Abstract
Introduction: Lafora disease (LD) is a rare form of progressive infantile epilepsy in which rapid neurological deterioration occurs as the disease advances, leading the patients to a vegetative state and then death, usually within the first decade of disease onset. Based on the capacity of the endogenous cannabinoid system (ECS) to modulate several cellular processes commonly altered in many neurodegenerative processes, as well as the antiepileptic properties of certain natural cannabinoids, the aim of this study was to evaluate the role of the ECS in LD progression.
Materials and Methods: We tested whether a natural cannabis extract highly enriched in cannabidiol (CBD) might be effective in curbing the pathological phenotype of malin knockout (KO) mice as an animal model of LD.
Results: Our results reveal for the first time that alterations in the ECS occur during the evolution of LD, mainly at the level of CB1, CB2, and G protein-coupled receptor 55 (GPR55) receptor expression, and that a CBD-enriched extract (CBDext) is able to reduce the cognitive impairment exhibited by malin KO mice. However, in contrast to what has previously been reported for other kinds of refractory epilepsy in childhood, the CBD-enriched extract does not reduce the severity of the epileptic seizures induced in this animal model of LD.
Conclusions: In summary, this study reveals that the ECS might play a role in LD and that a CBD-enriched extract partially reduces the dementia-like phenotype, but not the increased vulnerability to epileptic seizures, exhibited by an animal model of such a life-threatening disease.
Keywords: cannabidiol, cannabinoids, epilepsy, Lafora disease, neurodegeneration
Introduction
Lafora disease (LD) is a rare, fatal, autosomal recessive form of infantile progressive myoclonus epilepsy. As the disease advances, rapid neurological deterioration occurs, leading to a vegetative state and death, usually within the first decade of disease onset.1,2 Unfortunately, existing treatments are only palliative and no curative drugs are available yet.3
The hallmark of LD is the accumulation of insoluble polyglucosan inclusions, called Lafora bodies (LBs), within not only neurons and astrocytes but also in heart, muscle, and liver cells.2,4 Recessive mutations in two main loci, EPM2A and EPM2B, are known to produce LD. EPM2A encodes the glucan phosphatase laforin; up to 60% of patients present mutations for this gene. EPM2B encodes E3-ubiquitin ligase malin, mutated in 20–30% of patients. Although it is known that the two proteins are involved in the regulation of glycogen metabolism and form a functional complex, the underlying molecular mechanisms of this pathology are not fully understood.
The existing animal models of LD are genetically engineered mice lacking functional EPM2A5 or EPM2B6 genes. Both models exhibit LBs and neuronal degeneration, although the malin model exhibits a more severe phenotype.6
Abundant scientific evidence shows that the activity of the endogenous cannabinoid system (ECS) can protect neurons against a variety of toxic stimuli and modulate various processes that are commonly altered in many neurodegenerative processes.7 This modulatory system is composed of at least two well-characterized cannabinoid receptors coupled to Gi/o proteins, namely CB1 and CB2 receptors, their endogenous ligands (anandamide and 2-arachidonoylglycerol, mainly), and the enzymes associated with the synthesis and degradation of these endocannabinoid compounds. Cannabinoids can also bind to other receptors such as the G protein-coupled receptor 55 (GPR55), the peroxisome proliferator-activated receptors PPARα and PPARγ, and transient receptor potential vanilloid-1 (TRPV1).8
To date, no information is available about the potential involvement of the ECS in LD onset and progression. However, considering that this disease shares several pathological processes that are common to most neurodegenerative diseases (excitotoxicity, oxidative stress, neuroinflammation, synaptic alterations, and neuronal death), we hypothesize that the ECS might play a role in LD and could become a potential therapeutic target against this neurodegenerative disorder. Further, the benefit of targeting the ECS in LD might be extended to a reduction in the incidence of epileptic seizures, since numerous cannabinoid compounds, especially cannabidiol (CBD), have shown interesting anticonvulsant properties in refractory infantile epilepsies.9,10 For these reasons, the aim of this study was to challenge this hypothesis by using malin knockout (KO) mice as an animal model of LD.
Materials and Methods
Animals
Mice homozygous for the EPM2B deletion6 and their corresponding wild-type (WT) littermates were used for the study. Experimental groups were composed of 6–11 animals. All animal procedures were carried out by following the guidelines of the European Communities Council Directive 2010/63/EU and with the approval of the local ethical committee of the University of Barcelona.
Chronic treatment with a CBD-enriched cannabis extract
Animals were treated 5 days/week for 2 months from 4 months of age (early symptomatic phase) and 10 months of age (advanced symptomatic phase). Each experimental group received the CBD-enriched natural cannabis extract (CBDext: 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of Δ9-tetrahydrocannabinol [THC], among other minor cannabinoids including 2.5 mg/kg/day of cannabigerol [CBG], 3.3 mg/kg/day of cannabinol [CBN], 2.7 mg/kg/day of cannabichromene [CBC], 4 mg/kg/day of cannabidiol acid [CBDA], and <1 μg/kg/day of Δ9-tetrahydrocannabinol acid [THCA]) provided by Hemp Solutions S.L. (Spain) or vehicle (olive oil) by oral gavage.
The selection of the dose was based on previous studies reporting anticonvulsant properties of CBD-enriched extracts in other refractory epilepsies in children.10 After 3 days of washing period, animals were subjected to behavioral evaluation.
Behavioral evaluation
Cognitive performance was evaluated in the two-object recognition test, as previously described.11 To evaluate motor function, we quantified spontaneous activity and motor coordination. Spontaneous locomotor activity was measured by quantifying the total distance traveled by the animals during 10 min spent in a slightly illuminated open field (30 cm long×30 cm wide). Alterations in motor coordination were evaluated with the coat hanger test. The apparatus consists of a coat hanger (30 cm in length, 2 mm diameter) suspended 32 cm above a padded surface. The fall latency was recorded as the time from when the animal was attached to the hanger with its front paws until its fall, with a maximum time of 1 min. Each animal was subjected to four sessions, separated in time by at least 1 h.
At the end of the behavioral testing, animals were killed and their brains were rapidly removed from the skull and processed for study. One hemisphere was dissected on ice, immediately frozen, and stored at −80°C until use. The other hemisphere was fixed in 4% paraformaldehyde and processed for morphological studies.
Pilocarpine-induced seizures
During the daily handling of animals over 8 weeks of chronic treatment, no spontaneous seizures occurred. However, sporadic seizures were observed in their home cage in animals older than 15 months. For these reasons, the potential anticonvulsant properties of the CBDext were evaluated in malin KO and WT mice aged 16 months in the pilocarpine-induced seizure model.12
Animals were pretreated with the muscarinic antagonist scopolamine (1 mg/kg, s.c.; Sigma-Aldrich) 30 min before pilocarpine injection to prevent peripheral effects of the drug, and with the CBDext (98.5 mg/kg, oral) or vehicle 15 min before the administration of a submaximal dose of pilocarpine (250 mg/kg, i.p., Sigma-Aldrich). Then, the mice were observed for 1 h and scored according to this scale: (1) tremor; (2) single myoclonic jerks; (3) clonus; (4) one tonic-clonic seizure; (5) two seizures; (6) three or more seizures; and (7) death, or status epilepticus.
Quantification of LBs
The LBs were visualized with PAS staining and immunohistochemical techniques. Fixed tissue samples were embedded in paraffin, and 4-μm coronal sections were cut with a microtome. De-waxed sections were incubated with 1% periodic acid for 15 min, followed by an additional 15-min incubation with Schiff reagent. Sections were lightly counterstained with hematoxylin. After staining, the sections were dehydrated and cover-slipped for observation under a Nikon Eclipse E800 microscope (20×objective). LB density was calculated as the percentage of the area of PAS staining with respect to the total brain area. Pictures of 1 section per case were taken from the motor and retrospenial cortex, hippocampus (molecular layer), brainstem, and cerebellum, corresponding to the main regions in which LB deposition is observed in malin KO mice.3
Nissl staining and cell counting
De-waxed sections were stained with 10% cresyl violet at 56°C for 1 h, dehydrated, and cover-slipped for observation under a Nikon Eclipse E800 microscope (40×objective). The cell density in the motor cortex and CA1 region of the hippocampus was calculated in one representative picture taken from each animal by using the Count and Analysis tools of the Adobe® Photoshop® CS4 software.
Quantification of gene expression
The gene expression of CB1, CB2, GPR55 cannabinoid receptors, TRPV1 receptor, endocannabinoid-degrading enzymes fatty acid amide hydrolase (FAAH), and monoacylglycerol lipase (MAGL) and several components of the inflammatory signaling (the pro-inflammatory cytokines TNFA, interleukin (IL)-1B, IL-6, and the markers of microglial and astroglial reactivity IBA1 and GFAP, respectively) were quantified from frozen sensorimotor cortex and hippocampus samples of malin KO and WT mice with a quantitative real-time polymerase chain reaction by using TaqMan® system (Applied Biosystems). Samples were analyzed with the double delta CT method by using vehicle-treated WT samples as control and Hprt and Xpnpep1 as house-keeping genes.
Quantification of protein levels
The levels of postsynaptic density protein-95 (PSD-95; 1:500; Invitrogen), synaptophysin (1:4,000; Novocastra), GFAP (1:500; Dako), and IBA1 (1:1,000; Wako) proteins were evaluated in the sensorimotor cortex and hippocampus of mice by western blotting, as previously described.11 Densitometric quantification was carried out with TotalLab v2.01 software (Pharmacia) by using β-actin (1:10,000; Abcam) as protein loading control.
Statistical analysis
Statistical analysis was performed with the SPSS® Statistics v21.0 software. Data were analyzed with two-way analysis of variance (ANOVA) with genotype and treatment as between factors, followed by Tukey's post hoc when required (treated mice), Student's t-test (gene expression of ECS components), or Chi-square test (viability after pilocarpine-induced seizures). In all the experiments, the significance level was set at p<0.05.
Results and Discussion
Quantification of the ECS components in malin KO mice
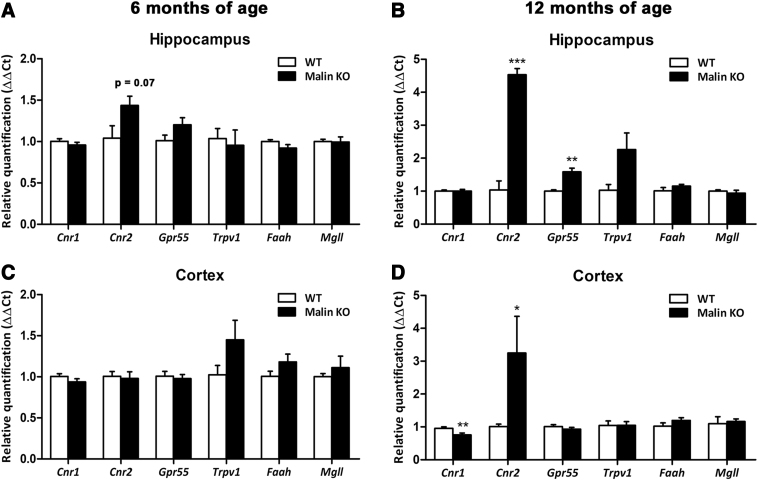
To assess the potential involvement of the ECS in LD progression, we evaluated the gene expression levels of CB1, CB2, GPR55, and TRPV1 receptors, and FAAH and MAGL endocannabinoid degradative enzymes, in the sensorimotor cortex and the hippocampus of malin KO mice at different stages of the neurodegenerative process. Our results reveal, for the first time, clear overexpression of the CB2 receptor in the cortex (p<0.05) and the hippocampus (p<0.001) of malin KO mice at 12 months of age (Fig. 1B, D), but no significant differences at 6 months of age despite a tendency (p=0.07) to increase in the hippocampus (Fig. 1A), as well as a significant reduction in the expression of CB1 (p<0.01) in the cortex of malin KO mice (Fig. 1D) and a significant increase of GPR55 in the hippocampus of malin KO mice at 12 months of age (Fig. 1B).
FIG. 1.
Gene expression quantification of the main ECS components (CB1, CB2, GPR55, and TRPV1 receptors, and the endocannabinoid degradative enzymes FAAH and MAGL) in the hippocampus (upper panels) and sensorimotor cortex (lower panels) of WT and malin KO mice at 6 months (left panels) or 12 months of age (right panels). No significant alterations in the expression of any of the ECS components evaluated were observed in mice at 6 months of age in the hippocampus (A) or in the cortex (B) in spite of a tendency to increase the expression of the CB2 receptor in the hippocampus of malin KO mice. In contrast, malin KO mice showed a significant increase in the CB2 receptor expression in the hippocampus (C) and cortex (D), as well as higher expression of Gpr55 in the hippocampus and reduced expression levels of CB1 receptor in the cortex at 12 months of age. Data are expressed as the mean values±SEM. *p<0.05, **p<0.01, ***p<0.001 compared with WT. ECS, endogenous cannabinoid system; Cnr1, gene coding for CB1 receptor; Cnr2, gene coding for CB2 receptor; Faah, gene coding for fatty acid amide hydrolase; Gpr55, gene coding for G protein-coupled receptor 55; KO, knockout; Mgll, gene coding for monoacylglycerol lipase; SEM, standard error of the mean; TRPV1, gene coding for transient receptor potential vanilloid-1; WT, wild-type.
Similar alterations in the two main cannabinoid receptors were also reported in other neurodegenerative diseases such as Alzheimer's disease and Huntington's disease.13 According to previous evidence, these changes in the expression of the main cannabinoid receptors might reveal a loss of the capacity of CB1 receptor to protect neurons against toxic stimuli and the participation of the CB2 receptor in the inflammatory process occurring in LD.14
GPR55 function in the brain is still poorly understood but some previous evidence indicates a relevant role of this receptor in memory processing, neurogenesis, and immune responses in the hippocampus.15,16 Thus, our results revealing increased levels of GPR55 in the hippocampus of malin KO mice at advanced age suggest a role for this receptor in the progression of the disease and point GPR55 out as a potential therapeutic target against LD. Other ECS components evaluated were not modified in malin KO mice at any of the ages analyzed.
Cognitive and motor evaluation of treated malin KO mice
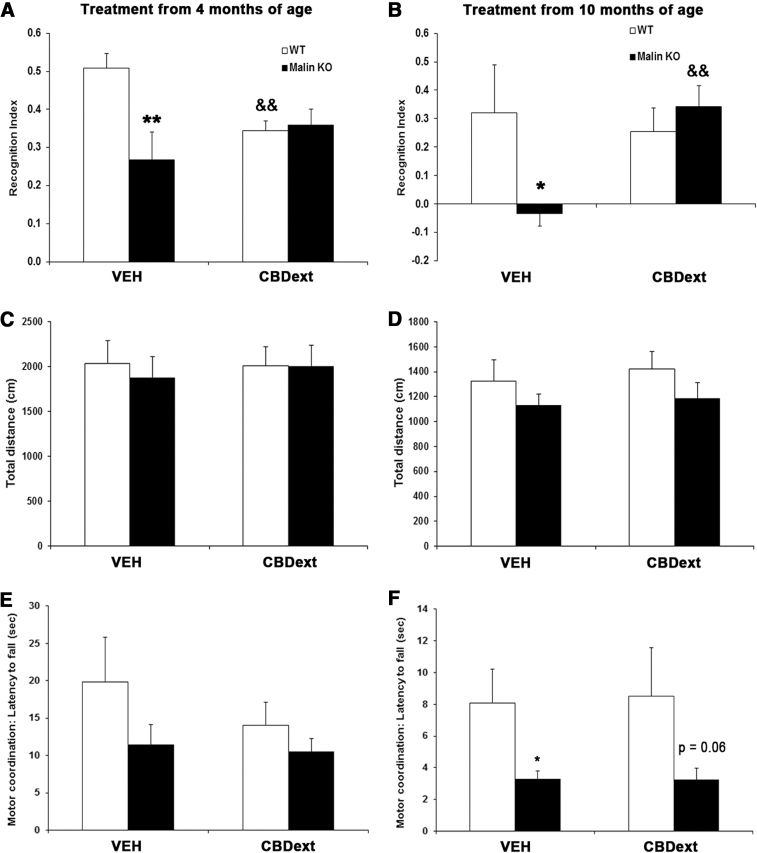
To challenge whether the cannabinoids might have potential utility in treating this orphan disease, we treated malin KO mice with a CBD-enriched cannabis extract, because of the neuroprotective, anti-inflammatory, and anti-epileptic properties exhibited by this compound in other animal models and human studies.9,10,17,18 Control malin KO mice exhibited memory impairment at 6 months (Fig. 2A, p<0.01) and 12 months of age (Fig. 2B, p<0.05) when compared with WT mice, as revealed by the significant reduction of the recognition index in the two-object recognition test. Interestingly, chronic treatment with the CBDext improved the cognitive performance in aged malin KO mice when compared with vehicle-treated littermates (p<0.01, Fig. 2B). However, CBDext induced a significant reduction in the memory performance of WT mice when administered from 4 months of age (p<0.01, Fig. 2A), raising questions about the potential side effects of this natural cannabis extract in healthy animals. This undesired effect could be related to the Δ9-THC contents of the cannabis extract used (4.8 mg/kg/day), but further research is needed to corroborate this assumption.
FIG. 2.
Behavioral evaluation of WT (white bars) and malin KO (black bars) mice after chronic treatment during 2 months with the CBD-enriched extract (98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids) from 4 (left panels) and 10 (right panels) months of age. (A, B) CBDext reduced the memory impairment of malin KO when administered from 10 months of age but it induced a reduction of the memory performance in WT aged 4 months at the beginning of treatment. (C, D) Spontaneous locomotor activity was not significantly modified by treatment or genotype at any age. (E, F) CBDext was not able to reduce motor coordination impairment of aged malin KO mice in the coat hanger test. Data are expressed as the mean values±SEM. *p<0.05, **p<0.01, p=0.06 compared with WT. &&p<0.01 compared with VEH. CBD, cannabidiol; CBDext, CBD-enriched extract; THC, tetrahydrocannabinol; VEH, vehicle.
As shown in Figure 2C and D, spontaneous locomotor activity measured during 10 min in the open field was not significantly modified by treatment or genotype at any age. Motor coordination was evaluated with the coat hanger test. No significant motor coordination impairment was observed in malin KO mice treated from 4 months of age, despite a tendency to decrease in motor coordination (Fig. 2E). In contrast, vehicle-treated malin KO mice aged 10 months at the beginning of the treatment exhibited a significant reduction in their motor coordination, as revealed by the decrease in fall latency from the coat hanger (Fig. 2F, p<0.05 compared with WT mice). However, CBDext was not able to reduce this motor coordination impairment. Specific details about the statistical analysis of behavioral evaluation are included in Table 1.
Table 1.
Statistical Details of the Behavioral Evaluation of Malin Knockout Mice Treated During 8 Weeks from 4 or 10 Months of Age with Cannabidiol-Enriched Extract or Vehicle
| Two-way ANOVA |
Tukey's post hoc test |
||||||
|---|---|---|---|---|---|---|---|
| Genotype | Treatment | Interaction | WT VEH vs. KO VEH | WT CBD vs. KO CBD | WT VEH vs. WT CBD | KO VEH vs. KO CBD | |
| Two-way ANOVA (treatment from 4 months of age) | |||||||
| Memory | F(1,35)=5.763, p<0.05 | F(1,35)=0.612, N.S. | F(1,35)=7.366, p<0.01 | p<0.01 | N.S. | p<0.01 | N.S. |
| Locomotor activity | F(1,16)=0.102, N.S. | F(1,16)=0.041, N.S. | F(1,16)=0.104, N.S. | — | — | — | — |
| Motor coordination | F(1,39)=2.529, N.S. | F(1,39)=0.801, N.S. | F(1,39)=0.523, N.S. | — | — | — | — |
| Two-way ANOVA (treatment from 10 months of age) | |||||||
| Memory | F(1,24)=1.879, N.S. | F(1,24)=2.592, N.S. | F(1,24)=5.226, p<0.05 | p<0.05 | N.S. | N.S. | p<0.01 |
| Locomotor activity | F(1,25)=2.347, N.S. | F(1,25)=0.758, N.S. | F(1,25)=0.003, N.S. | — | — | — | — |
| Motor coordination | F(1,25)=9.376, p<0.01 | F(1,25)=0.017, N.S. | F(1,25)=0.022, N.S. | p<0.05 | p=0.06 | — | — |
Two-way ANOVA with genotype and treatment as between-subjects factors was applied for the analysis of memory performance, locomotor activity, and motor coordination. When one factor or interaction between factors was significant, comparisons between groups were performed by Tukey's post hoc test. See the Materials and Methods section for details.
ANOVA, analysis of variance; CBD, cannabidiol; KO, knockout; N.S., not significant; VEH, vehicle; WT, wild-type.
Neuroprotective and neuroinflammatory properties of the CBD-enriched extract
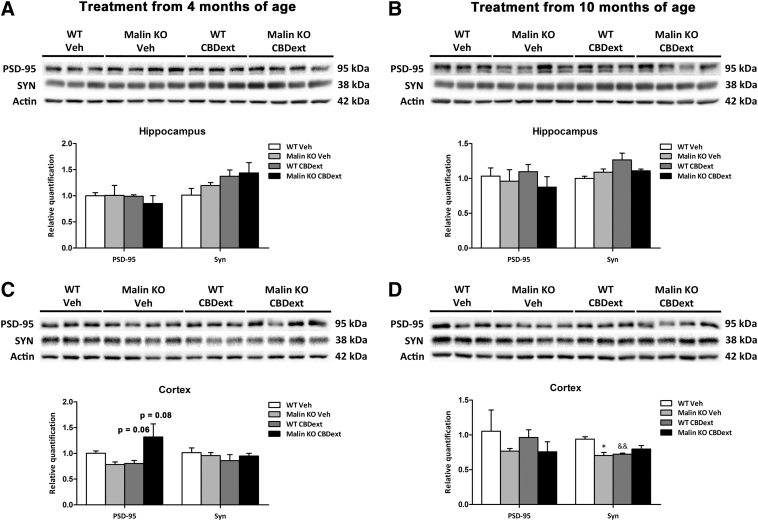
We first hypothesized that the distinct cognitive alterations produced by CBDext in WT and malin KO mice might be associated with the modulation of synaptic plasticity in brain areas that are relevant for cognition. Because of this, we evaluated the levels of PSD-95, one of the major scaffolding proteins, in the excitatory postsynaptic density and a potent regulator of synaptic strength,19 and the levels of the presynaptic component synaptophysin in cortical and hippocampal samples of treated mice.
No significant differences in synaptophysin or PSD-95 protein levels were observed in the hippocampus at any age (Fig. 3A, B). In contrast, two-way ANOVA revealed a significant interaction [F(1,10)=5.515, p<0.05], but no genotype or treatment effect, in the levels of PSD-95 in cortical samples from mice treated from 4 months of age (Fig. 3C). Subsequent Tukey's post hoc test showed a tendency to decrease in PSD-95 levels in CBD-treated WT mice (p=0.06) and to increase in CBD-treated malin KO mice (p=0.08), suggesting that the modulation of PSD-95 might be related to the distinct cognitive alterations produced by CBDext in both genotypes at early stages. However, no significant modulation of PSD-95 levels was observed in mice treated from 10 months of age (Fig. 3D).
FIG. 3.
Synaptic density in the hippocampus (upper panels) and sensorimotor cortex (lower panels) of WT (white bars) and malin KO (black bars) after chronic treatment with VEH or CBD-enriched extract (98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids) from 4 months of age (left panels) or from 10 months of age (right panels). (A, B) Representative immunoblots and densitometry quantification for PSD-95 and synaptophysin (Syn) proteins in the hippocampus of treated mice revealed no statistical differences between groups at 6 months (A) or 12 months of age (B). (C, D) Representative immunoblots and densitometry quantification for PSD-95 and synaptophysin (Syn) proteins in the cortex of treated mice. No significant differences between groups were observed in PSD-95 levels at any age, despite a tendency to decrease in WT and to increase in malin KO mice treated with CBD-enriched extract from 4 months of age (C). Densitometry quantification of synaptophysin immunoblots revealed no difference due to genotype and/or treatment in animals treated from 4 months of age (C), but a significant reduction in vehicle malin KO and CBDext WT mice treated from 10 months of age (D). Data are expressed as mean values±SEM. *p<0.05, compared with WT; &&p<0.01 compared with vehicle. PSD-95, postsynaptic density protein-95.
Moreover, a significant interaction [F(1,10)=13.248, p<0.01], but no genotype or treatment effect, was observed in cortical synaptophysin levels of mice treated from 10 months of age (Fig. 3D). Subsequent Tukey's post hoc test revealed a significant decrease in the synaptophysin levels of vehicle-treated malin KO (p<0.05) and of CBD-treated WT mice (p<0.01) when compared with vehicle-treated WT mice (Fig. 3D), suggesting presynaptic impairment as a substrate for the cognitive decline in aged animals. Nevertheless, the CBDext did not significantly modulate synaptophysin levels in treated malin KO mice, which indicates that other mechanisms must underlie the cognitive improvement induced by this cannabis extract at advanced stages.
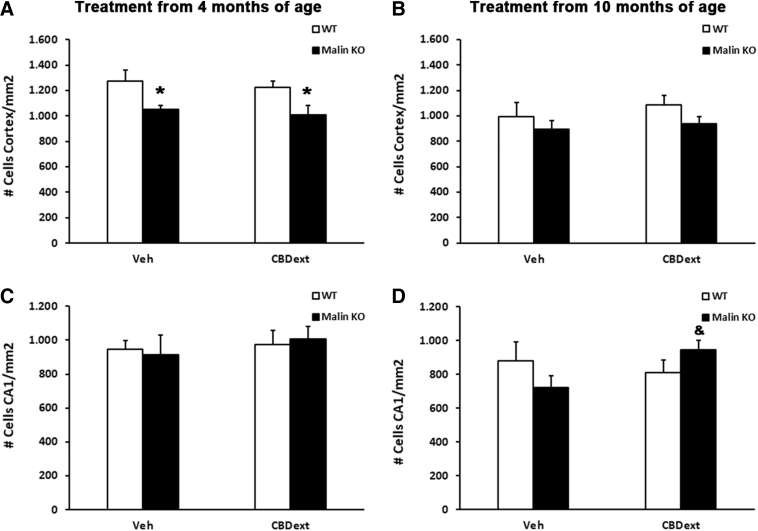
Among other potential mechanisms involved in the memory improvement induced by the CBDext in malin KO mice, we focused on cellular viability and neuroinflammation based on the already known protective and anti-inflammatory properties of CBD.17,18 Our findings reveal that the CBDext does not halt the cellular loss occurring in the cortex of malin KO mice from the early stages of the neurodegenerative process but it reduces the loss of neurons in the CA1 hippocampal area observed in malin KO mice at 12 months of age, which might contribute to the memory alterations observed. Specifically, cell counts from Nissl staining in the motor cortex of animals treated from 4 months of age, but not from 10 months of age, revealed a significant genotype effect [F(1,22)=11.621, p<0.01], but no treatment effect or interaction between the two factors. Subsequent Tukey's post hoc test showed a reduction in cellular density in the cortex of malin KO mice, both those treated with vehicle (p<0.05) and those treated with CBDext (p<0.05) when compared with WT animals (Fig. 4A, B). No significant difference due to genotype or treatment was observed on the cellular density in CA1 area of the hippocampus of mice treated from 4 months of age (Fig. 4C). In contrast, a significant interaction [F(1,22)=4.712, p<0.05], but no genotype or treatment effect, was observed in the density of cells in CA1 area of the hippocampus of mice treated from 10 months of age (Fig. 4D). Thus, subsequent Tukey's post hoc test showed an increase in cellular density in the hippocampus of CBD-treated malin KO when compared with vehicle-treated malin KO mice (p<0.05).
FIG. 4.
Quantification of the cellular density in the motor cortex (upper panels) and the CA1 region of the hippocampus (lower panels) of WT (white bars) and malin KO (black bars) after chronic treatment with VEH or CBD-enriched extract (CBDext 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids) from 4 months (left panels) or 10 months of age (right panels). (A) Malin KO mice treated from 4 months of age showed a significant cellular loss in the motor cortex when compared with WT animals, but no effect of CBDext was revealed. (B) In spite of a tendency, no statistical difference due to genotype and/or treatment was revealed in the motor cortex of older animals. (C) Cellular density in the CA1 region of the hippocampus was not modified by genotype and/or treatment in animals treated from 4 months of age. (D) In contrast, malin KO mice treated with CBDext from 10 months of age exhibited a higher cellular density in the hippocampus than vehicle-treated littermates. Data are expressed as the mean values±SEM. *p<0.05, compared with WT; &p<0.05 compared with VEH.
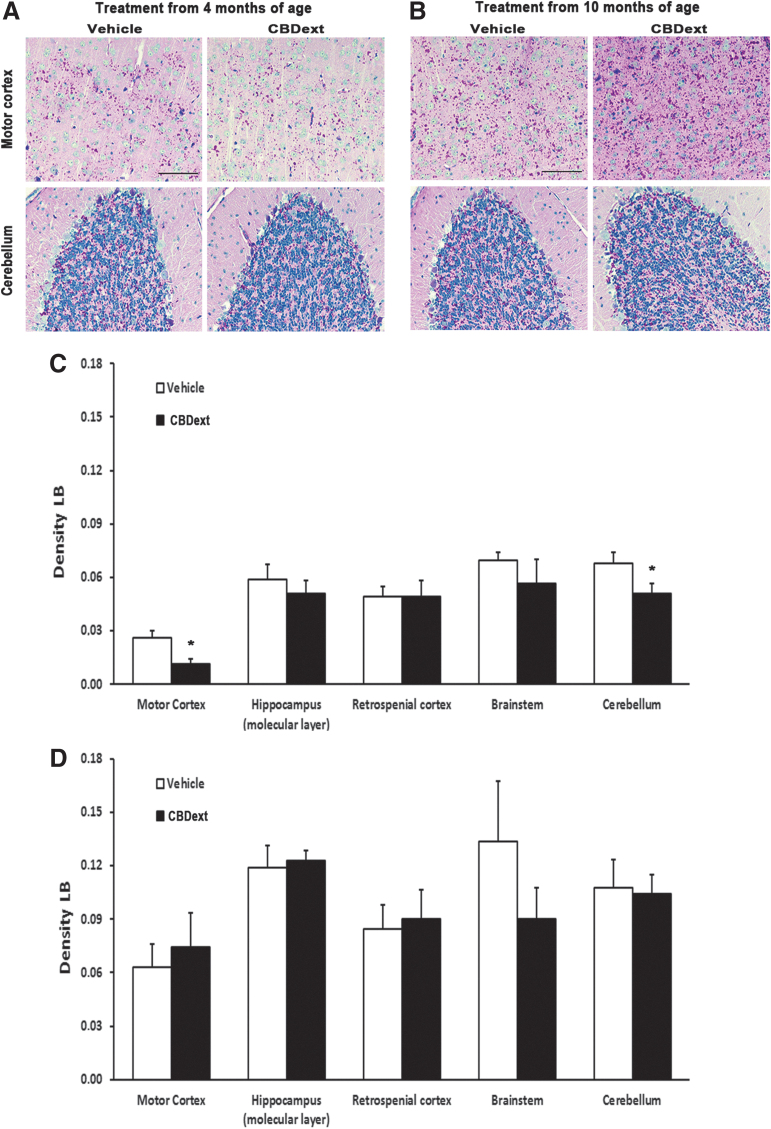
Interestingly, the CBDext reduced, when administered from 4 months of age, the accumulation of neurotoxic LB in discrete brain areas. Densitometry quantification of PAS staining revealed a significant decrease in the density of LB in the motor cortex (p<0.05) and cerebellum (p<0.05) of malin KO mice chronically treated with the CBDext from 4 months of age (Fig. 5A, C), but not from 10 months of age (Fig. 5B, D). However, the mechanisms underlying this CBDext-induced effect and its contribution to the cognitive improvement observed remain to be elucidated.
FIG. 5.
(A, B) Representative images of LBs in the motor cortex (upper panels) and cerebellum (bottom panels) of malin KO mice after chronic treatment with vehicle (left panels) or CBD-enriched extract (CBDext: 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids, right panels) from 4 months (A) and 10 months of age (B). Magenta staining indicates LB formation. Nuclei are counterstained in blue with hematoxylin. Scale bar represents 50 μm. (C, D) LB density quantification in motor and retrospenial cortex, hippocampus (molecular layer), brainstem, and cerebellum of animals treated from 4 months (C) or 10 months of age (D). Malin KO mice treated with CBDext from 4 months of age exhibited a reduction in LB density in motor cortex and cerebellum. Data are expressed as the mean values±SEM. *p<0.05, compared with vehicle. LB, Lafora body.
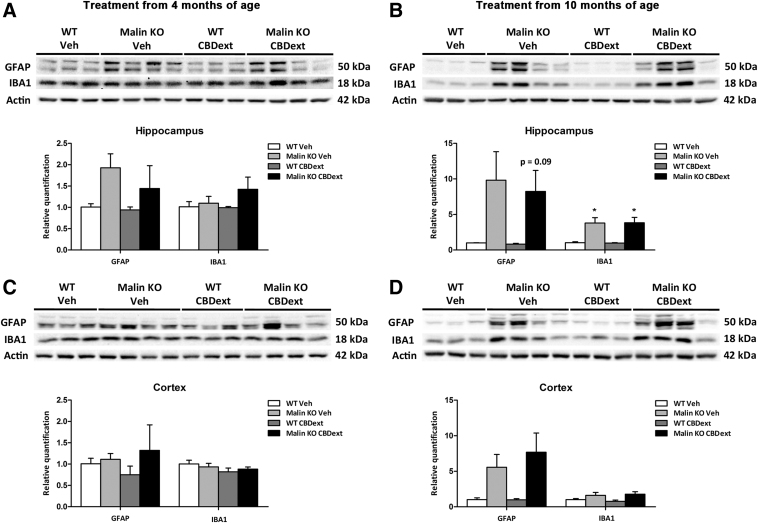
Next, we explored the potential anti-inflammatory properties of the CBDext in malin KO mice. The protein levels of astroglial (GFAP) and microglial (IBA1) reactivity markers were evaluated in sensorimotor cortical and hippocampal samples by western blotting. As shown in Figure 6A and C, no significant alterations in IBA1 or GFAP levels due to treatment or genotype were found in brain hippocampal or cortical samples of animals treated from 4 months of age. In contrast, a genotype effect was observed in the levels of IBA1 [Hippocampus: F(1,10)=19.150, p<0.01; Cortex: F(1,10)=5.871, p<0.05] and GFAP [Hippocampus: F(1,10)=7.573, p<0.05; Cortex: F(1,10)=8.491, p<0.05] in the animals treated from 10 months of age. Tukey's post hoc test revealed no significant differences between WT and malin KO mice treated with vehicle or CBDext in the cortex (Fig. 6D) but a significant increase in the hippocampal levels of IBA1 of malin KO mice treated with vehicle (p<0.05) or CBDext (p<0.05) when compared with WT animals (Fig. 6B).
FIG. 6.
Glial reactivity in the hippocampus (upper panels) and cortex (lower panels) of WT (white bars) and malin KO (black bars) after chronic treatment with VEH or CBD-enriched extract (CBDext: 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids) from 4 months of age (left panels) or from 10 months of age (right panels). (A, B) Representative immunoblots and densitometry quantification for GFAP (astroglial marker, upper band) and IBA1 (microglial marker, middle band) proteins and corresponding actin (lower band) loading control from hippocampal samples. No significant alterations in IBA1 or GFAP levels due to treatment or genotype were found in the brain hippocampus of animals treated from 4 months of age (A). In contrast, a significant increase in the hippocampal levels of IBA1 and GFAP was observed in malin KO mice treated from 10 months of age when compared with WT animals (B). (C, D) Representative immunoblots and densitometry quantification for GFAP (upper band) and IBA1 (middle band) proteins and corresponding actin (lower band) loading control from cortical samples. No statistical differences between groups were observed in animals treated from 4 months (C) or 10 months of age (D), despite a tendency to increase GFAP levels in malin KO mice treated from 10 months of age when compared with WT animals. Data are expressed as the mean values±SEM. *p<0.05, compared with WT.
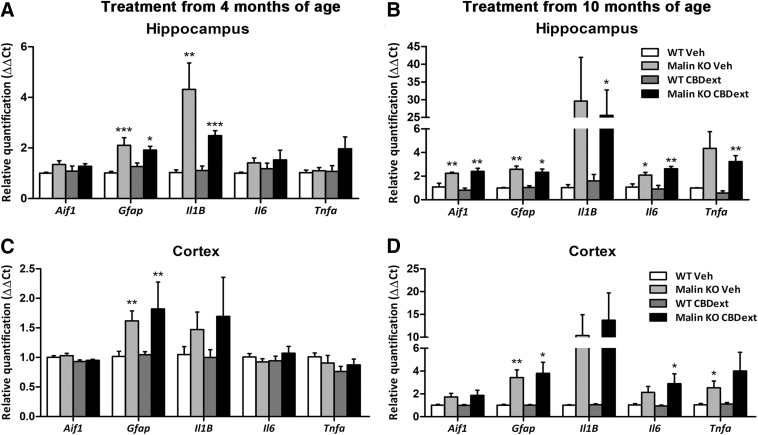
We also quantified the gene expression of various components of inflammatory signaling, including IBA1, GFAP, TNF-α, IL-1β, IL-6, and IL-10. As expected, malin KO mice treated from 10 months of age expressed higher levels of all the inflammatory signaling components when compared with WT mice in both the cortex and hippocampus, but no significant effect of treatment was observed in any case (Fig. 7B, D). At earlier stages, such differences were restricted to GFAP in the cortex and hippocampus and IL-1β in the cortex (Fig. 7A, C). Specific details about statistical analysis of the gene expression of inflammatory signaling components are included in Table 2. These results confirm the previously described increase of the inflammatory response during disease progression in this LD model,14 but they fail to demonstrate an anti-inflammatory effect of the CBDext at any age.
FIG. 7.
Gene expression of several inflammatory components in the hippocampus (upper panels) and sensorimotor cortex (lower panels) of WT (white bars) and malin KO (black bars) after chronic treatment with CBD-enriched extract (CBDext: 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of THC, among other minor cannabinoids) from 4 months (left panels) and 10 months of age (right panels). (A, B) Malin KO mice treated from 4 months of age (A), independently of the treatment received, exhibited increased expression of the genes coding for GFAP and IL-1β; whereas those treated from 10 months of age (B) expressed higher levels of all the evaluated inflammatory signaling components than WT mice in the hippocampus. No effect of treatment was observed in any of the genes analyzed in the hippocampus. (C, D) Similar to what occurred in the hippocampus, malin KO mice expressed in the cortex higher levels of GFAP than WT mice when treated from 4 months of age (C) or from 10 months of age (D), as well as increased expression levels of the IBA1 and the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α coding genes in older animals. No anti-inflammatory effect of the CBDext was observed at any age. Data are expressed as mean values±SEM. *p<0.05, **p<0.01, and ***p<0.001, compared with WT. Aif1, gene coding for IBA1; Gfap, gene coding for GFAP; Il1B, gene coding for IL1beta; Il6, gene coding for IL6; Tnfa, gene coding for TNFalpha; IL, interleukin.
Table 2.
Statistical Details of the Inflammatory Signaling Components Gene Expression in Cortex and Hippocampus of Malin Knockout Mice Treated During 8 Weeks from 4 or 10 Months of Age with Cannabidiol-Enriched Extract or Vehicle
| Two-way ANOVA |
Tukey's post hoc test |
||||||
|---|---|---|---|---|---|---|---|
| Genotype | Treatment | Interaction | WT VEH vs. KO VEH | WT CBDext vs. KO CBDext | WT VEH vs. WT CBDext | KO VEH vs. KO CBDext | |
| Hippocampus | |||||||
| Two-way ANOVA (treatment from 4 months of age) | |||||||
| Aif1 | F(1,18)=3.667, N.S. | F(1,18)=0.001, N.S. | F(1,18)=0.321, N.S. | — | — | — | — |
| Gfap | F(1,18)=25.130, p<0.001 | F(1,18)=0.030, N.S. | F(1,18)=1.592, N.S. | p<0.001 | p<0.05 | — | — |
| Il1B | F(1,16)=28.910, p<0.001 | F(1,16)=4.040, N.S. | F(1,16)=4.833, p<0.05 | p<0.01 | p<0.001 | ||
| Il6 | F(1,18)=2.659, N.S. | F(1,18)=0.415, N.S. | F(1,18)=0.014, N.S. | — | — | — | — |
| Tnfa | F(1,18)=3.516, N.S. | F(1,18)=3.189, N.S. | F(1,18)=2.533, N.S. | — | — | — | — |
| Two-way ANOVA (treatment from 10 months of age) | |||||||
| Aif1 | F(1,12)=35.340, p<0.001 | F(1,12)=0.062, N.S. | F(1,12)=0.870, N.S. | p<0.01 | p<0.01 | — | — |
| Gfap | F(1,13)=27.400, p<0.001 | F(1,13)=0.152, N.S. | F(1,13)=0.287, N.S. | p<0.01 | p<0.05 | — | — |
| Il1B | F(1,11)=6.010, p<0.05 | F(1,11)=0.262, N.S. | F(1,11)=0.046, N.S. | N.S. | p<0.05 | — | — |
| Il6 | F(1,12)=29.980, p<0.001 | F(1,12)=0.544, N.S. | F(1,12)=1.869, N.S. | p<0.05 | p<0.01 | — | — |
| Tnfa | F(1,12)=5.611, p<0.05 | F(1,12)=0.373, N.S. | F(1,12)=0.074, N.S. | N.S. | p<0.01 | — | — |
| Cortex | |||||||
| Two-way ANOVA (treatment from 4 months of age) | |||||||
| Aif1 | F(1,19)=0.746, N.S. | F(1,19)=6.166, p<0.05 | F(1,19)=0.004, N.S. | — | — | N.S. | N.S. |
| Gfap | F(1,20)=7.670, p<0.05 | F(1,20)=0.218, N.S. | F(1,20)=0.125, N.S. | p<0.01 | N.S. | — | — |
| Il1B | F(1,20)=2.240, N.S. | F(1,20)=0.053, N.S. | F(1,20)=0.133, N.S. | — | — | — | — |
| Il6 | F(1,20)=0.076, N.S. | F(1,20)=0.260, N.S. | F(1,20)=1.734, N.S. | — | — | — | — |
| Tnfa | F(1,19)=0.001, N.S. | F(1,19)=2.035, N.S. | F(1,19)=1.231, N.S. | — | — | — | — |
| Two-way ANOVA (treatment from 10 months of age) | |||||||
| Aif1 | F(1,20)=7.698, p<0.05 | F(1,20)=0.055, N.S. | F(1,20)=0.072, N.S. | p=0.06 | N.S. | — | — |
| Gfap | F(1,20)=19.288, p<0.001 | F(1,20)=0.090, N.S. | F(1,20)=0.096, N.S. | p<0.01 | p<0.05 | — | — |
| Il1B | F(1,20)=8.525, p<0.01 | F(1,20)=0.204, N.S. | F(1,20)=0.193, N.S. | p=0.06 | p=0.06 | — | — |
| Il6 | F(1,20)=9.155, p<0.01 | F(1,20)=0.457, N.S. | F(1,20)=0.694, N.S. | p=0.06 | p<0.05 | — | — |
| Tnfa | F(1,20)=6.323, p<0.05 | F(1,20)=0.776, N.S. | F(1,20)=0.645, N.S. | p<0.05 | N.S. | — | — |
Two-way ANOVA with genotype and treatment as between-subjects factors was applied for the analysis of the gene expression levels of several components of the inflammatory signaling. When one factor or interaction between factors was significant, comparisons between groups were performed by Tukey's post hoc test. See the Materials and Methods section for details.
CBDext, CBD-enriched extract.
Anticonvulsant properties of the CBD-enriched extract
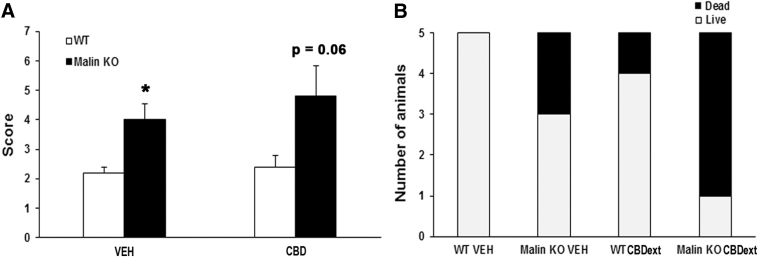
Finally, we evaluated the hypothetical anticonvulsant properties of the CBDext in malin KO mice. This animal model does not replicate the severe epileptic phenotype occurring in LD patients from early childhood, but it does manifest sporadic seizures from 15 months of age. Moreover, we observed that malin KO mice have greater susceptibility to pilocarpine-induced seizures.
Thus, we tested the anticonvulsant properties of the CBDext in malin KO and WT mice aged 16 months by using a submaximal dose of pilocarpine to induce seizures. Two-way ANOVA revealed a significant genotype effect [F(1,16)=11.455, p<0.01], but no treatment effect [F(1,16)=0.649, not significant (N.S.)] or interaction between the two factors [F(1,16)=0.234, N.S.], in the score calculated for pilocarpine-induced seizures (Fig. 8A). Subsequent Tukey's post hoc test indicated that malin KO mice exhibited a higher score than WT littermates in both the vehicle (p<0.05) and CBD-treated groups (p=0.06). Higher susceptibility to pilocarpine in malin KO mice was also revealed by the increased mortality associated with the acute administration of a submaximal dose of pilocarpine when compared with WT mice [χ2(1)=5.495, p<0.05] (Fig. 8B).
FIG. 8.
Increased susceptibility to pilocarpine-induced seizures in malin KO with respect to WT mice. (A) Score obtained 1 h after the administration of a submaximal dose of pilocarpine (250 mg/kg, i.p.) according to this scale: (1) tremor; (2) single myoclonic jerks; (3) clonus; (4) one tonic-clonic seizure; (5) two seizures; (6) three or more seizures, and (7) death, or status epilepticus. No significant difference due to the treatment (CBDext: 98.5 mg/kg/day, containing 35 mg/kg/day of CBD and 4.8 mg/kg/day of Δ9-THC, among other minor cannabinoids) was observed. Data are expressed as the mean values±SEM. *p<0.05, p=0.06 compared with WT after pilocarpine treatment. (B) Mortality after pilocarpine injection was increased in malin KO mice when compared with WT. CBDext pretreatment tends to increase the lethality of pilocarpine in mice, although no statistical difference due to the treatment was observed.
It is worthy to note that the dose of CBD used here (35 mg/kg) was significantly lower than the ones used to reveal the anticonvulsant properties of CBD in an animal model of Dravet syndrome (100–200 mg/kg),20 suggesting that testing higher doses of CBD might result in better results in our animal model. However, the fact that CBDext pretreatment, even at the low dose evaluated in this study, tends to increase the severity of pilocarpine effects in terms of lethality (Fig. 8B) [χ2(1)=1.978, p=0.15] dampens the interest to test higher doses of CBDext in malin KO mice.
These results, although unexpected, are not completely surprising, since recent studies have revealed that up to 15.4% of the patients enrolled in a study to evaluate long-term safety, tolerability, and efficacy of purified CBD in children with refractory epilepsy reported an increase in the number of seizures after exposure to doses of CBD equivalent to that included in this study.21 Moreover, considering the complexity of endocannabinoid signaling in epilepsy,22 we cannot rule out that other cannabinoids present in the natural extract might contribute to the apparent increased lethality observed in CBDext-treated mice after pilocarpine exposure.
Conclusions
In summary, this study reveals that the ECS might play a role in LD since the levels of the main cannabinoid receptors are altered during the disease progression in malin KO mice. Moreover, a CBD-enriched extract partially reduces the dementia-like phenotype in these animals. However, this extract does not halt the increased vulnerability to epileptic seizures exhibited by this animal model of such a life-threatening disease.
Acknowledgments
The authors thank Hemp Solutions Group S.L. for kindly providing the CBD-enriched natural extract. This work was supported by CIBERNED (Institute of Health Carlos III, Spanish Ministry of Economy and Competitiveness), Unión Iberoamericana de Universidades (NL01/2017), and Hemp Solutions Group S.L. The authors also thank T. Yohannan for editorial assistance. This work was partially supported by Hemp Solutions Group S.L.
Abbreviations Used
- ANOVA
analysis of variance
- CBD
cannabidiol
- CBDext
CBD-enriched extract
- ECS
endogenous cannabinoid system
- FAAH
fatty acid amide hydrolase
- GPR55
G protein-coupled receptor 55
- IL
interleukin
- KO
knockout
- LB
Lafora body
- LD
Lafora disease
- N.S.
not significant
- PSD-95
postsynaptic density protein-95
- THC
tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid-1
- WT
wild-type
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Aso E, Andrés-Benito P, Grau-Escolano J, Caltana L, Brusco A, Sanz P, Ferrer I (2020) Cannabidiol-enriched extract reduced the cognitive impairment but not the epileptic seizures in a Lafora disease animal model, Cannabis and Cannabinoid Research 5:2, 150–163, DOI: 10.1089/can.2019.0005.
References
- 1. Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248 [DOI] [PubMed] [Google Scholar]
- 2. Turnbull J, Tiberia E, Striano P, et al. Lafora disease. Epileptic Disord. 2016; 8:38–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berthier A, Payá M, García-Cabrero AM, et al. Pharmacological interventions to ameliorate neuropathological symptoms in a mouse model of Lafora disease. Mol Neurobiol. 2016;53:1296–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubio-Villena C, Viana R, Bonet J, et al. Astrocytes: new players in progressive myoclonus epilepsy of Lafora type. Hum Mol Genet. 2018;27:1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganesh S, Delgado-Escueta AV, Sakamoto T, et al. Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet. 2002;11:1251–1262 [DOI] [PubMed] [Google Scholar]
- 6. Criado O, Aguado C, Gayarre J, et al. Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum Mol Genet. 2012;21:1521–1533 [DOI] [PubMed] [Google Scholar]
- 7. Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci Ther. 2011;17:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–278 [DOI] [PubMed] [Google Scholar]
- 11. Aso E, Andrés-Benito P, Ferrer I. Genetic deletion of CB1 cannabinoid receptors exacerbates the Alzheimer-like symptoms in a transgenic animal model. Biochem Pharmacol. 2018;157:210–216 [DOI] [PubMed] [Google Scholar]
- 12. Kow RL, Jiang K, Naydenov AV, et al. Modulation of pilocarpine-induced seizures by cannabinoid receptor 1. PLoS One. 2014;9:e95922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aymerich MS, Aso E, Abellanas MA, et al. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol. 2018;157:67–84 [DOI] [PubMed] [Google Scholar]
- 14. López-González I, Viana R, Sanz P, et al. Inflammation in Lafora disease: evolution with disease progression in laforin and malin knock-out mouse models. Mol Neurobiol. 2017;54:3119–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill JD, Zuluaga-Ramirez V, Gajghate S, et al. Activation of GPR55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation. Brain Behav Immun. 2019;76:165–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramar C, Loureiro M, Renard J, et al. Palmitoylethanolamide modulates GPR55 receptor signaling in the ventral hippocampus to regulate mesolimbic dopamine activity, social interaction, and memory processing. Cannabis Cannabinoid Res. 2017;2:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández-Ruiz J, Sagredo O, Pazos MR, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–1385 [DOI] [PubMed] [Google Scholar]
- 19. Chen X, Nelson CD, Li X, et al. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114:11229–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sands TT, Rahdari S, Oldham MS, et al. Long-term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs. 2019;33:47–60 [DOI] [PubMed] [Google Scholar]
- 22. Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017;70:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]