Abstract
Introduction: Cannabinoids have long been known for their ability to treat nausea and vomiting. Recent reports, however, have highlighted the paradoxical proemetic effects of cannabinoids. Cannabinoid hyperemesis syndrome (CHS) is characterized by cyclical episodes of nausea and vomiting, accompanied by abdominal pain following prolonged, high-dose cannabis use, which is alleviated by hot baths and showers. Little is known about the cause of this syndrome.
Discussion: Cannabinoids produce a biphasic effect on nausea and vomiting, with low doses having an antiemetic effect and high doses producing emesis. Presentation and treatment of CHS are similar to cyclical vomiting syndrome as well as chemotherapy-related anticipatory nausea and vomiting, suggesting that these phenomena may share mechanisms. The prevalence of CHS is not known because of the symptomatic overlap with other disorders and the lack of knowledge of the syndrome by the public and physicians. Treatment with typical antiemetic drugs is ineffective for CHS, but anxiolytic and sedative drugs, along with hot showers, seem to be consistently effective at reducing symptoms. The only known way to permanently end CHS, however, is abstinence from cannabinoids. Case studies and limited pre-clinical data on CHS indicate that prolonged high doses of the main psychotropic compound in cannabis, Δ9-tetrahydrocannabinol (THC), result in changes to the endocannabinoid system by acting on the cannabinoid 1 (CB1) receptor. These endocannabinoid system changes can dysregulate stress and anxiety responses, thermoregulation, the transient receptor potential vanilloid system, and several neurotransmitters systems, and are thus potential candidates for mediating the pathophysiology of CHS.
Conclusions: Excessive cannabinoid administration disrupts the normal functioning of the endocannabinoid system, which may cause CHS. More clinical and pre-clinical research is needed to fully understand the underlying pathophysiology of this disorder and the negative consequences of prolonged high-dose cannabis use.
Keywords: Δ9-Tetrahydrocannabinol (THC), cannabinoid hyperemesis syndrome, nausea, stress, vomiting
Introduction
The plant Cannabis Sativa has been used for centuries recreationally and medicinally, and remains one of the most commonly used drugs worldwide.1,2 Cannabis has several chemical constituents, termed cannabinoids, but the only compound that produces psychotropic effects is Δ9-tetrahydrocannabinol (THC).3 THC is a partial agonist of the cannabinoid 1 (CB1) receptor of the endocannabinoid system.4 These receptors are located throughout the brain and body, and are activated by endogenous ligands known as endocannabinoids, including anandamide (AEA)5 and 2-arachidonoylglycerol (2-AG).6 AEA and 2-AG are degraded by fatty acid amide hydrolase (FAAH)7 and monoacylglycerol lipase (MAGL),8 respectively. To better understand the endocannabinoid system, highly potent, synthetic full agonists of the CB1 receptor have been synthesized (i.e., JWH-018 and HU210). Slight modifications to their chemical structures have led to the development of synthetic cannabinoid designer drugs, including Spice and K2, and are becoming increasingly popular.9
One of the first approved medical uses of a synthetic version of THC was to treat chemotherapy-induced nausea and vomiting.10 Even though considerable pre-clinical and clinical evidence suggests that cannabinoids reduce nausea and vomiting,11,12 recent evidence suggests that high doses of cannabinoids can produce nausea and vomiting in laboratory animals13–19 and humans,20–22 and can lead to cannabinoid hyperemesis syndrome (CHS) in the latter.23
It is perhaps not surprising that cannabinoids produce seemingly paradoxical effects on nausea and vomiting because cannabinoids are known to produce biphasic effects, where low and high doses typically produce opposite effects.24–27 Information regarding the adverse effects of high-dose CB1 agonists is of particular importance because THC content in cannabis has been consistently increasing,28,29 and high potency THC concentrates30–32 and designer synthetic cannabinoids9 are becoming increasingly popular. This article will discuss the seemingly contradictory effects of cannabinoids on nausea and vomiting, and the prevailing theories about CHS' mechanisms.
Cannabinoid Hyperemesis Syndrome
In certain individuals, long-term cannabis use may induce CHS. This syndrome is characterized by cyclical nausea and vomiting, accompanied by abdominal pain following prolonged, high-dose cannabis use.23 The symptoms associated with CHS can be alleviated by high temperature baths or showers, sometimes resulting in burns.23 CHS can also develop following the long-term use of synthetic cannabinoid designer drugs,33–36 which tend to be full agonists at the CB1 receptor, as opposed to THC, which is a partial agonist, and can cause adverse effects at lower doses.37 Indeed, nausea and vomiting are common side effects of acute synthetic cannabinoid intoxication.37–39
CHS presents in three stages: the prodromal, the hyperemetic, and the recovery phase.23,40 The prodromal stage of CHS is characterized by anxiety, severe nausea, and an array of autonomic symptoms, such as, sweating, flushing, and increased thirst, with symptoms being more severe in the morning. The prodromal stage can last for months before any vomiting attacks occur. The hyperemetic phase follows with debilitating abdominal pain, nausea, and vomiting. It is during this stage that patients typically develop compulsive hot bathing or showering behaviors. The emetic phase will last until prolonged abstinence from cannabis has occurred and CHS patients enter a recovery phase where vomiting and bathing behavior subsides. Full recovery from symptoms can be weeks or months following the cessation of cannabis use.23 These phases of CHS follow a cyclical repetitive pattern, usually initiated by the reoccurrence of cannabis use.40
Diagnosis
The Rome IV criteria list CHS as a subset of cyclical vomiting syndrome (CVS). Table 1 represents the diagnostic criteria of CVS and CHS. Analogous to CHS, CVS is characterized by reoccurring episodes of nausea and vomiting.41 Due to the commonalities with CVS, CHS is often misdiagnosed.42,43 Some argue that these syndromes may not be distinct disorders, but rather CHS is a variant of CVS brought on by excessive cannabis use.41 Subtle differences may help with accurate diagnosis to guide treatment.
Table 1.
Rome IV Diagnostic Criteria for Cyclical Vomiting Syndrome and Cannabinoid Hyperemesis Syndrome41
| Cyclical vomiting syndrome (CVS) | Cannabinoid hyperemesis syndrome (CHS) |
| Acute onset of stereotypical vomiting episode that lasts less than 1 week | Stereotypical vomiting episodes similar to CVS |
| At least three episodes in the previous year, two episodes in the last 6 months at least 1 week apart | Presentation following sustained excessive cannabis use |
| No vomiting between episodes | Relief from vomiting after abstinence from cannabis |
| Symptoms must be present for 3 months with onset 6 months earlier | Symptoms must be present for 3 months with onset 6 months earlier |
| Supportive criteria: history of migraine headaches | Supportive criteria: pathological bathing behavior (prolonged hot baths or showers) |
Individuals with CHS will recover following sustained abstinence from cannabis, but this is not the case for CVS.44 It is of note that a high proportion of individuals with CVS are also cannabis users.45,46 Interestingly, Venkatesan et al.45 found that among 437 CVS patients surveyed, 81% were cannabis users. Yet, the information is not available to discern if CVS patients concurrently use cannabis to relieve emetic symptoms or if these are individuals with CHS.
The main distinction between CHS and CVS is the resolution of symptoms for CHS following the termination of cannabis use. Ongoing follow-up of patients after stopping cannabis use would help with accurate diagnosis. Other distinguishing features have been proposed to properly diagnose CHS and CVS. For example, individuals with CVS tend to have rapid gastric emptying, whereas CHS is associated with delayed gastric emptying.23,44,47,48 Delayed gastric emptying in CHS is not surprising given that THC and other CB1 receptor agonist have an inhibitory effect on gastric motility.49–52 Gastric emptying studies could, therefore, improve diagnosis.
Treatment
The only effective way to permanently alleviate symptoms of CHS is stopping cannabis use; however, during acute emetic phases, both CVS and CHS symptoms are managed in similar ways.44 It is important to note that typical antiemetic drugs, such as the 5-HT3 receptor antagonist ondansetron, are not effective at alleviating nausea and vomiting from CHS.53 Anxiolytic and sedative drugs, such as benzodiazepines (i.e., diazepam) and antipsychotics (i.e., haloperidol), seem to be the most effective for emetic phases of CVS and CHS44,53–55; yet it is still unclear whether these drugs are effective because they interfere with the mechanism of CHS or because of their sedating effects. Acute management of symptoms is important for preventing dehydration and adrenal injury, common complications of CHS that can be fatal.56,57
Following acute symptom management, physicians should encourage cannabis abstinence in patients; however, abstaining from cannabis use can be difficult if the individual has cannabis use disorder (CUD). Currently, there is no information on the percentage of CHS individuals with concurrent CUD. If CHS is suspected, it could be beneficial to assess individuals for CUD to determine appropriate treatment. There are research groups investigating the benefits of early detection and addiction therapy intervention for individuals with CHS showing promising results.58 Treating CUD in this population could aid in preventing reoccurrence of CHS. In addition to addiction therapies, current pharmacological treatment options that are under investigation for CUD include the following: antidepressants, CB1 receptor agonists, and FAAH inhibitors.59–61 While CB1 agonists can be beneficial at reducing withdrawal symptoms, they should be used cautiously in CHS as they may exacerbate symptoms by increasing CB1 receptor activation.
Prevalence of CHS
Prevalence of CHS among the general population is difficult to determine because of the clinical similarities between CHS and CVS. Moreover, individuals may be unwilling to disclose their cannabis use to physicians or be unaware of the emetic effects of cannabis, leading to misdiagnosis and underreporting of CHS. Therefore, there is a lack of recognition and knowledge of the syndrome by sufferers and physicians.
Publications about CHS have been consistently increasing since 2004 when the syndrome was coined.53,62 Several groups have tried to establish the prevalence of CHS by retrospective analyses of reports from hospitals or urgent care centers.62–65 Kim et al.65 found that CVS diagnosis, including CHS, has nearly doubled since the legalization of cannabis in Colorado, United States. It is unclear, however, if this was due to an increase in cannabis use or an increase in cannabis use reporting due to the increased acceptance and legalization of cannabis. Schreck et al.62 determined that the rate of reporting in France is similar to that reported in international literature. These studies only provide estimates, and rely on accurate diagnosis and disclosure of cannabis use; therefore, they are likely underestimations of the actual prevalence. Indeed, Habboushe et al.64 acknowledge the limitations of their estimation and conclude that CHS is much more prevalent than is recognized in society. Prevalence cannot accurately be determined until accurate diagnosis and classification are agreed upon. Most studies regarding the epidemiology of CHS lack information about patient histories, comorbid psychiatric illnesses, possible re-disposing factors, and type of cannabis consumed (i.e. THC percentage), which may aid the understanding of the etiology of CHS.
CHS: Potentially a Dysfunctional Endocannabinoid System
Most research to date regarding CHS is limited to case studies (e.g.,23,42,66–70). While these reports provide information about how the syndrome manifests and what treatments are effective for some patients, they do not provide information about the underlying neurobiological mechanism. However, we can make informed speculations about the pathophysiology of CHS by considering information from case studies and the pharmacology of cannabinoids. The prevailing theory about how CHS develops is that extended activation of the CB1 receptor by prolonged exposure to THC leads to desensitization and/or downregulation of the CB1 receptor and changes to endocannabinoid-related enzymes, resulting in endocannabinoid system dysregulation.71
Many studies have shown dose-dependent CB1 receptor desensitization and/or downregulation in rodents following administration of high doses of CB1 agonists.72–75 Similar findings in humans using positron emission tomography find that daily cannabis smokers had fewer CB1 receptors compared to nonsmokers in cortical regions, including the insular cortex,76 a brain region implicated in nausea.77 CB1 receptor downregulation was positively correlated with years of cannabis use.76 There was, however, no self-report data about side-effects of cannabis, medical history, or follow-up of the participants; therefore, it cannot be known if any of the participants had, or went on to develop CHS. It remains unknown how changes to the endocannabinoid system could lead to the development of CHS and more empirical research is needed to identify the mechanism.
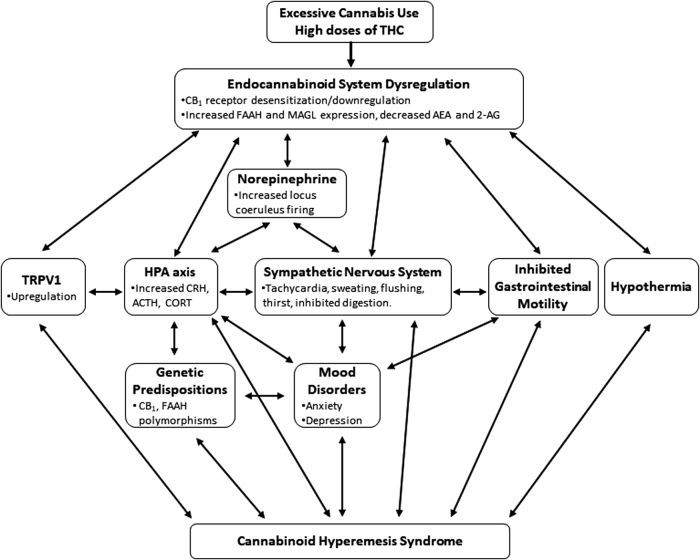
Given the ubiquitous nature of the endocannabinoid system, dysregulation of its normal functioning can have dramatic and potentially adverse outcomes. Indeed, along with acute and long-term high doses of CB1 agonists,13–22 CB1 antagonism/inverse agonism can also result in nausea and vomiting in humans78 and animals.13,79,80 Therefore, disrupting normal endocannabinoid signaling can influence nausea and vomiting. Figure 1 presents a schematic representation of factors that may contribute to CHS. Changes in the endocannabinoid system may lead to alterations in other systems that have the potential to promote nausea and vomiting. The impact of endocannabinoid system dysregulation on various neurobiological systems and their possible contribution to nausea and vomiting, and consequently CHS, are discussed in the following section.
FIG. 1.
Schematic representation of factors that may contribute to CHS. CHS, cannabinoid hyperemesis syndrome.
Acute cannabinoid-induced nausea and vomiting
One of the first approved and recognized medical uses of cannabis in modern medical history was for treatment of chemotherapy-induced nausea and vomiting.10 Recent research suggests that low doses of THC inhibit nausea by acting on CB1 receptors in the interoceptive insular cortex,81,82 and inhibit vomiting by activating CB1 receptors in the dorsal vagal complex (DVC).83,84 Despite the vast body of literature indicating that cannabinoids prevent nausea and vomiting, there are instances where higher doses of THC can produce vomiting in several species, including shrews,13,14 cats,15 dogs,16,17 monkeys,18 and humans.20–22,85–87
Work in our laboratory has investigated the anti-nauseating and pro-nauseating effects of cannabinoids using a pre-clinical rat model of nausea, conditioned gaping in the taste reactivity test.12,88 When a novel flavor, delivered intraorally, is paired with an emetic agent, such as Lithium Chloride (LiCl) or chemotherapy drugs, rats display a conditioned gaping response, characterized by the wide triangular opening of the mouth, when re-exposed to that flavor.89 Rats lack the motor output of vomiting, but do detect toxins the same way as species that can vomit.90–94 Therefore, rats provide an excellent model for studying neurobiological mechanism of nausea, without the confound of vomiting.
A low dose of THC prevents conditioned gaping produced by a flavor paired with LiCl in the taste reactivity paradigm.95 However, when THC is paired with a flavor in the taste reactivity paradigm, high doses of THC, but not low doses of THC, produce conditioned gaping reactions, indicating nausea.19,96 Pre-treatment with a CB1 antagonist prevented THC-induced nausea in rats19 and vomiting in shrews,14 further implicating the endocannabinoid system. We also found that repeated administration of the high dose that produced conditioned gaping in rats, also produced an upregulation of the gene for endocannabinoid degrading enzymes (both FAAH and MAGL,) in the hypothalamus.19 The resulting reduction in AEA and 2-AG, respectively (currently being evaluated), may produce dysregulation in processes regulated by the hypothalamus, such as stress and thermoregulation, in THC-induced nausea.
Dysregulated stress response in nausea and vomiting
Emesis is associated with an altered stress response (Fig. 1); however, this relationship is correlational and role of stress as an antecedent or a consequence of nausea and vomiting is unknown.97,98 Stress is hypothesized to be involved in the pathophysiology of various nausea and vomiting disorders, including CVS,55,99–101 and anticipatory nausea and vomiting associated with chemotherapy.102 CVS and anticipatory nausea and vomiting is correlated with anxiety and mood disorders.99,102 During the emetic phase of CVS in cannabis users and nonusers, there is an increase in circulating endocannabinoids,97 which is also seen during times of stress.103 There is also activation of the hypothalamic-pituitary-adrenal (HPA) axis during CVS, shown by elevated levels of glucocorticoid hormones and corticotropin-releasing hormone (CRH) during vomiting episodes.101 Anticipatory nausea and vomiting are the result of classically conditioned associations between the context where patients receive treatment and the nauseating and emetic effects of chemotherapy agents.104 Despite receiving first-line antiemetic therapy, ∼20% of chemotherapy patients still experience anticipatory nausea, and these individuals tend to have higher trait and state anxiety and experience more emotional distress than patients who do not experience anticipatory nausea.102,105
As with CHS and CVS, typical 5-HT3 receptor antagonists, which are effective in preventing acute vomiting and somewhat effective in preventing acute nausea in chemotherapy treatment,106,107 are ineffective at reducing the expression of anticipatory nausea and vomiting.108,109 For CHS, CVS, and anticipatory nausea and vomiting in chemotherapy, benzodiazepines are the most common treatment.110,111 Anticipatory nausea can be studied in pre-clinical models as well.109 Similar to conditioned gaping produced by a flavor paired with an emetic drug,12 rats and shrews gape when placed in a distinctive context paired with emetic agents.112–114 Low-dose cannabinoids, endocannabinoid manipulations, and benzodiazepines, but not ondansetron (5-HT3 antagonist), reduce contextually elicited conditioned gaping in rats and shrews, mirroring findings in humans with CHS.113–118
While stress and anxiety are common occurrences for many people, there are clear relationships between stress, anxiety, and several nausea and vomiting disorders. The direction and causality of these correlations have yet to be established and need to be investigated to understand how this might contribute to the development CHS in certain individuals.
Hypothalamic pituitary adrenal axis dysregulation
Nausea and vomiting are associated with dysregulated hypothalamic pituitary adrenal (HPA) axis activation,97,98,119 as well as changes in the endocannabinoid system (Fig. 1).97,119 The endocannabinoid system is essential for the allostasis of the HPA axis.120,121 Briefly, during basal stress, the hypothalamus becomes active and triggers the pituitary to send neuroendocrine signals to the adrenal glands to release glucocorticoid stress hormones.122 These hormones then provide negative feedback to the hypothalamus to terminate this process. When glucocorticoid hormones provide feedback to the hypothalamus, 2-AG is released to terminate the stress response by acting on CB1 receptors and inhibiting glutamate release.123–125 Conversely, AEA is tonically released in the hypothalamus to suppress the HPA axis and is reduced following exposure to a stressor, disinhibiting the stress response.124
Changes to the endocannabinoid system in the hypothalamus from cannabinoid use could lead to a dysregulated stress response, and perhaps contribute to the development of CHS. Alterations of the endocannabinoid system produced by exogenous cannabinoids are known to be dose-dependent and region-specific in the brain.76,126 Indeed, our laboratory has shown that repeated administration of a high dose of THC that produces nausea in rats, but not a lower antinausea dose, also causes an upregulation of the genes for the degrading enzymes of 2-AG, (MAGL) and of AEA(FAAH) in the hypothalamus, but not any other brain regions sampled (insular cortex, hippocampus, DVC, and nucleus accumbens).19 It is also intriguing that Tai et al.127 found that chronic exposure to THC or the full CB1 agonist JWH-018 produced downregulation, and reduced functionality in the hypothalamic, but not cortical, CB1 receptors in mice. This suggests that high doses of THC can lead to low levels of endocannabinoids within the hypothalamus, which may prevent inhibitory control of the HPA axis. More research is still needed to understand the impact of cannabinoids on hypothalamic endocannabinoid and endocannabinoid-related enzymes.
Endocannabinoids in the amygdala, specifically the basolateral amygdala (BLA), play a similar role for regulation of the stress response by inhibiting hypothalamic activity.122,128 Agonism of CB1 receptors on glutamatergic BLA neurons decreases glutamate release and inhibits HPA axis and autonomic activation.122 Stress-induced release of CRH from the hypothalamus causes an upregulation of FAAH in the BLA, leading to a reduction in AEA,129 which disinhibits this region, further activating the HPA axis.128,130 Under acute stress conditions, glucocorticoids provide feedback to increase 2-AG after a delay in the BLA129,131,132 and hypothalamus.123,124 Reduction of 2-AG in the BLA promotes anxiety behaviors130; therefore, it is possible that disruption of this mechanism by overactivation of the CB1 receptor could increase BLA excitability, and consequently promote stress and anxiety,133 potentiating nausea and vomiting in response to high-dose, long-term THC exposure.
High doses of cannabinoids are known to activate the stress response in humans and laboratory animals and cause increased CRH production75,134 and circulating glucocorticoid levels,135–137 and produce anxiety behavior.138 Research regarding HPA axis function in long-term cannabis users is less clear. Cuttler et al.139 demonstrated that when compared with nonusers, chronic cannabis users display a blunted cortisol increase in response to an acute stressor. Similarly, Somaini et al.140 found that cannabis users had a limited increase in plasma cortisol and adrenocorticotropin hormone (ACTH) compared to nonusers when shown unpleasant visual stimuli. On the other hand, some studies show that chronic cannabis users have higher basal cortisol and ACTH levels, and increased ratings of anxiety compared to nonusers.140,141 The authors suggest that altered stress reactivity in chronic cannabis users may be mediated by alterations in the endocannabinoid system required for regulation of HPA axis activation.121,122 Chronic cannabinoid administration alters endocannabinoid content in rodents142,143 and in humans, which differs between light and heavy users.144 Therefore, the blunted stress responses in chronic cannabis users may be the result of alterations in endocannabinoid signaling. More research is needed, however, to assess if the blunted stress response is an impairment in HPA activation or impaired negative feedback of the HPA axis. Moreover, research is needed to determine the impact of THC dose, length of use, and genetic or other pre-disposing factors on stress reactivity in cannabis users.140,145
Sympathetic nervous system dysregulation
Activation of the sympathetic nervous system (SNS) is also implicated in nausea and vomiting as indicated in Figure 1.77 Indeed, aberrant sympathetic activation is associated with CVS and CHS.99,146 Symptoms of CVS and CHS are more prominent in the morning, which is also when SNS and HPA axis activity is at its highest.99,146 CVS and CHS are often accompanied by sympathetic symptoms, including, sweating, flushing, thirst, hypertension, and tachycardia.40,99 Venkatesan et al.97 found that salivary alpha-amylase, a biomarker of SNS activity, was significantly higher in CVS patients who use cannabis compared to those who do not use cannabis, during the emetic phase. This finding suggests that cannabis potentiates the SNS dysfunction during emesis.
Prolonged cannabinoid use may dysregulate basal sympathetic signaling.99 Acutely, it is well established that THC increases heart rate and blood pressure.147,148 Studies have shown that chronic cannabis users have increased blood pressure and heart rate,149,150 and an increased skin conductance response in a fear conditioning paradigm151 compared to nonusers. Similarly, Schmid et al.152 found that cannabis users have greater heart rate variability than nonusers. Withdrawal from cannabis also leads to an increase in heart rate and blood pressure.153 These findings implicate the endocannabinoid system in SNS regulation.
Several brain structures send GABAergic projections that have CB1 receptors154 to the brainstem regions involved in SNS activation, the rostral ventrolateral medulla (rVLM), and vomiting, the nucleus tractus solitarius (NTS) located in the DVC.99,155,156 Disinhibition of the rVLM and NTS by CB1 receptor activation could increase the likelihood of SNS activation and vomiting. Similarly, CB1 receptor agonists increase NE release by activating CB1 receptors on GABAergic terminals in the locus coeruleus (LC), reducing GABA release, and disinhibiting this region.157,158 This effect is dose-dependent, with higher doses of THC producing greater LC activity.158 Indeed, THC dose-dependently activates the SNS through altered NE signaling, with larger doses producing greater activation through its effects on NE transmission, an important neurotransmitter for SNS activity.147,148,152,159,160
Propranolol, a β-adrenergic receptor antagonist that reduces SNS activity and inhibits HPA activation by high-dose cannabinoids,135 is also effective in alleviating CHS symptoms in human case studies.161 Other anxiolytic and sedating drugs, such as benzodiazepines and antipsychotics, can alleviate the nausea and vomiting associated with CHS as well.53 Given the link between SNS and nausea and vomiting, the sympathetic symptoms (i.e., hypertension, tachycardia, and sweating) associated with CHS episodes, the impact of THC on the SNS, and acute treatments, it is possible that a dysregulated SNS may contribute to the pathophysiology of CHS (Fig. 1).
TRPV1 and temperature regulation
The endocannabinoid system in the hypothalamus is a critical brain region involved in thermoregulation.162,163 Low doses of THC cause hyperthermia, whereas high doses produce hypothermia26,164 by activating CB1 receptors in the hypothalamus.165 As previously mentioned, hot baths and showers consistently decrease nausea and vomiting symptoms for individuals experiencing CHS.23,43,66,166 Nausea and vomiting are associated with a decline in body temperature in humans167–169 and animals.170 The hot bathing behavior associated with CHS could be overcoming the combined hypothermia of nausea and high doses of THC, improving symptoms.
High temperatures, such as during a hot shower, also activate transient receptor potential vanilloid 1 (TRPV1) receptors located on nociceptive neurons. These receptors are activated by noxious stimuli, high heat, or agonists like capsaicin,171 the chemical in chili peppers that makes them spicy, to send pain signals to the brain.172 The TRPV1 receptors are also implicated in nausea and vomiting pathways, where TRPV1 activity in the NTS leads to nausea and vomiting.173 Likewise, the endocannabinoid system is interconnected with TRPV1 systems. AEA and 2- AG are endogenous agonists of the TRPV1 receptor, and CB1 and TRPV1 receptors are often co-expressed on neurons, but have opposite effects, whereby CB1 receptors inhibit neuronal activity and TRPV1 receptor activates neuronal activity.171 While THC has minimal efficacy at the TRPV1 receptors, other cannabinoids found in the cannabis plant, such as cannabidiol and cannabinol, along with AEA and 2-AG, activate and desensitize the TRPV1 receptor.174,175
There is also a relationship between TRPV1 signaling and stress. While chronic stress leads to a downregulation of CB1 receptors, there is an upregulation of TRPV1 receptors, which can lead to visceral hyperalgesia.171 Dysregulation of the endocannabinoid system could lead to dysregulation of TRPV1 receptors, and their associations with nausea, vomiting, and stress could indicate why individuals with CHS experience relief from symptoms when they take hot baths or use capsaicin cream.
Topical capsaicin cream applied to the abdomen is reported to be effective and safe for alleviating symptoms of CHS.176–178 Topical capsaicin activates and then desensitizes the TRPV1 receptor, thus inhibiting pain signals, resulting in analgesic and anti emetic effects.171,173 Therefore, capsaicin could be beneficial in an emergency room setting to prevent dehydration and kidney failure.171,178 A retrospective chart analysis by Wagner et al.178 found that capsaicin cream reduced the overall number of medications used to treat individuals with CHS in the emergency room, but did not reduce the overall length of stay in the hospital. While it is effective, other medications are still needed in conjunction with topical capsaicin for total symptom resolution.178 The mechanism of how capsaicin reduces CHS symptoms is not known; therefore, its exact role in CHS treatment is not yet clear.
It is unclear if the relief felt by individuals with CHS by these therapies is because of direct alteration of TRPV1 receptors or because of alternative indirect mechanisms. Given the relationships between TRPV1 receptors, the endocannabinoid system, stress, pain, nausea, and the benefits of capsaicin cream and hot baths in CHS, it is possible that endogenous activation and function of TRPV1 receptors could be altered due to dysregulation of the endocannabinoid system, contributing to the development of CHS. Alternatively, high-temperature baths and capsaicin cream may produce a “cutaneous steal” syndrome.146 Topical capsaicin and hot baths cause vasodilation in the skin and may cause redirection of blood flow from the gut to the skin, alleviating abdominal pain and nausea.171 This theory would explain why symptom relief with these methods is only temporary with capsaicin and hot baths. Information regarding changes to the TRPV1 system and function of capsaicin following chronic cannabis exposure, and TRPV1 receptor function in individuals with CHS is still needed to confirm its contribution to CHS symptoms.
Gut motility regulation
Another possible contributing factor to the pathophysiology of CHS is the effect of cannabinoids on gastrointestinal function.179 Patients with CHS typically have delayed gastric emptying,23,47,66 which, as discussed earlier, could help to distinguish CHS from CVS, which typically presents with an increase in gastric emptying.48 Delayed gastric emptying without an obstruction, known as gastroparesis, produces nausea and vomiting.180,181 The proemetic effects of inhibiting gut motility by cannabinoids may override the antiemetic effects of cannabinoids. This could be another contributing factor in the pathophysiology of CHS (Fig. 1). THC and synthetic designer cannabinoids have been shown to inhibit gut motility and gastric emptying in rats by acting on CB1 receptors in the gut.51,52,182 The inhibitory gastric effects are dose-dependent, where higher doses have a greater inhibitory effect on gastric motility and emptying,51,52,182 and tolerance does not develop.183,184 This is also seen in humans.49,50 Furthermore, CRH released from the hypothalamus also inhibits gastrointestinal function.101,185 These data highlight the connection between cannabinoids, endocannabinoid system, stress, and nausea and vomiting.
Pre-disposing factors
One characteristic of CHS that remains unresolved is that not every cannabis user develops CHS; therefore, it is likely that certain individuals are pre-disposed to develop CHS. Yet the prevalence of CHS among cannabis users is unknown. Genome-wide association studies may be beneficial in determining genetic anomalies in individuals with CHS. Indeed, polymorphisms of the CB1 receptor gene are linked to an increased likelihood for individuals to develop CVS,186 and differences in the FAAH gene are associated with an increased likelihood of irritable bowel syndrome,187 addiction,188 and anxiety disorders.128,188
Individuals with pre-existing heightened or dysregulated stress responses may be at a higher risk of developing CHS. Mood disorders, such as anxiety and depression, are associated with dysregulated stress responses, and are linked to an increased likelihood of developing nausea and vomiting disorders.55,99,101,102 In addition, there is a correlation between mood disorders and excessive cannabis use,189 as well as excessive cannabis use in CVS,45 which may provide a link to CHS. Some studies have indicated that CHS is more common in males than females.43,45,46 Sex differences should also be investigated to further understand what pre-disposes individuals to CHS. Also, some evidence suggests that most individuals with CHS began using cannabis in their teenage years.43 Expression of CB1 receptors is high during adolescence, and plays a very important role in this critical period for neuronal development. Heavy cannabis use in adolescence has been implicated in endocannabinoid system dysregulation to possibly impair proper neuronal development,190 potentially influencing the development of CHS later in life. More research is needed, however, to understand how genetics, mood disorders, sex, and other pre-disposing factors contribute to the development of CHS.
Cannabis contamination
It is sometimes thought, often from anecdotal reports in popular media, that CHS is a result of poisoning from contaminants such as pesticides on cannabis and not from the actual plant constituents themselves. However, support is lacking for this hypothesis in the empirical literature.191,192 Contaminants can be detected on cannabis plant samples32,193 and in smoke.194 The bioavailability of contaminants in smoked cannabis is still unknown192 and a significant amount of smoke is often lost in side stream. Indeed, the symptoms of pesticide poisoning are different than the symptoms of CHS. Pesticide poisoning typically leads to nausea and vomiting, pulmonary and cardiovascular symptoms, convulsions, and hepatic toxicity.195,196 The only symptoms that are consistently seen with CHS are nausea and vomiting.23Most markedly, compulsive hot bathing, a symptom used as supportive criteria for CHS diagnosis,23,66,166 is not present in instances of pesticide poisoning.196 The limited pre-clinical research on CHS does indicate that cannabis-induced nausea and vomiting is mediated by the action of THC at the CB1 receptor.14,19 Moreover, synthetic cannabinoids, which are produced in laboratories and therefore do not have pesticides, can also lead to CHS,34–36 further undermining the notion of contamination contributing to CHS. In addition, since pesticides are not exclusive to cannabis, there would be many similar cases that would not be solved with cannabis abstinence. While pesticides can lead to adverse health problems, this is likely not the cause of CHS.
Conclusions
Low doses of THC are effective antiemetic treatments, but high doses paradoxically produce nausea and vomiting, and may lead to the development of CHS.23 This complex syndrome may have many contributing factors, including downregulation of CB1 receptors, a dysfunctional stress response, altered thermoregulatory and TRPV1 systems, inhibited gastric motility, and genetic factors.71 The evidence regarding the mechanisms responsible for cannabinoid-induced nausea and vomiting is limited, and there is much left to investigate.
Despite the possible pharmacological and behavioral therapies, the only way to eliminate CHS is abstaining from cannabinoids. Acute treatment is necessary to avoid dehydration, renal failure, and death, but long-term treatments need to emphasize that sustained abstinence is required to permanently cease symptoms. Education regarding the potential effects of long-term high doses of cannabinoids should be disseminated to physicians and the public to better detect and manage this debilitating syndrome. Future research, both clinical and pre-clinical, should continue to investigate the underlying mechanism and pre-disposing factors of CHS, to further understand the consequences of high-dose cannabinoid use and dysregulation of the endocannabinoid system.
Abbreviations Used
- 2-AG
2-arachidonoylglycerol
- ACTH
adrenocorticotropin hormone
- AEA
anandamide
- BLA
basolateral amygdala
- CB1
cannabinoid 1
- CHS
cannabinoid hyperemesis syndrome
- CRH
corticotropin-releasing hormone
- CUD
cannabis use disorder
- CVS
cyclical vomiting syndrome
- DVC
dorsal vagal complex
- FAAH
fatty acid amide hydrolase
- HPA
hypothalamic-pituitary-adrenal
- LC
locus coeruleus
- LiCl
lithium chloride
- MAGL
monoacylglycerol lipase
- NTS
nucleus tractus solitarius
- rVLM
rostral ventrolateral medulla
- SNS
sympathetic nervous system
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid 1
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Information
This research was supported by a research grant from the Natural Sciences and Engineering Reserch Council of Canada (NSERC: 03629) to L.A.P. and an NSERC CGS D Scholarship to M.V.K.
Cite this article as: DeVuono MV, Parker LA (2020) Cannabinoid hyperemesis syndrome: a review of potential mechanisms, Cannabis and Cannabinoid Research 5:2, 132–144, DOI: 10.1089/can.2019.0059.
References
- 1. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47 [DOI] [PubMed] [Google Scholar]
- 2. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mechoulam R, Gaoni Y. A total synthesis of delta-9-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965;87:3273–5 [DOI] [PubMed] [Google Scholar]
- 4. Devane WA, Dysarz FA, Johnson MR, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613 [PubMed] [Google Scholar]
- 5. Devane WA, Hanuš L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949 [DOI] [PubMed] [Google Scholar]
- 6. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90 [DOI] [PubMed] [Google Scholar]
- 7. Cravatt BF, Giang DK, Mayfield SP, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87 [DOI] [PubMed] [Google Scholar]
- 8. Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–4 [DOI] [PubMed] [Google Scholar]
- 9. Vandrey R, Dunn KE, Fry JA, et al. A survey study to characterize use of Spice products (synthetic cannabinoids). Drug Alcohol Depen. 2012;120:238–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parker LA. Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol. 2014;722:122–133 [DOI] [PubMed] [Google Scholar]
- 13. Darmani NA. Delta-9-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24:198–203 [DOI] [PubMed] [Google Scholar]
- 14. Cluny NL, Naylor RJ, Whittle BA, et al. The effects of cannabidiol and tetrahydrocannabinol on motion-induced emesis in Suncus murinus. Basic Clin Pharmacol Toxicol. 2008;103:150–156 [DOI] [PubMed] [Google Scholar]
- 15. Hockman CH, Perrin RG, Kalant H. Electroencephalographic and behavioral alterations produced by delta-9-tetrahydrocannabinol. Science. 1971;172:968–970 [DOI] [PubMed] [Google Scholar]
- 16. Loewe S. Studies on the pharmacology and acute toxicity of compounds with marihuana activity. J Pharmacol Exp Ther. 1946:154–161 [PubMed]
- 17. Shannon HE, Martin WR, Silcox D. Lack of antiemetic effects of delta-9-tetrahydracannabinol in apomorphine-induced emesis in the dog. Life Sci. 1978;23:49–53 [DOI] [PubMed] [Google Scholar]
- 18. Scheckel CL, Boff E, Dahlen P, et al. Behavioral effects in monkeys of racemates of two biologically active marijuana constituents. Science. 1968;160:1467–1469 [DOI] [PubMed] [Google Scholar]
- 19. DeVuono M V., Hrelja KM, Sabaziotis L, et al. Conditioned gaping produced by high dose Δ9-tetrahydracannabinol: dysregulation of the hypothalamic endocannabinoid system. Neuropharmacology. 2018;141:272–282 [DOI] [PubMed] [Google Scholar]
- 20. Frytak S, Moertel C, O'Fallon JR, et al. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. Ann Intern Med. 1979;19:825–830 [DOI] [PubMed] [Google Scholar]
- 21. Orr LE, McKernan JF. Antiemetic effect of delta-9-tetrahydrocannabinol in chemotherapy-associated nausea ande emesis as compared to placebo and compazine. J Clin Pharmacol. 1981;21:76–80 [DOI] [PubMed] [Google Scholar]
- 22. Vaziri ND, Thomas R, Stearling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol. 1981;18:353–366 [DOI] [PubMed] [Google Scholar]
- 23. Allen JH, De Moore G, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacology. 1998;59:347–352 [DOI] [PubMed] [Google Scholar]
- 25. Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Be. 2005;81:331–342 [DOI] [PubMed] [Google Scholar]
- 26. Taylor DA, Fennessy MR. Biphasic nature of the effects of delta9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur J Pharmacol. 1977;46:93–99 [DOI] [PubMed] [Google Scholar]
- 27. Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsycopharmacol. 2013;16:2273–84 [DOI] [PubMed] [Google Scholar]
- 28. Elsohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last two decades (1995-2014) - analysis of current data in the united states. Biol Psychiatry. 2017;79:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med. 2015;45:3181–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loflin M, Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict Behav. 2014;39:1430–1433 [DOI] [PubMed] [Google Scholar]
- 31. Pierre JM, Gandal M, Son M. Cannabis-induced psychosis associated with high potency “wax dabs.” Schizophr Res. 2016;172:211–212 [DOI] [PubMed] [Google Scholar]
- 32. Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci. 2015;40:797–803 [DOI] [PubMed] [Google Scholar]
- 33. Lui X, Villamagna A, Yoo J. The importance of recognizing cannabinoid hyperemesis syndrome from synthetic marijuana use. J Med Toxicol. 2017:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bick BL, Szostek JH, Mangan TF. Synthetic cannabinoid leading to cannabinoid hyperemesis syndrome. Mayo Clin Proc. 2014;89:1168–1169 [DOI] [PubMed] [Google Scholar]
- 35. Hopkins CY, Gilchrist BL. A case of cannabinoid hyperemesis syndrome caused by synthetic cannabinoids. J Emerg Med. 2013;45:544–546 [DOI] [PubMed] [Google Scholar]
- 36. Ukaigwe A, Karmacharya P, Donato A. A gut gone to pot: a case of cannabinoid hyperemesis syndrome due to K2, a synthetic sannabinoid. J Emerg Med Case Rep. 2014;2014:167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;0:12–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spaderna M, Addy PH, D'Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacol. 2013;228:525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu MLRY, Agito MD. Cannabinoid hyperemesis syndrome: marijuana is both antiemetic and proemetic. Clev Clin J Med. 2015;82:429–434 [DOI] [PubMed] [Google Scholar]
- 41. Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal disorders. Gastroenterology. 2016;150):1380–1392 [DOI] [PubMed] [Google Scholar]
- 42. Simonetto DA, Oxentenko AS, Herman ML, et al. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorensen CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J Med Toxicol. 2017;13:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blumentrath CG, Dohrmann B, Ewald N. Cannabinoid hyperemesis and the cyclic vomiting syndrome in adults: recognition, diagnosis, acute and long-term treatment. Ger Med Sci. 2017;15:Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venkatesan T, Sengupta J, Lodhi A, et al. An internet survey of marijuana and hot shower use in adults with cyclic vomiting syndrome (CVS). Exp Brain Res. 2014;232:2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choung RS, Locke GR, Lee RM, et al. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroent Motil. 2012;24:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galli JA, Sawaya R, Friedenberg F. Cannabinoid hyperemesis syndrome. CNS Neurosci Ther. 2013;19:994–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cooper C, Said S, Bizet J, et al. Rapid or normal gastric emptying as new supportive criteria for diagnosing cyclic vomiting syndrome in adults. Med Sci Monit. 2014;20:1491–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharm Therap. 1999;13:77–80 [DOI] [PubMed] [Google Scholar]
- 50. Aviello G, Romano B, Izzo A. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharm Sci. 2008;12:81–93 [PubMed] [Google Scholar]
- 51. Izzo AA, Mascolo N, Capasso R, et al. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. N-S Arch Pharmacol. 1999;360:221–223 [DOI] [PubMed] [Google Scholar]
- 52. Krowicki ZK, Moerschbaecher JM, Winsauer PJ, et al. Δ9-Tetrahydrocannabinol inhibits gastric motility in the rat through cannabinoid CB1 receptors. Eur J Pharmacol. 1999;371:187–196 [DOI] [PubMed] [Google Scholar]
- 53. Richards JR, Gordon BK, Danielson AR, et al. Pharmacologic treatment of cannabinoid hyperemesis syndrome: a systematic review. Pharmacotherapy. 2017 [DOI] [PubMed] [Google Scholar]
- 54. Khattar N, Routsolias JC. Emergency department treatment of cannabinoid hyperemesis syndrome: a review. Am J Ther. 2017;5:1–5 [DOI] [PubMed] [Google Scholar]
- 55. Hayes WJ, VanGilder D, Berendse J, et al. Cyclic vomiting syndrome: diagnostic approach and current management strategies. Clin Exp Gastroenterol. 2018;11:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Habboushe J, Sedor J. Cannabinoid hyperemesis acute renal failure: a common sequela of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2014;32:690..e1-690.e2. [DOI] [PubMed] [Google Scholar]
- 57. Nourbakhsh M, Miller A, Gofton J, et al. Cannabinoid hyperemesis syndrome: reports of fatal cases. J Forensic Sci. 2018:1–5 [DOI] [PubMed] [Google Scholar]
- 58. Pélissier F, Claudet I, Gandia-Mailly P, et al. Cannabis hyperemesis syndrome in the emergency department: how can a specialized addiction team be useful? A pilot study. J Emerg Med. 2016;51:544–551 [DOI] [PubMed] [Google Scholar]
- 59. Sherman BJ, McRae-Clark AL. Treatment of cannabis use disorder: current science and future outlook. Pharmacotherapy. 2016;36:511–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sabioni P, Le Foll B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. F1000Research. 2018;7:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. D'Souza DC, Cortes-Briones J, Creatura G, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 2019;6:35–45 [DOI] [PubMed] [Google Scholar]
- 62. Schreck B, Wagneur N, Caillet P, et al. Cannabinoid hyperemesis syndrome: review of the literature and of cases reported to the French addictovigilance network. Drug Alcohol Depen. 2018;182:27–32 [DOI] [PubMed] [Google Scholar]
- 63. Hernandez JM, Paty J, Price I. Cannabinoid hyperemesis syndrome presentation to the emergency department: a two-year multi-centre retrospective study. Cjem. 2016;18(S1):S98–S99 [DOI] [PubMed] [Google Scholar]
- 64. Habboushe J, Rubin A, Liu H, et al. The prevalence of cannabinoid hyperemesis syndrome among regular marijuana smokers in an urban public hospital. Basic Clin Pharmacol Toxico. 2018;122:660–662 [DOI] [PubMed] [Google Scholar]
- 65. Kim HS, Anderson JD, Saghafi O, et al. Cyclic vomiting presentations following marijuana liberalization in Colorado. Acad Emerg Med. 2015;22:694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing case report. Mayo Clin Proc. 2009;84:76–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nicolson SE, Denysenko L, Mulcare JL, et al. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53:212–219 [DOI] [PubMed] [Google Scholar]
- 68. Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23:790–793 [DOI] [PubMed] [Google Scholar]
- 69. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the united states. Digest Dis Sci. 2010;55:3113–3119 [DOI] [PubMed] [Google Scholar]
- 70. Heise L. Cannabinoid hyperemesis syndrome. Adv Emerg Nurs J. 2015;37:95–101 [DOI] [PubMed] [Google Scholar]
- 71. Darmani NA. Cannabinoid-induced hyperemesis: a conundrum-from clinical recognition to basic science mechanisms. Pharmaceuticals. 2010;3:2163–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sim LJ, Hampson RE, Deadwyler SA, et al. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhuang S, Kittler J, Grigorenko E V, et al. Effects of long-term exposure to delta9-THC on expression of cannabinoid receptor (CB1) mRNA in different rat brain regions. Mol Brain Res. 1998;62:141–149 [DOI] [PubMed] [Google Scholar]
- 74. Romero J, Berrendero F, Manzanares J, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308 [DOI] [PubMed] [Google Scholar]
- 75. Corchero J, Romero J, Berrendero F, et al. Time-dependent differences of repeated administration with Δ9- tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by μ-opioid and CB1-cannabinoid receptors in the caudate-putamen. Mol Brain Res. 1999;67:148–157 [DOI] [PubMed] [Google Scholar]
- 76. Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiat. 2012;17:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Napadow V, Sheehan JD, Kim J, et al. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex. 2013;23:806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:43–65 [DOI] [PubMed] [Google Scholar]
- 79. Parker LA, Mechoulam R, Schlievert C, et al. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacol. 2003;166:156–162 [DOI] [PubMed] [Google Scholar]
- 80. McLaughlin PJ, Winston KM, Limebeer CL, et al. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacol. 2005;180:286–293 [DOI] [PubMed] [Google Scholar]
- 81. Limebeer CL, Rock EM, Mechoulam R, et al. The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. Br J Pharmacol. 2012;167:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sticht MA, Limebeer CL, Rafla BR, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmachology. 2016;102:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Van Sickle MD, Oland LD, Ho W, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774 [DOI] [PubMed] [Google Scholar]
- 84. Van Sickle MD, Oland LD, Mackie K, et al. Delta-9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J PhysiolGastrointest Liver Physiol. 2003;285:G566-76 [DOI] [PubMed] [Google Scholar]
- 85. Noyes R, Brunk SF, Baram DA, et al. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. J Clin Pharmacol. 1975;15:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meyer P, Langos M, Brenneisen R. Human pharmacokinetics and adverse effects of pulmonary and intravenous THC-CBD formulations. Med Cannabis Cannabinoids. 2018;1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Soc Adm Pharm. 2016;12:638–654 [DOI] [PubMed] [Google Scholar]
- 88. Parker LA, Limebeer CL. Conditioned gaping in rats: a selective measure of nausea. Auton Neurosci. 2006;129:36–41 [DOI] [PubMed] [Google Scholar]
- 89. Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279 [DOI] [PubMed] [Google Scholar]
- 90. Bernstein IL, Chavez M, Allen D, et al. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res. 1992;575:132–137 [DOI] [PubMed] [Google Scholar]
- 91. Eckel LA, Ossenkopp K-P. Area postrema mediates the formation of rapid, conditioned palatability shifts in lithium-treated rats. Behav Neurosci. 1996;110:202–212 [PubMed] [Google Scholar]
- 92. Endo T, Takahashi M, Minami M. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biogenic Amines. 1995;11:399–407 [Google Scholar]
- 93. Horn CC, Richardson EJ, Andrews PLR, et al. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81 [DOI] [PubMed] [Google Scholar]
- 94. Horn CC, Kimball BA, Wang H, et al. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Limebeer CL, Parker LA. Delta-9-tetrahydrocannabinol interferes with the establishment and the expression of conditioned rejection reactions produced by cyclophosphamide: a rat model of nausea. NeuroReport. 1999;10:3769–3772 [DOI] [PubMed] [Google Scholar]
- 96. Parker LA, Gillies T. THC-induced place and taste aversions in lewis and sprague-dawley rats. Behav Neurosci. 1995;109:71–78 [DOI] [PubMed] [Google Scholar]
- 97. Venkatesan T, Zadvornova Y, Raff H, et al. Endocannabinoid-related lipids are increased during an episode of cyclic vomiting syndrome. Neurogastroent Motil. 2016;28:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Otto B, Riepl RL, Klosterhalfen S, et al. Endocrine correlates of acute nausea and vomiting. Auton Neuroscie-Basic. 2006;129:17–21 [DOI] [PubMed] [Google Scholar]
- 99. Levinthal DJ, Bielefeldt K. Adult cyclical vomiting syndrome: a disorder of allostatic regulation? Exp Brain Res. 2014;232:2541–2547 [DOI] [PubMed] [Google Scholar]
- 100. Taché Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Digest Dis Sci. 1999;44(8 Suppl):79S.—86S. [PubMed] [Google Scholar]
- 101. Li BUK, Misiewicz L. Cyclic vomiting syndrome: a brain-gut disorder. Gastroenterol Clin N. 2003;32:997–1019 [DOI] [PubMed] [Google Scholar]
- 102. Andrykowski MA. The role of anxiety in the development of anticipatory nausea in cancer chemotherapy: a review and synthesis. Psychosom Med. 1990;52:458–475 [DOI] [PubMed] [Google Scholar]
- 103. Dlugos A, Childs E, Stuhr KL, et al. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacol. 2012;37:2416–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nesse RM, Carli T, Curtis GC, et al. Pretreatment nausea in cancer chemotherapy: a conditioned response? Psychosom Med. 1980;42:33–36 [DOI] [PubMed] [Google Scholar]
- 105. Aapro M. CINV: still troubling patients after all these years. Support Care Cancer. 2018;26:5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249–262 [DOI] [PubMed] [Google Scholar]
- 107. Roscoe JA, Morrow GR, Hickok JT, et al. Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manag. 2000;20:113–121 [DOI] [PubMed] [Google Scholar]
- 108. Kamen C, Tejani MA, Chandwani K, et al. Anticipatory nausea and vomiting due to chemotherapy. Eur J Pharmacol. 2014;722:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rock EM, Limebeer CL, Parker LA. Anticipatory nausea in animal models: a review of potential novel therapeutic treatments. Exp Brain Res. 2014;232:2511–2534 [DOI] [PubMed] [Google Scholar]
- 110. James A, Nair M, Abraham D, et al. Effect of lorazepam in reducing psychological distress and anticipatory nausea and vomiting in patients undergoing chemotherapy. J Pharmacol Pharmacother. 2017;8:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Richards JR. Cannabinoid hyperemesis syndrome: pathophysiology and treatment in the emergency department. J Emerg Med. 2018;54:354–363 [DOI] [PubMed] [Google Scholar]
- 112. Limebeer CL, Krohn JP, Cross-Mellor S, et al. Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav Brain Res. 2008;187:33–40 [DOI] [PubMed] [Google Scholar]
- 113. Parker LA, Kwiatkowska M, Mechoulam R. Delta-9-tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: an animal model of anticipatory nausea and vomiting. Physiol Behav. 2006;87:66–71 [DOI] [PubMed] [Google Scholar]
- 114. Parker LA, Kemp SWP. Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: an animal model of anticipatory nausea and vomiting (ANV). NeuroReport. 2001;12:749–751 [DOI] [PubMed] [Google Scholar]
- 115. Limebeer CL, Hall G, Parker LA. Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol Behav. 2006;88:398–403 [DOI] [PubMed] [Google Scholar]
- 116. Rock EM, Limebeer CL, Navaratnam R, et al. A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology. 2014;231:3207–3215 [DOI] [PubMed] [Google Scholar]
- 117. Rock EM, Limebeer CL, Mechoulam R, et al. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology. 2008;196:389–395 [DOI] [PubMed] [Google Scholar]
- 118. Rock EM, Limebeer CL, Ward JM, et al. Interference with acute nausea and anticipatory nausea in rats by fatty acid amide hydrolase (FAAH) inhibition through a PPARα and CB 1 receptor mechanism, respectively: a double dissociation. Psychopharmacology. 2015;232:3841–3848 [DOI] [PubMed] [Google Scholar]
- 119. Choukèr A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS One. 2010;5:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hill MN, Patel S, Campolongo P, et al. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010;30:14980–14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Behav Rev. 2014;42:116–131 [DOI] [PubMed] [Google Scholar]
- 122. Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Di S, Malcher-Lopes R, Halmos KC, et al. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Patel S, Roelke CT, Rademacher DJ, et al. Endocannabinoid signaling negatively modulates stress- induced activation of thehypothalamic-pituitary- adrenal axis. Endocrinology. 2004;145:5431–5438 [DOI] [PubMed] [Google Scholar]
- 125. Evanson NK, Tasker JG, Hill MN, et al. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sim-selley LJ. Regulation of Cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:1–12 [DOI] [PubMed] [Google Scholar]
- 127. Tai S, Hyatt WS, Gu C, et al. Repeated administration of phytocannabinoid Δ9-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res. 2015;102:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gunduz-Cinar O, Hill MN, McEwen BS, et al. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gray JM, Vecchiarelli HA, Morena M, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bedse G, Hartley ND, Neale E, et al. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol Psychiat. 2017:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hill MN, Mclaughlin RJ, Morrish AC, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary- adrenal axis. Neuropsychopharmacol. 2009;34:2733–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Di S, Itoga XCA, Fisher XMO, et al. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neruosci. 2016;36:8461–8470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Morena M, Leitl KD, Vecchiarelli HA, et al. Emotional arousal state influences the ability of amygdalar endocannabinoid signaling to modulate anxiety. Neuropharmacology. 2016;111:59–69 [DOI] [PubMed] [Google Scholar]
- 134. De Fonseca FR, Gorriti MA, Fernández-Ruiz JJ, et al. Downregulation of rat brain cannabinoid binding sites after chronic Δ9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40 [DOI] [PubMed] [Google Scholar]
- 135. McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2009;624:71–76 [DOI] [PubMed] [Google Scholar]
- 136. Murphy LL, Muñoz RM, Adrian BA, et al. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446 [DOI] [PubMed] [Google Scholar]
- 137. Puder M, Weidenfeld J, Chowers I, et al. Corticotrophin and corticosterone secretion following Δ9-tetrahydrocannabinol, in intact and in hypothalamic deafferentated male rats. Exp Brain Res. 1982;46:85–88 [DOI] [PubMed] [Google Scholar]
- 138. Rey AA, Purrio M, Viveros M-P, et al. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cuttler C, Spradlin A, Nusbaum AT, et al. Blunted stress reactivity in chronic cannabis users. Psychopharmacol. 2017;234:2299–2309 [DOI] [PubMed] [Google Scholar]
- 140. Somaini L, Manfredini M, Amore M, et al. Psychobiological responses to unpleasant emotions in cannabis users. Eur Arch Psychiatry Clin Neurosci. 2012;262:47–57 [DOI] [PubMed] [Google Scholar]
- 141. King GR, Ernst T, Deng W, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci. 2011;31:17923–17931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Di Marzo V, Berrendero F, Bisogno T, et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of Δ9- tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–1635 [DOI] [PubMed] [Google Scholar]
- 143. González S, Fernández-Ruiz J, Di Marzo V, et al. Behavioral and molecular changes elicited by acute administration of SR141716 to Δ9-tetrahydrocannabinol-tolerant rats: an experimental model of cannabinoid abstinence. Drug Alcohol Depend. 2004;74:159–170 [DOI] [PubMed] [Google Scholar]
- 144. Morgan CJA, Page E, Schaefer C, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202:381–382 [DOI] [PubMed] [Google Scholar]
- 145. Chao T, Radoncic V, Hien D, et al. Stress responding in cannabis smokers as a function of trauma exposure, sex, and relapse in the human laboratory. Drug Alcohol Depend. 2018;185:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Richards JR. Cannabinoid hyperemesis syndrome: a disorder of the HPA axis and sympathetic nervous system? Med Hypotheses. 2017;103:90–95 [DOI] [PubMed] [Google Scholar]
- 147. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S–63S [DOI] [PubMed] [Google Scholar]
- 148. Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol. 2002;42:64–70 [DOI] [PubMed] [Google Scholar]
- 149. Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: united States national health and nutrition examination survey, 2005-2012. J Hypertens. 2016;34:1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Muniyappa R, Sable S, Ouwerkerk R, et al. Metabolic effects of chronic cannabis smoking. Diabetes Care. 2013;36:2415–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Papini S, Ruglass LM, Lopez-Castro T, et al. Chronic cannabis use is associated with impaired fear extinction in humans. J Abnorm Psychol. 2017;126:117–124 [DOI] [PubMed] [Google Scholar]
- 152. Schmid K, Schönlebe J, Drexler H, et al. The effects of cannabis on heart rate variability and well-being in young men. Pharmacopsychiatry. 2010;43:147–150 [DOI] [PubMed] [Google Scholar]
- 153. Vandrey R, Umbricht A, Strain EC. Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med. 2011;5:16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Puente N, Elezgarai I, Lafourcade M, et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS One. 2010;5:e8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. McCorry LK. Teachers' topics: physiology of the Autonomic Nervous System. Am J Pharm Educ. 2007;71:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bowman BR, Kumar NN, Hassan SF, et al. Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J Comp Neurol. 2013;521:213–232 [DOI] [PubMed] [Google Scholar]
- 157. Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci. 2009;1:87–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Muntoni AL, Pillolla G, Melis M, et al. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394 [DOI] [PubMed] [Google Scholar]
- 159. Mooventhan A, Nivethitha L. Scientific evidence-based effects of hydrotherapy on various systems of the body article. N Am J Med Sci. 2014;6:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Bachman JA, Benowitz NL, Herning RI, et al. Dissociation of autonomic and cognitive effects of THC in man. Psychopharmacology. 1979;61:171–175 [DOI] [PubMed] [Google Scholar]
- 161. Richards JR, Dutczak O. Propranolol treatment of cannabinoid hyperemesis syndrome. J Clin Psychopharmacol. 2017;37:482–484 [DOI] [PubMed] [Google Scholar]
- 162. Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol. 2011;305:1207–1228 [DOI] [PubMed] [Google Scholar]
- 163. Wenger T, Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. PLEFA. 2002;66:301–307 [DOI] [PubMed] [Google Scholar]
- 164. Fennessy MR, Taylor DA. Antagonism of the effects on thermoregulation of Δ9-tetrahydrocannabinol By clomipramine in the rat. Br J Pharmacol. 1978;63:267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rawls SM, Cabassa J, Geller EB, et al. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)- 6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968 [DOI] [PubMed] [Google Scholar]
- 166. Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15:156–158 [DOI] [PubMed] [Google Scholar]
- 167. Cheung B, Nakashima AM, Hofer KD. Various anti-motion sickness drugs and core body temperature changes. Aviat Space Environ Med. 2011;82:409–415 [DOI] [PubMed] [Google Scholar]
- 168. Mekjavic IB, Tipton MJ, Gennser M, et al. Motion sickness potentiates core cooling during immersion in humans. J Physiol. 2001;535:619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nobel G, Tribukait A, Mekjavic IB, et al. Efects of motion sickness on thermoregulatory responses in a thermoneutral air environment. Eur J App Physiol. 2012;112:1717–1723 [DOI] [PubMed] [Google Scholar]
- 170. Ngampramuan S, Cerri M, Vecchio F Del, et al. Thermoregulatory correlates of nausea in rats and musk shrews. Oncotarget. 2014;5:1565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Richards JR, Lapoint JM, Burillo-Putze G. Cannabinoid hyperemesis syndrome: potential mechanisms for the benefit of capsaicin and hot water hydrotherapy in treatment. Clin Toxicol. 2018;56:15–24 [DOI] [PubMed] [Google Scholar]
- 172. Rudd JA, Nalivaiko E, Matsuki N, Wan C, et al. The involvement of TRPV1 in emesis and anti- emesis. Tempurature. 2015;2:258–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sharkey KA, Cristino L, Oland LD, et al. Arvanil, anandamide and N -arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neruosci. 2007;25:2773–2782 [DOI] [PubMed] [Google Scholar]
- 174. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pertwee RG, Thomas A.. Therapeutic applications for agents that act at CB1 and CB2 Receptors. In: The Cannabinoid Receptors. Humana Press: Totowa, NJ, 2009, pp. 361–392 [Google Scholar]
- 176. Dezieck L, Hafez Z, Conicella A, et al. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: a case series. Clin Toxicol. 2017;55:908–913 [DOI] [PubMed] [Google Scholar]
- 177. Waterson Duncan R, Maguire M. Capsaicin topical in emergency department treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2017;35:1977–1978 [DOI] [PubMed] [Google Scholar]
- 178. Wagner S, Hoppe J, Zuckerman M, et al. Efficacy and safety of topical capsaicin for cannabinoid hyperemesis syndrome in the emergency department. Clin Toxicol. 2019;0:1–5 [DOI] [PubMed] [Google Scholar]
- 179. Camilleri M. Cannabinoids and gastrointestinal motility: pharmacology, clinical effects, and potential therapeutics in humans. Neurogastroenter Moti. 2018;30:e13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Camilleri M, Parkman HP, Shafi M, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972–1978 [DOI] [PubMed] [Google Scholar]
- 182. Izzo AA, Mascolo N, Pinto L, et al. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384:37–42 [DOI] [PubMed] [Google Scholar]
- 183. Abalo R, Cabezos PA, López-Miranda V, et al. Selective lack of tolerance to delayed gastric emptying after daily administration of WIN 55,212-2 in the rat. Neurogastroenter Moti. 2009;21:22–24 [DOI] [PubMed] [Google Scholar]
- 184. Abalo R, Cabezos PA, Vera G, et al. Cannabinoid-induced delayed gastric emptying is selectively increased upon intermittent administration in the rat: role of CB1 receptors. Neurogastroenter Moti. 2011;23:457–468 [DOI] [PubMed] [Google Scholar]
- 185. Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2009;117:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wasilewski A, Lewandowska U, Mosinska P, et al. Cannabinoid receptor type 1 and mu-opioid receptor polymorphisms are associated with cyclic vomiting syndrome. Am J Gastroenterol. 2017;112:933–939 [DOI] [PubMed] [Google Scholar]
- 187. Camilleri M, Katzka DA. Irritable Bowel Syndrome: methods, mechanisms, and pathophysiology. Genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1075–G1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cheung JTW, Mann RE, Ialomiteanu A, et al. Anxiety and mood disorders and cannabis use. Am J Drug Alcohol Abuse. 2010;36:118–122 [DOI] [PubMed] [Google Scholar]
- 190. Bossong MG, Niesink RJM. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385 [DOI] [PubMed] [Google Scholar]
- 191. McLaren J, Swift W, Dillon P, et al. Cannabis potency and contamination: a review of the literature. Addiction. 2008;103:1100–1109 [DOI] [PubMed] [Google Scholar]
- 192. Dryburgh LM, Bolan NS, Grof CPL, et al. Cannabis contaminants: sources, distribution, human toxicity and pharmacologic effects. Br J Clin Pharmacol. 2018;84:2468–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Schneider S, Bebing R, Dauberschmidt C. Detection of pesticides in seized illegal cannabis plants. Anal Methods. 2014;6:515–520 [Google Scholar]
- 194. Sullivan N, Elzinga S, Raber JC. Determination of pesticide residues in cannabis smoke. J Toxicol. 2013;2013:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Elinav E, Shapira Y, Ofran Y, et al. Near-fatal amitraz intoxication: the overlooked pesticide. Basic Clin Pharmacol Toxicol. 2005;97:185–187 [DOI] [PubMed] [Google Scholar]
- 196. Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107–115 [DOI] [PubMed] [Google Scholar]