Abstract
Introduction: Obsessive-compulsive disorder (OCD) is a disabling illness that is associated with significant functional impairment. Although evidence-based pharmacotherapies exist, currently available medications are ineffective in some patients and may cause intolerable side effects in others. There is an urgent need for new treatments.
Discussion: A growing body of basic and clinical research has showed that the endocannabinoid system (ECS) plays a role in anxiety, fear, and repetitive behaviors. At the same time, some patients with OCD who smoke cannabis anecdotally report that it relieves their symptoms and mitigates anxiety, and several case reports describe patients whose OCD symptoms improved after they were treated with cannabinoids. Taken together, these findings suggest that the ECS could be a potential target for novel medications for OCD. In this study, we review evidence from both animal and human studies that suggests that the ECS may play a role in OCD and related disorders. We also describe findings from studies in which cannabinoid drugs were shown to impact symptoms of these conditions.
Conclusions: An emerging body of evidence suggests that the ECS plays a role in OCD symptoms and may be a target for the development of novel medications. Further exploration of this topic through well-designed human trials is warranted.
Keywords: anxiety, cannabinoid, endocannabinoid system, OCD, repetitive behavior
Introduction
Obsessive-compulsive disorder (OCD) is a disabling illness with an approximate lifetime prevalence of 2–3% worldwide.1 The disorder is marked by intrusive repetitive thoughts and behaviors, which must be significantly time consuming, distressing, or functionally impairing to meet DSM 5 criteria.2 There is a bimodal distribution of age at onset, with peaks in childhood and early adulthood, and roughly equal distribution in adults between males and females. The illness typically follows a chronic course, with symptoms waxing and waning over time. OCD is associated with significant functional disability and impairment,3 and many patients do not achieve remission despite evidence-based treatments. Evidence suggests that abnormalities in cortico-striatal-thalamic-cortical circuitry and specific gene variants contribute to the pathogenesis of the disorder.4
The only medications approved by, for example, the Food and Drug Administration (FDA) for the treatment of OCD are serotonin reuptake inhibitors (SRIs, e.g., clomipramine and the selective SRIs).5 However, these drugs often provide only limited relief of symptoms, and are ineffective in some patients. Furthermore, SRIs typically require at least 6 weeks of sustained treatment before clinically meaningful improvement can be seen.5,6 Antipsychotics may be effective when added to SRIs for a subset of patients, but are ineffective in others. Although novel (e.g., second-generation) antipsychotic drugs there is not strong evidence suggesting that these are more effective in OCD. Moreover, antipsychotics as a class are associated with an increased risk for side effects, including weight gain, metabolic syndrome, Parkinsonism, tardive dyskinesia, and even neuroleptic malignant syndrome.7–9 Thus, there is an urgent need for new targets for treatment development.
One potential target is the endocannabinoid system (ECS). Recent studies in both humans and animals have shown a critical role for the ECS in anxiety, stress, fear, and repetitive/habitual behaviors.10 Moreover, many patients with OCD who use cannabis anecdotally report that it improves their symptoms and reduces anxiety. However, to date, few studies have systematically investigated this connection.
Methods
In this review, we discuss evidence linking the ECS to the neurobiology underlying OCD, and consider the potential role of this system as a target for pharmacological intervention. Given the heterogeneity in the OCD phenotype as well as evidence of both symptom overlap and shared neurobiological underpinnings in OCD and anxiety, tic, and impulse control disorders,11,12 the scope of our aims was broad. Thus, we elected to complete a narrative review, including a discussion of relevant data from studies on OC spectrum illnesses.
We searched the PubMed database from inception to 2018 using the search terms [cannabis], [cannabinoid], and [endocannabinoid] paired with broad terms (Table 1) to identify relevant articles. We reviewed titles and abstracts, examining full text for articles that provided data on original studies or reviews discussing the ECS and/or cannabinoids in relation to anxiety and OC spectrum illnesses. To identify other relevant articles, we reviewed references of articles identified in the initial search. As this was a narrative review, we did not record the total number of articles screened, identified, or reviewed, although over 150 articles were referenced in an initial draft. This list was then paired to limit redundancy and maximize the overall quality of evidence cited.
Table 1.
Search Strategy
| PubMed search terms | |
|---|---|
| General terms | Paired terms |
| Cannabis, cannabinoid, endocannabinoid | “obsessive compulsive disorder”, OCD, obsession, compulsion, obsessive, compulsive anxiety, fear, stress, trauma, “post-traumatic” tic, “Tourette Syndrome”, trichotillomania, excoriation repetitive, habit, habitual |
Neural Correlates of OCD: Current Models
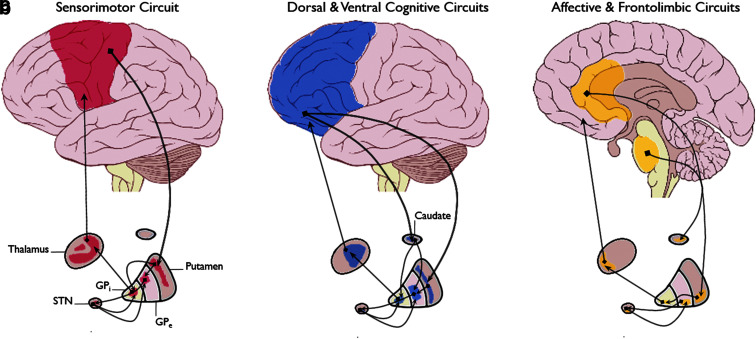
Aberrant activity in cortico-striato-thalamo-cortical circuits has been linked to OCD symptoms.13–16 Dysfunction in these circuits, which comprise a system of multiple loops through which information is passed in a coordinated fashion (Fig. 1), could bias an individual toward excessive selection of particular actions or inhibit screening out of inappropriate behaviors.
FIG. 1.
Schematic of CSTC Loops. (a) Sensorimotor circuit, (b) dorsal and ventral cognitive circuits, (c) affective and frontolimbic circuits. (Image is a derivative of “Brain human lateral view” and “Brain human sagittal section,” both by Patrick J. Lynch. The originals are licensed under the Creative Commons Attribution 2.5 Generic License and can be found at https://commons.wikimedia.org/wiki/File:Brain_human_lateral_view.svg and https://commons.wikimedia.org/wiki/File:Brain_human_sagittal_section.svg respectively). CSTC, cortico-striato-thalamo-cortical.
One area of focus has been the orbitofrontal cortex (OFC). For example, imaging studies in OCD patients have shown a shift in the balance of activity between the lateral and medial OFC, such that the lateral region (associated with responsiveness to threat and ritualized behaviors) becomes hyperactive, while the medial (associated with emotional regulation and reward processing) is hypoactive.17
The OFC also facilitates the flexible use of goal-directed and habitual behaviors. Data suggest that OCD patients may have deficient control over the balance between these competing action strategies, resulting in a bias toward habit formation.17–19 Rodent models have demonstrated that OFC regulates this balance through its projections to the dorsal medial striatum (DMS), which promotes goal-directed activity, and the dorsal lateral striatum (DLS), which supports habitual behaviors. Thus, some hypothesize that OCD symptoms result in part from a decrease in OFC-mediated control over the striatum, leading to an excess of DLS relative to DMS activity.18
Other brain regions have also been implicated in OCD. These include the dorsal anterior cingulate cortex (dACC), which has been associated with deficient error processing, and the hippocampus and amygdala, which are thought to impact fear and anxiety responses in OCD patients.17
The ECS
Overview
The ECS is widely distributed throughout the central and peripheral nervous systems (CNS/PNS). Maintenance of homeostasis is thought to be one of the ECS' major functions, and it has also been implicated in caloric energy balance, immune function, neurogenesis, pain, arousal, sleep, stress reactivity, and reward processing. ECS activity within the CNS prevents the development of excessive neuronal activitation,20 producing downstream effects which, broadly speaking, have been described as supporting the goal to “relax, sleep, forget, and protect”.19
Although efforts to study the properties of tetrahydrocannabinol (THC, the primary psychoactive ingredient in the cannabis plant) began in the early 1940s, its endogenous receptor, the cannabinoid receptor type 1 (CB1R), was not identified until 1988.20 Since then, there have been increasing efforts to characterize the components of this system. This work resulted in the discovery of a second receptor type (CB2R), endogenous lipid-based ligands for these receptors (known as “endocannabinoids” [eCBs]), and enzymes which synthesize and degrade eCBs, such as fatty acid amide hydroxylase (FAAH) and monoacylglycerol lipase (MAGL).10 Altogether, eCBs, their receptors, and the enzymes involved in their synthesis and degradation comprise what is now considered the ECS.
Cannabinoid receptors
CB1/2R are G protein-coupled receptors which, when bound by eCBs, initiate a cellular signaling cascade leading to changes in gene transcription, synaptic function, and cell migration during development. Although primarily targeting CB1/2R, eCBs activate a variety of noncannabinoid receptors, including the transient receptor potential vanilloid 1 (TRPV1) receptor, peroxisome proliferator-activated receptor (PPAR) γ, and the orphan G protein-coupled 55 receptor (GPCR55),21,22 which may account for some of the ECS' diverse functions.
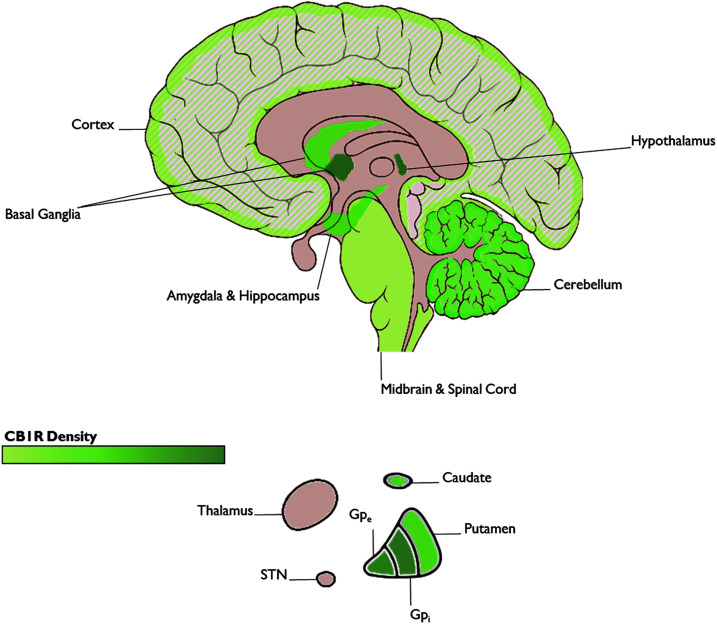
CB1R is the major receptor of the ECS in the CNS, and as such its presence is indicative of ECS activity in a given region. At the neuroanatomical level, high densities of CB1R have been identified in the basal ganglia, cerebellum, hippocampus, prefrontal cortex, and amygdala (Fig. 2). These regions have been implicated in OCD, suggesting that the ECS plays a role in OCD-relevant neural circuitry.10
FIG. 2.
CB1R distribution in the brain. (Image is a derivative of “Brain human sagittal section” by Patrick J. Lynch. The original is licensed under the Creative Commons Attribution 2.5 Generic License and can be found at https://commons.wikimedia.org/wiki/File:Brain_human_sagittal_section.svg). CB1R, cannabinoid receptor type 1.
CB1R is found in a variety of cell types, but is most highly expressed in the presynaptic axon terminals of inhibitory GABAergic interneurons. CB1R is expressed to a lesser degree in glutamatergic interneurons, where it localizes similarly to presynaptic axon terminals.10 CB1R is also expressed in other cell types, including astrocytes, where it impacts control over working memory and other cognitive functions,23,24 and mitochondria, where it participates in the regulation of adenosine triphosphate production and respiration.10
CB2R is rarely seen in the CNS in nonpathological states, but is ubiquitous throughout the PNS. CB2R is known to impact immune activity, inflammation, and response to injury.25,26
Endogenous cannabinoids
The first discovered and best-described eCBs are 2-arachidonoyl glycerol (2-AG) and N-arachidonoylethanolamine (AEA; also known as anandamide).22 These are metabolized by the enzymes, MAGL and FAAH, respectively.25 AEA can alternatively be degraded by N-acylethanolamine-hydrolizing acid amidase (NAAA) or by cyclooxygenase-2 (COX-2), with variant downstream effects on cell functioning depending on the metabolic pathway to which AEA is shunted.27 2-AG is a potent agonist of both CB1R and CB2R, whereas AEA is a relatively weak agonist of both receptors, and in the presence of an agent with stronger affinity may act as an antagonist.25
As described above, CB1R concentrates predominantly at presynaptic axon terminals of cortical GABAergic and glutamatergic neurons. However, it is also found throughout the cholinergic, serotonergic, and noradrenergic systems of the CNS. eCBs act as retrograde messengers, binding to presynaptic CB1R to cause suppression of neurotransmitter release, leading over time to both short- and long-term depression of synaptic transmission.26,28 eCBs can also regulate neurotransmission at neighboring synapses by both diffusing into the extracellular space and activating CB1R in nearby astrocytes.10
eCBs are unusual neurotransmitters in that their synthesis occurs “on demand”.10,21,28 Rather than being stored in synaptic vesicles, eCBs are synthesized from cellular membrane lipids in response to stimulation of the postsynaptic neuron. They are then released into the extracellular space,10,26 after which they are rapidly hydrolyzed and inactivated by FAAH, MAGL, and other metabolic enzymes.10,29 This unique mechanism enables an activity-driven functionality (e.g., increased when neural activity is high). By activating transiently at a particular synapse when the cellular constituents of that system have been stimulated beyond a given threshold, the ECS may serve as a brake mechanism, fine-tuning activity within specific neural circuits.
Exogenous cannabinoids
Exogenous cannabinoids can be subdivided into two categories (Table 2). Phytocannabinoids consist of over 108 compounds which are found in the cannabis plant, including THC and cannabidiol (CBD).10,30,31 Phytocannabinoids have been isolated in the laboratory, producing purified compounds or mixtures such as nabiximol (THC and CBD in a 1:1 ratio, administered in an oromucosal spray). Nabiximol is approved in Canada and several European countries.10 Synthetic cannabinoids have also been created. Nabilone (synthetic THC) and dronabinol (an alternate form of plant-derived THC) are both FDA approved for cancer-related nausea and vomiting.
Table 2.
Currently Available Exogenous Cannabinoids
| Agent | Class | US legal status | Method of delivery | Approved use |
|---|---|---|---|---|
| THC | Phytocannabinoids | Same as cannabis (FDA Schedule I) | Smoked/vaporized, oromucosal spray, capsules | None |
| CBD | Phytocannabinoids | Same as cannabis (FDA Schedule I), capsule form recently approved | Smoked/vaporized, oromucosal spray (not approved for use); capsules | Pediatric epilepsy |
| Nabiximol (THC & CBD) | Phytocannabinoids | Approved in Canada and parts of Europe, not in the US | Oromucosal spray | Adjunct treatment for spasticity, pain, nausea/vomiting |
| Dronabinol (THC) | Phytocannabinoids | FDA Approved | Capsules, oral liquid | Cancer-associated nausea/vomiting |
| Nabilone (Synthetic THC) | Synthetic Cannabinoid | FDA approved | Capsules | HIV, cancer-associated nausea/vomiting |
CBD, cannabidiol; THC, tetrahydrocannabinol; FDA, Food and Drug Administration.
CBD has garnered increasing interest as a potential treatment for a variety of neuropsychiatric conditions, including anxiety. Although both THC and CBD are phytocannabinoids, CBD is distinct in that it exhibits little to no activity at CB1R.30 Based on its ability to antagonize the receptor under certain conditions, CBD has been characterized as a negative allosteric modulator at CB1R.30,32 However, CBD may indirectly stimulate CB1R by inhibiting FAAH, thereby increasing levels of AEA and other eCBs.30 CBD may also inhibit adenosine reuptake, which is notable given that facilitation of adenosine signaling produces anxiolytic effects.32 Finally, CBD appears to have activity at a number of different receptor targets, including the 5HT1A receptor, TRPV1, and GPCR55.21 An oral formulation of CBD called Epidiolex was recently approved by the FDA for the treatment of seizures associated with Dravet and Lennox–Gastaut syndromes, two rare forms of pediatric epilepsy.
The ECS and OCD symptoms: From animal studies to human trials
Several lines of evidence suggest an association between the ECS and OCD symptoms. First, as reviewed above, CB1R are found in high densities in regions thought to be implicated in OCD, including the prefrontal cortex, basal ganglia, hippocampus, and amygdala.10 Second, preclinical studies (primarily in rodents) suggest that cannabinoid signaling may impact OCD-relevant neurocognitive functions, including fear extinction and the balance between goal-directed and habitual action strategies. Moreover, cannabinoids can improve symptoms in animal models of anxiety and compulsive behavior. Finally, preliminary studies of cannabinoids in patients with OCD and related disorders (e.g., anxiety, tic disorders) suggest the potential clinical utility of cannabinoid agents. This evidence is briefly reviewed below.
Animal studies
The ECS, anxiety, and fear
CB1R agonists have a biphasic effect on anxiety symptoms, with low doses producing anxiolysis, and high doses, anxiogenesis. This effect is reliably demonstrated in rodents and appears to be driven in part by activation of TRPV1 by high (but not low) doses of CB1R agonists.10,21,28 In contrast, direct activation of TRPV1 increases anxiety, suggesting that the balance between activation of CB1R versus TRPV1 may regulate anxiety-like behaviors.21,33
CB1R activation also impacts anxiety by modulating activity in excitatory and inhibitory outputs from the cortex.34 In mouse studies, CB1R agonists at low doses (which are anxiolytic) primarily activate CB1R in cortical glutamatergic neurons. In contrast, high doses of CB1R agonists are anxiogenic and are associated with a relative increase in activity in forebrain GABAergic neurons.35,36 In addition to dose dependence knockout, mice have also demonstrated that CB1R's impact on anxiety is responsive to environmental cues. Specifically, deleting CB1R from the cortex reduces exploratory behavior in mice (thought to reflect anxiety) under mildly stressful conditions, but not in highly aversive settings; on the other hand, loss of CB1R from forebrain GABAergic neurons increases exploratory behavior under mildly aversive conditions but does not impact responsiveness to highly aversive settings.36,37
Studies have also demonstrated that the ECS plays a complex role in the neuroendocrine response to stress. These findings are summarized in detail in a 2018 review by Hill et al.38 Generally speaking, release of glucocorticoids promotes the release of AEA and 2-AG. eCBs bind to amygdalar CB1R to dampen the stress response, promote adaptation to prolonged stress, and reduce catecholamine release from sympathetic terminals. In contrast, THC and exogenous cannabinoids decrease the stress response at low doses while increasing it at high doses.38 Taken together, these findings suggest that CB1R acts as a buffer on activity within anxiety circuits, exerting opposing effects depending on the dose of agonist and aversiveness of the local environment.10
Cannabinoids can also modulate extinction to conditioned fear, which is notable given that impaired fear extinction has been reported in OCD.17 Studies using knockout mice and pharmacological antagonists suggest that CB1R is required for extinction of fear memories to occur and that extinction occurs through ECS-mediated long-term depression of local GABAergic inhibitory networks within the basolateral amygdala.39 During fear extinction, eCB levels increase in the basolateral amygdala (which has been associated with extinction of aversive memories).40 Presynaptic CB1R activity can also regulate the behavioral responsiveness of mice to aversive memories by modulating cholinergic signaling in projections from the medial habenula to the interpeduncular nucleus.41 Exogenous cannabinoids, including THC and CBD, have been shown to reduce rodent responsiveness to conditioned fear,42,43 while selective CB1R antagonists appear to inhibit the extinction process.39
Animal studies also show that the metabolic enzymes of the ECS can impact anxiety symptoms. A human variant of the gene encoding FAAH has been identified, which results from a common single nucleotide polymorphism carried by 38% of individuals of European descent.10,44,45 A 2015 study of humans and mice with an analogous FAAH variant found that in both groups, FAAH expression was reduced, resulting in increased AEA levels in the CNS. Both groups also demonstrated decreased anxiety behaviors and an increased rate of fear extinction relative to control subjects. Resting-state functional magnetic resonance imaging (fMRI, in humans) and tract-tracing methods (in mice) revealed increased connectivity between frontal areas and the amygdala among individuals with the FAAH variant of both species, with mice also displaying reduced amygdala responsiveness to threatening stimuli, compared with controls.45 These parallel findings across species provide strong evidence that the ECS modulates anxiety and fear response in humans, and posit that this effect may result from increased fronto-amygdala connectivity. The presence of a common gene variant which impacts both anxiety symptoms and capacity to extinguish conditioned fear may also help to explain the differential response to exposure-based psychotherapy among patients with anxiety disorders and OCD.
Pharmacological studies of drugs that inhibit eCB metabolism have also been conducted. FAAH inhibitors in mice appear to enhance AEA signaling, which is associated with reduced anxiety-like behavior, decreased responsiveness to stress, and facilitation of fear extinction. Similarly, pharmacological blockade in mice of MAGL appears to have anxiolytic effects under a variety of conditions.10,46
The ECS and habitual/repetitive behaviors
Rodent studies have linked the ECS to habit learning and repetitive behavior. One mouse study found that intrastriatal administration of a CB1R antagonist impaired the ability to “unlearn” previously acquired procedural memories in response to changing reward contingencies, whereas intrahippocampal administration facilitated this process. These findings suggest that habit learning is affected by the interplay between hippocampal and striatal CB1R activity. For example, insufficient CB1R activity in projection neurons to striatum might lead to perseveration on previously learned behaviors and difficulty adapting to environmental changes.47
In a series of studies by Gremel et al.,18 ECS activity in corticostriatal circuitry was shown to modulate the shift between goal-directed and habitual action strategies. Using optogenetic techniques, the authors demonstrated that activating CB1R within OFC-DMS projection neurons decreased excitatory transmission, as measured by whole-cell patch clamp electrophysiology. CB1R deletion, both from OFC neurons in general and specifically from OFC-DMS projections, prevented habitual pressing of an instrumental lever during an outcome devaluation paradigm, leaving mice reliant on goal-directed strategies. Conversely, chemogenetic inhibition of transmission by OFC-DS neurons prevented mice from using goal-directed strategies. Taken together, these findings suggest that ECS activity in OFC impacts the balance between goal-directed and habitual action control. Loss of ECS-mediated plasticity within these circuits could thus contribute to OCD symptoms, perhaps by causing a relative shift toward activity in OFC-DLS relative to OFC-DMS circuitry resulting in excessive reliance on habit.
Other studies have focused on the impact of the ECS on repetitive behavior. For example, mouse studies found that either CB1R agonists or an inhibitor of AEA metabolism reduced the number of buried marbles on the marble-burying test, an animal model of compulsivity.48 In another mouse study, AEA decreased marble-burying behavior (MBB) at low doses, and increased MBB at high doses, an effect which was mediated by increased TRPV1 activation with high doses of AEA.49 Pretreatment with a TRPV1 agonist prevented the reduction in MBB at low doses, while capsazepine (a TRPV1 antagonist) mitigated the increase in MBB with high doses of AEA. Notably, the effect of capsazepine itself on MBB was comparable to fluoxetine, suggesting that TRPV1 may also be a viable target for novel anticompulsive agents.
Systemically delivered CBD has also been shown to reduce MBB in rodent models.50–52 Although CBD appears to be able to activate 5HT receptors (among a number of targets),32 these studies suggest that its effect on MBB is driven specifically by CB1R activation, as (1) 5HT1A receptor antagonists failed to prevent the effects of CBD52 and (2) CB1R antagonists reversed the effects of both fluoxetine and CBD on MBB. Neither fluoxetine nor CBD impacted motor or exploratory behavior in these studies, suggesting that their effects were specific to MBB rather than reflecting a nonspecific decrease in anxiety or locomotion. CBD also reversed the effects of meta-Chlorophenylpiperazine, a nonspecific serotonin receptor agonist, which increases repetitive grooming behaviors and MBB.50 CBD's effect on MBB was also shown to persist over 7 days of daily CBD administration,51 as compared with diazepam, which showed decreasing efficacy over time.43
Other work has explored the impact of cannabinoid agents on the serotonin system. A mouse study comparing a FAAH inhibitor, AEA, and an AEA reuptake inhibitor found that the cannabinoid agents impacted MBB in a biphasic fashion (decreased at low doses and increased at high doses), whereas fluoxetine reduced MBB in a linear fashion. Subeffective doses of fluoxetine also potentiated the effect of subeffective doses of all three cannabinoids on MBB, and a CB1R antagonist blocked the effects of both fluoxetine and the cannabinoids.53 In a separate study, paracetamol (acetaminophen, an analgesic/antipyretic), produced dose-dependent decreases in MBB that were comparable to those produced by fluoxetine. This effect was driven by paracetamol's effect at both 5HT receptors and CB1R. Furthermore, coadministration of paracetamol and fluoxetine had a synergistic effect toward reducing MBB.54 Taken together, these data suggest that compulsive-like behaviors can be modulated through interactions between the ECS and serotonin system.
Studies in Humans
Substantial evidence from the nonpsychiatric literature supports the safety and efficacy of cannabinoids for treating symptoms of medical conditions, including HIV, chronic pain, cancer, and multiple sclerosis.31 However, to date, few clinical studies have examined the role of these agents in psychiatric conditions. Relevant findings from these studies are discussed below.
Anxiety and fear
The relationship between cannabinoids, and anxiety and fear has been of particular interest in clinical research.17,42 In imaging studies of healthy controls, CBD and dronabinol attenuated amygdala response to fear-inducing stimuli. Dronabinol also facilitated fear extinction in laboratory studies of healthy adults,42 and was associated with increased vmPFC activity in a follow-up study of the same subjects.55 In laboratory settings, CBD has been shown to reduce experimentally induced anxiety and enhance the extinction of fear memories in healthy adults.44
Clinical trials in patients with posttraumatic stress disorder (PTSD) have shown that dronabinol and nabilone can reduce symptoms, including the response to fearful stimuli. Several studies have also demonstrated that nabilone may improve PTSD-associated nightmares and insomnia.44,56–59
CBD has also been studied as a potential anxiety treatment, although the results of clinical studies are mixed. As described above, CBD acts at a range of pharmacological targets, including CB2R, TRPV1, the serotonin 5HT1A receptor, GPCR55, and PPAR.27,60 Moreover, CBD may inhibit FAAH, thereby increasing AEA levels (and indirectly activating CB1R).30 CBD lacks toxic effects, even at high doses. Furthermore, as opposed to THC, CBD is nonpsychotogenic, and has been shown to reverse some of THC's negative effects, including paranoia and memory impairment.21,27,30,32,44,60 Although trials of CBD have shown little impact on baseline levels of anxiety, it may reduce experimentally induced anxiety and fear.21,23,32 For example, one study in healthy subjects and those with social anxiety disorder found that CBD reduced task-related anxiety with an effect comparable to a serotonin agonist or diazepam.21 Neuroimaging studies suggest that CBD reduces amygdala activation in response to threat and alters functional connectivity between prefrontal regions and the amygdala,32 which may explain its potential anxiolytic effects. However, a recent evaluation of the literature by the National Academy of Sciences highlighted problematic design aspects of the above studies (for example, providing just one dose of CBD61). Thus, further studies are needed.
Repetitive behaviors
Both case reports and survey data from patients with Tourette Syndrome (TS) indicate that smoked cannabis can reduce motor tics and urges to perform compulsive behaviors.62 Dronabinol has also been studied as a treatment for TS in two small clinical trials. In the first trial, a pilot study using a single-dose crossover design, 12 patients with TS showed a decrease in tic severity on the Tourette Syndrome Symptom List (TSSL) and reduced obsessive-compulsive behavior, relative to placebo, on a nonvalidated self-report scale developed by the investigators. A second trial in 24 adult patients with TS used a parallel design with daily dosing over 6 weeks. Dronabinol led to significant improvements compared with placebo on the Tourette Syndrome Clinical Global Impressions scale (TS-CGI), Shapiro Tic Syndrome Scale (STSS), and TSSL. Although the results of these studies are intriguing given the phenotypic overlap between OCD and tic disorders, these trials were limited by small sample sizes and high drop-out rates, and a recent Cochrane review found that there was insufficient evidence to recommend the clinical use of cannabinoids for TS.63,64
In a 2011 trial, 14 female subjects with trichotillomania received open treatment with dronabinol over a 12-week period. Although the trial lacked a placebo control, of the 12 subjects who completed the trial, 9 qualified as “responders” as measured by a reduction on the MGH Hairpulling Scale (MGH-HPS) of ≥35%.65
Obsessive-compulsive disorder
To date, only three case reports describe the effects of cannabinoids on OCD symptoms. In the first, a 38-year-old woman with major depression and OCD received dronabinol after responding poorly to paroxetine, clomipramine, and CBT, but reported that smoking cannabis usually relieved her symptoms. Over the course of 10 days in which clomipramine was augmented with dronabinol (10 mg thrice daily), the patient's score on the Yale–Brown Obsessive Compulsive Scale (YBOCS) decreased from 20 to 10 (i.e., from “moderate” to “mild” severity).
In the second case, a 36-year-old man with schizophrenia and OCD who was hospitalized for worsening psychotic and obsessive symptoms was treated with dronabinol after his OCD symptoms failed to respond to SSRIs and antipsychotics (including a year-long trial of clozapine and paroxetine, a 10-week trial of clozapine and clomipramine, and ECT). Dronabinol was added to clozapine and clomipramine at 10 mg twice daily, and within 2 weeks a decrease in the patient's YBOCS score from 25 to 15 was observed. The authors note that neither patient reported any side effects in response to adding dronabinol, nor was any deterioration of psychotic or mood symptoms observed.66 The latter case is notable given that 20–30% of patients with schizophrenia experience OCD symptoms, and a body of literature linking clozapine treatment to the development of OCD symptoms. No single theory has explained the overlap between OCD and psychosis, but this evidence supports additional study of the ECS' role in patients with symptoms of both conditions.
In a third case, a 24-year-old man developed persistent obsessions and compulsions following a left thalamic stroke, but did not respond to several high-dose medication and augmentation trials, including SRIs, antipsychotics, benzodiazepines, glutamate modulators, and mood stabilizers. The patient requested neurosurgical consultation for deep brain stimulator placement, but elected to receive dronabinol (20 mg daily) before surgery. Over the course of 2 weeks, the patient's YBOCS score decreased from 39 to 10. The authors note that this correlated with a significant improvement in the patient's quality of life and his ability to participate in CBT, which had previously been intolerable for him.67
Summary and Future Directions
The ECS is ubiquitous throughout the CNS and, through various mechanisms, acts to modulate neural activity across multiple circuits and neurotransmitter systems. As summarized in this study, convergent streams of data from neuroimaging studies, animal models, and preliminary clinical studies have revealed associations between the ECS, anxiety, and compulsive/repetitive behaviors.
Animal models indicate that cannabinoid agents have both anxiolytic and anticompulsive effects, which are mediated by their activity at multiple receptor sites, including CB1R, 5HT1A, and TRPV1. Broadly speaking, the ECS appears to regulate anxiety and OCD symptoms through its effects on top-down control by frontal cortical over striatal and limbic regions. More specifically, the ECS can modulate response to fearful stimuli, possibly by influencing signaling between frontal regions and the amygdala. Additionally, CB1R-mediated changes in frontal-striatal circuitry may impact the balance between goal-directed and habitual action selection. Altogether, these findings suggest that abnormalities within the ECS could underlie, at least in part, anxiety and compulsions in OCD populations, and that interventions targeting this system may be fertile ground for the development of new treatments.
Although preliminary, the available clinical data indicate that cannabinoids influence OCD-relevant processes, impacting anxiety symptoms, enhancing fear extinction, and reducing certain repetitive behaviors. To date, only case reports detail how cannabinoids affect OCD symptoms specifically, although the effects reported are promising. Further testing is warranted.
When considering the design of future studies, several key questions arise. First, which agents should be tested? THC analogs (e.g., dronabinol) and other CB1R agonists have generated the most positive results, but require careful monitoring for side effects (e.g., intoxication, increased anxiety at certain doses). Moreover, given the ubiquity of cannabinoid receptors throughout the CNS, agents that activate CB1R globally may have multiple, at times unwanted or competing, effects.28 Nonetheless, preliminary data from trials of CB1R agonists in PTSD are promising and support testing of these agents in patients with anxiety and OCD.56–59
CBD also has a potential role in treatment given its favorable side effect profile, lack of psychotogenic/anxiogenic properties, and ability to enhance fear extinction in humans and to reduce compulsive-like behavior in mice. The challenge with CBD, however, is knowing which of its many targets might be responsible for its clinical effects32,68
Given that the synthesis (and subsequent degradation) of eCBs increases on-demand in response to increased neural activity, drugs that effect metabolic enzymes may provide more targeted control over ECS activity than drugs that broadly activate cannabinoid receptors, thereby reducing the risk for side effects, anxiogenesis, and addiction/abuse.44,69 Thus, studies of FAAH and/or MAGL inhibitors are also of interest. Indeed, early evidence from rodent studies indicates that FAAH and MAGL inhibitors can reduce repetitive behaviors and anxiety. However, these compounds are not readily available for human research.
Other potential candidate drugs include cannabinoid reuptake inhibitors69 and agents that target TRPV1. Alternatively, compounds that interact indirectly with the ECS such as capsazepine,53 paracetamol,54 or COX2 inhibitors,32 may be useful. In addition to utility as monotherapy, novel agents could also be used in augmentation, as suggested by rodent models demonstrating synergistic effects with cannabinoids and SRIs.26,32,53,54,70 Finally, agents with dual activity like N-arachidonoyl-serotonin, which inhibits FAAH (thereby activating CB1R) and antagonizes TRPV1 (preventing its anxiogenic effect),32 are worth further exploration once compounds that are safe for human use become available.
After choosing a cannabinoid to test, selecting an appropriate measurement of its effects will be key. Although clinically validated, standard measures such as the YBOCS may not be sufficiently sensitive to the effects of cannabinoids. Designed to assess for OCD symptoms and distress over the past week, the YBOCS is also unable to detect acute changes in symptoms following drug administration. While the YBOCS remains the gold standard for use in large-scale treatment trials, behavioral paradigms that focus on underlying neurocognitive mechanisms could be an alternative means of assessing cannabinoid effects. For example, the two-step task, which uses computational modeling to differentiate goal-directed vs. habit-based learning, has been used to reveal a bias toward habit acquisition in disorders of compulsive behavior (e.g., binge eating, substance use, and OCD).71 Such a task might more directly tap the effects of cannabinoids on brain function.
Modern neuroimaging techniques may also clarify how cannabinoids impact neural circuitry, which will be critical given the ECS' complex array of functions within the CNS. PET ligands for CB1R are available72 and have been used to quantify its role in other psychiatric disorders.72–75 Task-based and resting-state fMRI have also been used to measure cannabinoid effects in healthy76,77 and psychiatrically ill subjects.32,78 Nonetheless, to facilitate large-scale clinical trials of cannabinoids, there remains a need to develop and validate noninvasive measures of target engagement.
Despite decades of research since the development of SSRIs, our pharmacological armamentarium for OCD remains largely unchanged. The emerging body of research summarized in this study indicates that the ECS may impact the neural circuitry underlying OCD and could be a target for novel treatments. Moving forward, further exploration of the complex relationship between OCD symptoms and the ECS will be critical to determining the utility of cannabinoids in treating OCD. Well-designed placebo-controlled studies are also needed to demonstrate the efficacy and tolerability of cannabinoid drugs in OCD populations, and to measure their impact on relevant neural targets, including frontostriatal and frontolimbic circuits. ECS-targeted drugs have the potential to yield novel pharmacotherapies which are long overdue for those who suffer the debilitating effects of OCD. Only further exploration of this topic will determine whether cannabinoids pass the most important test: Helping more patients with OCD to achieve wellness.
Abbreviations Used
- 2-AG
2-arachidonoyl glycerol
- AEA
N-arachidonoylethanolamine
- CBD
cannabidiol
- CNS
central nervous systems
- COX-2
cyclooxygenase-2
- dACC
dorsal anterior cingulate cortex
- DLS
dorsal lateral striatum
- DMS
dorsal medial striatum
- eCB
endocannabinoid
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydroxylase
- FDA
Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- MAGL
monoacylglycerol lipase
- MBB
marble-burying behavior
- NAAA
N-acylethanolamine-hydrolizing acid amidase
- OCD
obsessive-compulsive disorder
- OFC
orbitofrontal cortex
- PNS
peripheral nervous systems
- PTSD
posttraumatic stress disorder
- SRI
serotonin reuptake inhibitors
- STSS
shapiro tic syndrome scale
- THC
tetrahydrocannabinol
- TS
tourette syndrome
- TS-CGI
tourette syndrome clinical global impressions scale
- TSSL
tourette syndrome symptom list
- YBOCS
yale-brown obsessive compulsive scale
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Kayser RR, Snorrason I, Haney M, Lee FS, Simpson HB (2019) The endocannabinoid system: a new treatment target for obsessive compulsive disorder? Cannabis and Cannabinoid Research 4:2, 1–11, DOI: 10.1089/can.2018.0049.
References
- 1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005 [DOI] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2015 [Google Scholar]
- 3. Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and morbidity from disease, injuries, and risk factors in 1990 and projected to 2020. World Health Organization, Harvard, 1996 [Google Scholar]
- 4. Koran LM. Obsessive-compulsive disorder: an update for the clinician. Focus (Madison). 2007;V:299–313 [Google Scholar]
- 5. Hollander E, Rowland C, Stein DJ, et al. A pharmaco-economic and quality of life study of obsessive compulsive disorder. Psychopharmacol Bull. 1995;31:526 [Google Scholar]
- 6. Bloch MH, Green C, Kichuk SA, et al. Long-term outcome in adults with obsessive-compulsive disorder. Depress Anxiety. 2013;30:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloch MH, Kelmendi B, Coric V, et al. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11:622–632 [DOI] [PubMed] [Google Scholar]
- 8. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D, Ryba NL, Kalabalik J, et al. Critical review of the use of second-generation antipsychotics in obsessive-compulsive and related disorders. Drugs R D. 2018;18:167–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutz B, Marsicano G, Maldonado R, et al. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huisman-van Dijk HM, Schoot R van de, Rijkeboer MM, et al. The relationship between tics, OC, ADHD and autism symptoms: a cross- disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Res. 2016;237:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodwin GM. The overlap between anxiety, depression, and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2015;17:249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGuire PK, Bench CJ, Frith CD, et al. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994;164:459–468 [DOI] [PubMed] [Google Scholar]
- 14. Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586 [DOI] [PubMed] [Google Scholar]
- 15. Saxena S, Brody AL, Schwartz JM, et al. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. 1998;173:26–37 [PubMed] [Google Scholar]
- 16. van den Heuvel OA, van Wingen G, Soriano-Mas C, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–827 [DOI] [PubMed] [Google Scholar]
- 17. Milad MR, Rauch SL. Obsessive Compulsive Disorder: beyond Segregated Cortico- striatal Pathways. Trends Cogn Sci. 2012;16:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gremel CM, Chancey JH, Atwood BK, et al. Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron. 2016;90:1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E, et al. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology. 2008;55:55–62 [DOI] [PubMed] [Google Scholar]
- 20. Mechoulam R, Hanus L, Pertwee R, et al. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–764 [DOI] [PubMed] [Google Scholar]
- 21. Blessing EM, Steenkamp MM, Manzanares J, et al. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159 [DOI] [PubMed] [Google Scholar]
- 23. Han J, Kesner P, Metna-Laurent M, et al. Acute cannabinoids impair working memory through astroglial CB 1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050 [DOI] [PubMed] [Google Scholar]
- 24. Oliveira da Cruz JF, Robin LM, Drago F, et al. Astroglial type-1 cannabinoid receptor (CB1): a new player in the tripartite synapse. Neuroscience. 2016;323:35–42 [DOI] [PubMed] [Google Scholar]
- 25. Lu HC, MacKie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. 2012;367:3353–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bisogno T, Hanus L, De Petrocellis LD, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronan PJ, Wongngamnit N, Beresford TP. Molecular mechanisms of cannabis signaling in the brain. Prog Mol Biol Transl Sci. 2016;137:123–147 [DOI] [PubMed] [Google Scholar]
- 29. Moreira FA. Serotonin, the prefrontal cortex, and the antidepressant-like effect of cannabinoids. J Neurosci. 2007;27:13369–13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schrot RJ, Hubbard JR. Cannabinoids: medical implications. Ann Med. 2016;48:128–141 [DOI] [PubMed] [Google Scholar]
- 32. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aguiar DC, Moreira FA, Terzian AL, et al. Modulation of defensive behavior by Transient Receptor Potential Vanilloid Type-1 (TRPV1) channels. Neurosci Biobehav Rev. 2014;46:418–428 [DOI] [PubMed] [Google Scholar]
- 34. Katona I, Freund TF. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu Rev Neurosci. 2014;35:529–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rey AA, Purrio M, Viveros MP, et al. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Häring M, Kaiser N, Monory K, et al. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS One. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lafenêtre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology. 2009;57:715–721 [DOI] [PubMed] [Google Scholar]
- 38. Hill MN, Campolongo P, Yehuda R, et al. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43:80–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534 [DOI] [PubMed] [Google Scholar]
- 40. Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soria-Gómez E, Busquets-Garcia A, Hu F, et al. Habenular CB1 receptors control the expression of aversive memories. Neuron. 2015;88:306–313 [DOI] [PubMed] [Google Scholar]
- 42. Rabinak CA, Angstadt M, Sripada CS, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Mello Schier AR, de Oliveira Ribeiro NP, de Oliveira e Silva AC, et al. Cannabidiol, a cannabis sativa constituent, as an anxiolytic drug. Rev Bras Psiquiatr. 2012;34:219–232 [DOI] [PubMed] [Google Scholar]
- 44. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dincheva I, Drysdale AT, Hartley CA, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gunduz-Cinar O, MacPherson KP, Cinar R, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology, LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomes FV, Casarotto PC, Resstel LBM, et al. Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:434–438 [DOI] [PubMed] [Google Scholar]
- 49. Umathe SN, Manna SSS, Jain NS. Endocannabinoid analogues exacerbate marble-burying behavior in mice via TRPV1 receptor. Neuropharmacology. 2012;62:2024–2033 [DOI] [PubMed] [Google Scholar]
- 50. Nardo M, Casarotto PC, Gomes FV, et al. Cannabidiol reverses the mCPP-induced increase in marble-burying behavior. Fundam Clin Pharmacol. 2014;28:544–550 [DOI] [PubMed] [Google Scholar]
- 51. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), delta9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behavior. Psychopharmacology (Berl). 2012;219:859–873 [DOI] [PubMed] [Google Scholar]
- 52. Casarotto PC, Gomes F V, Resstel LBM, et al. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav Pharmacol. 2010;21:353–358 [DOI] [PubMed] [Google Scholar]
- 53. Umathe SN, Manna SSS, Jain NS. Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behav Brain Res. 2011;223:125–134 [DOI] [PubMed] [Google Scholar]
- 54. Manna SSS, Umathe SN. Paracetamol potentiates the antidepressant-like and anticompulsive-like effects of fluoxetine. Behav Pharmacol. 2015;26:268–281 [DOI] [PubMed] [Google Scholar]
- 55. Rabinak CA, Angstadt M, Lyons M, et al. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem. 2014;113:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bowers ME, Ressler KJ. An overview of translationally informed treatments for posttraumatic stress disorder: animal models of Pavlovian fear conditioning to human clinical trials. Biol Psychiatry. 2015;78:e15–e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588 [DOI] [PubMed] [Google Scholar]
- 59. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34:559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McPartland JM, Duncan M, Di Marzo V, et al. Are cannabidiol and delta9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. National Academies of Sciences, Engineering and Medicine. The health effects of cannabis and cannabinoids. National Academies Press, Washington, DC, 2017 [PubMed] [Google Scholar]
- 62. Müller-Vahl KR. Treatment of Tourette syndrome with cannabinoids. Behav Neurol. 2013;27:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Curtis A, Clarke CE, Rickards HE. Cannabinoids for Tourette's syndrome (review). Cochrane Database Syst Rev. 2009;4:CD006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koppel BS. Cannabis in the treatment of dystonia, dyskinesias, and tics. Neurotherapeutics. 2015;12:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grant JE, Odlaug BL, Chamberlain SR, et al. Dronabinol, a cannabinoid agonist, reduces hair pulling in trichotillomania: a pilot study. Psychopharmacology (Berl). 2011;218:493–502 [DOI] [PubMed] [Google Scholar]
- 66. Schindler F, Anghelescu I, Regen M, et al. Improvement in refractory obsessive-compulsive disorder with dronabinol. Am J Psychiatry. 2008;165:536–537 [DOI] [PubMed] [Google Scholar]
- 67. Cooper JJ, Grant J. Refractory OCD due to thalamic infarct with response to dronabinol. J Neuropsychiatry Clin Neurosci. 2017;29:77–78 [DOI] [PubMed] [Google Scholar]
- 68. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213–218 [DOI] [PubMed] [Google Scholar]
- 69. Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455 [DOI] [PubMed] [Google Scholar]
- 70. Imperatore R, Morello G, Luongo L, et al. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB1R signaling and anxiety-like behavior. J Neurochem. 2015;135:799–813 [DOI] [PubMed] [Google Scholar]
- 71. Voon V, Derbyshire K, Rück C, et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry. 2015;20:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Burns HD, Van Laere K, Sanabria-Bohorquez S, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci USA. 2007;104:9800–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wong DF, Kuwabara H, Horti AG, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ceccarini J, De Hert M, Van Winkel R, et al. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage. 2013;79:304–312 [DOI] [PubMed] [Google Scholar]
- 75. Neumeister A, Normandin MD, Murrough JW, et al. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–2109 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 76. Klumpers LE, Cole DM, Khalili-Mahani N, et al. Manipulating brain connectivity with δ9-tetrahydrocannabinol: a pharmacological resting state FMRI study. Neuroimage. 2012;63:1701–1711 [DOI] [PubMed] [Google Scholar]
- 77. Bhattacharyya S, Falkenberg I, Martin-Santos R, et al. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40:1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Whitfield-Gabrieli S, Fischer AS, Henricks AM, et al. Understanding marijuana's effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: a pilot investigation. Schizophr Res. 2018;194:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]