Abstract
Background
Chronic kidney disease is a common comorbid condition among persons living with human immunodeficiency virus (PWH). We characterized baseline kidney function in the REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) trial cohort.
Methods
REPRIEVE enrolled PWH with low to moderate cardiovascular risk based on traditional risk factors to evaluate the effect of statin therapy on cardiovascular events. We determined baseline estimated glomerular filtration rate (eGFR) with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), Modification of Diet in Renal Disease, and Cockcroft-Gault equations, and we evaluated baseline factors associated with eGFR <90 mL/min/1.73 m2 by logistic regression. We performed Bland-Altman plots and scatterplots to assess agreement between equations.
Results
Among 7770 participants enrolled, the median age was 50 years, 31% were female (natal sex), 43% black or African American and 15% Asian, the median body mass index (calculated as calculated as weight in kilograms divided by height in meters squared) was 25.8, and the median CD4 cell count 620/µL. The median CKD-EPI eGFR was 97 mL/min/1.73 m2, and 38% had an eGFR <90 mL/min/1.73 m2. In the adjusted model, factors associated with eGFR <90 mL/min/1.73 m2 included white race, older age, higher body mass index, high-income region of enrollment, hypertension, and tenofovir disoproxil fumarate. The CKD-EPI and Modification of Diet in Renal Disease equations demonstrated strong agreement, particularly at lower eGFR values. Overall, there was 56% concordance between the 3 equations (categories <60, 60 to <90, ≥90 mL/min), improving to 73% after accounting for individual body surface area.
Conclusions
REPRIEVE enrolled a diverse cohort including a substantial number of PWH with reduced kidney function. Factors associated with reduced eGFR included traditional risk factors and tenofovir disoproxil fumarate exposure. Three commonly used equations have only fair agreement, with potential implications for both clinical care and epidemiologic studies.
Clinical Trials Registration
Keywords: HIV, chronic kidney disease, kidney function, creatinine
With advances in human immunodeficiency virus (HIV) therapy, the incidence of HIV-associated nephropathy and subsequent end-stage renal disease have declined substantially [1–3]. Still, 20%–30% of persons living with HIV (PWH) are estimated to have chronic kidney disease (CKD) that is stage 1 or higher [4–6], and HIV infection remains an established risk factor for CKD, even after adjustment for traditional risk factors [7]. Black race, diabetes mellitus and hypertension are common among PWH and associated with an increased risk for CKD [8, 9]. In fact, the effect of HIV may be additive to traditional risk factors [10].
The increased risk of CKD among PWH may be mediated in part by HIV-associated inflammation [11]. Identifying adjunctive therapies that target inflammatory pathways has the potential to prevent CKD in this population. Data from randomized controlled trials suggest that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (ie, statins) have a beneficial effect on the kidney among risk groups in the general population, including patients with diabetes mellitus, hypertension, and preexisting atherosclerotic cardiovascular disease (ASCVD) [12–14]. That the kidney protective effect of statins is thought to be mediated in part through anti-inflammatory pathways is highly relevant in the setting of HIV infection [15, 16]. The REPRIEVE trial (Randomized Trial to Prevent Vascular Events in HIV; NCT02344290) is an ongoing randomized controlled trial evaluating whether pitavastatin calcium (referred to as pitavastatin hereafter) will prevent major adverse cardiovascular events in PWH with low to moderate traditional risk for cardiovascular disease. The design of the REPRIEVE trial has been described elsewhere [17]. An ancillary study embedded within the REPRIEVE trial is focused on whether pitavastatin slows or prevents the decline in kidney function among the cohort.
The primary objective of the current analysis was to describe the baseline kidney function in the REPRIEVE population. Secondary objectives were to evaluate differences across race and region of enrollment in estimated glomerular filtration rate (eGFR) by 3 eGFR estimation equations used to screen for trial eligibility, evaluate the agreement of the 3 equations, and investigate factors associated with reduced kidney function.
METHODS
Study Participants
REPRIEVE enrolled 7770 participants between March 2015 and July 2019 at >100 sites globally [18]. Inclusion criteria included PWH between 40 and 75 years of age, receiving antiretroviral therapy (ART) with CD4 cell counts >100/ µ L, low to moderate ASCVD risk according to the American College of Cardiology/American Hospital Association risk calculator, and preserved kidney function (eGFR ≥60 mL/min/1.73 m2 or creatinine clearance [CrCl] ≥60 mL/min). Key exclusion criteria included clinical ASCVD, diabetes with low-density lipoprotein cholesterol levels >70 mg/dL, decompensated cirrhosis, active cancer within 12 months, and ongoing statin use. The full inclusion and exclusion criteria and others details of the REPRIEVE design are available elsewhere [17]. A history of CKD was defined based on the medical history of participants at the time of enrollment. The rationale for including only persons with preserved kidney function was related to recommendations regarding pitavastatin dosing in persons with significantly reduced kidney function.
Ethics Statement
The REPRIEVE kidney ancillary study is fully embedded in the REPRIEVE trial. Each clinical research site obtained institutional review board/ethics committee approval and any other applicable regulatory entity approvals. Participants were provided with study information, including discussion of risks and benefits, and were asked to sign the approved declaration of informed consent.
Measures of Kidney Function
REPRIEVE allowed sites to use 3 commonly used creatinine-based equations to assess kidney function for trial eligibility: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR, Modification of Diet in Renal Disease (MDRD) eGFR, and Cockcroft-Gault CrCl [19–21]. All 3 equations were developed in North America and were designed with the aim of estimating glomerular filtration rate (GFR) levels <90 mL/min/1.73 m2 or mL/min (see Supplementary Table 1 or the equations). These creatinine-based equations were developed to identify and stage CKD and appropriately adjust the dose of medications that are cleared by the kidney. The Cockcroft-Gault equation was derived from 60 hospitalized white men with normal body weight to estimate CrCl as a surrogate for GFR [19]. The equation has no adjustment for body surface area (BSA), has not been updated to reflect the newer techniques used to measure creatinine, and tends to overestimate kidney function [22]. The MDRD equation was developed in a cohort of 1628 predominantly white individuals with CKD and has replaced the Cockcroft-Gault equation for most clinical laboratory reporting [20]. The MDRD equation performs well for persons with CKD but poorly among persons with GFR >90 mL/min/1.73 m2 [23]. Neither of these equations seem to perform well for persons outside of North America, and they particularly overestimate kidney function for persons of Asian descent [24, 25].
Based on the limitations of these 2 equations, the CKD-EPI equation was developed specifically to estimate kidney function more accurately in persons with GFR >60 mL/min/1.73 m2 [21]. Data from 26 studies were used to develop and validate the equation and included persons with normal kidney function. The CKD-EPI equation performs as well as the MDRD equation at GFRs <60 mL/min/1.73 m2 and moderately better in persons with normal GFR [26]. However, precision of the 2 equations is not significantly different [27]. In this analysis, we evaluated eGFR by each of the 3 equations as continuous and categorized according to clinically meaningful cutoffs (<60, 60 to <90, and ≥90 mL/min/1.73 m2 or mL/min) based on CKD classification [28]. Evaluation of factors associated with reduced kidney function (eGFR <90 mL/min/1.73 m2) used the CKD-EPI equation, which has been shown to yield more accurate estimates of kidney function in the general population and is the recommended equation for use in PWH [29, 30]. For description of additional methods, see the Supplementary Methods section in this supplement [18].
Statistical Analysis
Distributions of eGFR by CKD-EPI, eGFR by MDRD, and Cockcroft-Gault CrCl were evaluated by race and region, categorized using the Global Burden of Disease (GBD) “super-regions” that group countries based on epidemiologic similarity and geographic proximity [31]. The concordance between the 3 equations was evaluated over the full distribution, using Bland-Altman plots and scatterplots and clinically meaningful cutoff points (<60, 60 to <90, and ≥90 mL/min). For the Bland-Altman plots and scatterplots, we grouped the participants into 2 groups: persons from high-income regions and those from other regions. For comparison of the 3 estimates, an adjustment for individual BSA (calculated using the DuBois and DuBois formula) was made to eGFR by the MDRD and CKD-EPI equations by multiplying the eGFR by BSA/1.73 m2 to provide consistent units across the 3 equations.
Baseline characteristics were individually assessed for their association with eGFR <90 mL/min/1.73 m2 by the CKD-EPI equation using logistic regression models that adjusted for natal sex and race. Characteristics with a very low type 1 error probability (P < .001) and clinically meaningful estimated effect sizes were chosen for inclusion in the fully adjusted model. Odds ratios and associated 95% confidence intervals summarize the direction and magnitude of associations between the characteristics and eGFR <90 mL/min/1.73 m2 for each model. Given the large sample size, inference is based on a nominal .001 significance level and clinically meaningful effect sizes. The analysis was conducted using SAS software, version 9.4 for UNIX (SAS Institute).
RESULTS
Baseline Characteristics
Among the 7770 participants enrolled, the median age was 50 years, 69% were male, 43% were black or African American, 35% were white, and the median body mass index (BMI; calculated as calculated as weight in kilograms divided by height in meters squared) was 25.8. In North America (Canada, United States, and Puerto Rico), 18% were of Hispanic/Latino ethnicity. The prevalences of relevant comorbid conditions included 23% for hypertension, 5% for chronic active viral hepatitis B or C, 1% for diabetes mellitus, and <0.5% for history of CKD. The median baseline CD4 cell count was 620/ µ L, with 49% having a nadir <200/ µ L. Of the persons with HIV viral load data available, 98% had HIV plasma viral loads <400 copies/mL. The median total ART exposure was 10 years. and 85% had prior tenofovir disoproxil fumarate (TDF) exposure with a median duration of 5.5 years. Common ART combinations included nonnucleoside reverse-transcriptase inhibitors plus nucleoside reverse-transcriptase inhibitors (NRTIs) (47%), integrase strand transfer inhibitor plus NRTIs (25%), and protease inhibitors plus NRTIs (19%) [32]. The baseline characteristics of the population are provided in Table 1.
Table 1.
Baseline Characteristics Overall and by Categories of Estimated Kidney Function Based on Chronic Kidney Disease Epidemiology Collaboration Equation
| eGFR Based on CKD-EPI, mL/min per 1.73 mm2 | ||||
|---|---|---|---|---|
| Characteristicsa | Total (N = 7770) | <60 (n = 202) | 60 to <90 (n = 2738) | ≥90 (n = 4826) |
| Demographic and Behavioral | ||||
| Age, median (Q1, Q3), y | 50 (45–55) | 54 (49–58) | 52 (47–56) | 49 (44–54) |
| Natal sex, no. (%) | ||||
| Male | 5352 (69) | 148 (73) | 2072 (76) | 3130 (65) |
| Female | 2418 (31) | 54 (27) | 666 (24) | 1696 (35) |
| Race, no. (%)b | ||||
| Black or African American | 3378 (43) | 58 (29) | 923 (34) | 2395 (50) |
| White | 2701 (35) | 121 (60) | 1364 (50) | 1214 (25) |
| Asian | 1139 (15) | 6 (3) | 273 (10) | 860 (18) |
| Other | 552 (7) | 17 (8) | 178 (7) | 357 (7) |
| Ethnicity, no. (%)c | ||||
| Hispanic or Latino | 698 (18) | 24 (13) | 309 (16) | 365 (20) |
| Not Hispanic or Latino | 3187 (81) | 154 (86) | 1600 (83) | 1429 (79) |
| Unknown | 34 (1) | 2 (1) | 21 (1) | 11 (1) |
| Enrollment region, no. (%) | ||||
| High income | 4096 (53) | 183 (91) | 2025 (74) | 1884 (39) |
| Other regionsd | 3674 (47) | 19 (9) | 713 (26) | 2942 (61) |
| Smoking status, no. (%) | ||||
| Current | 1933 (25) | 41 (20) | 664 (24) | 1227 (25) |
| Former | 1906 (25) | 72 (36) | 790 (29) | 1043 (22) |
| Never | 3923 (51) | 89 (44) | 1281 (47) | 2552 (53) |
| Cardiovascular and Metabolic | ||||
| History of hypertension, no. (%) | 1785 (23) | 88 (44) | 705 (26) | 991 (21) |
| History of diabetes, no. (%) | 66 (1) | 8 (4) | 19 (1) | 39 (1) |
| Use of antiplatelet therapy, no. (%)e | 275 (4) | 14 (7) | 138 (5) | 123 (3) |
| Weight, median (Q1, Q3), kg | 75 (64–87) | 87 (78–97) | 79 (69–90) | 72 (61–84) |
| BMI, median (Q1, Q3)f | 25.8 (22.8–29.4) | 28.7 (25.3–31.6) | 26.3 (23.7–29.8) | 25.2 (22.1–29.0) |
| BMI, no. (%)f | ||||
| <18.5 | 288 (4) | 1 (<0.5) | 40 (1) | 247 (5) |
| 18.5–24.9 | 3115 (40) | 45 (22) | 986 (36) | 2083 (43) |
| 25–29.9 | 2664 (34) | 80 (40) | 1054 (39) | 1528 (32) |
| ≥30 | 1696 (22) | 75 (37) | 657 (24) | 963 (20) |
| BSA, median (Q1, Q3), m2 | 1.9 (1.7–2.0) | 2.0 (1.9–2.1) | 1.9 (1.8–2.1) | 1.8 (1.7–2.0) |
| BP, median (Q1, Q3) mm Hg | ||||
| Systolic | 122 (113–132) | 125 (116–133) | 122 (114–132) | 122 (112–132) |
| Diastolic | 80 (72–85) | 80 (74–85) | 80 (72–85) | 80 (71–86) |
| HIV-Related Health Status | ||||
| Nadir CD4 count | ||||
| <200/ µ L | 3792 (49) | 96 (48) | 1348 (49) | 2347 (49) |
| ≥200/ µ L | 3716 (48) | 95 (47) | 1279 (47) | 2340 (48) |
| Unknown | 262 (3) | 11 (5) | 111 (4) | 139 (3) |
| CD4 cell count, median (Q1, Q3), cells/ µ L)g | 620 (447–826) | 621 (448–855) | 619 (444–840) | 621 (449–816) |
| HIV-1 RNA, copies/mL | ||||
| <400 | 5865 (98) | 189 (99) | 2401 (98) | 3272 (97) |
| ≥400 | 133 (2) | 2 (1) | 42 (2) | 89 (3) |
| Total ART duration, median (Q1, Q3), y | 10 (5–15) | 11 (6–18) | 10 (6–16) | 9 (5–13) |
| Tenofovir exposure duration, median (Q1, Q3), y | 5.5 (3.0–8.7) | 6.1 (2.9–10.0) | 6.0 (3.0–9.3) | 5.1 (2.9–8.1) |
| Tenofovir exposure duration, no. (%) | ||||
| 0 y | 1185 (15) | 22 (11) | 295 (11) | 867 (18) |
| <5 y | 2836 (37) | 70 (35) | 933 (34) | 1831 (38) |
| 5 to <10 y | 2609 (34) | 63 (31) | 976 (36) | 1569 (33) |
| ≥10 y | 1127 (15) | 46 (23) | 526 (19) | 555 (12) |
| ART regimen class, no. (%) | ||||
| NRTI + INSTI | 1978 (25) | 111 (55) | 1021 (37) | 842 (17) |
| NRTI + NNRTI | 3676 (47) | 29 (14) | 833 (30) | 2814 (58) |
| NRTI + PI | 1439 (19) | 23 (11) | 537 (20) | 879 (18) |
| NRTI sparing | 199 (3) | 10 (5) | 110 (4) | 79 (2) |
| Other NRTI containing | 476 (6) | 29 (14) | 235 (9) | 212 (4) |
| Other Comorbidities | ||||
| History of kidney disease, no. (%) | 29 (<0.5) | 8 (4) | 17 (1) | 4 (<0.5) |
| Chronic viral hepatitis, no. (%) | 361 (5) | 15 (7) | 161 (6) | 185 (4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure; BSA, body surface area; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aAll statistics are calculated based on participants with data collected. Statistical comparisons between eGFR groupings are not made because the cohort size provides very high power to detect very small differences of no clinical relevance. Four participants missing eGFR by CKD-EPI are included in the overall total. Missing data include smoking status (n = 8), weight (n = 4), BMI (n = 7), CD4 cell count (n = 22), HIV-1 RNA level (n = 1772), total ART use (n = 2), ART regimen class (n=2), and medical history (n = 6). ART regimen class (n=2).
b“Other” race includes self-identification as native or indigenous to the enrollment region, >1 race (with no single race noted as predominant), or unknown race.
cEthnicity presented per National Institutes of Health definition for participants in United States (including Puerto Rico) and Canada only.
dIncludes Latin American and Caribbean, South East and East Asia, South Asia, and sub-Saharan Africa. Statistical comparisons between eGFR groupings are not made because the cohort size provides very high power to detect very small differences of no clinical relevance.
eIncludes aspirin.
fBMI was calculated as weight in kilograms divided by height in meters squared.
gRecorded at entry.
Distribution of Kidney Function
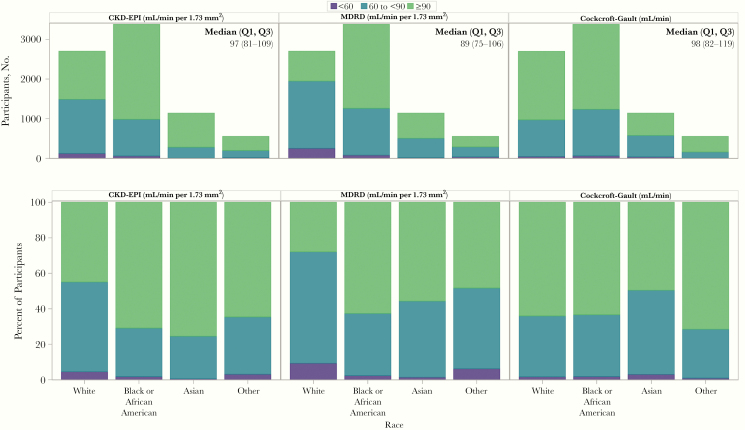
Among 7766 participants with complete data to estimate CKD-EPI eGFR, the distribution of kidney function using the 3 equations by race is provided in Figure 1. Overall, the median CKD-EPI eGFR was 97 mL/min/1.73 m2, and 38% had an eGFR <90 mL/min/1.73 m2. The eGFR tended to be lower in high-income regions than other GBD regions, with 53% of persons from high-income regions having an eGFR <90 mL/min/1.73 m2, versus ≤26% in other GBD regions (Supplementary Table 2), with median eGFRs of 88 (Q1, Q3: 75–101) and 106 (93–118) mL/min/1.73 m2, respectively (Figure 2A). Notably, weight, BMI, and BSA were all higher for participants from high-income regions than for those from other GBD regions (Supplementary Table 2). Similar differences in kidney function were evident when comparing by race, with median CKD-EPI eGFRs of 87, 104, and 102 mL/min/1.73 m2 for white, black or African American, and Asian participants, respectively. Overall 55% of white participants, compared with 29% of black or African American and 25% of Asian participants, had an eGFR <90 mL/min/1.73 m2. Eighteen participants (0.2%) were enrolled into the trial despite having estimated kidney function <60 mL/min by all 3 equations (Table 2).
Figure 1.
Distribution of estimated kidney function by the 3 equations by racial groups. The distribution of estimated kidney function by racial groups (white [n = 2701], black or African American [n = 3378], Asian [n = 1139], or other [n = 552]). The upper panels present the absolute numbers of persons within each category (<60, 60 to <90, or ≥90 mL/min/1.73 m2 or mL/min) for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), Modification of Diet in Renal Disease (MDRD), or Cockcroft-Gault equations. The median (Q1, Q3) CKD-EPI estimated glomerular filtration rate (eGFR) was 97 (81–109) mL/min/1.73 m2; the median MDRD eGFR, 89 (75–106) mL/min/1.73 m2; and the median Cockcroft-Gault creatinine clearance (CrCl), 98 (82–119) mL/min. The lower panels present the data as percentages of participants within each racial group.
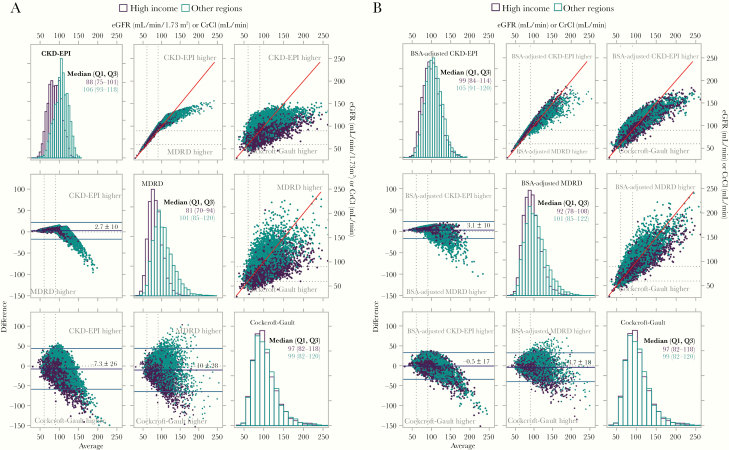
Figure 2.
Histograms, Bland-Altman plots and scatterplots for 3 kidney function equations by Global Burden of Disease (GBD) super-regions (high-income versus other regions). A, Data presented without body surface area (BSA) adjustment for Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) equations. Estimated glomerular filtration rate (eGFR) distributions by each equation are presented in histograms on the diagonal. Data from high-income regions is represented by purple, and data from other GBD regions in teal. Panel 1 (top row left) for CKD-EPI: median eGFR, 88 and 106 mL/min/1.73 m2 for high-income and other GBD regions, respectively. Panel 5 (middle row center) for MDRD: median eGFR 81 and 101 mL/min/1.73 m2 for high-income and other GBD regions, respectively. Panel 9 (bottom row right) for Cockcroft-Gault: median creatinine clearance (CrCl) 97 and 99 mL/min for high-income and other GBD regions, respectively. Pairwise Bland-Altman plots below the diagonal present plots of the average of the 2 equations on the x-axis versus the difference of the 2 equations on the y-axis. Panel 4 (middle row left) presents MDRD versus CKD-EPI equation with the CKD-EPI yielding an overall average 2.7 units higher, with notably higher values for the MDRD equation for persons with eGFR > 120 mL/min/1.73 m2. Panel 7 (bottom row left) presents Cockcroft-Gault versus CKD-EPI equation with the Cockcroft-Gault yielding an overall average 7.3 units higher, with notable variation between the 2 equations for any given value. Panel 8 (bottom row center) presents Cockcroft-Gault versus MDRD equation with the Cockcroft-Gault yielding an overall average 10 units higher, with notable variation between the 2 equations for any given value which is more pronounced at higher values. Simple scatterplots are presented in panels 2 (top row center) (MDRD vs CKD-EPI), 3 (top row right) (Cockcroft-Gault vs CKD-EPI) and 6 (middle row right) (Cockcroft-Gault vs MDRD). These data confirm the findings in the Bland-Altman plots. B, Data presented with adjustment for individual BSA for CKD-EPI and MDRD equations, in the same format as in A. The discrepancy between the eGFR values for high-income and other GBD regions is partially attenuated by adjusting for BSA, with an increased eGFR estimate for high-income regions. The variation in values generated by the equations is decreased. This attenuation is best represented in the scatterplots presented in B, panels 2, 3, and 6.
Table 2.
Three-Way Classification of Kidney Function Equations: Comparison of Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease Equations, Without Adjustment for Body Surface Area
| Participants, No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| eGFR by MDRD, mL/min/1.73 m2 | ||||||||||
| <60 | 60 to <90 | ≥90 | ||||||||
| CrCl by CG, mL/min | CrCl by CG, mL/min | CrCl by CG, mL/min | ||||||||
| eGFR by CKD-EPI, mL/min/1.73 m2 | <60 | 60 to <90 | ≥90 | <60 | 60 to <90 | ≥90 | <60 | 60 to <90 | ≥90 | All Participants |
| <60 | 18 (0.2) | 167 (2.2) | 16 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 201 (2.6) |
| 60 to <90 | 14 (0.2) | 131 (1.7) | 31 (0.4) | 99 (1.3) | 1381 (17.8) | 1080 (13.9) | 0 (0) | 1 (0.0) | 0 (0) | 2737 (35.3) |
| ≥90 | 0 (0) | 0 (0) | 0 (0) | 4 (0.1) | 353 (4.5) | 696 (9.0) | 8 (0.1) | 757 (9.8) | 3006 (38.7) | 4824 (62.1) |
Abbreviations: CG, Cockcroft-Gault; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
The eGFR distributions and agreement of the 3 equations by GBD region are presented with Bland-Altman plots and scatterplots in Figure 2. For both the CKD-EPI and MDRD equations, there are distinct eGFR distributions by region, with lower eGFR values from the high-income versus other GBD regions. The data demonstrate good agreement between the CKD-EPI and MDRD eGFR up to 120 mL/min/1.73 m2, above which the MDRD equation yields consistently higher values (Figure 2A, panel 4). The discrepancy between the 2 equations is heightened for participants from the other GBD regions.
In contrast, the Cockcroft-Gault equation yielded similar results for high-income and other GBD regions, with median CrCl values of 97 and 99 mL/min, respectively. Compared with the CKD-EPI and MDRD estimates, the Cockcroft-Gault CrCl estimates tended to be higher in high-income regions, whereas CKD-EPI and MDRD tended to be higher in other GBD regions (Figure 2A, panels 7 and 8). The net result was that the distributions of the Cockcroft-Gault estimates do not exhibit the same regional differences observed with the CKD-EPI and MDRD equations.
After adjustment of the CKD-EPI and MDRD equations for individual BSA, the agreement between the equations is notably improved, particularly in the clinically relevant range <90 mL/min (Figure 2B). The improvement appears to be driven by the resulting increase in eGFR in high-income regions where median weights, and therefore BSAs, were higher, and thus adjustment had a greater impact. Similar analyses by sex and race are shown in Supplementary Figures 1 and 2. These suggest that the BSA-adjusted MDRD and CKD-EPI equations tend to give higher estimates of kidney function than the Cockcroft-Gault equation in men than in women (Supplementary Figure 1) and in black or African American persons compared with other races (Supplementary Figure 2).
When the equations were compared over the clinically significant ranges of the data, 38% of the cohort had eGFRs <90 mL/min/1.73 m2 or mL/min by the CKD-EPI and Cockcroft-Gault equations, whereas 52% had eGFR <90 mL/min/1.73 m2 by the MDRD equation. The overall concordance (<60, 60 to <90, ≥90 mL/min/1.73 m2) between the 3 equations was 56% (Table 2), which improved to 73% when the CKD-EPI and MDRD equations were adjusted for individual BSA (Table 3).
Table 3.
Three-Way Classification of Kidney Function Equations: Comparison of Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease Equations, With Adjustment for Body Surface Area
| Participants, No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| eGFR by MDRD with BSA Adjustment, mL/min | |||||||||
| <60 | 60 to <90 | ≥90 | |||||||
| CrCl by CG, mL/min | CrCl by CG, mL/min | CrCl by CG mL/min | |||||||
| eGFR by CKD-EPI With BSA Adjustment, mL/min | <60 | 60 to <90 | <60 | 60 to <90 | ≥90 | <60 | 60 to <90 | ≥90 | All Participants |
| <60 | 37 (0.5) | 48 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 85 (1.1) |
| 60 to <90 | 19 (0.2) | 91 (1.2) | 85 (1.1) | 1660 (21.4) | 308 (4.0) | 0 (0) | 32 (0.4) | 5 (0.1) | 2200 (28.4) |
| ≥90 | 0 (0) | 0 (0) | 1 (0.0) | 349 (4.5) | 539 (6.9) | 1 (0.0) | 609 (7.8) | 3975 (51.2) | 5474 (70.6) |
Abbreviations: BSA, body surface area; CG, Cockcroft-Gault; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Factors Associated With Reduced Kidney Function
Factors associated with reduced kidney function (CKD-EPI eGFR <90 mL/min/1.73 m2) were evaluated using logistic regression among participants without history of kidney disease who had CKD-EPI eGFRs available. Single variable analyses were adjusted for sex and race. Enrollment in high-income regions, male sex, white race, older age, higher BMI, higher BSA, smoking history, diagnosis of hypertension, diagnosis of chronic active viral hepatitis, greater total antiretroviral use in years, TDF exposure, and use of antiplatelet therapy were associated with an increased odds of eGFR <90 mL/min/1.73 m2 in single variable analyses (Table 4). In the fully adjusted analysis, white race, enrollment in high-income regions, older age, higher BMI, diagnosis of hypertension, diagnosis of chronic active viral hepatitis, and TDF exposure remained associated with an increased odds of eGFR <90 mL/min/1.73 m2 (Table 4). We performed similar analyses using estimates from the MDRD and Cockcroft-Gault equations. The analysis using the MDRD equation yielded very similar results, whereas the analysis using the Cockcroft-Gault equation yielded different results for covariates associated with weight in our cohort, including race and sex (data not shown).
Table 4.
Adjusted and Fully Adjusted Logistic Regression to Assess Baseline Factors Associated With Estimated Glomerular Filtration Rate <90 mL/min/1.73 mm2 by Chronic Kidney Disease Epidemiology Collaboration Equation
| Adjustedb | Fully Adjustedc | |||
|---|---|---|---|---|
| Covariatea | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Natal sex, female vs male | 0.60 (.54–.67)d | <.001 | 0.93 (.82–1.05) | .22 |
| Race | ||||
| Asian vs White | 0.27 (.23–.31)d | <.001 | 1.06 (.87–1.28) | .58 |
| Black or African American vs White | 0.33 (.30–.37)d | <.001 | 0.45 (.40–.51) | <.001 |
| Other vs White | 0.44 (.36–.53)d | <.001 | 0.76 (.62–.95) | .01 |
| Age (y), per 5 y | 1.36 (1.31–1.41) | <.001 | 1.40 (1.34–1.46) | <.001 |
| Enrollment region, other regionse vs High Income | 0.23 (.21–.26) | <.001 | 0.25 (.22–.29) | <.001 |
| Smoking status | ||||
| Current vs never | 0.95 (.84–1.07) | .36 | … | … |
| Former vs never | 1.25 (1.11–1.41) | <.001 | … | … |
| History of hypertension, yes vs no | 1.56 (1.40–1.75) | <.001 | 1.26 (1.12–1.43) | <.001 |
| History of diabetes yes vs no | 1.12 (.67–1.86) | .67 | … | … |
| Use of antiplatelet therapy, yes vs nof | 1.76 (1.36–2.26) | <.001 | 0.98 (.75–1.28) | .88 |
| Weight (kg), per 5 kg | 1.09 (1.08–1.11) | <.001 | … | … |
| BMIg | ||||
| <18.5 vs 18.5–24.9 | 0.43 (.30–.61) | <.001 | 0.46 (.32–.66) | <.001 |
| 25–29.9 vs 18.5–24.9 | 1.40 (1.25–1.57) | <.001 | 1.34 (1.18–1.51) | <.001 |
| ≥30 vs 18.5–24.9 | 1.66 (1.45–1.90) | <.001 | 1.39 (1.20–1.61) | <.001 |
| BSA (m2), per 0.5 mm2 | 2.33 (2.07–2.62) | <.001 | … | … |
| BP (mm Hg), per 10 mm Hg | ||||
| Systolic | 1.01 (.98–1.05) | .44 | … | … |
| Diastolic | 1.00 (.95–1.05) | .98 | … | … |
| Duration of ART exposure (y) | ||||
| 5–10 vs <5 | 1.02 (.89–1.17) | .75 | … | … |
| ≥10 vs <5 | 1.42 (1.25–1.61) | <.001 | … | … |
| Nadir CD4 count (cells/mm2) | ||||
| <200 vs ≥200 | 1.12 (1.02–1.24) | .02 | … | … |
| Unknown vs ≥200 | 1.46 (1.13–1.90) | .004 | … | … |
| CD4 count (cells/ µ L), per 100 cells | 1.01 (.99–1.02) | .54 | … | … |
| Tenofovir exposure (y) | ||||
| 0 to <5 vs 0 | 1.52 (1.30–1.78) | <.001 | 1.83 (1.54–2.16) | <.001 |
| 5 to <10 vs 0 | 1.87 (1.60–2.18) | <.001 | 1.62 (1.37–1.91) | <.001 |
| ≥10 vs 0 | 2.50 (2.09–3.00) | <.001 | 1.66 (1.37–2.01) | <.001 |
| Chronic active viral hepatitis, yes vs no | 1.70 (1.37–2.12) | <.001 | 1.26 (1.00–1.58) | .051 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure, BSA, body surface area; CI, confidence interval; OR, odds ratio.
aParticipants with a history of kidney disease (n = 29) and missing estimated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration equation (n = 4) have been excluded.
bEstimates from univariate models, adjusted for natal sex and race.
cEstimates from multivariable logistic regression; covariates with P values <.001 and clinically meaningful estimated effect sizes were chosen for inclusion in the fully adjusted model.
dEstimate from a univariate model.
eIncludes Latin American and Caribbean, South East and East Asia, South Asia, and sub-Saharan Africa.
fIncludes aspirin.
gBMI was calculated as weight in kilograms divided by height in meters squared.
DISCUSSION
Overall, the REPRIEVE trial enrolled PWH who are representative of the global HIV epidemic and includes persons with a wide range of kidney function. The proportion of persons with reduced eGFR (<90 mL/min or mL/min/1.73 m2) ranged from 38% to 52%, depending on the 3 commonly used equations allowed for eligibility screening in the trial. The 3 equations demonstrated only fair concordance in classifying participants by accepted eGFR strata.
Given that the REPRIEVE trial recruited persons receiving ART, the majority with suppressed HIV, and with low to moderate risk of ASCVD, it is not surprising that the cohort is reflective of the aging HIV epidemic. Certain traditional risk factors were associated with an increased odds of reduced eGFR, including older age, higher BMI, history of hypertension, history of chronic viral hepatitis, and exposure to TDF-based ART. Notably, white race was associated with an increased odds of having decreased eGFR compared with other racial groups, particularly black or African American participants. This effect is likely related to the fact that black or African American participants were more likely than white participants to be female (43% vs 14%, respectively). Furthermore, the MDRD equation has previously been demonstrated to underestimate the prevalence of impaired kidney function for persons of black or African American race [33]. In addition, the risk factors used in the ASCVD pooled risk calculator overlap with CKD risk factors that are likely more prevalent among black or African American persons than among white individuals of similar age. This may have resulted in the exclusion of some persons of black race or African American persons or men with lower eGFRs from trial participation.
The increased odds of reduced eGFR among PWH with TDF exposure was expected (Table 4). The impact of tenofovir use on kidney function has been well established, with the greatest effect being reported in persons receiving TDF with a ritonavir-boosted protease inhibitor [5, 30, 34]. In fact, the Infectious Disease Society of America clinical practice guidelines for the management of kidney disease stress the potential nephrotoxicity of TDF and the importance of monitoring kidney function for PWH receiving TDF-based regimens [1]. In 2015, the Food and Drug Administration approved tenofovir alafenamide (TAF), a tenofovir prodrug that accumulates in nucleated cells rather than the plasma and thus may have less effect on kidney function, although there remains debate about whether there are true benefits of TAF over TDF [35, 36]. Since then, an increase in TAF use in HIV regimen has been recognized, including among REPRIEVE participants [32]. Because REPRIEVE enrollment began before the approval of TAF for HIV treatment, we will be able to evaluate a differential effect of these antiretroviral agents in future analyses. For the current analysis, tenofovir exposure specifically targeted reported exposure to TDF, given limited exposure to the TAF formulation.
These data indicate good agreement between the CKD-EPI and MDRD equations in the REPRIEVE sample, particularly for eGFR <90 mL/min/1.73 m2, but poor agreement >120 mL/min/1.73 m2. This finding is reassuring that both equations provide a reasonable determination of whether PWH have mild impairment in kidney function. Conversely, the Cockcroft-Gault equation had poor agreement with either the CKD-EPI or MDRD equations, particularly at higher eGFR values. For the comparisons between the equations, adjusting for individual BSA to report all estimates in the same units (milliliters per minute) improved the concordance (Figure 2B). The impact of this adjustment was greatest in the high-income regions, where obesity and high BSA were common. Although adjustment for BSA is not routinely performed in clinical practice, it has been recommended to allow use of the CKD-EPI estimate for drug dosing, which is currently based on the Cockcroft-Gault equation [37]. The current study was not designed to evaluate the accuracy of the different GFR equations in the absence of a direct measure of GFR; however, the CKD-EPI is generally considered a more accurate estimate of directly measured GFR,
This analysis is limited by the fact that participants with eGFR <60 mL/min/1.73 m2 or Cr/Cl <60 mL/min were excluded from the parent trial; <3% of participants had a CKD-EPI eGFR <60 mL/min/1.73 m2, and only 0.2% fell below this threshold by all 3 equations. We relied on creatinine-based estimates and did not include a direct GFR measure or a cystatin-C based equation in this analysis given that most clinicians use only the creatinine-based equations for clinical care of PWH. Because we used the data available at time of entry, we cannot be certain that these estimations reflect stable kidney function; nonetheless, all participants were required to be engaged in HIV care and on stable ART, indicative of overall stable clinical status.
We did not discriminate between TDF and TAF exposure for this analysis, but exposure to TAF was limited in the cohort at time of entry. We will evaluate the differential effect of TAF versus TDF on kidney function, as well as the effect of other ART agents, in future analyses once greater exposure to TAF has accrued. Future analyses will also examine the urinary protein and albumin within this cohort, as these data are not yet available to the research team. We will also have data regarding host genetic factors, including APOL1 risk variants and other polymorphisms that have been linked to progression of CKD. We also preferentially presented analyses based on the CKD-EPI formula. Studies comparing these estimates to a direct measure of GFR have demonstrated that the CKD-EPI eGFR is more accurate than the other 2 equations in most populations, including PWH receiving stable ART. The adjustment for black race may introduce some error in African populations, but overall we made the assumption that the CKD-EPI equation provides the most accurate and least biased estimate of measured GFR.
In summary, the REPRIEVE trial has recruited a diverse cohort of PWH who represent the ongoing global HIV epidemic. The trial includes a large number of individuals with reduced estimates of kidney function and is well positioned to determine whether pitavastatin therapy can prevent the decline in kidney function in PWH. The use of any of these creatinine-based equations for estimate of kidney function has limitations that must be considered, particularly when making medication dosing recommendations. Future analyses will evaluate changes in kidney function over time in this well-characterized cohort and whether pitavastatin can prevent the loss of kidney function.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. They also thank the AIDS Clinical Trial Group (ACTG) for clinical site support; the ACTG’s clinical trials specialists, notably Barbara Bastow, Laura Moran, and Jhoanna Roa, for regulatory support; the data management center, Frontier Science Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support. The team also thanks Emma Kileel for her coordination on many aspects of REPRIEVE, and E. T. O. recognizes Teri Kennedy for her ongoing and tireless logistical support of this work.
Disclaimer. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI) or the National Institute of Allergy and Infectious Diseases, the National Institutes of Health (NIH), or the US Department of Health and Human Services.
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grant R01 DK108438), the NHLBI (grants U01 HL123336 and U01 HL123339), the National Institute of Allergy and Infectious Diseases (grants UM1 AI068636, UM1 AI106701, and UM1AI069452), and the University of Alabama at Birmingham Center for AIDS Research (grant P30AI02267).
Supplement sponsorship. This supplement is sponsored by Massachusetts General Hospital, through NIH funding including U01HL123336.
Potential conflicts of interest. E. T. O. reports grants from the NIH during the conduct of the study; grant support through his institution from ViiV Healthcare, Gilead Sciences, Janssen, and Bavarian Nordic, outside the submitted work; and personal fees from Gilead Sciences, TheraTechnologies, ViiV Healthcare, and Merck, outside the submitted work. A. K. reports grants from NIH/NIDDK and NIH/NHLBI during the conduct of the study. K. V. F. reports an educational grant from Gilead Sciences, outside the submitted work. K. S. reports grants from the NIH during the conduct of the study. L. M. reports grants from the NIH and nonfinancial support from Kowa Pharmaceuticals America and from Gilead Sciences, during the conduct of the study; and grants from Janssen Pharmaceutical, Merck Sharp & Dohme, ViiV Healthcare, Johnson & Johnson, Pfizer Pharmaceuticals, and Bristol-Myers Squibb and nonfinancial support from Sanofi-Aventis, outside the submitted work. G. M. reports personal fees from Merck, ViiV Healthcare, and Gilead Sciences, outside the submitted work. J. A. A. reports grants from Massachusetts General Hospital during the conduct of the study; and grants from Frontier Technologies and Gilead Sciences, grants and personal fees from Janssen, Merck, and GlaxoSmithKline–ViiV Healthcare, and personal fees from Medicure and Theratechnologies, outside the submitted work. P. S. D. reports grant support from Kowa Pharmaceuticals America and Gilead Sciences for the conduct of the study. S. K. G. reports grant support through his institution from Kowa Pharmaceuticals America and Gilead Sciences for the conduct of the study and from Theratechnologies, Navidea, and ViiV Healthcare, outside the submitted work. H. J. R. reports grants from NIH/NIDDK, NIH/NHLBI, and Kowa Pharmaceuticals America during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lucas GM, Ross MJ, Stock PG, et al. ; HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis 2008; 197:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. [DOI] [PubMed] [Google Scholar]
- 4. Overton ET, Patel P, Mondy K, et al. ; Sun Study Investigators. Cystatin C and baseline renal function among HIV-infected persons in the SUN Study. AIDS Res Hum Retroviruses 2012; 28:148–55. [DOI] [PubMed] [Google Scholar]
- 5. Calza L, Sachs M, Colangeli V, et al. Prevalence of chronic kidney disease among HIV-1-infected patients receiving a combination antiretroviral therapy. Clin Exp Nephrol 2019; 23:1272–9. [DOI] [PubMed] [Google Scholar]
- 6. Cristelli MP, Trullàs JC, Cofán F, et al. ; CKD-H. Clinical Investigators. Prevalence and risk factors of mild chronic renal failure in HIV-infected patients: influence of female gender and antiretroviral therapy. Braz J Infect Dis 2018; 22:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Althoff KN, McGinnis KA, Wyatt CM, et al. ; Veterans Aging Cohort Study (VACS). Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis 2012; 59:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gathogo E, Jose S, Jones R, et al. End-stage kidney disease and kidney transplantation in HIV-positive patients: an observational cohort study. J Acquir Immune Defic Syndr 2014; 67:177–80. [DOI] [PubMed] [Google Scholar]
- 10. Medapalli RK, Parikh CR, Gordon K, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr 2012; 60:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallipattu SK, Liu R, Zhong Y, et al. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 2013; 83:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amarenco P, Callahan A 3rd, Campese VM, et al. Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial. Stroke 2014; 45:2974–82. [DOI] [PubMed] [Google Scholar]
- 13. Colhoun HM, Betteridge DJ, Durrington PN, et al. ; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–9. [DOI] [PubMed] [Google Scholar]
- 14. Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G; Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 2005; 68:237–45. [DOI] [PubMed] [Google Scholar]
- 15. Ascher SB, Scherzer R, Nishtala A, et al. Association of statin use with kidney damage and function among HIV-infected men. J Acquir Immune Defic Syndr 2019; 82:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis 2014; 59:1148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grinspoon S, Douglas P, Hoffman U, Ribaudo H. Leveraging a landmark trial of primary CVD prevention in HIV: introduction from the REPRIEVE Co-PI’s. J Inf Dis 2020; 222(Suppl 1)S1–7. [DOI] [PMC free article] [PubMed]
- 19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130:461–70. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005; 16:459–66. [DOI] [PubMed] [Google Scholar]
- 23. Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens 2006; 15:276–84. [DOI] [PubMed] [Google Scholar]
- 24. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol 2005; 16:1413–9. [DOI] [PubMed] [Google Scholar]
- 25. Imai E, Horio M, Nitta K, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis 2007; 50:927–37. [DOI] [PubMed] [Google Scholar]
- 26. Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 2010; 56:486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012; 156:785–95. [DOI] [PubMed] [Google Scholar]
- 28. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63:713–35. [DOI] [PubMed] [Google Scholar]
- 29. Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010; 55:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swanepoel CR, Atta MG, D’Agati VD, et al. ; Conference Participants. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2018; 93:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Institute for Health Metrics and Evaluation. Frequently asked questions http://www.healthdata.org/gbd/faq. Accessed 22 April 2020.
- 32. Fictenbaum C, Ribaudo H, Leon-Cruz MS, et al. Patterns of antiretroviral therapy use and immunologic profiles at enrollment in the REPRIEVE Trial. J Inf Dis 2020; 222(Suppl 1):S8–19. [DOI] [PMC free article] [PubMed]
- 33. Arora P, Rajagopalan S, Patel N, Nainani N, Venuto RC, Lohr JW. The MDRD equation underestimates the prevalence of CKD among blacks and overestimates the prevalence of CKD among whites compared to the CKD-EPI equation: a retrospective cohort study. BMC Nephrol 2012; 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen SD, Kopp JB, Kimmel PL. Kidney Diseases Associated with Human Immunodeficiency Virus Infection. N Engl J Med 2017; 377:2363–74. [DOI] [PubMed] [Google Scholar]
- 35. DeJesus E, Haas B, Segal-Maurer S, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retroviruses 2018; 34:337–42. [DOI] [PubMed] [Google Scholar]
- 36. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad 2018; 4:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Institute of Diabetes and Digestive and Kidney Diseases. CKD & drug dosing: information for providers https://www.niddk.nih.gov/health-information/professionals/advanced-search/ckd-drug-dosing-providers. Accessed 22 April 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.