Abstract
Introduction: Highly purified cannabidiol (CBD) (approved as Epidiolex® in the United States) has demonstrated efficacy with an acceptable safety profile in patients with Lennox–Gastaut or Dravet syndrome in four randomized controlled trials. CBD possesses affinity for many target classes with functional effects relevant to the pathophysiology of many disease types, including epilepsy. Although the mechanism of action of CBD underlying the reduction of seizures in humans is unknown, transient receptor potential vanilloid 1 (TRPV1) represents a plausible target because (1) CBD activates and then desensitizes TRPV1, (2) TRPV1 is overexpressed in models of temporal lobe epilepsy and patients with epilepsy, (3) and TRPV1 modulates neuronal excitability.
Methods: To investigate a potential role of TRPV1 in the anticonvulsive effects of CBD, the effect of CBD on seizure threshold was assessed using a mouse maximal electroshock threshold model of generalized seizure in TRPV1 knockout and wildtype mice. The dose dependence of the CBD effect was determined and compared with that of the positive comparator diazepam and vehicle.
Results: At 50 and 100 mg/kg, CBD significantly (p<0.0001) increased seizure threshold in wildtype mice compared with TRPV1 knockout and vehicle controls. This effect was observed only at 100 mg/kg in TRPV1 knockout mice compared with knockout vehicle mice, in which gene deletion partially attenuated the CBD-increased seizure threshold. The effect of high-dose CBD in wildtype mice was nevertheless significantly different from vehicle-treated TRPV1 knockout mice (p<0.0001). Bioanalysis confirmed that genotype-specific differential brain exposure to CBD was not responsible for the observed effect on seizure threshold.
Conclusion: These data strongly implicate TRPV1 in the potential mechanisms of action for the anticonvulsive effects of CBD. The partial inhibition of the anticonvulsive effect of high-dose CBD in TRPV1 knockout mice may indicate the involvement of targets other than TRPV1. Further characterization of TRPV1 in the anticonvulsive effect of CBD in validated models of seizure is warranted, as is pharmacological investigation of the molecular interaction between CBD and TRPV1.
Keywords: epilepsy, seizures, anticonvulsant, mechanism of action, TRPV1, CBD
Introduction
The transient receptor potential vanilloid 1 (TRPV1) channel consists of six transmembrane domains with a nonselective hydrophobic pore between the fifth and sixth transmembrane domains that is responsive to chemical and physical stimuli.
Activation of TRPV1 by the vanilloid capsaicin, noxious heat, low pH, various lipids,1 or other agents including phytocannabinoids2 leads to Ca2+ influx through the channel. Depending on the degree and duration of influx, the increase in intracellular Ca2+ can desensitize the channel, representing a protective negative feedback mechanism. TRPV1 channel activation is thought to be a sensor of physiological and pathological processes including itch, temperature, cancers, genetic disorders, pain, and seizures.3,4 TRPV1 receptors are expressed widely throughout the central nervous system,5 where they promote neuronal depolarization, firing of action potentials, and generation of postsynaptic currents.6
The anticonvulsive efficacy of cannabidiol (CBD) has been described in a range of preclinical models of seizure7 and in clinical trials wherein amelioration of seizure in Dravet and Lennox–Gastaut syndromes has been established.8,9 The demonstration that the anticonvulsive effect of CBD may involve TRP channels was demonstrated by reversal of CBD-dependent inhibition of pentylenetetrazol-induced seizures by the TRPV1 antagonist, SB366791.10
Bisogno et al.11 were the first to describe the affinity of CBD for TRPV1 and its action as an agonist, with subsequent validation and extension to include evidence of CBD-induced rapid desensitization.2 Recent evidence suggests that TRPV1 may contribute to the onset and progression of some forms of epilepsy.12–14 The possibility that TRPV1 is involved in the anticonvulsive activity of CBD is based upon the observations that CBD can activate and rapidly desensitize TRPV1 receptors at low micromolar concentrations in recombinant systems and in in vitro experimental models of epileptiform activity.15 Patch clamp recordings from human TRPV1-expressing HEK293 cells demonstrated rapid, concentration-dependent activation, and desensitization of TRPV1 by CBD. Both effects were sensitive to the TRPV1 receptor antagonist, capsazepine, confirming that CBD was acting specifically at TRPV1 receptors.
Collectively, these data prompted the present investigation of the possible contribution of TRPV1 channel activity to the anticonvulsive effects of CBD in a model of generalized seizures shown to be responsive to CBD treatment.
Methods
TRPV1 knockout mice (B6.126X1-trpv1tm1Jul/J) were obtained from Jackson Laboratories and wildtype C57Bl6 mice from Charles River, United Kingdom. Studies were conducted in accordance with national and institutional guidance. The use of animals in experiments and testing is regulated under the Animals (Scientific Procedures) Act 1986. Animals were randomly assigned to either vehicle or CBD (n=12 per group) and received intraperitoneal (i.p.) injection of either plant-derived highly purified CBD (10–100 mg/kg; GW Pharmaceuticals) or CBD vehicle (ethanol:Kolliphor® EL:saline, 1:1:18 ratio) 60 min before testing, based upon the pharmacokinetic data previously described.15
The positive control diazepam (2.5 mg/kg in saline; Sigma) was administered 30 min before testing. Diazepam was selected as a positive control on the basis that its primary mechanism of anticonvulsive action is TRVP1 independent.
Mice were individually assessed for production of a tonic hind limb extensor seizure after a single corneal delivered electroshock of 0.1 sec duration, using an “up and down method” of shock titration. Thus, the first mouse within a treatment group was shocked at the expected or estimated CC50 current (current producing tonic hind limb extensor seizures in 50% of animals). For subsequent animals, the stimulus intensity was lowered or raised (usually in 1–5 mA intervals) if the preceding mouse did or did not show tonic hind limb extension. This procedure was continued for all mice within a treatment group. Using the method originally described by Kimball et al.,16 the data generated in a single group of animals were used to calculate CC50, which is presented as mean±standard deviation.
For assessment of brain and plasma CBD concentrations, a satellite group of wildtype and knockout mice (n≤5 per group) received plant-derived highly purified CBD (10, 25, 50, or 100 mg/kg; i.p.) under isoflurane anesthesia, and plasma was collected into lithium heparin tubes through cardiac puncture 60 min postdose. Samples were centrifuged (4°C; 1500 g; 10 min) and the resultant plasma (∼400 μL) was added to the same volume of ascorbic acid (100 mg/mL). Immediately after blood collection, animals were decapitated and brains were removed, rinsed in physiological saline, and snap frozen in liquid nitrogen. Plasma and brain samples were stored at −80°C until use. Bioanalytical samples were prepared and analyzed using liquid chromatography/tandem mass spectrometry for CBD according to a validated method.
Planned statistical comparisons were conducted to determine the statistical significance of the difference in seizure threshold between wildtype and TRPV1 knockout mice within and between given treatment groups, using a two-way ANOVA with Tukey post hoc test on the basis that the data were normally distributed. Similarly, two-way ANOVA with Sidak post hoc test was conducted to make planned comparisons of plasma and brain exposure between wildtype and TRPV1 knockout mice within each dose group.
Results
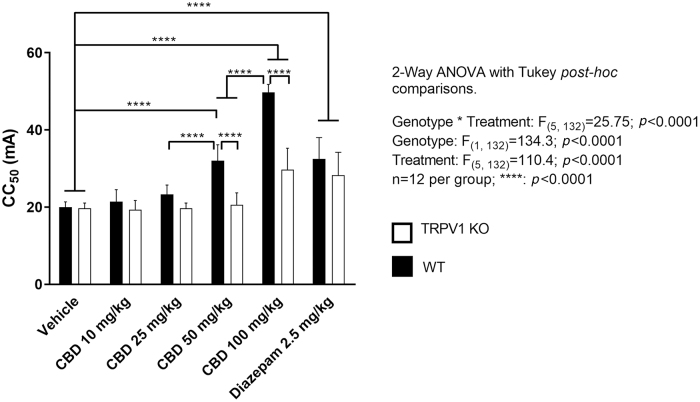
Two-way ANOVA identified a significant interaction (F(5,132)=25.75; p<0.0001) between genotype and treatment upon seizure threshold (CC50), as defined by an increase in current required to induce seizure in 50% of animals within a given dose group. In addition, significant main effects of genotype (F(1,132)=134.3; p<0.0001) and treatment (F(5,132)=110.4; p<0.0001) were also observed (Fig. 1).
FIG. 1.
Comparison of the effects of CBD in TRPV1 KO and WT mice in the maximal electroshock threshold test. Generalized seizures were induced by a constant current stimulus (1–300 mA; 0.1 sec duration) through corneal electrodes in WT and TRPV1 KO mice. Differences between CC50 values (mA) were assessed using a two-way ANOVA with post hoc Turkey test: ****p<0.0001; n=12 animals per group. Data represent mean±standard deviation. CBD, cannabidiol; CC50, current producing tonic hind limb extensor seizures in 50% of animals; TRPV1 KO, transient receptor potential vanilloid 1 knockout; WT, wildtype.
Significant increases in CC50 were detected in wildtype animals treated with CBD at both 50 and 100 mg/kg (each p<0.0001), when compared with the wildtype vehicle group. In contrast, significant increases in CC50 were observed in TRPV1 knockout mice treated with CBD only at 100 mg/kg (p<0.0001) compared with the knockout vehicle group. In addition, wildtype mice treated with 50 mg/kg CBD had significantly increased seizure threshold compared with TRPV1 knockout mice at the same dose and were not significantly different from TRPV1 knockout mice treated with 100 mg/kg CBD.
As predicted by previous examination of purified CBD in a range of animal models, including a maximal electroshock model of generalized seizure, a dose-dependent increase in seizure threshold was observed in wildtype mice wherein each stepwise dose increase from 25 to 50 mg/kg and 50 to 100 mg/kg resulted in significant increases in seizure threshold (p<0.0001).
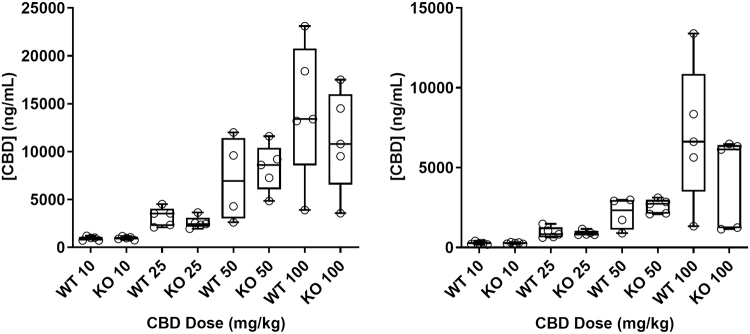
Bioanalytical assessment of brain and plasma concentrations of CBD revealed no interaction between genotype and dose group (brain: F(3,30)=0.5453; p=0.6551; plasma: F(3,31)=0.6402; p=0.5949), nor was a main effect of genotype detected (brain: F(1,30)=0.1740; p=0.6976; plasma: F(1,31)=0.3211; p=0.5750). A main effect of dose group was detected (brain: F(3,30)=18.55; p<0.0001; plasma: F(3,31)=21.38; p<0.0001). Therefore, median exposures were dose dependent but not genotype dependent, indicating that the degree of efficacy observed between doses but not between wildtype and TRPV1 knockout animals was attributable to exposure (Fig. 2).
FIG. 2.
CBD exposure in WT and TRPV1 KO mice in plasma and brain. CBD exposure was measured 60 min after intraperitoneal injection of CBD in mice. n≤5 animals per group. Median CBD exposure is represented by a horizontal line and 25th and 75th percentiles by the box, whiskers indicate minimum and maximum values, and open symbols describe individual data points. Differences in exposure between genotype were assessed using a two-way ANOVA.
Conclusion
Highly purified botanically derived CBD dose-dependently increased seizure threshold in wildtype mice: an effect that was significantly curtailed with TRPV1 gene deletion. Concentrations of CBD measured in the plasma and brain at doses shown to increase seizure threshold were consistent with those required to activate TRPV1 channels, further validating the relevance of the present findings and those of Vilela et al.10 Interestingly, the higher dose of CBD (100 mg/kg) significantly (p<0.001), but partially, increased seizure threshold in TRPV1 knockout mice when compared with the knockout vehicle group, suggesting the engagement of mechanisms independent of TRPV1. Importantly, no effect of TRPV1 gene deletion on either plasma or brain exposure to CBD was observed.
The present observation that the anticonvulsive effect of diazepam was marginally attenuated in TRPV1 knockout mice is in accordance with the findings of other studies wherein exposure to diazepam can result in elevated concentrations of the endogenous signaling lipid anandamide in the brain.17 These mediators, like CBD, both activate and desensitize TRPV1 channels.18 Thus, the effect of diazepam on GABA signaling may result in the release of these mediators, which may then increase diazepam actions through interaction with their receptors, including TRPV1. Therefore, the inhibition of diazepam effect in mice lacking functional TRPV1 channels is consistent with both this previous hypothesis and, indirectly, the present suggestion of a TRPV1-mediated mechanism of action of CBD.
Further examination of the molecular interaction of CBD with TRPV1 and other potential targets involved in the mechanism of anticonvulsive action is warranted, as is the assessment of the role of TRPV1 in the efficacy of CBD in other well-validated models of seizure, including those in which the involvement of TRPV1 in seizure has been previously described and the efficacy of CBD confirmed. Given the diverse pharmacology of CBD, the modulation of targets beyond TRPV1 (including GPR55) involved in the normalization of aberrant inhibitory-excitatory imbalance and calcium signaling associated with conditions that include multiple types of epilepsies have been described and investigated further. In summary, the data presented are consistent with previous studies in which the effect of CBD on TRPV1 function has been identified and strongly implicates TRPV1 as one of multiple targets underlying the anticonvulsive mechanism of action of CBD.
Abbreviations Used
- CBD
cannabidiol
- i.p.
intraperitoneal
- MEST
maximal electroshock threshold
- TRPV1
transient receptor potential vanilloid 1
Author Disclosure Statement
All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship. R.A.G., C.G.S., N.A.J., and B.J.W. are employees of GW Research Ltd. and stock shareholders of GW Pharmaceuticals plc. V.D.M. receives research grants from, and is a consultant for, GW Research Ltd.
Funding Information
This study was funded by GW Research Ltd. Copyediting assistance was provided by Dr. Jennifer Kahle (BPS Intl) and funded by Greenwich Biosciences, Inc.
Cite this article as: Gray RA, Stott CG, Jones NA, Di Marzo V, Whalley BJ (2020) Anticonvulsive properties of cannabidiol in a model of generalized seizure are transient receptor potential vanilloid 1 dependent, Cannabis and Cannabinoid Research 5:2, 145–149, DOI: 10.1089/can.2019.0028.
References
- 1. Rosenbaum T, Simon SA. TRPV1 receptors and signal transduction. In: Liedtke WB, Heller S, eds. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. CRC Press/Taylor & Francis Group, LLC: Boca Raton, FL, 2007 [PubMed]
- 2. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165:787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menigoz A, Boudes M. The expression pattern of TRPV1 in brain. J Neurosci. 2011;31:13025–13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xing J, Li J. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J Neurophysiol. 2007;97:503–511 [DOI] [PubMed] [Google Scholar]
- 7. Patra PH, Barker-Haliski M, White HS, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020 [DOI] [PubMed] [Google Scholar]
- 9. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096 [DOI] [PubMed] [Google Scholar]
- 10. Vilela LR, Lima IV, Kunsch ÉB, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017;75:29–35 [DOI] [PubMed] [Google Scholar]
- 11. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CY, Li W, Qu KP, et al. Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur J Pharmacol. 2013;714:288–294 [DOI] [PubMed] [Google Scholar]
- 13. Manna SS, Umathe SN. A possible participation of transient receptor potential vanilloid type 1 channels in the antidepressant effect of fluoxetine. Eur J Pharmacol. 2012;685:81–90 [DOI] [PubMed] [Google Scholar]
- 14. von Rűden EL, Jafari M, Bogdanovic RM, et al. Analysis in conditional cannabinoid 1 receptor-knockout mice reveals neuronal subpopulation-specific effects on epileptogenesis in the kindling paradigm. Neurobiol Dis. 2015;73:334–347 [DOI] [PubMed] [Google Scholar]
- 15. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141 [DOI] [PubMed] [Google Scholar]
- 16. Kimball AW, Burnett WT Jr., Doherty DG. Chemical protection against ionizing radiation. I. Sampling methods for screening compounds in radiation protection studies with mice. Radiat Res. 1957;7:1–12 [PubMed] [Google Scholar]
- 17. Micale V, Cristino L, Tamburella A, et al. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology. 2009;34:593–606 [DOI] [PubMed] [Google Scholar]
- 18. Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457 [DOI] [PubMed] [Google Scholar]