an Italian study that compared pollen counts and meteorological data in conjunction with allergen sensitization patterns of patients56 concluded that climate change, i.e., increased temperatures, could modify global pollen load and the rate of allergic sensitization across extended periods. At the national level, Upperman et al.57 used National Health Interview Survey data to show a clear link between exposure to extreme heat events and increased prevalence of hay fever among US adults. Sapkota et al.58 did a US county assessment and showed that counties that experienced earlier spring onsets had a 14% increase in hay fever, indicating climate effects on changing plant phenology and the prevalence of AR. Overall, these studies are consistent with climate and/or CO2 induced changes in aeroallergen production.
UNMET NEEDS, FUTURE DIRECTIONS AND OPPORTUNITIES
Our understanding of climatic change in the context of aeroallergen biology and the implications for respiratory health is becoming clearer. However, there are a number of specific research issues, as well as a number of current practices, that need to be assessed in order to improve health care outcomes related to climate change and allergic disease.
Aeroallergen demographics
There is an immediate need to understand and document the role of climate/CO2 in changing demography and evolutionary potential of known sources of pollen or spores that are known to be allergenic. Of particular note is the need to provide additional insight into mycological allergen sources both in regard to seasonality and sporulation as it relates to climatic change. Such information would provide valuable data regarding climate driven fluxes in bio-geographical distribution and phenological patterns of allergen release and distribution.
Standardization of pollen metrics
At present, there is no uniform standard for collecting pollen. The 2 most widely applied collection methodologies, a Burkard Hirst type volumetric pollen trap and a Rotorod volumetric sampler, can differ in their sensitivity, depending on particle size59 similarly, site pollen collection is often listed as “rooftop”, with less detail about height or surrounding vegetation. Similarly, there is no uniform standard as what constitutes a pollen “season” with different groups applying different metrics. Yet, acceptance of a uniform standard would be invaluable in evaluating climate impacts. Traditional methods of elaborating pollen and spore counts do not necessarily reflect the airborne allergen load that an individual may be exposed to over a given time; standardization could help determine threshold levels for sensitization and for symptom elicitation across different regions.
Automation and increasing sampling sites
There is an obvious need to provide relevant pollen information in the context of changing climate or population demographics (e.g., city vs. rural). However, such a need contrasts with the number of trained counters and site availability. For example, New York City, with an estimated metro population of 20 million, has 2 pollen collection sites.
Establishment of long-term aerobiological networks and pollution monitoring must be a goal for all countries if we are to monitor and assess the effects of climate change on respiratory health. This, in turn, will necessitate the development of more cost-effective and portable monitoring methodology to obtain these data. Automated pollen monitoring using recognition software has been suggested as one means to bridge the gap. In 1 report, a comparison of a Burkard system with a fully automated image recognition based pollen monitoring system (BAA500) for daily pollen counts was highly correlated (r = 0.98); however, some specific pollen sources (Salix) were not identified satisfactorily.60 Yet, there is obvious potential for upgrading software and automation for such systems (www.pollensense.com).
Environmental interactions
There are recognized complex interactions between air pollution and allergies; and links to allergen sensitization, inflammatory markers, lung development in children and other allergic conditions such as atopic dermatitis and insect allergies are certainly worthy of additional research.4 However, what is the role of other climatic consequences in air quality? Australia during the summer of 2019-2020 has experienced an unprecedented wildfire season, exacerbated by climatic change, with an estimated loss of approximately 40,000 square miles. How will concurrent changes in fire outbreaks and climate alter pollen seasonality and load? How will future extreme events alter allergen sensitivity and allergic disease?
In addition, there are multiple interactions among known climate variables that require further elucidation. Initial data61 suggested that rising CO2 per se could increase Amb a 1 allergenicity in ragweed, and these findings are consistent with those of another study for ragweed for recent and projected CO2 changes.62 Integrated assessments that account for urban vs. rural microclimates and land use changes (e.g., afforestation) will also be necessary to delineate and quantify environmental factors related to spatial and temporal changes in pollen or spore production.
Clinical trials
While there are initial clinical studies that corroborate observations regarding climate aspects in the context of aeroallergen production, much more needs to be done. There is an obvious demand to understand threshold levels for sensitization and symptom elicitation related to aeroallergen exposure and the role that climate change could play in that regard. A clear need to determine how to projected climatic changes are likely to affect other forms of allergic diseases such as food allergy, atopic dermatitis and stinging insect allergies. Finally, there is a shortage of broad-based studies that can directly correlate climate- or CO2-induced change in pollen or spore exposure to clinical symptoms and health outcomes.
Final thoughts
There is overwhelming scientific consensus that ongoing increases in CO2 and other anthropogenic infra-red absorbing gases are and will continue to alter a number of climatic variables. If, as expected, climate change impacts continue to intensify, there is a critical need to assess these impacts on aeroallergens, allergic disease and overall respiratory health.
Physicians, especially allergists and pulmonologists, are the first line of defense in helping individuals in coping and recovering from environmental factors that impact air quality. However, the science is multi-disciplinary, and should include modelers, climate experts, botanists and clinicians. There is a compelling need to continue and expand research, not only in regard to elucidating the underlying links between climate CO2 and projected temporal and seasonal changes in aeroallergens, but to addressing the degree to which human actions can manage changes in vegetation and aeroallergen exposure.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Gamble JL, Reid CE, Post E, Sacks J. Review of the impacts of climate variability and change on aeroallergens and their associated effects. Washington, D.C.: U.S. Environmental Protection Agency; 2008. [Google Scholar]
- 2.Ozdoganoglu T, Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis. 2012;6:11–23. doi: 10.1177/1753465811431975. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Prevention of allergy and allergic asthma. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Katelaris CH, Beggs PJ. Climate change: allergens and allergic diseases. Intern Med J. 2018;48:129–134. doi: 10.1111/imj.13699. [DOI] [PubMed] [Google Scholar]
- 5.Tohidinik HR, Mallah N, Takkouche B. History of allergic rhinitis and risk of asthma; a systematic review and meta-analysis. World Allergy Organ J. 2019;12:100069. doi: 10.1016/j.waojou.2019.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mudarri DH. Valuing the economic costs of allergic rhinitis, acute bronchitis, and asthma from exposure to indoor dampness and mold in the US. J Environ Public Health. 2016;2016:2386596. doi: 10.1155/2016/2386596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey C. CO2 emissions will break another record in 2019 [Internet] Boston: Scientific American; 2019. [cited 2020 Feb 10]. Available from: https://www.scientificamerican.com/article/co2-emissions-will-break-another-record-in-2019/ [Google Scholar]
- 8.Wuebbles DJ, Fahey DW, Hibbard KA, Dokken DJ, Stewart BC, Maycock TK. Climate science special report: fourth national climate assessment, volume I. Washington, D.C.: U.S. Global Change Research Program; 2017. [Google Scholar]
- 9.Willis KJ, MacDonald GM. Long-term ecological records and their relevance to climate change predictions for a warmer world. Annu Rev Ecol Evol Syst. 2011;42:267–287. [Google Scholar]
- 10.Stein RA, Sheldon ND, Smith S. Rapid response to anthropogenic climate change by Thuja occidentalis: implications for past climate reconstructions and future climate predictions. PeerJ. 2019;7:e7378. doi: 10.7717/peerj.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolte A, Czajkowski T, Kompa T. The north-eastern distribution range of European beech-a review. Forestry. 2007;80:413–429. [Google Scholar]
- 12.Richter R, Berger UE, Dullinger S, Essl F, Leitner M, Smith M, et al. Spread of invasive ragweed: climate change, management and how to reduce allergy costs. J Appl Ecol. 2013;5:1422–1430. [Google Scholar]
- 13.Nguyen T, Bajwa AA, Navie S, O'Donnell C, Adkins S. Parthenium weed (Parthenium hysterophorus L.) and climate change: the effect of CO2 concentration, temperature, and water deficit on growth and reproduction of two biotypes. Environ Sci Pollut Res Int. 2017;24:10727–10739. doi: 10.1007/s11356-017-8737-7. [DOI] [PubMed] [Google Scholar]
- 14.Chew GL, Saha S. Impacts of climate change on indoor allergens. In: Beggs PJ, editor. Impacts of climate change on allergens and allergic diseases. Cambridge: Cambridge University Press; 2016. pp. 113–136. [Google Scholar]
- 15.Barne C, Alexis NE, Bernstein JA, Cohn JR, Demain JG, Horner E, et al. Climate change and our environment: the effect on respiratory and allergic disease. J Allergy Clin Immunol Pract. 2013;1:137–141. doi: 10.1016/j.jaip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17:284–296. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 17.Cecchi L, D'Amato G, Ayres JG, Galan C, Forastiere F, Forsberg B, et al. Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy. 2010;65:1073–1081. doi: 10.1111/j.1398-9995.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- 18.Peden D, Reed CE. Environmental and occupational allergies. J Allergy Clin Immunol. 2010;125:S150–60. doi: 10.1016/j.jaci.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 19.Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, Rantio-Lehtimäki A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol. 2002;46:159–170. doi: 10.1007/s00484-002-0139-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Bielory L, Mi Z, Cai T, Robock A, Georgopoulos P. Allergenic pollen season variations in the past two decades under changing climate in the United States. Glob Change Biol. 2015;21:1581–1589. doi: 10.1111/gcb.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziska L, Knowlton K, Rogers C, Dalan D, Tierney N, Elder MA, et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc Natl Acad Sci U S A. 2011;108:4248–4251. doi: 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sindt C, Besancenot JP, Thibaudon M. Airborne Cladosporium fungal spores and climate change in France. Aerobiologia. 2016;32:53–68. [Google Scholar]
- 23.Frei T, Gassner E. Climate change and its impact on birch pollen quantities and the start of the pollen season an example from Switzerland for the period 1969–2006. Int J Biometeorol. 2008;52:667–674. doi: 10.1007/s00484-008-0159-2. [DOI] [PubMed] [Google Scholar]
- 24.Galán C, García-Mozo H, Vázquez L, Ruiz L, Díaz De La Guardia C, Domínguez-Vilches E. Modeling olive crop yield in Andalusia, Spain. Agron J. 2008;100:98–104. [Google Scholar]
- 25.Damialis A, Halley JM, Gioulekas D, Vokou D. Long-term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmos Environ. 2007;41:7011–7021. [Google Scholar]
- 26.Clot B. Trends in airborne pollen: an overview of 21 years of data in Neuchâtel (Switzerland) Aerobiologia. 2003;19:227–234. [Google Scholar]
- 27.Corden JM, Millington WM. The long-term trends and seasonal variation of the aeroallergen Alternaria in Derby, UK. Aerobiologia. 2001;17:127–136. [Google Scholar]
- 28.Frei T, Gassner E. Trends in prevalence of allergic rhinitis and correlation with pollen counts in Switzerland. Int J Biometeorol. 2008;52:841–847. doi: 10.1007/s00484-008-0178-z. [DOI] [PubMed] [Google Scholar]
- 29.Ladeau SL, Clark JS. Pollen production by Pinus taeda growing in elevated atmospheric CO2 . Funct Ecol. 2006;20:541–547. [Google Scholar]
- 30.Darbah JN, Kubiske ME, Nelson N, Oksanen E, Vapaavuori E, Karnosky DF. Effects of decadal exposure to interacting elevated CO2 and/or O3 on paper birch (Betula papyrifera) reproduction. Environ Pollut. 2008;155:446–452. doi: 10.1016/j.envpol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Kim KR, Oh JW, Woo SY, Seo YA, Choi YJ, Kim HS, et al. Does the increase in ambient CO2 concentration elevate allergy risks posed by oak pollen? Int J Biometeorol. 2018;62:1587–1594. doi: 10.1007/s00484-018-1558-7. [DOI] [PubMed] [Google Scholar]
- 32.Ziello C, Sparks TH, Estrella N, Belmonte J, Bergmann KC, Bucher E, et al. Changes to airborne pollen counts across Europe. PLoS One. 2012;7:e34076. doi: 10.1371/journal.pone.0034076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Isukapalli S, Bielory L, Georgopoulos P. Bayesian analysis of climate change effects on observed and projected airborne levels of birch pollen. Atmos Environ (1994) 2013;68:64–73. doi: 10.1016/j.atmosenv.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziska LH, Makra L, Harry SK, Bruffaerts N, Hendrickx M, Coates F, et al. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: a retrospective data analysis. Lancet Planet Health. 2019;3:e124–31. doi: 10.1016/S2542-5196(19)30015-4. [DOI] [PubMed] [Google Scholar]
- 35.Celenza A, Fothergill J, Kupek E, Shaw RJ. Thunderstorm associated asthma: a detailed analysis of environmental factors. BMJ. 1996;312:604–607. doi: 10.1136/bmj.312.7031.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbeau DN, Grimsley LF, White LE, El-Dahr JM, Lichtveld M. Mold exposure and health effects following hurricanes Katrina and Rita. Annu Rev Public Health. 2010;31:165–178. doi: 10.1146/annurev.publhealth.012809.103643. [DOI] [PubMed] [Google Scholar]
- 37.Damialis A, Mohammad AB, Halley JM, Gange AC. Fungi in a changing world: growth rates will be elevated, but spore production may decrease in future climates. Int J Biometeorol. 2015;59:1157–1167. doi: 10.1007/s00484-014-0927-0. [DOI] [PubMed] [Google Scholar]
- 38.Roetzer T, Wittenzeller M, Haeckel H, Nekovar J. Phenology in central Europe--differences and trends of spring phenophases in urban and rural areas. Int J Biometeorol. 2000;44:60–66. doi: 10.1007/s004840000062. [DOI] [PubMed] [Google Scholar]
- 39.Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111:290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- 40.Wolf J, O'Neill NR, Rogers CA, Muilenberg ML, Ziska LH. Elevated atmospheric carbon dioxide concentrations amplify Alternaria alternata sporulation and total antigen production. Environ Health Perspect. 2010;118:1223–1228. doi: 10.1289/ehp.0901867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehrig R. The influence of the hot and dry summer 2003 on the pollen season in Switzerland. Aerobiologia. 2006;22:27–34. [Google Scholar]
- 42.Price C. Thunderstorms, lightning and climate change. In: Betz HD, Schumann U, Laroche P, editors. Lightning: principles, instruments and applications. Dordrecht: Springer; 2009. pp. 521–535. [Google Scholar]
- 43.Likhvar VN, Pascal M, Markakis K, Colette A, Hauglustaine D, Valari M, et al. A multi-scale health impact assessment of air pollution over the 21st century. Sci Total Environ. 2015;514:439–449. doi: 10.1016/j.scitotenv.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Sujaritpong S, Dear K, Cope M, Walsh S, Kjellstrom T. Quantifying the health impacts of air pollution under a changing climate-a review of approaches and methodology. Int J Biometeorol. 2014;58:149–160. doi: 10.1007/s00484-012-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinney P, Weinberger K, Miller R. Interactions among climate change, air pollutants, and aeroallergens. In: Beggs P, editor. Impacts of climate change on allergens and allergic diseases. Cambridge: Cambridge University Press; 2016. pp. 113–136. [Google Scholar]
- 46.Muranaka M, Suzuki S, Koizumi K, Takafuji S, Miyamoto T, Ikemori R, et al. Adjuvant activity of diesel-exhaust particulates for the production of IgE antibody in mice. J Allergy Clin Immunol. 1986;77:616–623. doi: 10.1016/0091-6749(86)90355-6. [DOI] [PubMed] [Google Scholar]
- 47.Howlett BJ, Knox RB. Allergic interactions. In: Linskens HF, Heslop-Harrison J, editors. Cellular interactions. Berlin: Springer; 1984. pp. 655–673. [Google Scholar]
- 48.Knox RB, Suphioglu C, Taylor P, Desai R, Watson HC, Peng JL, et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997;27:246–251. [PubMed] [Google Scholar]
- 49.Steinsvik TE, Ormstad H, Gaarder PI, Aaberge IS, Bjønness U, Løvik M. Human IgE production in hu-PBL-SCID mice injected with birch pollen and diesel exhaust particles. Toxicology. 1998;128:219–230. doi: 10.1016/s0300-483x(98)00075-4. [DOI] [PubMed] [Google Scholar]
- 50.Burnett RT, Cakmak S, Brook JR, Krewski D. The role of particulate size and chemistry in the association between summertime ambient air pollution and hospitalization for cardiorespiratory diseases. Environ Health Perspect. 1997;105:614–620. doi: 10.1289/ehp.97105614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McConnell R, Berhane K, Yao L, Lurmann FW, Avol E, Peters JM. Predicting residential ozone deficits from nearby traffic. Sci Total Environ. 2006;363:166–174. doi: 10.1016/j.scitotenv.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 52.Gordian ME, Haneuse S, Wakefield J. An investigation of the association between traffic exposure and the diagnosis of asthma in children. J Expo Sci Environ Epidemiol. 2006;16:49–55. doi: 10.1038/sj.jea.7500436. [DOI] [PubMed] [Google Scholar]
- 53.Sarfaty M, Bloodhart B, Ewart G, Thurston GD, Balmes JR, Guidotti TL, et al. American Thoracic Society member survey on climate change and health. Ann Am Thorac Soc. 2015;12:274–278. doi: 10.1513/AnnalsATS.201410-460BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Amato G, Holgate ST, Pawankar R, Ledford DK, Cecchi L, Al-Ahmad M, et al. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ J. 2015;8:25. doi: 10.1186/s40413-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito K, Weinberger KR, Robinson GS, Sheffield PE, Lall R, Mathes R, et al. The associations between daily spring pollen counts, over-the-counter allergy medication sales, and asthma syndrome emergency department visits in New York City, 2002–2012. Environ Health. 2015;14:71–78. doi: 10.1186/s12940-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ariano R, Canonica GW, Passalacqua G. Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann Allergy Asthma Immunol. 2010;104:215–222. doi: 10.1016/j.anai.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Upperman CR, Parker JD, Akinbami LJ, Jiang C, He X, Murtugudde R, et al. Exposure to extreme heat events is associated with increased hay fever prevalence among nationally representative sample of US adults: 1997–2013. J Allergy Clin Immunol Pract. 2017;5:435–441.e2. doi: 10.1016/j.jaip.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapkota A, Murtugudde R, Curriero FC, Upperman CR, Ziska L, Jiang C. Associations between alteration in plant phenology and hay fever prevalence among US adults: implication for changing climate. PLoS One. 2019;14:e0212010. doi: 10.1371/journal.pone.0212010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frenz DA. Comparing pollen and spore counts collected with the Rotorod Sampler and Burkard spore trap. Ann Allergy Asthma Immunol. 1999;83:341–347. doi: 10.1016/S1081-1206(10)62828-1. [DOI] [PubMed] [Google Scholar]
- 60.Oteros J, Pusch G, Weichenmeier I, Heimann U, Möller R, Röseler S, et al. Automatic and online pollen monitoring. Int Arch Allergy Immunol. 2015;167:158–166. doi: 10.1159/000436968. [DOI] [PubMed] [Google Scholar]
- 61.Singer BD, Ziska LH, Frenz DA, Gebhard DE, Straka JG. Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct Plant Biol. 2005;32:667–670. doi: 10.1071/FP05039. [DOI] [PubMed] [Google Scholar]
- 62.Choi YJ, Oh HR, Oh JW, Kim KR, Kim MJ, Kim BJ, et al. Chamber and field studies demonstrate differential Amb a 1 contents in common ragweed depending on CO2 levels. Allergy Asthma Immunol Res. 2018;10:278–282. doi: 10.4168/aair.2018.10.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]