Abstract
Purpose
It remains unknown whether allergen-specific immunotherapy (AIT) could attenuate airway inflammatory response triggered by allergen exposure.
Methods
We performed Dermatophagoides pteronyssinus (Der-p) nasal provocation tests (NPTs) in allergic rhinitis (AR) and/or asthma patients without AIT (non-AIT), or at 16, 52, 104, or 156 weeks after Der-p AIT. Rhinitis and asthma visual analog scale (VAS; VAS of nasal symptoms [VAS-NS], VAS of asthma symptoms), the rhinoconjunctivitis quality of life questionnaire (RQLQ), nasal lavage, sputum induction, fractional exhaled nitric oxide (FeNO), nasal airway resistance, pulmonary function, and airway hyperresponsiveness were performed before and after NPT.
Results
Non-AIT subjects demonstrated significantly higher VAS-NS before and after NPT compared to AIT subjects (P < 0.05). NPT response was positive in 14 (100%) non-AIT, 7 (70%) 16 weeks-AIT, 6 (60%) 52 weeks-AIT, 6 (60%) 104 weeks-AIT, and 2 (20%) 156 weeks-AIT subjects. The NPT grade significantly correlated with AIT duration and baseline RQLQ score (r = −0.561, P < 0.001 and r = 0.525, P < 0.001, respectively). Sputum and nasal lavage eosinophil count, and FeNO in non-AIT subjects were significantly increased 6 hours after NPT (P < 0.05). AIT subjects did not change their sputum or nasal lavage eosinophil count before and after NPT. Subjects with 156 weeks-AIT demonstrated significantly lower levels of sputum and nasal lavage eosinophil count before and after NPT when compared with non-AIT patients (P < 0.05). Sputum eosinophil counts positively correlated with nasal lavage eosinophil counts at baseline and 6 hours after NPT (r = 0.719, P = 0.006 and r = 0.823, P < 0.001, respectively) in non-AIT patients.
Conclusion
Our results show that AIT can attenuate both upper and lower airway immune response to nasal allergen exposure in patients with AR and/or asthma.
Keywords: Asthma, allergic rhinitis, immunotherapy, allergy, inflammation, allergen, house dust mite
INTRODUCTION
Allergen-specific immunotherapy (AIT) is the only disease-modifying therapy that is effective for the treatment of allergic diseases including allergic rhinitis (AR) and asthma.1,2 AIT builds tolerance towards the allergen through long-term repeated exposure to defined doses of allergen, together with drug therapy and allergen avoidance.3 The efficacy of AIT for both children and adults in pollen, pet dander, and house dust mite (HDM) has been confirmed by recent systematic meta-analyses and real-world practice.4,5,6
AIT can achieve substantial clinical results by improving nasal and respiratory symptoms by reducing medication need.7 AIT is also associated with an improvement in quality of life, and prevents the progression of AR to asthma by reducing new sensitization. Clinical efficacy can persist over a long period of time after discontinuation.8 Symptom and medication score, the visual analog scale (VAS), and the rhinoconjunctivitis quality of life questionnaire (RQLQ) are the most commonly used indices to evaluate the efficacy of AIT both in clinical trials and in clinical practice.9,10 However, these subjective parameters cannot offer solid evidence to indicate the effect of AIT on attenuating inflammation in the upper or lower airway in response to allergens.
The nasal provocation test (NPT) is an objective tool to diagnose AR and has been used to evaluate AIT efficacy in clinical trials.11 Both titrated and single-dose nasal challenge protocols targeting grass, birch, HDM, and cat allergens have been found to be well correlated with immunological outcomes12,13 Response to nasal challenge could serve as a surrogate for the reaction occurring during natural exposure to allergens, while allowing dose control and real-time recording of symptoms.14 We have previously reported that NPT can increase lower airway eosinophilic inflammation in AR patients without asthma symptoms.15 Although it is reported that the nasal response to NPT is attenuated after AIT, it remains unknown whether it could decrease lower airway inflammation triggered by NPT.
Methods that evaluate lower airway inflammation, such as bronchial biopsy and alveolar lavage, are invasive. Induced sputum cell counts and fractional exhaled nitric oxide (FeNO) are noninvasive markers of lower airway inflammation, and their levels correlate well with eosinophil levels in bronchial biopsies and lavage.16
In the current study, we compared changes in lower airway inflammation as indicated by sputum eosinophil count and upper airway inflammation as assessed by nasal lavage fluid eosinophil count, before and after 6 hours of NPT. The chosen timing to assess changes in inflammation was based on the evidence that allergic reactions typically develop after 2-6 hours and peak 6-9 hours after allergen exposure while recruiting and activating immune cells, especially eosinophils.17
MATERIALS AND METHODS
Study design and subjects
The study was conducted at the Department of Allergy and Clinical Immunology at The First Affiliated Hospital of Guangzhou Medical University from December 2018 to December 2019. We recruited patients with AR and/or asthma treated with medications but not AIT (non-AIT) as well as those treated with 16 weeks-, 52 weeks-, 104 weeks-, and 156 weeks-AIT. All subjects fulfilled the diagnostic criteria of the AR and its Impact on Asthma guideline for AR and/or the Global Initiative for Asthma guideline for mild-to-moderate asthma.18,19 Non-AIT patients were treated with regular symptom-control medications. Patients with AIT received Der-p AIT and finished with an up-dosing phase (16 weeks) or a maintenance dose at 52, 104, and 156 weeks following a standard dosing protocol (Denmark, ALK-Abello, Alutard-SQ). Patients were excluded if they had chronic or recurrent sinusitis, a history of smoking, and a forced expiratory volume in 1 second (FEV1) < 70% as predicted at screening. Participants did not use nasal corticosteroids or any other anti-histamine or anti-leukotriene receptor medication at least 2 weeks prior to pre-NPT assessment. The study was approved by the ethical committee of the first affiliated hospital of Guangzhou Medical University (Institutional Review Board No. 81370129) and we obtained written informed consent from all subjects.
Before performing NPT, all subjects were given a pre-NPT clinical assessment, including pulmonary function and methacholine bronchial challenge testing, allergen skin prick tests, total and specific immunoglobulin E (IgE) measurements, FeNO measurements, anterior rhinomanometry for nasal airway resistance (NAR), and eosinophil count in nasal lavage and induced sputum. In addition, subjects were offered questionnaires, including the RQLQ, the asthma control test (ACT), and VAS of nasal symptoms (VAS-NS), including rhinorrhea, sneezing, itching, and congestion. Asthma symptoms were assessed with the VAS-AS, including coughing, chest tightness, breathlessness and wheezing, in order to assess the severity of symptoms as well as the influence of diseases on perceived quality of life.
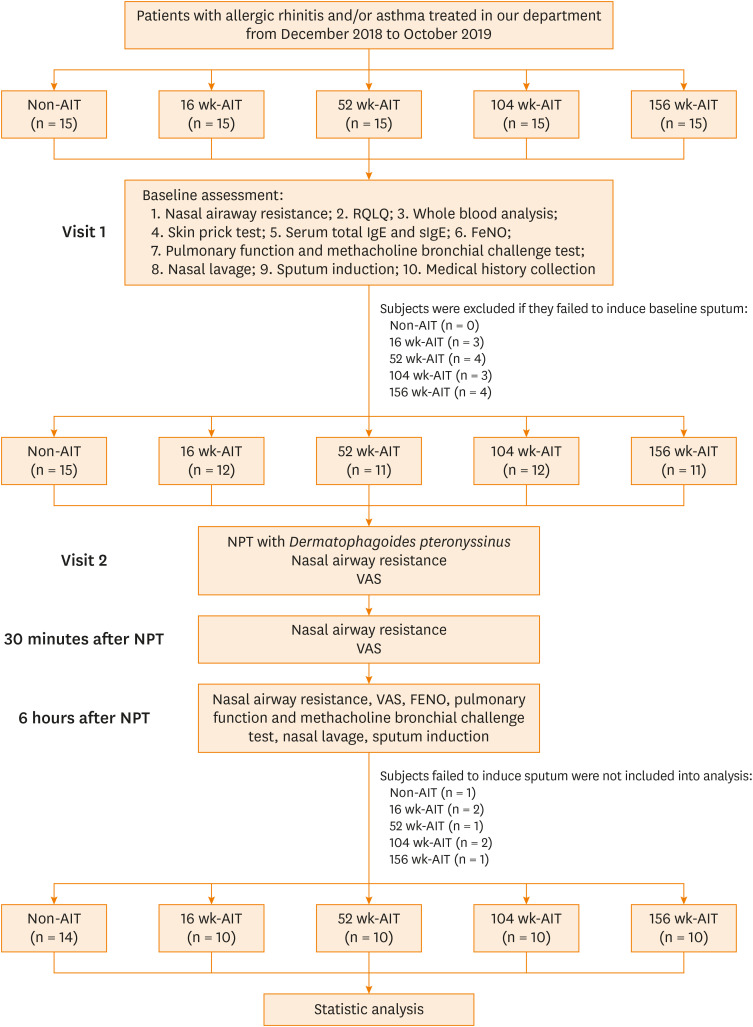
Subjects were excluded if they failed to induce sputum at baseline assessment. On the following day, the subjects included underwent a Der-p NPT. NAR, VAS-NS, and VAS-AS were documented at the endpoint, post-30 minutes, and post-6 hours of NPT, respectively. Additionally, after 6 hours of NPT, subjects were provided a post-NPT clinical assessment, which included FeNO measurement, assessment of pulmonary function, methacholine bronchial challenge test, and eosinophil count in nasal lavage and induced sputum. Subjects with 2 successful sputum inductions were included in the final statistical analysis (Fig. 1).
Fig. 1. Flow diagram of study progression.
AIT, allergen-specific immunotherapy; NPT, nasal provocation test; RQLQ, rhinoconjunctivitis quality of life questionnaire; IgE, immunoglobulin E; sIgE, specific immunoglobulin E; FeNO, fractional exhaled nitric oxide; VAS, visual analogue scale.
VAS
The visual analog scale evaluation system comprised a 10-cm-long segment with markings at 1-cm intervals, upon which the subject was asked to indicate his/her actual perception of symptoms by marking a point along the segment.20 In this study, 0 corresponded to optimal symptom-free, whereas 10 corresponded to the most severe symptoms.
Der-p NPT procedure
NPT was performed according to the guidelines of the German Society for Allergy and Clinical Immunology, using a Rhino V-4.53 rhinomanometer (Jaeger™; CareFusion, Hoechberg, German).21 All diluent and Der-p solution was delivered to the inferior turbinate with a nebulizer with a fixed volume of 100 µL per puff. Solutions were applied to the subjects with breath holding after a deep inspiration to avoid the allergen being inhaled into the lower airways. NAR was repeatedly measured at a transnasal pressure difference of 150 Pa with active rhinomanometry. After a 30-minute adaptation period, the subjects were given 100 µL of diluent, followed by rhinometry to exclude nasal hyper-reactivity. In the absence of a nonspecific response, increasing concentrations of Der-p allergen extract (10, 100, 1,000, and 10,000 BU/mL) (Inmunotek SL, Madrid, Spain) were applied in both nostrils at 10-minute intervals. After each application, clinical symptoms were determined and rhinomanometry was performed. Total nasal symptom scores represented the sum of the scores for: sneezing (0-2 sneezes = 0; 3-5 = 1; >5 = 2); rhinorrhea (moderate = 1; severe = 2); and tearing and/or itching (eyes and/or throat = 1; conjunctivitis, cough, urticaria and/or dyspnea = 2). NPT was considered positive if 1) the total nasal symptom score was ≥4; 2) nasal airflow reduced 60% or more compared to the baseline level; and 3) the symptom score was ≥3 plus nasal airflow was reduced by 20% or more.22 Grade for nasal responsiveness (NPT-grade) was classified according to the Der-p allergen dosage (BU/mL) of endpoint of NPT and categorized as follows: Der-p > 10,000 BU/mL = 0; 1,000 BU/mL < Der-p ≤ 10,000 BU/mL = 1; 100 BU/mL < Der-p ≤ 1,000 BU/mL = 2; 10 BU/mL < Der-p ≤ 100 BU/mL = 3; 1 BU/mL < Der-p ≤ 10 BU/mL = 4; and diluent and Der-p ≤ 1 BU/mL = 5.
Lung function test and FeNO measurement
Assessment of pulmonary function and the methacholine bronchial challenge test were conducted by the trained operators with a spirometer (MasterScreen PFT; Jaeger™; CareFusion), in accordance with the American Thoracic Society/European Respiratory Society guidelines.23 Bronchial hyperresponsiveness was defined as FEV1 decreasing to ≥20% of its baseline level when ≤2.504 mg of a cumulative dose of methacholine (PD20) was administered. FeNO was measured with a portable rapid response chemiluminescent analyzer at an expiratory flow rate of 50 mL/sec (NIOX System; Aerocrine, Stockholm, Sweden).24
Nasal lavage, sputum induction, and inflammatory cell differentiation
Both nasal lavage and sputum induction were performed before NPT and 6 hours after NPT. During nasal lavage, subjects sat upright with head posed forward. Ten milliliters of sterile saline solution (0.9% NaCl room temperature in a syringe with a sponge adapter) was then infused into the nostrils. The lavage fluid was immediately centrifuged for 10 minutes at 3,000 g and 4°C, and then the supernatant was aspirated. Then, dithiothreitol solution was added to the sample for 15 minutes to solubilize mucus and cells were re-suspended for cytology staining. Cytospin slides with the cell pellet were air-dried, fixed, and stained with hematoxylin and eosin (H&E). The cell pellet was observed under a light microscope (×200), and the number of eosinophils was recorded as the average absolute value of the number of eosinophils in 20 non-overlapping microscope fields. Sputum induction was performed according to a method previously described.25 Briefly, sputum was induced from each subject by inhalation of 3% hypertonic saline solution for 15 minutes. Patients rinsed their mouth with 0.9% saline solution before sputum expectoration in order to minimize oral contamination and blew their nose. They expectorated sputum into a cup. The first portion of sputum was discarded, and the inhalation procedure was continued for a further 15 minutes. The collected sample was treated with dithioerythritol, vortexed for 15 seconds, and incubated for 15 minutes at 37°C. The samples were then centrifuged for 10 minutes at 3,000 g and 4°C. Cytospin slides were prepared and stained with H&E, and a differential cell count was obtained from 400 non-squamous cells. Only samples with cell viability > 70% and squamous cell contamination < 20% were analyzed.
Statistical analysis
Statistical analyses were performed using the SPSS software package (version 22.0; IBM Corp., Armonk, NY, USA). Continuous variables are expressed as number (%), mean ± standard deviation, median, and interquartile range. The NPT positivity and grades of nasal responsiveness were numerical data and compared between groups with the chi-square test and Fisher's exact test. Comparisons between groups were made using the t test or Mann-Whitney U test for continuous endpoints and the χ2 test for categorical endpoints. Correlation analysis was assessed using Pearson's correlation or Spearman's correlation. P values < 0.05 were considered statistically significant.
RESULTS
Demographics and pre-NPT clinical characteristics of the subjects recruited
Fourteen non-AIT subjects and 10 AIT subjects at each of the AIT time points (16 weeks-, 52 weeks-, 104 weeks-, and 156 weeks-AIT) were included in the data analysis. The detailed demographic and clinical characteristics of the subjects are presented in Table 1. Briefly, there was no significant difference among the 5 groups in sex, age, body mass index, age of rhinitis onset, lung function indices, or FeNO value (all P > 0.05). Subjects in the AIT groups exhibited significantly smaller skin prick testing wheal size against Der-p (P < 0.001) as well as a smaller proportion of nasal medication use (P < 0.05). There was no significant difference in total IgE or Der-p specific IgE serum levels between the groups. Furthermore, the RQLQ scores of the subjects were significantly lower in 104 weeks- and 156 weeks-AIT groups than in the non-AIT group (P < 0.05).
Table 1. Demographic and clinical characteristics of all subjects before NPT.
| Subjects' characteristics | Non-AIT subjects (n = 14) | AIT subjects | P value | ||||
|---|---|---|---|---|---|---|---|
| 16 wk (n = 10) | 52 wk (n = 10) | 104 wk (n = 10) | 156 wk (n = 10) | ||||
| Age (yr) | 24.71 ± 9.17 | 27.70 ± 9.13 | 28.80 ± 9.05 | 30.60 ± 12.94 | 34.70 ± 10.51 | 0.215 | |
| Sex (female) | 35.70 | 40.00 | 40.00 | 40.00 | 30.00 | 0.987 | |
| Body mass index (kg/m2) | 21.01 ± 2.73 | 21.12 ± 3.52 | 21.05 ± 3.51 | 22.33 ± 3.54 | 22.29 ± 2.71 | 0.735 | |
| Age of rhinitis onset (yr) | 13.57 ± 7.39 | 17.90 ± 10.83 | 17.70 ± 8.41 | 18.30 ± 14.09 | 13.20 ± 6.91 | 0.570 | |
| Combined with asthma | 92.90 | 70.00 | 70.00 | 90.00 | 100.00 | 0.220 | |
| FeNO (ppb) | 47.85 ± 20.19 | 37.40 ± 20.68 | 37.30 ± 14.91 | 36.30 ± 14.15 | 36.80 ± 13.27 | 0.386 | |
| Parameters of lung function | |||||||
| FEV1 (% predicted) | 92.09 ± 6.49 | 92.38 ± 6.32 | 88.46 ± 7.61 | 90.20 ± 6.43 | 88.08 ± 7.42 | 0.747 | |
| FVC (% predicted) | 97.72 ± 8.65 | 99.58 ± 11.75 | 104.00 ± 15.17 | 102.59 ± 8.62 | 105.92 ± 11.31 | 0.410 | |
| FEV1/FVC (% predicted) | 94.66 ± 13.12 | 91.60 ± 9.40 | 86.73 ± 10.60 | 92.50 ± 10.93 | 84.02 ± 6.23 | 0.124 | |
| FEF25–75 (% predicted) | 76.21 ± 30.32 | 62.65 ± 16.50 | 61.92 ± 27.28 | 66.78 ± 22.61 | 49.20 ± 7.860 | 0.101 | |
| BHR (PD20 mg) | 0.99 ± 0.91 | 1.46 ± 1.11 | 1.41 ± 1.18 | 1.20 ± 0.99 | 1.26 ± 0.99 | 0.619 | |
| Total IgE (kU/l) | 230.50 (155.00–718.50) | 447.00 (278.00–1,636.00) | 311.50 (130.25–609.00) | 753.50 (433.75–1,314.50) | 315.50 (112.40–744.25) | 0.145 | |
| sIgE of Der-p (kU/l) | 33.55 (20.18–62.57) | 77.92 (27.55–174.50) | 34.40 (9.54–95.20) | 51.75 (33.65–114.58) | 34.85 (9.13–69.60) | 0.240 | |
| sIgE of Der-f (kU/l) | 28.30 (26.10–59.50) | 65.70 (45.40–143.23) | 32.95 (20.85–85.21) | 65.25 (42.98–122.52) | 35.30 (6.48–59.80) | 0.064 | |
| Wheal of Der-p SPT (mm) | 11.32 ± 3.91 | 6.38 ± 1.80* | 5.05 ± 1.42* | 4.60 ± 1.47* | 4.55 ± 1.09* | <0.001 | |
| Wheal of Der-f SPT (mm) | 9.54 ± 5.50 | 6.08 ± 1.98* | 4.78 ± 1.51* | 5.25 ± 1.55* | 5.25 ± 1.64* | 0.003 | |
| Peripheral neutrophil (109/L) | 3.72 ± 1.71 | 3.92 ± 1.36 | 3.45 ± 1.25 | 3.71 ± 1.13 | 3.53 ± 1.46 | 0.738 | |
| Peripheral eosinophil count (109/L) | 0.42 ± 0.29 | 0.32 ± 0.23 | 0.29 ± 0.14 | 0.37 ± 0.23 | 0.25 ± 0.11 | 0.373 | |
| Nasal lavage eosinophils (/200 H&E) | 19.76 ± 16.31 | 16.00 ± 13.61 | 14.70 ± 10.52 | 18.50 ± 12.00 | 7.65 ± 4.97* | 0.229 | |
| Induced sputum | |||||||
| Neutrophil | 31.84 ± 18.75 | 28.40 ± 23.65 | 35.40 ± 16.30 | 35.62 ± 21.87 | 48.99 ± 16.73 | 0.144 | |
| Eosinophil | 11.54 ± 4.81 | 7.93 ± 4.52 | 8.52 ± 5.94 | 7.43 ± 5.92 | 3.51 ± 2.74* | 0.009 | |
| Lymphocyte | 2.58 ± 3.72 | 1.81 ± 4.27 | 1.83 ± 1.65 | 1.53 ± 1.86 | 1.82 ± 2.69 | 0.942 | |
| Macrophage | 54.05 ± 21.97 | 61.86 ± 30.00 | 54.25 ± 20.00 | 55.42 ± 27.56 | 45.68 ± 16.29 | 0.727 | |
| Overall RQLQ score | 48.86 ± 19.90 | 38.60 ± 34.29 | 36.70 ± 29.27 | 23.30 ± 14.06* | 21.30 ± 16.50* | <0.001 | |
| Asthma control test score | 23.71 ± 1.38 | 24.4 ± 0.97 | 24.8 ± 0.42 | 24.9 ± 0.31 | 24.5 ± 0.53 | 0.108 | |
| Current medications | |||||||
| ICS | 74.42 | 30.00 | 40.00 | 30.00 | 10.00 | 0.037 | |
| LTRA | 21.43 | 10.00 | 10.00 | 0.00 | 0.00 | <0.001 | |
| Antihistamine | 42.86 | 10.00 | 0.00 | 0.00 | 0.00 | 0.006 | |
| Intranasal CS | 57.14 | 20.00 | 20.00 | 20.00 | 0.00 | 0.024 | |
Data are expressed as mean ± standard deviation, median (interquartile range), or number (%). P value was calculated from analysis of variance, Fisher's exact test, or Kruskal-Wallis test among the 5 groups.
AIT, allergen-specific immunotherapy; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25–75, forced expiratory flow between 25% and 75% of vital capacity; ICS, inhaled corticosteroid; CS, corticosteroid; IgE, immunoglobulin E; sIgE, specific immunoglobulin E; FeNO, fractional exhaled nitric oxide; LTRA, leukotriene receptor antagonist; BHR, bronchial hyperresponsiveness; H&E, hematoxylin and eosin; RQLQ, rhinoconjunctivitis quality of life questionnaire; SPT, skin prick testing; Der-f, Dermatophagoides farinae; Der-p, Dermatophagoides pteronyssinus.
*P < 0.05 compared to non-AIT subjects.
Nasal responsiveness to NPT, NAR, and VAS
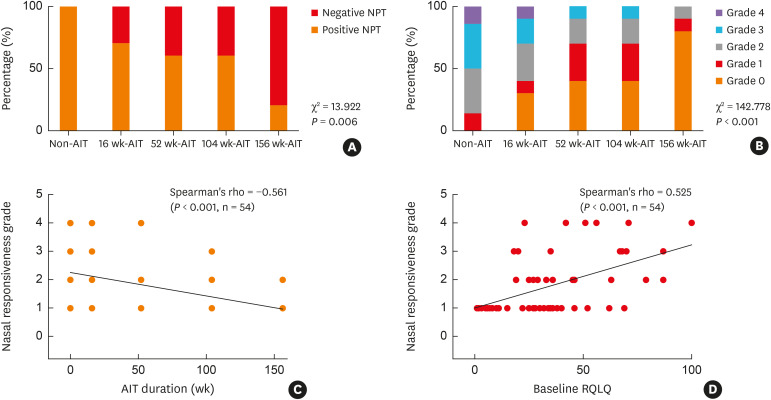
The percentage of positive responsiveness to NPT was significantly higher in non-AIT subjects (14, 100%), than in the 16 weeks-AIT (7, 70%), 52 weeks-AIT (6, 60%), 104 weeks-AIT (6, 60%), and 156 weeks-AIT (2, 20%) groups (χ2 = 13.922, P = 0.006, Fig. 2A ). Additionally, non-AIT subjects exhibited highest NPT-grades with 7 (50%) subjects exceeding grade 3 compared to 3 patients (30%) in the 16 weeks-, 1 (10%) in the 52 weeks-, 1 (10%) in the 104 weeks-, and 0 (0%) in the 156 weeks-AIT group (χ2 = 142.778, P < 0.001, Fig. 2B). NPT grade positively correlated with baseline RQLQ score (r = 0.327, P = 0.016) and negatively correlated with AIT duration (r = −0.561, P < 0.001) (Fig. 2C and D). There was no significant correlation between NPT grade and either baseline nasal or sputum eosinophil counts (data were not shown).
Fig. 2. NPT results. (A) The percentage of a positive response to NPT in non-AIT subjects and in those subjected to 16 weeks, 52 weeks, 104 weeks, or 156 weeks of AIT. (B) The percentage of different nasal responsiveness grade in the 5 groups. (C) Correlation between nasal responsiveness grade and AIT duration (D) Correlation between nasal responsiveness grade and baseline RQLQ score.
NPT, nasal provocation test; AIT, allergen immunotherapy; RQLQ, rhinoconjunctivitis quality of life questionnaire.
After NPT, all subjects showed increased NAR compared to baseline and there were no differences in NAR among the 5 groups. Before NPT, VAS-NS was found to be significantly higher in non-AIT subjects compared to those treated with AIT (P < 0.05). At the end of NPT, VAS-NS significantly increased for both non-AIT and AIT subjects. However, non-AIT subjects presented worse nasal symptoms of congestion and itching, with significantly higher VAS-NS that those treated with AIT (P < 0.05). In addition, non-AIT subjects maintained a significantly higher VAS-NS compared to those treated with AIT both at 30 minutes and 6 hours after NPT (P < 0.05) (Table 2). Baseline and post-NPT VAS-AS was not different between the subjects.
Table 2. NAR and VAS at baseline and after NPT.
| Parameters at different time points | Non-AIT subjects (n = 14) | AIT subjects | ||||
|---|---|---|---|---|---|---|
| 16 wk (n = 10) | 52 wk (n = 10) | 104 wk (n = 10) | 156 wk (n = 10) | |||
| NAR (Pa/cm3/sec) | ||||||
| Baseline | 0.28 ± 0.11 | 0.26 ± 0.04 | 0.25 ± 0.07 | 0.29 ± 0.10 | 0.27 ± 0.09 | |
| End-point of NPT | 0.39 ± 0.21 | 0.38 ± 0.17 | 0.33 ± 0.10 | 0.44 ± 0.32 | 0.33 ± 0.14 | |
| Post-30 min of NPT | 0.47 ± 0.32 | 0.47 ± 0.21 | 0.39 ± 0.10 | 0.21 ± 0.11 | 0.34 ± 0.17 | |
| Post-6 hr of NPT | 0.38 ± 0.33 | 0.34 ± 0.18 | 0.29 ± 0.23 | 0.36 ± 0.13 | 0.27 ± 0.12 | |
| P value | < 0.001 | < 0.001 | 0.012 | 0.018 | 0.020 | |
| Congestion | ||||||
| Baseline | 2.79 ± 2.47 | 1.60 ± 2.10 | 1.60 ± 2.31 | 1.10 ± 1.52 | 1.80 ± 1.68 | |
| End-point of NPT | 6.07 ± 1.94 | 4.30 ± 2.54 | 3.20 ± 2.89* | 3.42 ± 1.37 | 3.50 ± 2.12 | |
| Post-30 min of NPT | 5.21 ± 1.96 | 3.10 ± 1.85* | 2.50 ± 2.32* | 2.15 ± 2.00* | 2.70 ± 1.83* | |
| Post-6 hr of NPT | 3.14 ± 1.66 | 2.50 ± 1.58* | 1.90 ± 2.85* | 1.00 ± 1.16* | 0.80 ± 3.07* | |
| P value | < 0.001 | 0.038 | 0.016 | 0.003 | 0.016 | |
| Itching | ||||||
| Baseline | 0.79 ± 1.89 | 0.20 ± 0.63 | 0.00 ± 0.00 | 0.20 ± 0.63 | 0.30 ± 0.67 | |
| End-point of NPT | 4.43 ± 3.01 | 2.80 ± 2.97 | 1.80 ± 1.81* | 3.23 ± 2.50 | 1.70 ± 1.77* | |
| Post-30 min of NPT | 2.57 ± 0.71 | 0.80 ± 1.14* | 1.15 ± 1.92 | 0.90 ± 1.29* | 1.00 ± 1.49* | |
| Post-6 hr of NPT | 1.07 ± 1.38 | 0.20 ± 0.42 | 0.40 ± 1.26 | 0.60 ± 1.26 | 0.00 ± 0.00* | |
| P value | < 0.001 | 0.003 | 0.044 | < 0.001 | 0.015 | |
| Rhinorrhea | ||||||
| Baseline | 0.57 ± 1.09 | 0.20 ± 0.63 | 0.30 ± 0.67 | 0.00 ± 0.00 | 0.40 ± 0.84 | |
| End-point of NPT | 3.79 ± 2.26 | 3.00 ± 3.23 | 2.60 ± 3.69 | 2.55 ± 2.50 | 2.00 ± 2.16 | |
| Post-30 min of NPT | 2.29 ± 2.44 | 0.60 ± 1.26* | 1.50 ± 2.27 | 0.00 ± 0.00* | 0.50 ± 0.85* | |
| Post-6 hr of NPT | 0.43 ± 0.76 | 0.30 ± 0.95 | 0.10 ± 0.32 | 0.20 ± 0.63 | 0.00 ± 0.00 | |
| P value | < 0.001 | 0.004 | 0.005 | < 0.001 | 0.005 | |
| Sneezing | ||||||
| Baseline | 0.21 ± 0.80 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| End-point of NPT | 4.11 ± 3.34 | 3.10 ± 3.63 | 2.30 ± 3.20 | 2.95 ± 2.95 | 1.50 ± 2.55 | |
| Post-30 min of NPT | 0.00 ± 0.00 | 0.60 ± 1.35 | 0.30 ± 0.95 | 0.00 ± 0.00 | 0.40 ± 0.84 | |
| Post-6 hr of NPT | 0.43 ± 1.60 | 0.20 ± 0.63 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| P value | < 0.001 | 0.004 | 0.010 | < 0.001 | < 0.001 | |
| Total VAS-NS | ||||||
| Baseline | 4.57 ± 2.53 | 2.60 ± 2.63* | 2.35 ± 2.47* | 1.40 ± 1.58* | 2.90 ± 2.88 | |
| End-point of NPT | 20.67 ± 10.05 | 13.80 ± 10.28 | 12.00 ± 7.94* | 12.15 ± 9.85* | 9.10 ± 6.70* | |
| Post-30 min of NPT | 10.5 ± 5.63 | 5.50 ± 4.53* | 6.30 ± 6.91* | 3.15 ± 1.97* | 4.50 ± 3.31* | |
| Post-6 hr of NPT | 5.71 ± 4.79 | 3.60 ± 2.63 | 2.70 ± 3.80* | 2.00 ± 2.54* | 1.01 ± 1.73* | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Coughing | ||||||
| Baseline | 0.43 ± 0.51 | 0.20 ± 0.42 | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.10 ± 0.32 | |
| End-point of NPT | 0.53 ± 0.65 | 0.23 ± 0.49 | 0.14 ± 0.44 | 0.13 ± 0.41 | 0.14 ± 0.44 | |
| Post-30 min of NPT | 0.45 ± 0.55 | 0.22 ± 0.47 | 0.13 ± 0.41 | 0.12 ± 0.38 | 0.12 ± 0.38 | |
| Post-6 hr of NPT | 0.43 ± 0.51 | 0.20 ± 0.42 | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.10 ± 0.32 | |
| P value | NS | NS | NS | NS | NS | |
| Breathlessness | ||||||
| Baseline | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| End-point of NPT | 0.03 ± 0.11 | 0.05 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-30 min of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-6 hr of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| P value | NS | NS | NS | NS | NS | |
| Chest tightness | ||||||
| Baseline | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| End-point of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-30 min of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-6 hr of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| P value | NS | NS | NS | NS | NS | |
| Wheezing | ||||||
| Baseline | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| End-point of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-30 min of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Post-6 hr of NPT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| P value | NS | NS | NS | NS | NS | |
| Total VAS-AS | ||||||
| Baseline | 0.43 ± 0.51 | 0.20 ± 0.42 | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.10 ± 0.32 | |
| End-point of NPT | 0.57 ± 0.67 | 0.28 ± 0.59 | 0.14 ± 0.44 | 0.13 ± 0.41 | 0.14 ± 0.44 | |
| Post-30 min of NPT | 0.45 ± 0.55 | 0.22 ± 0.47 | 0.13 ± 0.41 | 0.12 ± 0.38 | 0.12 ± 0.38 | |
| Post-6 hr of NPT | 0.43 ± 0.51 | 0.20 ± 0.42 | 0.10 ± 0.32 | 0.10 ± 0.32 | 0.10 ± 0.32 | |
| P value | NS | NS | NS | NS | NS | |
Data are expressed as mean ± standard deviation. P value was calculated from the Mann-Whitney U test or Kruskal-Wallis test among groups.
AIT, allergen-specific immunotherapy; NAR, nasal airway resistance; NPT, nasal provocation test; VAS, visual analogue scale; VAS-NS, visual analogue scale of nasal symptoms; VAS-AS, visual analogue scale of asthmatic symptoms; NS, not significant.
*P < 0.05 compared to non-AIT subjects.
Changes of lung function, FeNO, sputum and nasal lavage eosinophil counts 6 hours after NPT
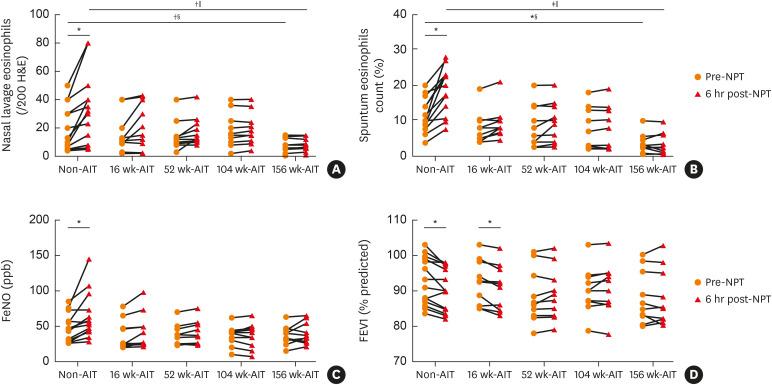
Compared to baseline of NPT, nasal lavage and sputum eosinophil counts increased significantly in non-AIT subjects (P = 0.028 and P = 0.039, respectively), but not in AIT subjects at each timepoint, (Fig. 3A and B). Similarly, FeNO significantly increased only in non-AIT individuals 6 hours after NPT (P = 0.015) (Fig. 3C). Furthermore, FEV1% of non-AIT and 16 weeks-AIT subjects, but not in subjects of other AIT timepoints, significantly decreased after 6 hours of NPT (P = 0.020 and P = 0.019, respectively) (Fig. 3D). Furthermore, subjects of non-AIT had significantly higher level of nasal lavage (P = 0.023 and P = 0.028, respectively) and sputum eosinophil counts (P = 0.028 and P = 0.03, respectively) as compared to those of 156 weeks-AIT before and 6 hours after NPT (Fig. 3A and B).
Fig. 3. Changes in clinical parameters before and after 6 hours of NPT in non-AIT and AIT subjects. (A) Changes in nasal eosinophil counts. (B) Changes in sputum eosinophil counts. (C) Changes in FeNO. (D) Changes in FEV1%.
NPT, nasal provocation test; AIT, allergen immunotherapy treatment; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; H&E, hematoxylin and eosin.
*P < 0.05; †P < 0.01; ‡P < 0.001, comparisons in parameters before and after 6 hours after NPT were calculated by Wilcoxon signed-rank test; §Comparison between non-AIT and 156 weeks AIT groups (Mann-Whitney U test); ‖Comparison among non-AIT and AIT groups (Kruskal-Wallis test).
Correlation between upper and lower airway eosinophilic parameters
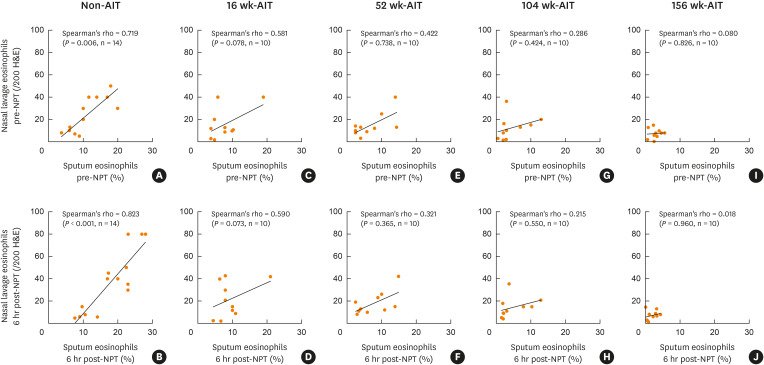
Sputum eosinophil counts at baseline and 6 hours after NPT positively correlated with nasal lavage eosinophil counts in non-AIT subjects (r = 0.719, P = 0.006 and r = 0.823, P < 0.001, respectively) (Fig. 4A and B), but did not in any AIT subjects (Fig. 4C-J). Eosinophils in nasal lavage did not correlate with FeNO at baseline or 6 hours after NPT either in any subjects regardless of AIT status (data not shown).
Fig. 4. Correlation between nasal lavage eosinophil counts and sputum eosinophil counts before and 6 hours post NPT. (A, B) non-AIT subjects. (C, D) 16 weeks-AIT subjects. (E, F) 52 weeks-AIT subjects. (G, H) 104 weeks-AIT subjects. (I, J) 156 weeks-AIT subjects.
NPT, nasal provocation test; AIT, allergen immunotherapy treatment; H&E, hematoxylin and eosin.
DISCUSSION
In the current study, we investigated the effects of Der-p AIT on upper and lower airway eosinophilic inflammation by using Der-p allergen NPT. Our findings support the beneficial role of AIT in relieving AR symptoms, decreasing medication use, and improving quality of life. Furthermore, patients with AIT showed increased nasal allergen tolerance compared to those without. With prolongation of AIT duration, both positive NPT rate and the grade of nasal responsiveness to allergen challenge decreased. Most intriguingly, we found that subjects without AIT showed increased nasal lavage and sputum eosinophil counts as well as a decline in FEV1 after 6 hours of NPT, while none of the AIT subjects, even those that were treated for 16 weeks, did not exhibit increased nasal lavage or sputum eosinophil counts after NPT. Finally, we observed that there was no correlation between upper and lower airway inflammation in subjects with AIT, whereas subjects without AIT presented significant associations between nasal and bronchial eosinophilic inflammation. Overall, our work establishes that AIT can attenuate both upper and lower airway inflammations in response to nasal allergen exposure and might play a beneficial role in interfering between nasal and bronchial inflammatory interactions.
It is well recognized that AIT is the only clinical treatment option that has a disease-modifying effect in patients with allergic diseases.26 The efficacy of AIT for AR and/or asthma has been confirmed in recent systematic meta-analyses.27,28,29 Indeed, subjects receiving AIT in our study exhibited better AR symptoms compared to subjects without AIT as assessed by self-scored questionnaires. NPT is an objective tool to diagnose AR and has been used for evaluating AIT efficacy in clinical trials.11 In a phase II study, 36 participants with HDM-induced AR treated with AIT demonstrated a significant increase in nasal tolerance at 24 months compared to placebo as assessed by NPT.12 Similarly, in a more recent phase I study using NPT as an evaluation method, 23 HDM-allergic participants showed a significant reduction in nasal symptoms compared to placebo in week 12.30 Our results are in line with these findings, in that subjects in our study with as little as 16 weeks of AIT showed a significant increased nasal tolerance against allergen challenge compared to subjects without AIT. Moreover, both the positive NPT rate and the nasal responsiveness grade were reduced when the duration of AIT was prolonged. The lowest levels of these indices observed in our study were in patients with 156 weeks (3 years) of AIT. Indeed, international guidelines have reached the consensus that an AIT duration of 3 to 5 years is sufficient to reach an optimal allergen tolerance.1 To date, there is no suitable parameter offering solid evidence that indicates the AIT duration that could maintain optimal allergen tolerance for a long period after discontinuation. The ACT and RQLQ are commonly used indices to evaluate the outcome of AIT. We found that the RQLQ score significantly correlated with the grade of nasal allergen responsiveness. Although self-reported questionnaires are needed to show the influence of reduced allergenicity on allergic disease, only tolerance to allergen exposure can provide an objective estimate of patients' allergies. Thus, we suggest that NPT is needed to monitor the efficacy of AIT. Repeated negative responsiveness to NPT might be the ideal measure of optimal AIT efficacy.
Previously, we observed that NPT could simultaneously increase upper and lower airway inflammation and decrease lung function in AR patients without asthma symptoms.15 Additionally, Braunstahl et al.31 found that NPT induces adhesion molecule expression and tissue eosinophilia in the upper and lower airways of AR patients. Therefore, it seems likely that allergen exposure in the upper airway could induce a lower airway inflammatory response. Clinical trials have used NPT to report that AIT effectively attenuates nasal response to allergen.14 However, it remained unclear whether the impact of AIT on lower airway inflammatory response triggered by upper allergen exposure. In our current work, we observed that subjects with AIT did not show an increase in either upper or lower airway eosinophilic inflammation 6 hours post-NPT, or a decrease in FEV1% as compared to subjects without AIT. To our knowledge, this is the first report showing that AIT can attenuate both upper and lower airway eosinophilic inflammatory responses triggered by allergen provocation in the upper airways. AR is a major comorbidity of asthma, and poor AR control contributes to worsening clinical expression of asthma.32 Our data showing that AIT suppresses eosinophilic inflammation in the upper and lower airways are associated with fewer nasal symptoms and increased airway tolerance against allergen exposure. Drainage of upper airway inflammatory mediators is a likely mechanism underlying the impact of nasal inflammation on lower airway disorders.33,34 There is evidence showing that the production of nasal inflammatory mediators, including interleukin (IL)-4, IL-9, and eotaxin was suppressed after 3 years of AIT.35 Less aspiration of inflammatory mediators from postnasal discharge into the bronchi leads to less impact on inflammations in the lower airways.
The concept of combined airways or “one airway, one disease” is well known and supported by the observation that AR and asthma are often associated and share similar immunopathological features.36,37,38 In our previous work, we found that the nasal lavage eosinophil count correlated with sputum eosinophil count, FeNO, FEV1%, and PD20 6 hours after NPT.15 Similarly, in the current study, we found that sputum eosinophil count was significantly correlated with nasal lavage eosinophil count in non-AIT subjects both at baseline and after 6 hours of NPT. However, there was no correlation between upper and lower eosinophilic inflammation in AIT subjects at any AIT time point, regardless of whether measurements were taken before or after 6 hours of NPT. In combination with our previous findings, our data show that there was a strong interaction between upper and lower airway inflammation after nasal allergen challenge in patients with AR and/or asthma even after regular medication treatment. However, the present study proved that such strong interaction could be interfered with by establishing allergen tolerance with AIT. We assumed that reduced response to allergen exposure in nasal mucosa after AIT would further reduce the impact of inflammatory mediators on hyperreactivity of lower airways. In this scenario, the correlation between nasal and bronchial inflammation was interrupted in patients with AIT. The findings offer a new perspective on the effect of AIT in preventing AR from developing into asthma.
The current study has some limitations. Our findings were based on a cross-sectional perspective, which might be less powerful compared to the results of a longitudinal study. Conducting a longitudinal study with kinetic design for 3 years or longer is needed to validate the effect of AIT on upper and lower airway inflammation modification. In addition, the design of the current study does not allow us to make conclusions about the underlying mechanism of AIT on the attenuation of both upper and lower airway inflammatory responses to NPT. It would have been ideal to include more detailed assessments on structural and immune effector cells, such as functions of airway epithelial cells, Th2, T, and B regulatory cells before and after NPT.39 Biomarkers present in nasal lavage fluid and sputum, such as allergen specific IgE and IgG4 antibodies, can serve as allergic and inhibitory indicators40 and might be helpful for further investigations into the effect of AIT on upper and lower airway immune responses.
In conclusion, our results show that AIT can attenuate both upper and lower airway immune responses to nasal allergen exposure and interferes with the interaction between nasal and bronchial inflammation. Further studies with a larger sample size and a longitudinal design with detailed immunological assessments are needed to validate the findings of the benefits of AIT treatment in patients with AR and/or asthma.
ACKNOWLEDGMENTS
We thank to nurses Shiyan Fu and Hualian Luo for recruiting the patients, Dr. Yufang Tang for inducing sputum and performing FeNO and Ms. Jiayin An for performing pulmonary function tests.
This study was supported by the following projects: 1) “Precision medicine research program” of National key research and development project of China (2016YFC0905800); 2) Special funding for science and technology development of Guangdong province (Frontier and key technology innovation direction - major science and technology special project) (2017B020226006); 3) Key project of National natural science foundation of China- Guangdong united fund (U1801286).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2019;11:156–169. doi: 10.4168/aair.2019.11.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296.e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014;133:612–619. doi: 10.1016/j.jaci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Soyka MB, van de Veen W, Holzmann D, Akdis M, Akdis CA. Scientific foundations of allergen-specific immunotherapy for allergic disease. Chest. 2014;146:1347–1357. doi: 10.1378/chest.14-0049. [DOI] [PubMed] [Google Scholar]
- 6.Rhyou HI, Nam YH. Efficacy of allergen immunotherapy for allergic asthma in real world practice. Allergy Asthma Immunol Res. 2020;12:99–109. doi: 10.4168/aair.2020.12.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roxbury CR, Lin SY. Efficacy and safety of subcutaneous and sublingual immunotherapy for allergic rhinoconjunctivitis and asthma. Otolaryngol Clin North Am. 2017;50:1111–1119. doi: 10.1016/j.otc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Calderón MA, Bousquet J, Canonica GW, Cardell LO, Fernandez de Rojas DH, Kleine-Tebbe J, et al. Guideline recommendations on the use of allergen immunotherapy in house dust mite allergy: time for a change? J Allergy Clin Immunol. 2017;140:41–52. doi: 10.1016/j.jaci.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 11.Soliman M, North ML, Steacy LM, Thiele J, Adams DE, Ellis AK. Nasal allergen challenge studies of allergic rhinitis: a guide for the practicing clinician. Ann Allergy Asthma Immunol. 2014;113:250–256. doi: 10.1016/j.anai.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Rondón C, Campo P, Salas M, Aranda A, Molina A, González M, et al. Efficacy and safety of D. pteronyssinus immunotherapy in local allergic rhinitis: a double-blind placebo-controlled clinical trial. Allergy. 2016;71:1057–1061. doi: 10.1111/all.12889. [DOI] [PubMed] [Google Scholar]
- 13.Tenn MW, Rawls M, Ellis AK. Nasal challenges in allergen immunotherapy trials. Curr Opin Allergy Clin Immunol. 2018;18:489–494. doi: 10.1097/ACI.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 14.Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317:615–625. doi: 10.1001/jama.2016.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Xian M, Xie Y, Zheng J, Li J. Aggravation of airway inflammation and hyper-responsiveness following nasal challenge with Dermatophagoides pteronyssinus in perennial allergic rhinitis without symptoms of asthma. Allergy. 2016;71:378–386. doi: 10.1111/all.12808. [DOI] [PubMed] [Google Scholar]
- 16.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129:S9–23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2019. Available from: www.ginasthma.org. [Google Scholar]
- 20.Dhand R, Kalra S, Malik SK. Use of visual analogue scales for assessment of the severity of asthma. Respiration. 1988;54:255–262. doi: 10.1159/000195533. [DOI] [PubMed] [Google Scholar]
- 21.Riechelmann H, Bachert C, Goldschmidt O, Hauswald B, Klimek L, Schlenter WW, et al. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German Society for Allergology and Clinical Immunology (ENT section) in cooperation with the Working Team for Clinical Immunology. Laryngorhinootologie. 2003;82:183–188. doi: 10.1055/s-2003-38411. [DOI] [PubMed] [Google Scholar]
- 22.Gosepath J, Amedee RG, Mann WJ. Nasal provocation testing as an international standard for evaluation of allergic and nonallergic rhinitis. Laryngoscope. 2005;115:512–516. doi: 10.1097/01.MLG.0000149682.56426.6B. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 25.Qin R, Long F, Xiao X, Xiao J, Zheng Z, Feng M, et al. Sputum Autoantibodies are more relevant in autoimmune responses in asthma than are serum autoantibodies. Allergy Asthma Immunol Res. 2019;11:406–421. doi: 10.4168/aair.2019.11.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132:1322–1336. doi: 10.1016/j.jaci.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Mösges R, Valero Santiago A, Allekotte S, Jahed N, Astvatsatourov A, Sager A, et al. Subcutaneous immunotherapy with depigmented-polymerized allergen extracts: a systematic review and meta-analysis. Clin Transl Allergy . 2019;9:29. doi: 10.1186/s13601-019-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631. doi: 10.1111/all.13201. [DOI] [PubMed] [Google Scholar]
- 29.Asamoah F, Kakourou A, Dhami S, Lau S, Agache I, Muraro A, et al. Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:25. doi: 10.1186/s13601-017-0160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunawardana NC, Zhao Q, Carayannopoulos LN, Tsai K, Malkov VA, Selverian D, et al. The effects of house dust mite sublingual immunotherapy tablet on immunologic biomarkers and nasal allergen challenge symptoms. J Allergy Clin Immunol. 2018;141:785–788.e9. doi: 10.1016/j.jaci.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–476. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 32.Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012;26:187–190. doi: 10.2500/ajra.2012.26.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7–13. doi: 10.1097/01.all.0000010978.62527.4e. [DOI] [PubMed] [Google Scholar]
- 34.Togias A. Mechanisms of nose-lung interaction. Allergy. 1999;54(Suppl 57):94–105. doi: 10.1111/j.1398-9995.1999.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 35.Scadding GW, Eifan AO, Lao-Araya M, Penagos M, Poon SY, Steveling E, et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy. 2015;70:689–696. doi: 10.1111/all.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasano MB. Combined airways: impact of upper airway on lower airway. Curr Opin Otolaryngol Head Neck Surg. 2010;18:15–20. doi: 10.1097/MOO.0b013e328334aa0e. [DOI] [PubMed] [Google Scholar]
- 37.Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci (Basel) 2019;7:E27. doi: 10.3390/medsci7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourdin A, Gras D, Vachier I, Chanez P. Upper airway x 1: allergic rhinitis and asthma: united disease through epithelial cells. Thorax. 2009;64:999–1004. doi: 10.1136/thx.2008.112862. [DOI] [PubMed] [Google Scholar]
- 39.Renand A, Shamji MH, Harris KM, Qin T, Wambre E, Scadding GW, et al. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J Allergy Clin Immunol. 2018;141:1750–1760.e1. doi: 10.1016/j.jaci.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng M, Su Q, Lai X, Xian M, Shi X, Wurtzen PA, et al. Functional and immunoreactive levels of IgG4 correlate with clinical responses during the maintenance phase of house dust mite immunotherapy. J Immunol. 2018;200:3897–3904. doi: 10.4049/jimmunol.1701690. [DOI] [PubMed] [Google Scholar]