ABSTRACT
Renal replacement therapy (RRT) for acute kidney injury (AKI) patients in an intensive care unit (ICU) presents unique problems of providing biochemical and fluid removal in patients with unstable circulations, inotropes, and increased capillary permeability. Although no individual modality has been shown to confer a mortality benefit, it is assumed that continuous therapies like peritoneal dialysis (PD) and venovenous hemofiltration or hemodiafiltration may be better tolerated by the patient with hemodynamic instability, raised intracranial pressure (ICP), and liver failure. An individual patient may require more than one treatment in the course of his/her illness. The therapies offered may reflect available resources, local expertise, and cost constraints.
How to cite this article
Lobo VA. Renal Replacement Therapy in Acute Kidney Injury: Which Mode and When? Indian J Crit Care Med 2020;24(Suppl 3):S102–S106.
Keywords: Continuous renal replacement therapy, Peritoneal dialysis, Sustained low-efficiency daily dialysis
INTRODUCTION
The successful dialysis of a patient with acute kidney injury (AKI) in 1946 by Wilhem Kolf reduced the mortality of AKI by 50% and revolutionized the way we manage AKI. Since then it remains an undeniable fact that patients with AKI should receive renal replacement therapy (RRT) though controversy exists about the optimal time for starting, the best modality, and the dose of dialysis to be delivered to patients.
Recent trials have also suggested that in a significant number of patients in whom RRT was delayed or deferred, it actually improved without the replacement therapy. Furthermore, life-threatening complications such as thrombosis, infection related to the dialysis access, hemorrhage hypotension, and membrane bioincompatibility may theoretically aggravate renal and other vital organ injury in critically ill patients with AKI. The RRT device and process itself thus may contribute to adverse outcomes. A meta-analysis of eight studies including both intermittent and continuous therapies in fact suggested that higher the intensity of RRT, slower the recovery from AKI.1 Vanmassenhove et al.2 have suggested that RRT may be the second hit that delays or prevents recovery from AKI. The nephrologist and intensivist delivering RRT to the critically ill patient with AKI therefore need to select the modality of RRT that saves lives without prolonging AKI, the principle of “primum non nocere (first, do no harm)”.
Unique about the ICU patients with AKI is that multiple indications for RRT exist simultaneously. The clinician may have to balance providing biochemical correction of hyperkalemia, acidosis, and uremia; remove fluid for pulmonary edema; and maintain blood pressure in a patient with increased extravascular volume, inotrope need, and increased permeability of capillaries and veins.
The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline 5.6.1 states, Use continuous RRT (CRRT) and intermittent RRT as complementary therapies in AKI patients. (not graded)1
The acute dialysis quality initiative (ADQI) consensus3 for developing countries noted that the availability of appropriate technology and expertise including for the pediatric population may limit the use of CRRT and intermittent hemodialysis (IHD) leaving units to fall back on PD in some settings.
The ADQI statement underlines that that availability of technologies is determined by local regulations, local resources, including staff, their training/experience and laboratory support, and financial constraints. The choice of the technologies that should be made available must balance these issues.
A complete comparison of the advantages and disadvantages of the various modalities is provided in Table 1, while Table 2 compares the technology required to provide the different modalities of RRT to critically ill AKI patients.
Table 1.
Advantages and disadvantages of various renal replacement therapy modalities
| Modality | Advantages | Disadvantages | Appropriate setting |
|---|---|---|---|
| IHD | Rapid removal of toxins and low molecular weight substances | Rapid fluid removal leading to hypotension | Hemodynamically stable patients with hyperkalemia, metabolic acidosis, or poisoning with a dialyzable toxin |
| Dialysis disequilibrium and cerebral edema | |||
| Allows “down time” for diagnostic and therapeutic procedures | Requires treated water and concentrates | ||
| Reduced exposure to anticoagulation; hence, lower bleeding risk | Not possible to combine with other organ support systems | ||
| Lower costs than CRRT (around INR 2,000 daily in India) | |||
| CRRT | Continuous removal of toxins | Slower clearance of toxins | Hemodynamically unstable patients with pulmonary edema, liver disease, or increased intracranial pressure |
| Less hypotension and need for escalation of vasopressors | Need for prolonged anticoagulation | Can be easily and appropriately coupled with other extracorporeal organ support systems | |
| Easy control of fluid balance because of unlimited fluid removal | Dedicated filter sets and sterile fluid bags required | ||
| Allows adequate nutrition even in anuric patients | Patient immobilization or frequent interruptions compromising adequate solute and fluid removal | ||
| User-friendly interactive machines | Increased infection risks | ||
| Some middle-molecular-weight solute possible | High costs (around INR 25,000 to 30,000 daily for average adult) | ||
| SLED | Slower volume and solute removal | Slower clearance of toxins | Hemodynamically unstable |
| Hemodynamic stability | Can be coupled with other extracorporeal organ support systems | ||
| Successfully performed without anticoagulation | |||
| Allows “down time” for diagnostic and therapeutic procedures | |||
| Same machines may be used for more than one treatment per day, or for acute HD, SLED, or even maintenance HD | |||
| Lower cost (around INR 2,500–3,000 daily, upto 7,000 if SLEDD-f) | |||
| PD | Hemodynamic stability | Inadequate clearance in hypercatabolic patients | Hemodynamically unstable with coagulopathy, difficult access, increased risk of cerebral edema in underresourced regions |
| Technically simple | Protein loss | Stand-alone therapy not possible to combine with any other support system | |
| No anticoagulation | No control of rate of fluid removal | ||
| No need for vascular access | Risk of peritonitis | ||
| Lower cost (around INR 1,000–2,000 daily) | Hyperglycemia | ||
| Gradual removal of toxins | Requires intact peritoneal cavity | ||
| Impairs diaphragmatic movement, potential for respiratory problems |
Table 2.
Technical aspects of renal replacement therapy
| Modality | IHD | CRRT | SLED | PD |
|---|---|---|---|---|
| Blood flow | 250–300 mL/minute | 100–150 mL/minute | 150–200 mL/minute | NA |
| Dialysate flow | 500 mL/minute | 1000–3000 mL/hour | 100–300 mL/minute | 1000–2000 mL/hour |
| Clearance principle | Diffusion | Diffusion and convection | Largely diffusion (convection added in SLED-f) | Diffusion largely |
| Ultrafiltration rate | Around 500–600 mL/hour | Around 100–300 mL/hour | 100–300 mL/hour | Unpredictable |
| Replacement fluid | NA | Around 1000–2000 mL/hour | NA in SLED, 1000–6000 mL/hour in SLED-f | NA |
| Effluent volume (L/day) | NA | 36–72 | NA | 20–40 |
| Small solute clearance (mL/minute) | 200 | 15–30 | 100–150 | 15–20 |
| Daily clearance (L) | 48 | 36–72 | 54–60 | 20–36 |
The consensus statement stresses on the concept of supply and demand with critical illness AKI being a hypercatabolic state with no renal reserve.
Their consensus statement reads Selection of RRT modality depends on the capability/availability of the technology, its inherited risks and the current needs of the patient.3 The consensus statement recognizes the fact that in a limited resource setting all modalities may not be available and that different patients may require different modalities of RRT at the same time or the same patient may require different modalities during the course of his or her illness. In centers infrequently practicing a modality of RRT, the expertise is slow to develop and this is exacerbated by the financial burden of modalities like CRRT.
The KDIGO (2012) guidelines suggest using CRRT, rather than standard intermittent RRT, for hemodynamically unstable patients, while a more generalized statement has been provided by the 17th ADQI statement.4
Theoretically CRRT offers slower fluid removal than IHD, resulting in more hemodynamic stability and better control of fluid balance, the slower control of solute concentration, avoiding large fluctuations and fluid shifts and greater flexibility (allowing adaptation of the treatment to the patient's need at any time). Disadvantages include the need for immobilization, the greater need for anticoagulation, the risk of hypothermia, and, in some settings, higher costs.
Continuous types of RRT are recommended in situations where shifts in fluid balance and metabolic fluctuations are poorly tolerated. Intermittent and prolonged intermittent types of RRT have a role in situations where rehabilitation or mobilization is the priority, and fluid and metabolic fluctuations can be tolerated.4
Sustained low-efficiency dialysis (SLED) has been proposed as an alternative to other forms of RRT and is used in many centers worldwide for logistical reasons. Although a Cochrane review on this subject was unable to show a difference in the episodes of hypotension between CRRT and IHD, it did establish that the end treatment mean arterial pressure (MAP) was higher with CRRT despite a lesser escalation of inotropes. Even the ADQI 16 consensus statement fails to lay down a definite threshold of hypotension at which CRRT, SLED, and IHD become preferred treatment modalities but leaves the ultimate decision to the clinician. Marshall et al.5 at the University of Arkansas Medical Sciences (UAMS) found a higher MAP post-CRRT compared with SLED but also noted that those patients who could not tolerate-SLED also could not undergo CRRT. A recent survey including 60 centers from the USA and 48 from the Indian subcontinent and Latin America revealed a marked geographical variation in RRT practices. Limited availability, expertise, and high costs limit CRRT in developing countries. Sustained low-efficiency daily dialysis (SLEDD), however, was used in 25% of centers in developing countries as compared to 20% in developed countries.6
The rescue study by Schwenger7 was a study in 232 patients randomized to either SLED or CRRT which showed no difference in the 90-day mortality, ICU, or in-hospital mortality, and interestingly in treatment times. A small advantage in times of dialysis dependence was seen for SLEDD, and ventilator days in favor of CRRT, and the ultrafiltration rates in both SLED and CRRT were among the lowest ever in this study, which may have contributed to the excellent hemodynamic stability. Similarly Kitchlu et al.8 in a nonrandomized study showed no difference in the 30-day mortality in patients treated with either CRRT or SLEDD. A total of 1,564 patients from 18 studies were included in a systematic review conducted by Kovacs in 2017.9 No statistically significant difference was observed in the primary outcome, renal recovery [risk ratio (RR) 0.87, 95% confidence interval (CI) 0.63 to 1.20]. The secondary outcome of time to renal recovery (mean difference 1.33, 95% CI 0.23 to 2.88, I2 = 0%) also was not significantly different.
Mortality was marginally better for SLED over CRRT (RR 1.21, 95% CI 1.02 to 1.43, I2 = 47%); however, this diminished only when randomized controlled trials (RCTs) were included (RR 1.25, 95% CI 1.00 to 1.57).
The KDIGO also suggest using CRRT, rather than intermittent RRT, for AKI patients with acute brain injury or other causes of increased ICP or generalized brain edema.
The KDIGO guideline cites studies showing an increase in brain water and ICP with intermittent dialysis but not with CRRT. Wu et al.10 carried out a crossover trial in 12 end-stage renal disease patients with cerebral hemorrhage, ICP < 12 mm Hg, and anuria, in which subjects were randomized on day 1 to either CRRT or SLED with crossover on day 2 and ICP was continuously monitored. They found no significant change in ICP, MAP, or cerebral perfusion pressure from baseline at 8 hours of treatment and concluded that SLEDD was equivalent to CRRT in traumatic brain injury. Since patients with acute liver failure, acute on chronic liver failure, hepatic encephalopathy, and AKI also experience fluctuations in ICP, any of the modalities, PD, CRRT, or SLED may be offered to these patients while IHD is probably best avoided.
In the current era, patients in the ICU frequently develop AKI in the setting of multiorgan failure and may receive multiple extracorporeal supports including membrane oxygenation, therapeutic apheresis, plasma exchange, adsorbent sorbent therapies for liver failure, or sepsis. The ADQI takes note of this in its Consensus Statement 3.1: In situations where other extracorporeal therapies are required, CRRT is recommended and integrated systems are preferred over parallel systems.
Because of the continuous nature of CRRT and to a lesser extent SLED, these therapies are preferred for coupling with other extracorporeal supports. Figure 1 shows a patient with severe myocarditis on venoarterial (VA) extracorporeal membrane oxygenation (ECMO) also undergoing CRRT, while Figure 2 shows coupling of a cytokine removal filter, plasma exchange, and SLEDD.
Fig. 1.

Continuous renal replacement therapy coupled with VA ECMO. The Prismaflex circuit with an M60 filter is connected in parallel before the oxygenator. Because the centrifugal pump is driving the extracorporeal circuit, the access pressure will be positive
Figs 2A and B.
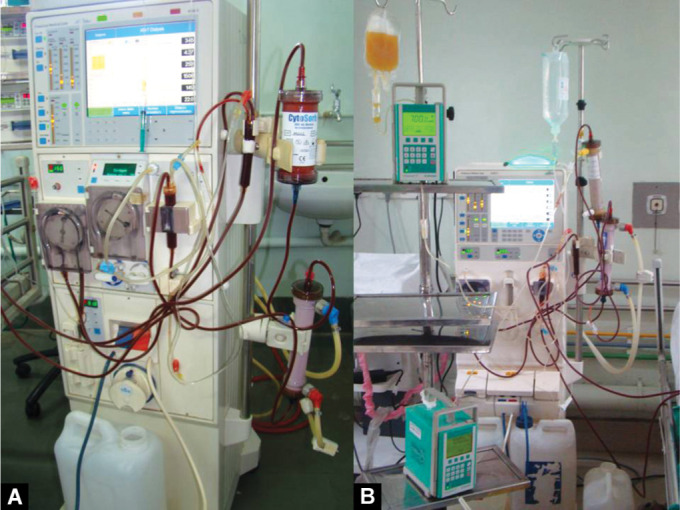
(A) The cytokine removal filter for sepsis and the hemodialyzer connected in series. The cytokine filter is before the dialyzer; (B) Coupled plasma exchange and sustained low-efficiency dialysis. Here the dialyzer is connected before the plasma filter in a series circuit
Table 3 lists all the recent head-to-head comparisons between intermittent and continuous therapies. Individual studies used different definitions of AKI and were underpowered. Most of the trials excluded patients with hypotension or maximized efforts to improve the hemodynamic tolerance of IHD. The high rate of crossover between the treatment modalities also complicates the interpretation of the results. In addition, in some of the trials, IHD patients were treated with bioincompatible membranes and studies were not standardized for treatment dose. A subsequent RCT not included in the Cochrane meta-analyses reported similar outcomes.
Table 3.
Summary of studies comparing intermittent hemodialysis with continuous renal replacement therapy
| Study | n | Weight (%) | OR and 95% CI for mortality with CRRT |
|---|---|---|---|
| Agustine (2004) | 80 | 5.12 | 0.96 (0.72–1.3) |
| Gasparovic (2003) | 104 | 6.66 | 1.19 (0.9–1.58) |
| SHAPS (2005) | 316 | 20.4 | 0.93 (0.78–1.12) |
| Mehta (2001) | 166 | 10.3 | 1.38 (1.05–1.82) |
| Noble (2006) | 94 | 6.2 | 0.94 (0.78–1.12) |
| Uehlinger (2005) | 125 | 8 | 0.93 (0.65–1.33) |
| Vinsoneau (2006) | 360 | 23.2 | 0.98 (0.05–1.13) |
| Shafs (2009) | 316 | 20.24 | 1.5 (0.8–2.2) |
| Total | 1,561 | 100 | 1.01 (0.92–1.12) |
PERITONEAL DIALYSIS
Peritoneal dialysis is generally the “Cinderella” of RRT modalities in AKI. In an open, randomized comparison of pumped venovenous hemofiltration and PD in patients with infection-associated acute renal failure in an infectious-disease referral hospital in Vietnam, 70 adult patients with severe falciparum malaria (48 patients) or sepsis (22 patients) were assigned to hemofiltration or PD.11 The mortality rate of 47% (17 patients) was three times higher in the group assigned to PD, as compared with 15% (5 patients) in the group assigned to hemofiltration (p = 0.005). Resolution of acidosis and decline in serum creatinine concentration was faster in the group assigned to hemofiltration than in the group assigned to PD (p < 0.005), and RRT was required for a significantly shorter period. In a multivariate analysis, the odds ratio (OR) for death was 5.1 (95% CI, 1.6 to 16) and for future dialysis was 4.7 (95% CI, 1.3 to 17). Cost of a life saved was also lower for hemofiltration. Despite its limitations, this study focused on the possible inadequacy of PD to provide appropriate fluid and solute clearance in very sick patients.
Cruz et al. conducted a systematic review on the effect of PD compared with extracorporeal therapies in AKI.12 From 983 possible citations, they analyzed 24 studies containing 1,556 patients. There were 13 descriptive studies with 597 patients, without a comparator group, 7 cohort studies, and 4 prospective RCTs from India, Nigeria, Vietnam, and Brazil, respectively. Except for the Brazilian study, the Jadad score even of the RCTs was <3.
Details of the PD technique were often not reported. The studies used either rigid catheters or flexible Tenckhoff catheters with automated cycler use in four studies and in one study each, lactate acetate and bicarbonate as a buffer. The comparator was IHD in seven studies, extended daily dialysis (EDD) in one study, and CRRT in four studies. Thus, the heterogeneity among the studies was large. Among observational studies, the pooled OR for mortality with PD was 0.96 (95% CI = 0.53 to 1.71, p = 0.18), I2 = 0.21 and among RCTs it was 1.50 (95% CI = 0.46 to 4.86, p = 0.50), I2 = 0.77 using a random effects model.
Clinicians in developing countries face additional challenges due to limited resources, reduced availability of trained staff and equipment, cultural and socioeconomic aspects, and administrative and governmental barriers, all of which affect patient selection, choice of RRT modality, and management of RRT. A summary statement from the 17th ADQI consensus on RRT in developing countries states: all RRT modalities have particular advantages and offer clinicians options to manage patients and optimize care.
Based on the existing evidence, the choice of RRT modality should be based on the clinical status of the patient (hemodynamic stability, catabolic state, need for removal of large amounts of fluid, presence of life-threatening complications, or acute brain injury), availability of modalities, clinical experience, and financial cost of therapy.
Transition of modalities should be considered if the demand–capacity imbalance or treatment priorities have changed and can be met better by an alternative technique.
The statement also makes a special mention of the need to provide RRT for children with AKI, recommending that units managing children who need acute RRT have the appropriate infrastructure, equipment, and trained personnel to provide appropriate standards of care.
Children usually receive hemodialysis or PD in adult units, rather than from a dedicated pediatric team involved in multidisciplinary care. For RRT to be safe and effective, a minimal infrastructure has to be available with full local commitment, viable finances, skilled staff, and equipment. All devices need to function properly at all times, and trained personnel and equipment should be available on a 24-hour basis. For young children (younger than 5 years), PD is often the first choice because of its availability and the ease of initiation. Transition of modality should be considered when the option exists, and adequate infrastructure and trained personnel are available.
The Indian Society of Nephrology 2019 draft guidelines on their website provide detailed recommendations to provide RRT to critically ill children with AKI, in India.13 While children <10 kg may still frequently be managed with PD, the availability of dedicated machines with volumetric or gravimetric ultrafiltration control and pediatric software allows even very small children and neonates to be managed with SLED and CRRT in expert hands. The FX Ped dialyzer, HF20, and AV Ped filters have surface areas of 0.2 m2 and priming volumes of 18 to 24 mL. Used with specialized tubings, these allow infants to be safely given extracorporeal RRTs.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.KDIGO Clinical Practice Guideline for Acute Kidney Injury, Kid Int; 2012;2(S1):1–138. March. [Google Scholar]
- 2.Vanmassenhove J, Kielstein J, Jörres A, Biesen WV. Management of patients at risk of acute kidney injury. Lancet. 2017;389(10084):2139–2151. doi: 10.1016/S0140-6736(17)31329-6. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Annigeri RA, Ostermann M, Tolwani A, Vazquez-Rangel A, Ponce D, Bagga A, et al. Renal support for acute kidney injury in the developing world. Kidney Int Rep. 2017;2(4):559–578. doi: 10.1016/j.ekir.2017.04.006. DOI: [DOI] [Google Scholar]
- 4.Ostermann M, Joannidis M, Pani A, Floris M, De Rosa S, Kellum JA, et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42(3):224–237. doi: 10.1159/000448506. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK. Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int. 2001;60(2):777–785. doi: 10.1046/j.1523-1755.2001.060002777.x. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Raina R, Chauvin AM, Bunchman T, Askenazi D, Deep A, Ensley MJ, et al. Treatment of AKI in developing and developed countries: An international survey of pediatric dialysis modalities. PLoS ONE. 2017;(5):1–9. doi: 10.1371/journal.pone.0178233. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwenger V, Weigand MA, Hoffmann O, Dikow R, Kihm LP, Seckinger J, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - a randomized interventional trial: the renal replacement therapy study in intensive care unit patients. Crit Care. 2012;16(4):R140. doi: 10.1186/cc11445. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitchlu A, Adhikari N, Burns KEA, Friedrich JO, Garg AX, Klein D, et al. Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study. BMC Nephrol. 2015;16(1):127. doi: 10.1186/s12882-015-0123-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs B, Sullivan K, Hiremath S, Patel R. Effect of sustained low efficient dialysis versus continuous renal replacement therapy on renal recovery after acute kidney injury in the intensive care unit: a systematic review and meta-analysis. Nephrology. 2017;22(5):343–353. doi: 10.1111/nep.13009. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Wu VC, Huang TM, Shiao CC, Lai CF, Tsai PR, Wang WJ, et al. NSARF Group. - The hemodynamic effects during sustained low-efficiency dialysis versus continuous veno-venous hemofiltration for uremic patients with brain hemorrhage: a crossover study. J Neurosurg. 2013;119(5):1288–1295. doi: 10.3171/2013.4.JNS122102. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895–902. doi: 10.1056/NEJMoa020074. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Chionh CY, Soni SS, Finkelstein FO, Ronco C, Cruz DN. Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol. 2013;8(10):1649–1660. doi: 10.2215/CJN.01540213. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ISN Guidelines on Hemodialysis Units; Indian Journal of Nephrology |. 2020;(Supplement 1) Volume 30 |. January. [Google Scholar]


