Abstract
Background
Reproductive aging may contribute to cardiometabolic comorbid conditions. We integrated data on gynecologic history with levels of an ovarian reserve marker (anti-müllerian hormone [AMH)] to interrogate reproductive aging patterns and associated factors among a subset of cisgender women with human immunodeficiency virus (WWH) enrolled in the REPRIEVE trial.
Methods
A total of 1449 WWH were classified as premenopausal (n = 482) (menses within 12 months; AMH level ≥20 pg/mL; group 1), premenopausal with reduced ovarian reserve (n = 224) (menses within 12 months; AMH <20 pg/mL; group 2), or postmenopausal (n = 743) (no menses within12 months; AMH <20 pg/mL; group 3). Proportional odds models, adjusted for chronologic age, were used to investigate associations of cardiometabolic and demographic parameters with reproductive aging milestones (AMH <20 pg/mL or >12 months of amenorrhea). Excluding WWH with surgical menopause, age at final menstrual period was summarized for postmenopausal WWH (group 3) and estimated among all WWH (groups 1–3) using an accelerated failure-time model.
Results
Cardiometabolic and demographic parameters associated with advanced reproductive age (controlling for chronologic age) included waist circumference (>88 vs ≤88 cm) (odds ratio [OR], 1.38; 95% confidence interval, 1.06–1.80; P = .02), hemoglobin (≥12 vs <12 g/dL) (2.32; 1.71–3.14; P < .01), and region of residence (sub-Saharan Africa [1.50; 1.07–2.11; P = .02] and Latin America and the Caribbean [1.59; 1.08–2.33; P = .02], as compared with World Health Organization Global Burden of Disease high-income regions). The median age (Q1, Q3) at the final menstrual period was 48 (45, 51) years when described among postmenopausal WWH, and either 49 (46, 52) or 50 (47, 53) years when estimated among all WWH, depending on censoring strategy.
Conclusions
Among WWH in the REPRIEVE trial, more advanced reproductive age is associated with metabolic dysregulation and region of residence. Additional research on age at menopause among WWH is needed.
Clinical Trials Registration
NCT0234429.
Keywords: HIV, Women, Sex, Reproductive Aging, Menopause, Cardiometabolic Risk
For women, reproductive aging may fuel risks of diverse cardiometabolic comorbid conditions traditionally associated with chronologic aging. Specifically, reproductive aging—characterized by depletion of primordial oocyte stores and, in turn, reduced endogenous estrogen production [1]—may predispose women to atherosclerotic cardiovascular disease (ASCVD), heart failure, dyslipidemia, glucose dysregulation, ectopic fat deposition, and bone fragility [2, 3]. General-population women’s studies exploring reproductive age-associated cardiometabolic risks typically focus on 2 key points: whether a woman is in menopause and when she reached this milestone. Overall, the earlier the menopausal transition, the higher a woman’s reproductive age–associated risk for comorbid conditions [3]. With respect to cardiometabolic risk, earlier menopause and/or reduced number of cycling years have been associated with increased risk for ischemic heart disease, stroke, and heart failure [4–6].
Accelerated reproductive aging has been postulated to contribute to heightened cardiometabolic risk among women with (vs without) human immunodeficiency virus (HIV) [7]. Nevertheless, studies have yielded conflicting results as to whether women with HIV (WWH) experience accelerated reproductive aging, including earlier age at menopause [8]. One potential explanation underlying conflicting findings relates to differing statistical methods used to describe or estimate age at menopause [9].
A second explanation underlying conflicting findings on age at menopause relates to the inherent challenge of categorizing WWH as premenopausal versus postmenopausal. World Health Organization (WHO) criteria define menopause as permanent cessation of menses, recognized after 12 months of consecutive, otherwise unexplained, amenorrhea [10]. Reference-standard “STRAW + 10” criteria define menopause analogously, while also considering levels of hypothalamic-pituitary-gonadal axis hormones (eg, follicle stimulating hormone and estradiol) [11, 12]. However, some WWH experience prolonged amenorrhea [13] followed by subsequent resumption of menses [14]. Indeed, among WWH, factors including severe concomitant illness, adherence with select psychotropic medications, and use of opioids may result in suppression of hypothalamic-pituitary hormones, provoking reversible menstrual irregularity [15].
Recent years have seen a surge in research on anti-müllerian hormone (AMH), a marker of ovarian reserve relevant to staging reproductive aging [16]. AMH is secreted by ovarian granulosa cells. Throughout a woman’s adult life, AMH levels progressively decline—in concert with oocyte stores—and drop to an undetectable level a few years before menopause [17, 18]. Levels of AMH, which tend to be consistent throughout the menstrual cycle [19], predict age at menopause, both in the general population [20–22] and among WWH [23].
Our research group previously described a classification scheme synthesizing menstrual history and AMH levels to categorize midlife WWH along a contiguous 3-group reproductive aging spectrum: premenopausal, premenopausal with reduced ovarian reserve, and postmenopausal [24]. We reasoned applying this system may help disentangle effects of reproductive versus chronologic aging on surrogates of cardiometabolic risk among WWH. We showed that in a small sample of asymptomatic WWH from the Northeast United States, more advanced reproductive age was associated with noncalcified coronary atherosclerotic plaque, even after controlling for traditional risk factors, including chronologic age. Furthermore, we demonstrated that measures of ASCVD risk increased across the reproductive aging spectrum, in advance of menopause [24].
Building on our previous work, we now apply an analogous system to explore the correlates and timing of reproductive aging transitions in a global cohort of midlife antiretroviral therapy (ART)–treated WWH enrolled in the REPRIEVE trial. As part of our REPRIEVE Women’s Objectives study, we analyzed baseline data from 1449 cisgender female REPRIEVE participants, investigating associations of cardiometabolic and demographic parameters with reproductive aging milestones (AMH <20 pg/mL or >12 months amenorrhea).
Furthermore, after excluding from our analysis cohort WWH with bilateral oophorectomy (with or without hysterectomy), we summarized age at final menstrual period (FMP) among those women deemed by our classification to be postmenopausal (group 3). We also estimated age at FMP among WWH across all 3 reproductive aging groups, using a time-to-events methods (ie, an accelerated failure-time model). Insights gleaned may have relevance for anticipating reproductive aging transitions in WWH and focusing cardiometabolic health preservation efforts on parameters related to more advanced reproductive age.
METHODS
Study Participants
The REPRIEVE trial (NCT02344290) enrolled 7770 individuals with HIV (31% natal female), aged 40–75 years, without prior cardiovascular disease (CVD) and with low-to-moderate traditional CVD risk. Each clinical research site obtained institutional review board/ethics committee approval, as well as any other applicable regulatory entity approvals. Participants were provided with study information, including a discussion of risks and benefits, and were asked to sign the approved declaration of informed consent. Associated publications include additional details about trial design, objectives, and methods [25, 26].
Our primary analysis on correlates of reproductive aging transitions centered on 1449 cisgender WWH (henceforth referred to as WWH) enrolled in the non–European Union (EU) component of REPRIEVE after integration of the REPRIEVE Women’s Objectives (protocol version 3, distributed to REPRIEVE sites on 2 February 2016). As noted in the article by Grinspoon et al, 213 European participants were recruited into a protocol parallel to the main REPRIEVE protocol (REPRIEVE-EU). REPRIEVE-EU participants’ data were not planned for in the original REPRIEVE Women’s Objectives proposal and were not included in this analysis.
Among women enrolled in the non-EU component of REPRIEVE after protocol version 3, participants were excluded from the analysis for parameters expected to confound classification in our reproductive scheme. Such parameters included (1) self-report of any of the following: history of hysterectomy without bilateral oophorectomy, history of uterine ablation, current use of a progesterone intrauterine device or implant, or current use of sex hormones or sex hormone blockers and/or (2) missing or invalid AMH levels (Supplementary Figure 1). For our secondary analysis on timing of (natural/nonsurgical) reproductive aging transitions, WWH who reported bilateral oophorectomy (with or without hysterectomy) were also excluded (n = 75).
Data Elements Assessed in REPRIEVE Trial Participants
See also in this Supplement the article by Grinspoon et al, which includes methods pertaining to collecting and describing data elements assessed among all REPRIEVE participants (including race, ethnicity, and region of enrollment. (inferred to represent region of residence, and grouped according to the WHO Global Burden of Disease super region scheme [27]). Of note, waist circumference >88 cm was classified as high in women, according to WHO guidelines [28]. Sex-specific thresholds for anemia—a potential cardiometabolic risk factor [29]—were defined according to WHO guidelines (hemoglobin <12 g/dL) [30]. Among cisgender women, gynecologic history focused on past gynecologic surgeries or procedures, current or past hormone use, and menstrual history, as elicited by investigator interview during the enrollment process.
Quantification of AMH Levels
AMH was evaluated from serum specimens obtained at the study entry visit, before study treatment initiation. Specimens were stored and frozen at −80°C and subsequently thawed and run in batches (BRAC Laboratory, Brigham and Women’s Hospital, Boston, Massachusetts). AMH levels were quantified by means of enzyme-linked immunosorbent assay (picoAMH assay; Ansh Labs; intra-assay variation, 2.3%–4.5%; interassay variation, 2.1%–3.7%).
Reproductive Aging Classification Scheme
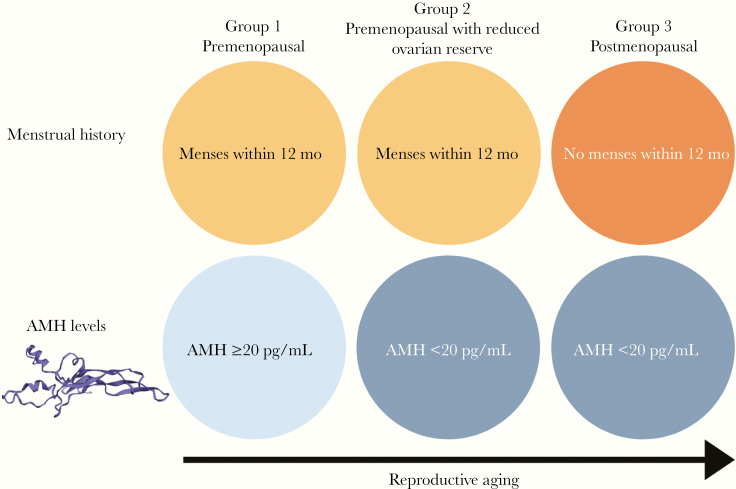
WWH were categorized along an ordinal 3-group reproductive aging spectrum, defined by time since last menstrual period (LMP) and by AMH level, as follows: premenopausal (menses within 12 months and AMH ≥20 pg/mL; group 1), premenopausal with reduced ovarian reserve (menses within 12 months and AMH <20 pg/mL; group 2), and postmenopausal (no menses within 12 months and AMH <20 pg/mL; group 3)) (Figure 1). Women with no menses within 12 months and with AMH levels ≥20 pg/mL were presumed to have prolonged amenorrhea in the absence of ovarian failure and were excluded. Women’s self-perception of menopause status was not incorporated into the aforementioned reproductive aging classification scheme. For this reproductive aging classification scheme, an AMH threshold of 20 pg/mL was set for consistency with our group’s previously published scheme [24].
Figure 1.
Reproductive aging classification scheme. Women with human immunodeficiency virus included in the present analysis were categorized along a contiguous 3-group reproductive aging spectrum defined by time since the last menstrual period and by anti-müllerian hormone (AMH) level: group 1, premenopausal (menses within 12 months and AMH ≥20 pg/mL); group 2, premenopausal with reduced ovarian reserve (menses within 12 months and AMH <20 pg/mL); and group 3, postmenopausal (no menses within 12 months and AMH <20 pg/mL).
Statistical Methods
Distributions of selected behavioral, traditional metabolic, and HIV-specific characteristics were summarized overall and by reproductive aging spectrum subgroups. Trends across the reproductive aging subgroups were assessed using Jonckheere-Terpstra and Kruskal-Wallis tests.
Baseline characteristics were assessed for association with more advanced reproductive age, controlling for chronologic age, using cumulative logit models. This type of ordinal response model jointly estimates covariate effects for the binary comparisons of having detectable versus undetectable AMH (ie, group 1 vs groups 2 and 3) and LMP within the last 12 months versus LMP >12 months earlier (ie, groups 1 and 2 vs group 3) (Figure 1). Nonhomogenous and homogeneous (proportional) effects were estimated and assessed for fit by score tests. Across all covariates, nonsignificance of these tests implied that a single common odds ratio (OR) was appropriate for both comparisons (ie, for each covariate, the OR for undetectable vs detectable AMH was the same as that for an LMP >12 months earlier vs an LMP within the last 12 months). For continuous covariates, linearity in the logit was assessed graphically using groups defined by quartiles.
When nonlinearity was observed, the model was refit using categories informed by both observed data and externally defined clinical categories. For parsimony, in cases in which neither model suggested an association, the continuous formulation of the covariate is reported. For covariates with >2 levels, pairwise comparison P values to the reference were provided when the overall P value was <.05. The OR estimates and respective 95% confidence intervals (CIs) summarize the direction and magnitude of associations between the characteristics and reproductive aging comparisons, controlling for chronologic age.
After WWH who reported bilateral oophorectomy (with or without hysterectomy) were excluded from the sample, age at FMP was assessed in 2 ways. First, age at LMP was summarized for the WWH deemed by our classification system to be postmenopausal (no menses within 12 months and AMH <20 pg/mL; group 3). This age was calculated from age at study entry and self-reported years since the last LMP. Because this group was deemed to be postmenopausal —based on synthesis of self-reported history and objective hormonal data—age at LMP was inferred to represent age at FMP in this group. Next, age at FMP among the group of WWH as a whole (ie, WWH in groups 1–3) was estimated using an accelerated failure-time model with Weibull distribution [31]. Among women with LMP within 12 months irrespective of AMH level (groups 1 and 2), age at FMP was censored at LMP. In a sensitivity analysis, age at FMP was censored only among women with detectable AMH (group 1), because the LMP may represent the FMP among some women with undetectable AMH. Age at menopause was inferred to be 1 year after age at FMP, consistent with standard definitions of menopause.
Inference was based on a nominal .05 significance level with no formal adjustment for multiple testing. All statistical analyses were performed using SAS software (for the UNIX platform), version 9.4.
RESULTS
Criteria for Inclusion in Analysis
A total of 2002 cisgender women enrolled in REPRIEVE at non-EU sites during the evaluation period for this investigation. Among this sample, 375 women were excluded for parameters preventing accurate classification in our reproductive aging scheme (see Methods). In addition, 170 women were excluded due to missing or invalid/uninterpretable AMH data, and 8 women were excluded based on LMP and AMH data suggesting prolonged amenorrhea in the absence of ovarian failure (no menses within 12 months and AMH ≥20 pg/mL). The final sample for the primary analysis on correlates of reproductive aging transitions included 1449 participants (Supplementary Figure 1). For the secondary analysis of timing of (natural/nonsurgical) reproductive aging transitions, WWH who reported bilateral oophorectomy (with or without hysterectomy) were also excluded (n = 75).
Baseline Characteristics of Analysis Cohort
The median age of participants was 49 years, and just over half (51%) met the criteria for group 3 of the reproductive aging spectrum (postmenopausal). Participants were from sub-Saharan Africa (35%), high-income regions (24%), Latin America and the Caribbean (23%), and South East/East/South Asia (18%). The predominant race was black or African American (63%), followed by Asian (18%), and white (13%). Thirteen percent of participants reported current cigarette smoking (15% former), and 13% reported current or former substance use (including methamphetamine, cocaine, or intravenous drugs). The median body mass index (calculated as weight in kilograms divided by height in meters squared) was 27.0, and 31% of participants had a body mass index ≥30. The median waist circumference was 93 cm, and 62% of participants had a waist circumference >88 cm. The median time since HIV diagnosis was 13 years, and the median duration of ART use was 9 years. The median CD4 cell count was 685/mm3, and 74% of participants had a CD4 cell count ≥500/mm3. HIV-1 RNA levels were below the assay lower limit of quantification in 89% of participants (Table 1).
Table 1.
Baseline Characteristics
| Reproductive Aging Spectrum | |||||
|---|---|---|---|---|---|
| Characteristicsa | Total(N = 1449) | Group 1(n = 482) | Group 2(n = 224) | Group 3(n = 743) | P Value |
| Demographic and Behavioral | |||||
| Age, median (Q1, Q3), y | 49 (45, 55) | 44 (41, 46) | 48 (46, 51) | 55 (51, 59) | <.001b |
| Age group, no. (%) | |||||
| 40 to <45 y | 354 (24) | 283 (59) | 43 (19) | 28 (4) | <.001b |
| 45 to <50 y | 383 (26) | 170 (35) | 106 (47) | 107 (14) | |
| ≥50 y | 712 (49) | 29 (6) | 75 (33) | 608 (82) | |
| Race, no. (%)c | |||||
| Black or African American | 914 (63) | 262 (54) | 137 (61) | 515 (69) | <.001d |
| Asian | 262 (18) | 135 (28) | 45 (20) | 82 (11) | |
| White | 185 (13) | 65 (13) | 21 (9) | 99 (13) | |
| Other | 88 (6) | 20 (4) | 21 (9) | 47 (6) | |
| Enrollment region, no (%)e | |||||
| High income | 346 (24) | 98 (20) | 55 (25) | 193 (26) | <.001d |
| Latin America and Caribbean | 330 (23) | 87 (18) | 53 (24) | 190 (26) | |
| Southeast, East, and South Asia | 261 (18) | 134 (28) | 45 (20) | 82 (11) | |
| Sub-Saharan Africa | 512 (35) | 163 (34) | 71 (32) | 278 (37) | |
| Smoking status, no. (%) | |||||
| Current | 195 (13) | 46 (10) | 29 (13) | 120 (16) | <.001b |
| Former | 214 (15) | 61 (13) | 31 (14) | 122 (16) | |
| Never | 1040 (72) | 375 (78) | 164 (73) | 501 (67) | |
| Substance use status, no. (%)f | |||||
| Current or former | 195 (13) | 38 (8) | 34 (15) | 123 (17) | <.001b |
| Never | 1254 (87) | 444 (92) | 190 (85) | 620 (83) | |
| Intravenous drug use status, no. (%) | |||||
| Former | 40 (3) | 9 (2) | 7 (3) | 24 (3) | .18b |
| Never | 1409 (97) | 473 (98) | 217 (97) | 719 (97) | |
| Cardiovascular and Metabolic | |||||
| History of hypertension, no. (%) | 406 (28) | 86 (18) | 64 (29) | 256 (34) | <.001d |
| History of diabetes, no. (%) | 6 (<0.5) | 1 (<0.5) | 0 (0) | 5 (1) | .17d |
| BMI, median (Q1, Q3)g | 27.0 (23.4, 31.3) | 26.4 (22.6, 31.0) | 28.0 (24.3, 32.5) | 27.1 (23.7, 31.3) | .14b |
| BMI category, no. (%)g | |||||
| <18.5 | 49 (3) | 19 (4) | 8 (4) | 22 (3) | .37b |
| 18.5–24.9 | 487 (34) | 177 (37) | 61 (27) | 249 (34) | |
| 25–29.9 | 459 (32) | 148 (31) | 73 (33) | 238 (32) | |
| 30–34.9 | 227 (16) | 56 (12) | 46 (21) | 125 (17) | |
| 35–39.9 | 152 (10) | 60 (12) | 22 (10) | 70 (9) | |
| ≥40 | 75 (5) | 22 (5) | 14 (6) | 39 (5) | |
| Waist circumference, median (Q1, Q3), cm | 93 (84, 103) | 90 (80, 100) | 93 (84, 103) | 94 (86, 104) | <.001b |
| Waist circumference category, no. (%) | |||||
| ≤88 cm | 546 (38) | 219 (46) | 80 (36) | 247 (33) | <.001b |
| >88 cm | 894 (62) | 261 (54) | 142 (64) | 491 (67) | |
| Laboratory values, median (Q1, Q3) | |||||
| Triglycerides, mg/dL | 103 (75, 144) | 98 (68, 134) | 100 (79, 149) | 107 (76, 151) | <.001b |
| Total cholesterol, mg/dL | 190 (167, 215) | 184 (162, 205) | 192 (170, 216) | 196 (172, 220) | <.001b |
| LDL-C, mg/dL | 112 (92, 132) | 107 (87, 127) | 109 (93, 133) | 115 (94, 136) | <.001b |
| HDL-C, mg/dL | 53 (44, 64) | 51 (43, 61) | 53 (45, 65) | 54 (45, 66) | .001b |
| eGFR (CKD-EPI), mL/min/1.73 mm2 | 104 (88, 117) | 108 (95, 124) | 105 (88, 119) | 100 (84, 112) | <.001b |
| Hemoglobin, g/dL | 13 (12, 14) | 13 (11, 14) | 13 (12, 14) | 13 (12, 14) | <.001b |
| Hemoglobin category, no. (%) | |||||
| <12 g/dL | 348 (24) | 175 (37) | 57 (25) | 116 (16) | <.001b |
| ≥12 g/dL | 1096 (76) | 304 (63) | 167 (75) | 625 (84) | |
| HIV-Related Health Status | |||||
| Time since HIV diagnosis, median (Q1, Q3), y | 13 (8, 18) | 12 (8, 17) | 12 (7, 17) | 13 (9, 18) | .055b |
| Nadir CD4 cell count, no. (%) | |||||
| <50 cells/mm3 | 217 (15) | 64 (13) | 35 (16) | 118 (16) | .03b |
| 50–199 cells/mm3 | 503 (35) | 152 (32) | 85 (38) | 266 (36) | |
| 200–349 cells/mm3 | 372 (26) | 131 (27) | 53 (24) | 188 (25) | |
| ≥350 cells/mm3 | 325 (22) | 126 (26) | 45 (20) | 154 (21) | |
| Unknown | 32 (2) | 9 (2) | 6 (3) | 17 (2) | |
| CD4 cell count, median (Q1, Q3), cells/mm3 | 685 (494, 905) | 646 (485, 873) | 689 (488, 894) | 702 (503, 924) | .01b |
| CD4 cell count, no. (%) | |||||
| <350 cells/mm3 | 143 (10) | 54 (11) | 26 (12) | 63 (8) | .29b |
| 350–499 cells/mm3 | 231 (16) | 75 (16) | 35 (16) | 121 (16) | |
| ≥500 cells/mm3 | 1075 (74) | 353 (73) | 163 (73) | 559 (75) | |
| HIV-1 RNA copies/mL, no. (%) | |||||
| below LLQ <20 | 338 (35) | 109 (34) | 52 (33) | 177 (36) | .72d |
| below LLQ <40 | 462 (47) | 157 (49) | 80 (51) | 225 (46) | |
| below LLQ <400 | 64 (7) | 20 (6) | 8 (5) | 36 (7) | |
| above LLQ | 109 (11) | 37 (11) | 17 (11) | 55 (11) | |
| Total ART duration, median (Q1, Q3), y | 9 (5, 14) | 9 (5, 13) | 9 (5, 14) | 10 (6, 14) | <.001b |
| ART regimen class, no. (%) | |||||
| NRTI + INSTI | 205 (14) | 51 (11) | 31 (14) | 123 (17) | .050d |
| NRTI + NNRTI | 890 (61) | 309 (64) | 134 (60) | 447 (60) | |
| NRTI + PI | 288 (20) | 103 (21) | 47 (21) | 138 (19) | |
| NRTI sparing | 24 (2) | 7 (1) | 4 (2) | 13 (2) | |
| Other NRTI containing | 42 (3) | 12 (2) | 8 (4) | 22 (3) | |
| Other Comorbidities | |||||
| History of depression, no. (%) | 271 (19) | 73 (15) | 37 (17) | 161 (22) | .003d |
| Chronic active HBV, no. (%) | 21 (1) | 8 (2) | 4 (2) | 9 (1) | .48d |
| Chronic active HCV, no. (%) | 17 (1) | 4 (1) | 5 (2) | 8 (1) | .88d |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol; LLQ, lower limit of quantification; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aAll statistics are calculated based on participants with data collected. Data were missing for waist circumference in 9 participants, for hemoglobin level in 5, for time since HIV diagnosis in 1, and for HIV-1 RNA levels below the LLQ in 476.
b P values based on Jonckheere-Terpstra test.
c“Other” race includes participants self-identifying as native or indigenous to the enrollment region, as >1 race (with no single race noted as predominant), or of unknown race.
d P values based on Kruskal-Wallis test.
eClassified according to Global Burden of Disease regions.
fSubstance use includes use of cocaine, methamphetamine, and intravenous drugs.
gBMI is calculated as weight in kilograms divided by height in meters squared.
Trends Across Groups Along the Reproductive Aging Spectrum
Several trends were observed across the successive reproductive aging groups (from premenopausal [group 1] to premenopausal with reduced ovarian reserve [group 2] to postmenopausal [group 3]) (Table 1). Clinically meaningful differences across the groups were apparent with respect to age, race, enrollment region, cigarette smoking status, substance use, history of hypertension, waist circumference, lipid levels, creatinine clearance, hemoglobin levels, duration of ART use, select immune parameters, and prevalence of select comorbid conditions. The magnitude of these associations is shown in Table 1. Because select trends were inferred to be influenced by increasing chronologic age across groups, modeling was next performed, controlling for this factor.
Factors Associated With More Advanced Reproductive Age in Age-Adjusted Models
Age-adjusted proportional odds models revealed associations between select cardiometabolic and demographic parameters and more advanced reproductive age. The proportionality assumption held for all models (score test P values > .05; data not shown), suggesting that all covariate effects were the same when comparing undetectable versus detectable AMH and LMP >12 months earlier versus LMP within the last 12 months. Consequently, only the results from the proportional odds models are presented in Table 2. Nonhomogenous effects are included in Supplementary Table 1. As expected, higher chronologic age was associated with more advanced reproductive age (OR, 1.49 per year older; 95% CI, 1.44–1.54; P < .01). Controlling for chronologic age, the following cardiometabolic parameters were associated with more advanced reproductive age: waist circumference (>88 vs ≤88 cm) (OR, 1.38; 95% CI, 1.06–1.80; P = .02) and hemoglobin (≥12 vs <12 g/dL) (2.32; 1.71–3.14; P < .01).
Table 2.
Chronologic Age–Adjusted Logistic Regression Models Assessing Relationships Between Covariates and More Advanced Reproductive Age
| Proportional Oddsa | |||
|---|---|---|---|
| Covariateb | OR (95% CI) | Pairwise P Valuec | Overall P Value |
| Age (per 1 y)d | 1.49 (1.44–1.54) | … | <.01 |
| Race | |||
| Asian vs White | 0.77 (.48–1.23) | … | 0.1 |
| Black or African American vs White | 1.10 (.73–1.65) | … | |
| Other vs White | 1.42 (.76–2.65) | … | |
| Enrollment region | |||
| Latin America and Caribbean vs High Income | 1.59 (1.08–2.33) | .02 | <.01 |
| Southeast, East, and South Asia vs High Income | 0.95 (.64–1.41) | .79 | |
| Sub-Saharan Africa vs High Income | 1.50 (1.07–2.11) | .02 | |
| Smoking status | |||
| Current vs never | 1.21 (.81–1.79) | … | .15 |
| Former vs never | 0.74 (.51–1.09) | … | |
| Intravenous drug use (former vs never) | 0.55 (.23–1.27) | … | .16 |
| BMIe | |||
| <18.5 vs 18.5–24.9 | 1.47 (.68–3.20) | … | .10 |
| 25–29.9 vs 18.5–24.9 | 1.01 (.73–1.38) | … | |
| 30–34.9 vs 18.5–24.9 | 1.54 (1.04–2.28) | … | |
| ≥35 vs 18.5–24.9 | 0.86 (.58–1.27) | … | |
| Waist circumference (>88 vs ≤88 cm) | 1.38 (1.06–1.80) | … | .02 |
| Total cholesterol (mg/dL) | |||
| 160–199 vs <160 | 0.99 (.69–1.42) | … | .14 |
| 200–239 vs <160 | 1.15 (.79–1.67) | … | |
| ≥240 vs <160 | 1.73 (1.01–2.95) | … | |
| HDL-C (per 10 mg/dL) | 1.06 (.98–1.14) | … | .16 |
| eGFR (CKD-EPI) (per 10 mL/min/1.73 mm2) | 1.06 (.99–1.13) | … | .11 |
| Hemoglobin (≥12 vs <12 g/dL) | 2.32 (1.71–3.14) | … | <.01 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; eGFR, estimated glomerular filtration rate; HDL-C, HDL-C, high-density lipoprotein cholesterol; OR, odds ratio.
aEstimates are from cumulative logistic regression models with proportional odds, ORs and CIs are shown. Under the proportional odds, the OR for women with undetectable versus detectable anti-müllerian hormone is the same as the OR for women with the last menstrual period >12 months earlier versus within the last 12 months. The proportional odds assumption was validated by the score test for all models. See the Statistical Methods section for more details.
bContinuous covariates were assessed for linearity and were presented categorically if the interpretation changed.
Only models with an overall P value < .2 are presented; the following covariates were considered and are not shown: substance use, history of hypertension, triglycerides, low-density lipoprotein cholesterol level, duration of human immunodeficiency virus infection (years), CD4 cell count, duration of ART exposure (years), ART regimen, depression medications, chronic active hepatitis B virus, and chronic active hepatitis C virus.
cFor covariates with >2 levels, pairwise P values for comparison to the reference were provided when the overall P value was <.05.
dEstimate from a univariate model.
eBMI is calculated as weight in kilograms divided by height in meters squared.
Select regions of enrollment were also associated with more advanced reproductive age. Women enrolled in sub-Saharan Africa or Latin America and the Caribbean had higher odds of more advanced reproductive age than women of the same chronologic age from high-income regions (OR [95% CI], 1.50 [1.07–2.11] for sub-Saharan Africa and 1.59 [1.08–2.33] for Latin America and the Caribbean; both P = .02) (Table 2). These regional relationships with more advanced reproductive age remained significant even after controlling for waist circumference, which differed by region (data not shown).
Age at FMP
Among postmenopausal women (group 3), the median (Q1, Q3) age at LMP (inferred to be FMP) was 48 (45, 51) years. For estimation of age at FMP in the group as a whole (censoring for age at FMP among WWH in groups 1 and 2), the median (Q1, Q3) of the predicted distribution of age at FMP was 50 (47, 53) years. In a sensitivity analysis that censored only WWH with detectable AMH (group 1), the predicted percentiles of age at FMP was 49 (46, 52) years.
DISCUSSION
Analysis of baseline data from a prospectively recruited, global cohort of midlife WWH enrolled in the REPRIEVE trial yielded the following insights regarding reproductive aging. First, among the full cohort of WWH, select cardiometabolic risk factors were associated with more advanced reproductive age, controlling for chronologic age. These included waist circumference >88 cm and hemoglobin levels ≥12 g/dL. Second, residence in sub-Saharan Africa or Latin America and the Caribbean (vs high-income regions) was associated with higher relative odds of more advanced reproductive age, while controlling for chronologic age. Finally, among postmenopausal WWH, the median age at FMP was 48 years, correlating with an age at menopause of 49 years. When estimated across the full group of WWH, the median predicted distribution of age at FMP was 49 or 50 years, depending on the censoring strategy applied, corresponding to an age at menopause of 50 or 51 years, respectively.
General-population studies have shown that staging reproductive aging by self-reported menstrual history alone may be less accurate than analogous staging achieved through a synthesis of self-reported menstrual history and hypothalamic-pituitary-gonadal axis hormones [32]. One strength of our analysis of factors related to reproductive aging milestones was that these milestones were defined not only by self-reported menstrual history but also by levels of AMH, a key biomarker of ovarian reserve. A second strength of our study includes the size and diversity of our cohort, as well as the standardized data collection methods across sites. Indeed, our study integrates menstrual history data and data on levels of AMH among nearly 1500 midlife WWH from across the globe to yield important, clinically relevant insights on correlates and timing of reproductive aging transitions.
In this group of WWH, cardiometabolic parameters, including high waist circumference >88 cm and hemoglobin level ≥12 g/dL, were associated with more advanced reproductive age, controlling for chronologic age. Previously published physiology studies among women in the general population have suggested relationships between reproductive aging and increased adiposity [33], as well as increased ectopic fat deposition (heart, viscera) [34, 35]. A small study has also highlighted an association between reproductive aging and ectopic fat deposition (heart) among WWH [36]. Intriguingly, a general-population study by Papadakis et al [37] revealed hormone replacement therapy decreased ectopic (visceral) fat volume among menopausal women, implying that reduced endogenous estrogen production may prompt ectopic fat deposition. By contrast, our finding that hemoglobin levels ≥12 g/dL are associated with advanced reproductive aging among WWH is unexpected, given that hemoglobin typically declines with age among women in the general population [38]. Theoretically, cessation of menses among WWH of more advanced reproductive age could be contributing to the association we observed. Additional work is needed to determine whether the relationships between reproductive aging milestones and cardiometabolic parameters observed in our study are causal, and, if so, the direction of causality.
Of note, our data on trends in cardiometabolic parameters observed across the successive reproductive aging groups suggest that select adverse metabolic changes (eg, those related to circulating lipid levels) occur across a continuum of reproductive aging. This finding is highly intuitive, given that hormonal changes among aging women also occur along a continuum, not abruptly after 12 months of amenorrhea. Moreover, this finding resonates with those of general-population studies showing that aging women analogously experience other endocrine/metabolic comorbid conditions (eg, accelerated bone loss) en route to menopause [39, 40]. It is worth noting, however, that in modeling controlling for chronologic age, no significant associations between lipid levels and more advanced reproductive age were observed. Nevertheless, taken together, findings from our study and others suggest that clinicians should engage midlife WWH in cardiometabolic risk assessment and cardiometabolic risk prevention before these women are determined to be postmenopausal.
Among this group of WWH, residence in sub-Saharan Africa or Latin America and the Caribbean (vs residence in high-income regions) was associated with higher odds of more advanced reproductive age, controlling for chronologic age. Of note, general-population studies have also highlighted apparent regional differences in age at menopause [41, 42]. Our finding that WWH in sub-Saharan Africa or Latin America and the Caribbean (vs WWH in high-income regions) have higher odds of more advanced reproductive age has broad implications, given that the majority of WWH globally reside in these regions. Further research is required to delineate the interplay of genetic and environmental factors potentially underlying this observation.
As noted above, the median age at FMP in our study (and thus inferences on age at menopause) differed depending on the group sampled and the statistical methods used. As reflected in a meta-analysis by Imai et al [8], previous studies aiming to characterize age at menopause among WWH have yielded conflicting results. Likely explanations for inconsistent findings include (1) differences in sampling and analytic methods, (2) singular reliance on self-reported menstrual history in the absence of relevant hormonal data, (3) differences in definitions of menopause, and (4) absence of thorough investigation for parameters that may predispose to prolonged amenorrhea in the absence of menopause. In contrast to the controversy on age at menopause among WWH, there seems to be consensus across studies on the point that women with HIV have lower age-adjusted levels of AMH than those without HIV [43–45], reflective of advanced reproductive aging. Harmonization of strategies used across studies to characterize age at menopause among WWH would be valuable.
The extent to which our findings can be generalized to WWH globally remains unclear. Our findings were derived from ART-treated WWH with low-to-moderate traditional CVD risk engaged in care and enrolled in a large-scale CVD prevention trial (REPRIEVE). That is, our studied population may be expected to be healthier than the global population of midlife WWH. Also of note, the cross-sectional design of our investigation precludes definitive inferences as to whether observed associations (eg, between advanced reproductive age and select parameters) are causal and limits the ability to assess the directionality of observed associations. And finally, our approaches to estimating age at menopause among WWH are limited, in part, by participant self-report of age at LMP as well as the relatively young age of the cohort (with only 51% postmenopausal). Continued follow-up of menstrual patterns in this cohort would allow for a more precise estimation of age at menopause in future analyses.
Our current work illustrates that, among midlife WWH, waist circumference >88 cm and hemoglobin level ≥12 g/dL are associated with more advanced reproductive age after controlling for chronologic age. Furthermore, in our study, WWH from sub-Saharan Africa and/or Latin America and the Caribbean demonstrated higher odds for more advanced reproductive age, compared with WWH from high-income regions. Finally, our estimates of median age at menopause varied depending on sampling and the analytic approaches used. Overall, our findings may help clinicians anticipate the menopausal transition in WWH and promote early engagement in efforts to characterize and mitigate cardiometabolic risk and other reproductive-age associated risks. In addition, through this investigation, we extend the application of a reproductive aging staging scheme potentially relevant to future studies focused on endocrine or cardiometabolic risks among midlife women. Our baseline characterization of reproductive aging among cisgender women in the REPRIEVE trial will enable future assessment of whether reproductive age influences the risk and timing of major adverse cardiovascular events, controlling for chronologic age.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. The study investigators would like to thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. We would also like to thank the AIDS Clinical Trial Group for clinical site support, the AIDS Clinical Trial Group Clinical Trials Specialists for regulatory support, the data management center, Frontier Science Foundation, for data support, and the Center for Biostatistics in AIDS Research for statistical support. Finally, we would like to acknowledge Steven Whitford and Thomas Ricker at the Brigham Research Assay Core, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Disclaimer. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), or the US Department of Health and Human Services.
Financial support. This work was supported by the NHLBI (grants U01 HL123336 and U01 HL123339), the NIAID (grants UM1 AI068636, UM1 AI106701, and R01 AI 123001 to M. V. Z. and S. E. L.).
Supplement sponsorship. This supplement is sponsored by Massachusetts General Hospital, through NIH funding including U01HL123336.
Potential conflicts of interest. M. V. Z. reports grant support through her institution from Gilead Sciences, for the conduct of the study. J. S. C. reports personal fees from Merck, outside the submitted work. A. K. reports grants from NIAID/NIH and NHLBI/NIH, during the conduct of the study. L. S. reports grants from NIAID/NIH, during the conduct of the study. J. T. reports funding from the German Research Foundation (Deutsche Forschungsmeinschaft [grant TA 1438/1-1]), outside the submitted work. T. H. B. reports equity of less than $10 000 from Excision Bio Therapeutics, outside the submitted work. M. T. L. reports grant support through his institution from Kowa Pharmaceuticals America, for the conduct of the study, and grants from AstraZeneca, outside the submitted work. K. V. F. reports an educational grant from Gilead Sciences, outside the submitted work. U. H. reports grant support through his institution from Kowa Pharmaceuticals America, for the conduct of the study, and grants from MedImmune/AstraZeneca, HeartFlow, and Duke University (Abbott) and other support from Duke University (through the NIH) and Recor Medical, outside the submitted work. S. K. G. reports grant support through his institution from Kowa Pharmaceuticals America and Gilead Sciences, for the conduct of the study, and from Theratechnologies, Navidea, and ViiV, outside the submitted work. P. S. D. reports grant support from Kowa Pharmaceuticals America and Gilead Sciences, for the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 2011; 38:455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009; 30:465–93. [DOI] [PubMed] [Google Scholar]
- 3. Chae CU, Derby CA. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am 2011; 38:477–88. [DOI] [PubMed] [Google Scholar]
- 4. Jung KJ, Kim MR, Yun YD, Kim HC, Jee SH. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: the Korean Heart Study. Menopause 2016; 23:60–6. [DOI] [PubMed] [Google Scholar]
- 5. de Kat AC, Verschuren WM, Eijkemans MJ, Broekmans FJ, van der Schouw YT. Anti-Müllerian hormone trajectories are associated with cardiovascular disease in women: results from the Doetinchem cohort study. Circulation 2017; 135:556–65. [DOI] [PubMed] [Google Scholar]
- 6. Ebong IA, Watson KE, Goff DC Jr, et al. Age at menopause and incident heart failure: the Multi-Ethnic Study of Atherosclerosis. Menopause 2014; 21:585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS 2017; 12:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imai K, Sutton MY, Mdodo R, Del Rio C. HIV and menopause: a systematic review of the effects of HIV infection on age at menopause and the effects of menopause on response to antiretroviral therapy. Obstet Gynecol Int 2013; 2013:340309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011; 38:425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Research on the menopause in the 1990s: report of a WHO Scientific Group. World Health Organ Tech Rep Ser 1996; 866:12–14. [PubMed] [Google Scholar]
- 11. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause 2001; 8:402–7. [DOI] [PubMed] [Google Scholar]
- 12. Harlow SD, Gass M, Hall JE, et al. ; STRAW + 10 Collaborative Group Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cejtin HE, Kalinowski A, Bacchetti P, et al. Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol 2006; 108:1423–31. [DOI] [PubMed] [Google Scholar]
- 14. Cejtin HE, Evans CT, Greenblatt R, et al. Prolonged amenorrhea and resumption of menses in women with HIV. J Womens Health (Larchmt) 2018; 27:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS 2019; 33:483–91. [DOI] [PubMed] [Google Scholar]
- 16. Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008; 93:3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 2011; 95:170–5. [DOI] [PubMed] [Google Scholar]
- 18. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014; 20:370–85. [DOI] [PubMed] [Google Scholar]
- 19. Seifer DB, Golub ET, Lambert-Messerlian G, et al. Biologic markers of ovarian reserve and reproductive aging: application in a cohort study of HIV infection in women. Fertil Steril 2007; 88:1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Disseldorp J, Faddy MJ, Themmen AP, et al. Relationship of serum antimüllerian hormone concentration to age at menopause. J Clin Endocrinol Metab 2008; 93:2129–34. [DOI] [PubMed] [Google Scholar]
- 21. Broer SL, Eijkemans MJ, Scheffer GJ, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 2011; 96:2532–9. [DOI] [PubMed] [Google Scholar]
- 22. Nair S, Slaughter JC, Terry JG, et al. Anti-mullerian hormone (AMH) is associated with natural menopause in a population-based sample: the CARDIA Women’s Study. Maturitas 2015; 81:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scherzer R, Greenblatt RM, Merhi ZO, et al. Use of antimüllerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol 2017; 216:46.e1–46.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS 2016; 30:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grinspoon S, Douglas PS, Hoffmann U, Ribaudo H. Leveraging a landmark trial of primary CVD prevention in HIV. J Infect Dis 2020; 222(Suppl 1):S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2018; 392:1684–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Waist circumference and waist-hip ratio: a report of a WHO expert consultation 2008. [Google Scholar]
- 29. Kaiafa G, Kanellos I, Savopoulos C, Kakaletsis N, Giannakoulas G, Hatzitolios AI. Is anemia a new cardiovascular risk factor? Int J Cardiol 2015; 186:117–24. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. Hemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011. [Google Scholar]
- 31. Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab 2013; 98:729–35. [DOI] [PubMed] [Google Scholar]
- 32. Johnston JM, Colvin A, Johnson BD, et al. Comparison of SWAN and WISE menopausal status classification algorithms. J Womens Health (Larchmt) 2006; 15:1184–94. [DOI] [PubMed] [Google Scholar]
- 33. Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019; 4:e124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Khoudary SR, Shields KJ, Janssen I, et al. Cardiovascular fat, menopause, and sex hormones in women: the SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab 2015; 100:3304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab 2007; 92:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toribio M, Neilan TG, Awadalla M, et al. Intramyocardial triglycerides among women with vs without HIV: hormonal correlates and functional consequences. J Clin Endocrinol Metab 2019; 104:6090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papadakis GE, Hans D, Gonzalez Rodriguez E, et al. Menopausal hormone therapy is associated with reduced total and visceral adiposity: the OsteoLaus cohort. J Clin Endocrinol Metab 2018; 103:1948–57. [DOI] [PubMed] [Google Scholar]
- 38. Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol 2015; 52:261–9. [DOI] [PubMed] [Google Scholar]
- 39. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008; 93:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greendale GA, Huang M, Cauley JA, et al. Trabecular bone score declines during the menopause transition: the Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab 2020; 105:e1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 2010; 13:419–28. [DOI] [PubMed] [Google Scholar]
- 42. Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014; 43:1542–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scherzer R, Bacchetti P, Messerlian G, et al. Impact of CD4+ lymphocytes and HIV infection on Anti-Müllerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol 2015; 73:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wessman M, Korsholm AS, Bentzen JG, et al. Anti-müllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case-control study from Copenhagen, Denmark. J Virus Erad 2018; 4:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santulli P, de Villardi D, Gayet V, et al. Decreased ovarian reserve in HIV-infected women. AIDS 2016; 30:1083–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.