Abstract
Background
Patterns of antiretroviral therapy (ART) use and immunologic correlates vary globally, and contemporary trends are not well described.
Methods
The REPRIEVE trial (Randomized Trial to Prevent Vascular Events in HIV) enrolled persons with human immunodeficiency virus (HIV) who were aged 40–75 years, receiving ART, and had low-to-moderate cardiovascular disease risk. ART use was summarized within Global Burden of Disease (GBD) super-regions, with adjusted linear and logistic regression analyses examining associations with immune parameters and key demographics.
Results
A total of 7770 participants were enrolled, with a median age of 50 years (interquartile range, 45–55 years); 31% were female, 43% were black or African American, 15% were Asian, 56% had a body mass index >25 (calculated as weight in kilograms divided by height in meters squared), and 49% were current or former smokers. The median CD4 T-cell count was 620/µL (interquartile range, 447–826/ µ L), and the median duration of prior ART use, 9.5 years (5.3–14.8) years. The most common ART regimens were nucleoside/nucleotide reverse-transcriptase inhibitor (NRTI) plus nonnucleoside reverse-transcriptase inhibitor (43%), NRTI plus integrase strand transfer inhibitor (25%), and NRTI plus protease inhibitor (19%). Entry ART varied by GBD region, with shifts during the trial enrollment period. In adjusted analyses, entry CD4 cell count and CD4/CD8 ratio were associated with GBD region, sex, entry regimen, duration of ART, and nadir CD4 cell count; CD4 and CD8 cell counts were also associated with body mass index and smoking status.
Conclusions
There were substantial variations in ART use by geographic region and over time, likely reflecting the local availability of specific medications, changes in treatment guidelines and provider/patient preferences. The analyses of CD4 cell counts and CD4/CD8 ratios may provide valuable insights regarding immune correlates and outcomes in people living with HIV.
Clinical Trials Registration
Keywords: HIV, statins, pitavastatin calcium, antiretroviral therapy, CD4 cell count, CD4/CD8 ratio, REPRIEVE, cardiovascular disease
Approximately 37 million people globally are living with human immunodeficiency virus (HIV) [1]. The success of antiretroviral therapy (ART) has resulted in longer survival for people with HIV (PWH) [2]. Worldwide, >24.5 million PWH are now receiving ART [3]. The current era of ART has seen major shifts in ART availability and prescribing patterns, along with changes in key patient characteristics, including body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). Moreover, the availability of new antiretrovirals is not uniform across worldwide regions, and relatively little is known regarding the changing patterns of ART use globally especially in the aging population. Finally, there is limited information on contemporary patterns of ART use and how such patterns relate to other factors like immune status.
The current analysis leveraged detailed data acquired on ART use and immune status across a global cohort of PWH. The REPRIEVE trial (Randomized Trial to Prevent Vascular Events in HIV) was designed as the first large-scale, long-term randomized trial to assess a primary cardiovascular disease (CVD) prevention strategy among PWH, with low-to-moderate risk for CVD according to traditional risk factors, using statin therapy [4, 5]. The trial was initiated to address the reported increased CVD and CVD mortality rates among PWH, contemporaneous with overall improvements in AIDS-related complications and life expectancy [6–8]. The trial enrolled 7770 PWH across 5 continents between 2015 and 2019. The large size of the trial and diversity of the enrolled population allows for the opportunity to assess differences in HIV-specific elements, such as patterns of ART usage, as well as immunologic characteristics and correlates. Further context for REPRIEVE and methods on ascertainment of key data elements is provided in the introductory article within this supplement [9].
The aims of this analysis were to characterize patterns of ART use and immunologic characteristics over the 4-year period of enrollment in REPRIEVE and to examine indices of immunologic health in relation to key participant characteristics across regions. We hypothesized that there would be differences in the patterns of ART usage over time and that sociodemographic and potentially modifiable health factors would be associated with different immunologic profiles. Specifically, we investigated whether there were regional differences in CD4 and CD8 T-cell counts and how these might relate to other health parameters, demographics and patterns of ART use. Understanding how specific ART regimens affect CVD risk is particularly germane because clinical outcomes are being documented within REPRIEVE. Furthermore, it is the complex interplay between host characteristics, environmental exposures, the immune system, HIV, antiretrovirals, and other factors that affects CVD risk and the development of CVD. Information ensuing from these analyses will not only prove critical to contextualizing the longitudinal results of the REPRIEVE trial but will also help inform our understanding of changing patterns of ART use and correlates of immune function among PWH across the globe.
METHODS
Sites and Study Population
The REPRIEVE trial (NCT02344290) is a prospective, double-blind, randomized, placebo-controlled, multicenter, phase III efficacy study with 2 arms (pitavastatin calcium [4 mg/d] vs placebo), enrolling PWH currently taking ART [4, 5], at >100 sites in 12 countries. The primary entry criteria included PWH ≥40 and ≤75 years of age, taking any combination ART regimen for ≥6 months before study entry, with CD4 cell counts >100/ µ L [4, 5].
At trial entry, information on medical history was ascertained, including cardiovascular risk history, physical examination, and medication history. Laboratory values were obtained as part of the trial screening and entry process or abstracted from clinical care records (see supplementary methods in Grinspoon et al [9]).
Antiretroviral Regimen Definitions
Antiretroviral regimen histories were collected at entry to include estimates of total duration of ART exposure, duration of exposure to protease inhibitors (PIs), thymidine analogues, tenofovir disoproxil fumarate (TDF), and abacavir. The details of the current ART regimen at trial enrollment (including estimated start date) is reported as the entry regimen. For this analysis, entry regimen classes were categorized by use of nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs), nonnucleoside reverse-transcriptase inhibitors (NNRTIs), PIs, and integrase strand transfer inhibitors (INSTIs). For simplicity, boosting agents for PIs, such as ritonavir and cobicistat, are not described.
Statistical Analyses
Distributions of entry regimens are shown graphically, stratified according to the Global Burden of Disease (GBD) super-region [10] and within key demographic factors, including duration of current regimen, self-reported natal sex and race, and calendar date of enrollment. Distributions of immunologic parameters at study entry—CD4 cell counts, CD8 cell counts, and CD4/CD8 ratio (≥1.0 or <1.0)—are examined descriptively and by means of adjusted linear (CD4 and CD8 cell counts) and logistic regression (low CD4/CD8 ratio) analyses to evaluate associations with enrollment region, ART exposures, and key participant characteristics.
Given the complex patterns of these covariates and their potential confounding and collinearity across and within enrollment regions, modeling was repeated in exploratory subgroup analyses by GBD region, natal sex, and total duration of prior ART exposure; targeted interaction tests were evaluated. Owing to collinearity between GBD region and race, multivariable analyses were adjusted only for race in the high-income regions. As a result of this parameterization, the reference comparator for enrollment region is high-income white race. Given the large sample size and high power to detect small effect sizes, formal statistical comparisons are limited to regression analyses, with inference based on very low type 1 error (P < .001) and clinically meaningful estimated effect sizes. Data analysis was conducted using SAS software, version 9.4 for UNIX (SAS Institute).
Ethics Statement
Each clinical research site obtained institutional review board/ethics committee approval and any other applicable regulatory entity approvals. Participants were provided with study information, including discussion of risks and benefits and were asked to sign the approved declaration of informed consent.
RESULTS
Study Population
From March 2015 through July 2019, a total of 7770 participants enrolled. The median age was 50 years (interquartile range [IQR], 45–55 years). Thirty-one percent of participants were natal female, and 2% reported identifying on the transgender spectrum. The overall racial distribution of participants was 43% black or African-American, 35% white, 15% Asian, and 7% other races. In North America (Canada, United States, and Puerto Rico), 18% were Latino or Hispanic. The median CD4 cell count at entry was 620/ µ L (IQR, 447–826/ µ L). The HIV-1 RNA level was below local assay quantification limits in 87% of participants, and of the remaining 13%, 75% had viral loads ≤200 copies/mL (See Grinspoon et al [9, table 1] for further description of the cohort).
ART Exposure
The median duration of prior oral ART exposure at REPRIEVE entry was 9.5 years (IQR, 5.3–15 years). Prior exposure to PIs was reported in 47% of participants (median 7.0 years; IQR, 3.6–11 years), to thymidine analogues in 49% (5.9 years; 3.0 – 9.9 years), and to TDF in 85% (5.5 years; 3.0–8.7 years) (Table 1).
Table 1.
Demographic and Cardiovascular Characteristics by Entry Antiretroviral Therapya
| Participants, No. (%)b | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (N = 7770) | NRTI + INSTI (n = 1978) | NRTI + NNRTI (n = 3676) | NRTI + PI (n = 1439) | NRTI Sparing (n = 199) | Other NRTI Containing (n = 476) |
| Demographic and Behavioral | ||||||
| Age, median (IQR), y | 50 (45–55) | 51 (46–55) | 49 (44–54) | 50 (46–55) | 51 (47–56) | 51 (47–55) |
| Natal sex | ||||||
| Male | 5352 (69) | 1563 (79) | 2293 (62) | 974 (68) | 150 (75) | 370 (78) |
| Female | 2418 (31) | 415 (21) | 1383 (38) | 465 (32) | 49 (25) | 106 (22) |
| Racec | ||||||
| Black or African American | 3378 (43) | 786 (40) | 1679 (46) | 630 (44) | 65 (33) | 218 (46) |
| White | 2701 (35) | 1064 (54) | 829 (23) | 487 (34) | 101 (51) | 218 (46) |
| Asian | 1139 (15) | 24 (1) | 893 (24) | 193 (13) | 20 (10) | 9 (2) |
| Other | 552 (7) | 104 (5) | 275 (7) | 129 (9) | 13 (7) | 31 (7) |
| Ethnicityd | ||||||
| Hispanic or Latino | 698 (18) | 302 (17) | 188 (20) | 117 (18) | 23 (16) | 68 (17) |
| Not Hispanic or Latino | 3187 (81) | 1472 (82) | 743 (79) | 532 (81) | 115 (82) | 324 (83) |
| Unknown | 34 (1) | 13 (1) | 14 (1) | 4 (1) | 3 (2) | 0 (0) |
| Smoking status | ||||||
| Current | 1933 (25) | 586 (30) | 764 (21) | 378 (26) | 52 (26) | 153 (32) |
| Former | 1906 (25) | 606 (31) | 752 (20) | 352 (24) | 54 (27) | 140 (30) |
| Never | 3923 (51) | 784 (40) | 2158 (59) | 708 (49) | 93 (47) | 180 (38) |
| Substance use | ||||||
| Current | 152 (2) | 52 (3) | 52 (1) | 34 (2) | 6 (3) | 8 (2) |
| Former | 2277 (29) | 958 (48) | 592 (16) | 431 (30) | 83 (42) | 213 (45) |
| Never | 5333 (69) | 967 (49) | 3030 (82) | 972 (68) | 110 (55) | 252 (53) |
| Cardiovascular and Metabolic | ||||||
| BMIe | ||||||
| <18.5 | 288 (4) | 19 (1) | 226 (6) | 34 (2) | 3 (2) | 6 (1) |
| 18.5–24.9 | 3115 (40) | 613 (31) | 1656 (45) | 585 (41) | 77 (39) | 184 (39) |
| 25–29.9 | 2664 (34) | 755 (38) | 1190 (32) | 490 (34) | 79 (40) | 148 (31) |
| ≥30 | 1696 (22) | 586 (30) | 603 (16) | 329 (23) | 40 (20) | 138 (29) |
| HIV-Related Health Status | ||||||
| Nadir CD4 T-cell count | ||||||
| <50/ µ L | 1406 (18) | 352 (18) | 513 (14) | 307 (21) | 57 (29) | 176 (37) |
| 50–199/ µ L | 2386 (31) | 490 (25) | 1193 (32) | 473 (33) | 69 (35) | 160 (34) |
| 200–349/ µ L | 2039 (26) | 501 (25) | 1031 (28) | 397 (28) | 33 (17) | 77 (16) |
| ≥350/ µ L | 1677 (22) | 541 (27) | 834 (23) | 224 (16) | 33 (17) | 45 (9) |
| Unknown | 262 (3) | 94 (5) | 105 (3) | 38 (3) | 7 (4) | 18 (4) |
| History of AIDS-defining event | 1849 (24) | 328 (17) | 874 (24) | 432 (30) | 65 (33) | 150 (32) |
| CD4 T-cell count, median (IQR), cells/ µ L | 620 (447–826) | 628 (456–845) | 633 (468–832) | 612 (422–820) | 605 (447–834) | 521 (348–720) |
| CD8 T-cell count median (IQR), cells/ µ L | 779 (564–1032) | 775 (555–1006) | 750 (547–992) | 838 (600–1129) | 840 (601–1083) | 886 (664–1112) |
| HIV-1 RNA level below LLQ | ||||||
| <20 copies/mL | 2819 (47) | 1207 (64) | 849 (37) | 442 (38) | 96 (51) | 223 (50) |
| <40 copies/mL | 2243 (37) | 407 (22) | 1131 (49) | 528 (45) | 56 (30) | 121 (27) |
| <400 copies/mL | 187 (3) | 31 (2) | 129 (6) | 21 (2) | 4 (2) | 2 (<0.5) |
| ≥LLQ | 750 (13) | 240 (13) | 202 (9) | 179 (15) | 31 (17) | 98 (22) |
| ART History | ||||||
| Total ART use median (IQR) | 9.6 (5.3–14.8) | 9.0 (4.8–15.6) | 8.3 (4.7–12.3) | 11.0 (6.5–16.0) | 17 (11–21) | 16.0 (10.4–20.3) |
| Total ART use | ||||||
| <5 y | 1709 (22) | 503 (25) | 968 (26) | 209 (15) | 6 (3) | 23 (5) |
| 5–10 y | 2305 (30) | 556 (28) | 1230 (33) | 406 (28) | 35 (18) | 78 (16) |
| ≥10 y | 3754 (48) | 918 (46) | 1478 (40) | 823 (57) | 158 (79) | 375 (79) |
| Unknown | 2 (<0.5) | 1 (<0.5) | 0 (0) | 1 (<0.5) | 0 (0) | 0 (0) |
| Protease exposure | 3624 (47) | 985 (50) | 615 (17) | 1400 (97) | 192 (96) | 430 (90) |
| Thymidine exposure | 3799 (49) | 601 (30) | 1870 (51) | 867 (60) | 137 (69) | 323 (68) |
| Abacavir exposure | 1618 (21) | 775 (39) | 297 (8) | 262 (18) | 74 (37) | 209 (44) |
| Tenofovir exposure | 6572 (85) | 1707 (86) | 3035 (83) | 1241 (86) | 151 (76) | 437 (92) |
| Duration of entry ART regimen, median (IQR), y | 2.3 (0.8–5.2) | 1.0 (0.5–1.9) | 3.6 (1.7–6.8) | 2.9 (0.9–6.2) | 1.8 (0.7–4.6) | 1.4 (0.6–3.8) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; LLQ, lower limit of quantification; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI , protease inhibitor.
aAll statistics are calculated based on participants with data collected. Missing data include smoking status (n = 8), substance use (n = 8), history of AIDS-defining event (n = 6), and HIV-1 RNA level below the LLQ (n = 1771). Two participants were missing an entry regimen at the time of analysis and are excluded from the total by entry regimen.
bData represent no. (%) of participants unless otherwise specified.
c“Other” race includes participants self-identifying as native or indigenous to the enrollment region, as >1 race (with no single race noted as predominant), or as of unknown race.
dEthnicity presented according to National Institutes of Health definition for participants in the United States (including Puerto Rico) and Canada only.
eBMI is calculated as weight in kilograms divided by height in meters squared.
Entry ART regimens included NRTIs plus NNRTIs (47%); NRTIs plus INSTIs (25%); and NRTIs plus PIs (with or without boosting agents) (19%); 6% and 3% of participants, respectively, were taking other NRTI-containing and NRTI-sparing regimens (these regimens primarily included either a PI or an NNRTI taken with an INSTI, with or without an NRTI). The median regimen duration at study entry was 2.3 years (IQR, 0.8–5.2 years); 10% of participants had been taking this regimen for >8.8 years (Table 1). The most commonly used agents in the NRTI class, were Lamivudine (3TC) or Emitricitabine (FTC) in combination with TDF (in 61%), tenofovir alafenamide (TAF) (15%), or and abacavir (12%), respectively. In the NNRTI class, the most commonly used agents were efavirenz (68%), rilpivirine (16%), and nevirapine (13%). The most commonly used PIs were darunavir (44%), atazanavir (40% (with or without boosting agents), and lopinavir-ritonavir (15%). In the INSTI class, dolutegravir (DTG) (50%), elvitegravir (34%), and raltegravir (14%) were used most commonly.
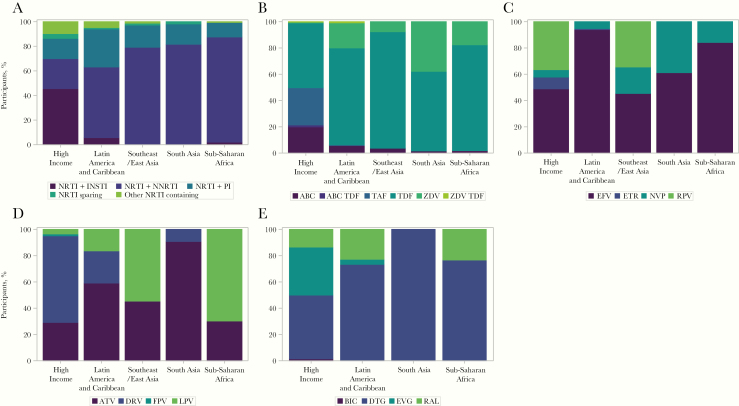
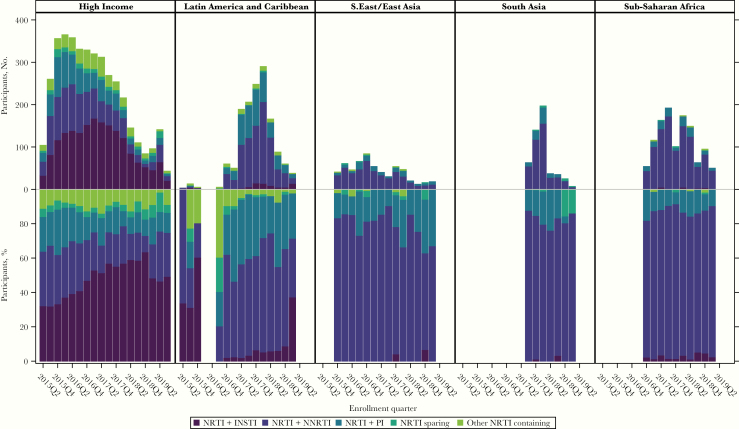
There were important differences in entry regimen by enrollment region (Figure 1). In particular, there was greater regimen diversity among participants enrolled in the high-income region, followed by the Latin America and Caribbean region, with clear evolution of regimens over time that was less apparent in other regions (Figure 2). The use of INSTIs was almost exclusive to the high-income region, with DTG and elvitegravir the most common choices. The amount of raltegravir use waned over the enrollment period, with emerging use of bictegravir (Supplementary Figure 1A). Within the NRTI class, TDF use predominated across all regions (Supplementary Figure 2). TAF was used increasingly over the course of enrollment in the high-income region (Supplementary Figure 1B). There were stark differences in PI use across enrollment regions, with darunavir far more common in the high-income region, with some emerging use in Latin America and the Caribbean and South Asia (India) (Figure 1D). Aazanavir was used across all regions and lopinavir/ritonavir predominated in sub-Saharan Africa. Efavirenz was the most commonly used NNRTI across all regions (Figure 1E). Rilpivirine use increased over the enrollment period in the high-income and Southeast Asia GBD super-regions (Supplementary Figure 3) and nevirapine was a common NNRTI choice in other regions.
Figure 1.
Class of entry regimen stratified by Global Burden of Disease region. Only regimens and agents with frequency >5 % of participants are shown. Abbreviations: ABC, abacavir; ATV, atazanavir; BIC, bictegravir; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, Etravirine; FPV, fos-amprenavir; INSTI, integrase strand transfer inhibitor; LPV, lopinavir; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Figure 2.
Changes in entry regimen class by time of enrollment. Top panels show the numbers of participants; lower panels, the percentages participants (calculated within each bar). Within each panel, enrollment quarter with a frequency <3 within a panel not shown. Abbreviations: INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; Q2, quarter 2; Q4, quarter 4.
Controlling for enrollment region, ART regimen at entry also varied by duration prior ART exposure, BMI and nadir CD4 cell count (Supplementary Figures 4–6). Given the current focus on INSTI use and its association with weight gain, the trend showing a higher percentage of INSTI-based regimens associated with higher BMI (Supplementary Figure 5) was examined further with stratification by duration of entry regimen. This analysis revealed the trend was strongest among those on their entry regimen for less than 1 year (Supplementary Figure 7). Because uptake of widespread INSTI use is relatively recent, it is noted that the numbers of participants on an INSTI regimen for >3 years at the time of REPRIEVE entry is very low, and no measure of precision is presented to allow any formal inference.
Immunologic Profiles
The median CD4 cell count at entry was 620/ µ L (IQR, 447–826/ µ L), the median CD8 cell count was 779/ µ L (564–1032/ µ L), and the median CD4/CD8 ratio was 0.81 (0.56–1.15) (Table 2). While overlapping across regions, the distributions of CD4 and CD8 cell counts were slightly higher in the high-income and Latin America and Caribbean regions. The CD4/CD8 ratios were highest among participants in Southeast and East Asia (Thailand), and lowest in South Asia and sub-Saharan Africa.
Table 2.
Immunologic Outcomes by Global Burden of Disease Super-region
| Outcome | Total (N = 7770) | High Income (n = 4096) | Latin America and Caribbean (n = 1423) | Southeast/East Asia (n = 590) | South Asia (n = 504) | Sub-Saharan Africa (n = 1157) |
|---|---|---|---|---|---|---|
| CD4 T-cell count, cells/ µ L | ||||||
| All participants | ||||||
| Median (IQR) | 620 (447–826) | 614 (446–835) | 658 (482–865) | 626 (476–784) | 591 (378–758) | 598 (433–802) |
| 10th to 90th percentile range | 309–1050 | 305–1070 | 339–1091 | 341–977 | 257–998 | 307–1010 |
| ART exposure <5 y | ||||||
| Median (IQR) | 590 (401–801) | 587 (400–793) | 629 (439–847) | 535 (333–691) | 542 (296–746) | 579 (403–796) |
| 10th to 90th percentile range | 262–1039 | 249–1022 | 310–1064 | 181–815 | 194–952 | 270–1038 |
| ART exposure 5–10 y | ||||||
| Median (IQR) | 614 (448–834) | 621 (456–859) | 649 (472–849) | 573 (400–714) | 591 (395–750) | 587 (436–788) |
| 10th to 90th percentile range | 319–1052 | 313–1096 | 366–1089 | 311–905 | 265–997 | 328–989 |
| ART exposure ≥10 y | ||||||
| Median (IQR) | 637 (470–834) | 623 (454–832) | 692 (523–909) | 656 (513–812) | 640 (433–788) | 618 (449–821) |
| 10th to 90th percentile range | 329–1053 | 319–1060 | 355–1126 | 396–1015 | 281–1024 | 328–1007 |
| CD8 T-cell count, cells/ µ L | ||||||
| All participants | ||||||
| Median (IQR) | 779 (564–1032) | 768 (546–1002) | 838 (601–1112) | 679 (505–879) | 891 (647–1244) | 772 (583–1016) |
| 10th to 90th percentile range | 427–1362 | 405–1304 | 468–1458 | 396–1101 | 483–1581 | 453–1352 |
| ART exposure <5 y | ||||||
| Median (IQR) | 794 (571–1066) | 743 (526–983) | 847 (612–1136) | 617 (473–809) | 879 (626–1270) | 803 (598–1042) |
| 10th to 90th percentile range | 439–1404 | 402–1286 | 487–1489 | 324–1024 | 463–1664 | 456–1379 |
| ART exposure 5–10 y | ||||||
| Median (IQR) | 770 (565–1003) | 729 (532–933) | 797 (586–1041) | 699 (530–858) | 925 (644–1267) | 807 (599–1055) |
| 10th to 90th percentile range | 425–1337 | 402–1252 | 434–1392 | 385–1089 | 515–1564 | 477–1357 |
| ART exposure ≥10 y | ||||||
| Median (IQR) | 777 (562–1030) | 786 (561–1050) | 868 (615–1133) | 679 (504–882) | 880 (683–1186) | 739 (564–960) |
| 10th to 90th percentile range | 426–1361 | 406–1355 | 469–1479 | 404–1120 | 488–1528 | 449–1302 |
| CD4/CD8 ratio | ||||||
| All participants | ||||||
| Median (IQR) | 0.81 (0.56–1.15) | 0.85 (0.56–1.20) | 0.81 (0.54–1.15) | 0.91 (0.68–1.22) | 0.63 (0.44–0.89) | 0.78 (0.55–1.06) |
| 10th to 90th percentile range | 0.36–1.53 | 0.37–1.63 | 0.34–1.57 | 0.52–1.52 | 0.29–1.15 | 0.37–1.37 |
| Ratio <1, no. (%) | 4214 (65) | 1841 (62) | 928 (66) | 330 (57) | 353 (82) | 762 (70) |
| ART exposure <5 y | ||||||
| Median (IQR) | 0.76 (0.47–1.10) | 0.84 (0.49–1.17) | 0.78 (0.46–1.12) | 0.78 (0.56–1.19) | 0.57 (0.34–0.80) | 0.74 (0.50–1.04) |
| 10th to 90th percentile range | 0.30–1.53 | 0.31–1.67 | 0.28–1.52 | 0.38–1.41 | 0.24–1.10 | 0.32–1.40 |
| Ratio <1, no. (%) | 1004 (68) | 299 (62) | 331 (68) | 30 (58) | 106 (84) | 238 (73) |
| ART exposure 5–10 y | ||||||
| Median (IQR) | 0.81 (0.56–1.14) | 0.89 (0.61–1.24) | 0.82 (0.58–1.21) | 0.84 (0.61–1.13) | 0.63 (0.46–0.91) | 0.73 (0.53–1.03) |
| 10th to 90th percentile range | 0.39–1.55 | 0.41–1.75 | 0.41–1.59 | 0.43–1.32 | 0.29–1.13 | 0.39–1.28 |
| Ratio <1, no. (%) | 1259 (66) | 468 (59) | 291 (64) | 81 (69) | 146 (81) | 273 (74) |
| ART exposure ≥10 y | ||||||
| Median (IQR) | 0.84 (0.59–1.17) | 0.83 (0.55–1.20) | 0.83 (0.58–1.12) | 0.94 (0.71–1.25) | 0.72 (0.52–0.94) | 0.84 (0.61–1.12) |
| 10th to 90th percentile range | 0.40–1.53 | 0.38–1.58 | 0.37–1.57 | 0.57–1.60 | 0.34–1.23 | 0.44–1.40 |
| Ratio <1, no. (%) | 1949 (63) | 1072 (63) | 306 (66) | 219 (54) | 101 (80) | 251 (64) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
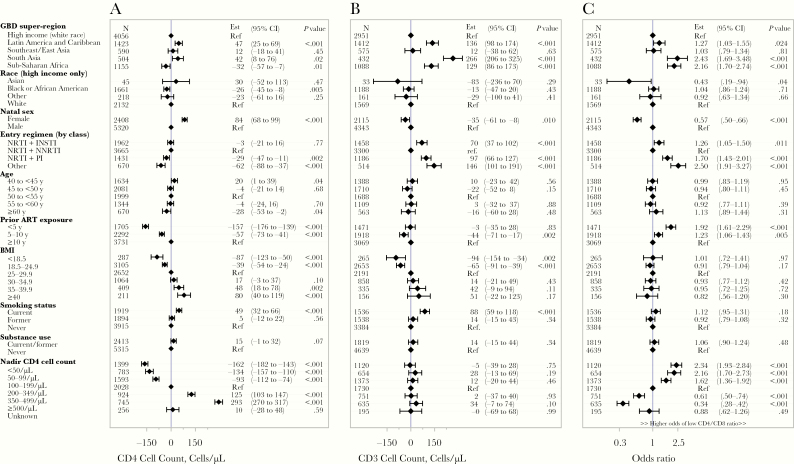
Sub-Saharan African enrollment, black or African American race (high-income region only), male natal sex, and NRTI-sparing or multiclass ART regimens were associated with lower CD4 cell counts. In contrast, enrollment from the Latin America and Caribbean region and current smoking were associated with higher CD4 cell counts. A positive association was noted between higher CD4 cell counts and longer ART exposure, higher BMI, and higher nadir CD4 cell counts across the distributions of these covariates (Figure 3). Sensitivity analyses restricted to those with HIV-1 RNA levels below the assay limit of quantification did not affect the results (data not shown).
Figure 3.
Adjusted regression analyses on CD4 T-cell count (A), CD8 T-cell count (B), and low (<1) CD4/CD8 ratio (C). Abbreviations: ART, antiretroviral therapy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CI, confidence interval; Est = Estimate, and Ref = Reference; GBD, Global Burden of Disease; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; Ref, reference.
The findings were also generally robust in analyses performed by enrollment region, natal sex, and duration of prior ART exposure (data not shown). Exceptions include the relationship between CD4 cell count and BMI that was not apparent in the Latin America and Caribbean region and the effect of nadir CD4 cell counts on current CD4 cell counts that was attenuated with increasing duration of prior ART exposure (Supplementary Figure 8). In further exploratory analyses, adjustment for white blood cell (WBC) counts at entry attenuated the CD4 cell count associations with BMI, sub-Saharan Africa and Latin America and Caribbean GBD enrollment, and smoking status (Supplementary Figure 9 and Supplementary Table 2). The addition of WBC counts into the adjusted model of CD4 cell counts did not affect the significance of the relationship with CD4 cell count nadir or natal female sex. In analysis restricted to high-income GBD region enrollment, Latino/Hispanic ethnicity was not associated with higher CD4 cell counts (data not shown).
Marked differences in CD8 cell counts across enrollment regions and by entry ART regimen were apparent, and there was a positive association between BMI and CD8 cell counts. There was no association between CD8 cell counts and nadir CD4 cell count. Male natal sex (compared with female) and current smoking (compared with former and never smoking) were associated with a higher CD8 cell count (Figure 3B). These findings were all robust in exploratory subgroup analyses by enrollment region, natal sex, and prior ART duration, and sensitivity was restricted to participants with HIV-1 RNA levels below the assay limit of quantification (data not shown). Additional adjustment by WBC counts attenuated the positive associations of CD8 cell counts with BMI and smoking status (Supplementary Figure 9).
There were several notable associations with entry CD4/CD8 ratios. South Asia and sub-Saharan African enrollment regions were associated with a higher odds of having a low (<1.0) CD4/CD8 ratio (Figure 3C). Male natal sex, NRTI plus PI and other entry regimens (ie, NRTI-sparing regimens, multiclass regimens), a shorter duration of total ART exposure, and a lower CD4 cell count nadir were associated with higher odds of a low CD4/CD8 ratio (Figure 3C). Again, these findings were robust in exploratory subgroup analyses performed by enrollment region, natal sex, and prior ART duration and sensitivity restricted to participants with HIV-1 RNA levels below the assay limit of quantification (data not shown).
DISCUSSION
The REPRIEVE trial provides an opportunity to investigate and compare patterns of contemporary use of ART and immunologic profiles evaluating associations with sociodemographic factors and select health characteristics across GBD super-regions, in a time of evolving patterns of global ART use from 2015 to 2019. The study population had extensive prior exposure to ART, with a median 9.5 years at the time of enrollment. The data demonstrate clear differences in the patterns of ART use by GBD regions with greater regimen variability in high-income regions along with more use of INSTIs and more NNRTI use in other GBD regions.
Furthermore, specific use of different antiretrovirals changed over the enrollment period with increased use of TAF, DTG, and rilpivirine and decreased use of atazanavir in the high-income GBD super-region. Changes in the use of specific antiretrovirals were observed in select other GBD super-regions, such as an increase in DTG in Thailand and TAF in the Latin America and Caribbean region. Amidst this variability, the use of tenofovir prodrugs was consistent across all regions. Analyses of immunologic profiles showed entry CD4 cell count and CD4/CD8 ratio were associated with region of enrollment, natal sex, total duration of ART, and nadir CD4 cell count. CD4 cell count was also associated with BMI and smoking status. The immunologic findings were consistently observed across GBD regions and in subgroup analyses by region, sex, and total duration of ART exposure.
Data from REPRIEVE provide a valuable snapshot of regional and temporal differences in the global use of antiretrovirals. Although the observed patterns of ART use likely reflect the availability of specific agents across different regions, changes in practice guidelines, and preferences by providers and patients, there are few published reports, to our knowledge, of patterns of ART use across regions [11]. Studies of contemporaneous populations across multiple global regions in the current era of ART are lacking though there are reports of first- and second-line therapy use [12, 13].
Analysis of the immunologic profiles of study participants yielded several key insights. As expected, nadir CD4 cell count was associated with CD4 at entry with lower nadirs tracking with lower entry CD4 cell counts, even in the subpopulation with extensive ART exposure. Higher CD4 cell counts were associated with female natal sex, longer exposure to ART, higher BMI, and current smoking across all regions. Higher CD4/CD8 ratios, a measure of immune reconstitution, were similarly associated with longer ART exposure, higher nadir CD4 cell counts, female natal sex, and higher BMI.
The addition of WBC counts to the adjusted models eliminated the association between CD4 cell counts and smoking. Similarly, there was no longer an association observed between CD4 cell counts with higher BMI and a higher CD4/CD8 ratio. We suspect that higher WBC counts correlate with more immune activation and may be more closely correlated than smoking, BMI and CD4/CD8 ratios. Indeed, we demonstrated that higher WBC counts are associated with higher CD4 and CD8 cell counts (Supplementary Table 2). There have been a number of observations in persons without HIV demonstrating higher leukocyte and CD4 cell counts associated with cigarette smoking [14–16]. The proinflammatory state induced by cigarette smoke is hypothesized to lead to both higher CD4 and higher CD8 cell counts.
Numerous studies have demonstrated that lower nadir CD4 cell counts are associated with incomplete recovery of CD4 cell counts in response to treatment with ART [17, 18]. Similarly, CD4 cell counts rise in a predictable manner over time with ART treatment [19–21]. There are a also number of studies indicating that women have higher CD4 cell counts than men, which is what we observed in the current study [22–24]. Nadir CD4 cell counts and CD4/CD8 ratios have been demonstrated to be important predictors of clinical outcomes in PWH [25, 26]. In particular, CVD outcomes are associated with lower nadir CD4 cell counts [27, 28].
Finally, higher CD8 cell counts were observed in sub-Saharan Africa, South Asia and Latin America and Caribbean GBD regions than in high-income and Southeast/East Asia regions. The explanation for these differences is not clear. It is possible that unmeasured inflammatory stimuli in some regions may amplify CD8 cell counts [29]. Petoumenos et al [30] reported similar CD8 cell counts between a cohort in Southeast Asia and a high-income country cohort in Australia. This is similar to what we observed. We were not able to find publications that compared CD8 cell counts between other GBD regions in the era of potent ART. Further investigation is required to understand these differences by GBD regions.
Recently, since 2019, weight gain and higher BMIs have been reported in PWH, particularly with the initiation of INSTI-based ART [31–33]. Interestingly, we noted greater proportions of participants receiving INSTI-based entry regimens with increasingly higher entry BMIs, which were most striking among participants starting their entry regimen within a year of REPRIEVE enrollment (Supplementary Figure 7). In the Women’s Interagency Study of HIV, women switching to INSTIs gained an average 2.1 kg with an increase of 0.8 in BMI, compared with women who remained on non-INSTI ART regimens [33]. DTG use was associated with more weight gain over 18 months in the Vanderbilt cohort (6- vs 2.6-kg increase with NNRTIs; P < .05) [31].
Of note, not all groups have documented increased weight gain with INSTI use [34, 35]. The observations within the REPRIEVE cohort are consistent with most of published reports of higher BMI with the use of INSTIs but must be interpreted with caution. ART in the REPRIEVE trial is not randomized, and these descriptive, cross-sectional analyses make no adjustments to control for confounding by indication, whereby heavier people may have been assigned to INSTI-based ART. Further prospective studies are needed, and REPRIEVE may be leveraged in the future to assess potential confounding factors associated with INSTI use and BMI. In addition, the relationships between INSTI use, weight gain, and major adverse cardiovascular events can be evaluated in REPRIEVE to determine whether there are cardiovascular consequences of weight gain in this population.
Another interesting observation was the association between BMI and immunologic profiles. Lower CD4 and CD8 cell counts were associated with lower BMI. For CD4 cell counts, there was an overall positive association across the full BMI distribution. Although the associations were most consistently seen in high-income and sub-Saharan African GBD super-regions, they were robust, remaining apparent in multivariable analyses controlling for GBD region, race, natal sex, ART entry regimen, prior ART exposure, and nadir CD4 cell counts, and in subgroup analyses by region, natal sex, and prior ART exposure.
Several other groups have evaluated the relationship between BMI and CD4 T-cell recovery. In the ALLRT trial, investigators reported a blunted CD4 cell recovery in those with lower BMI [36]. In contrast, the NA-ACCORD investigators found a more complicated relationship with attenuation of CD4 cell recovery in more obese populations [37]. A significant literature exists relating increasing weight to improved immune function [38]. Teleologically, greater weight provided a greater survival advantage to fight infectious diseases, such as tuberculosis [39, 40].
Moreover, existing data suggest an important relationship between adipose tissue and immune function. Some data suggest that leptin signaling can explain the link between adiposity and immune function [41–44]. For example, leptin deficiency is associated with a reduction in CD4 T cells and overall T-cell production, with consequential increase in childhood infections [43, 44]. Mice deficient in leptin have a reduction in circulating T cells, and leptin replacement has been shown to increase T-cell response, reversing starvation-induced immunosuppression [42]. The cohort enrolled in REPRIEVE have been taking ART for a median of almost 10 years, and the vast majority have HIV-1 RNA levels below the assay quantification limits or at very low quantifiable levels. This cohort likely reflects PWH who have experienced substantial CD4 cell recovery with the use of ART. Thus, the positive association between CD4 and CD8 cell counts and BMI may reflect the level of immunologic recovery in response to ART. The REPRIEVE trial is an ideal setting to improve our understanding of the cardiometabolic consequences of weight gain, BMI, and the interplay with the immune system.
The current analysis has a number of strengths and weaknesses. Although REPRIEVE carefully assessed ART use and other key immune and participant characteristics in a global population across GBD regions, in the current era of ART, it is a cross-sectional evaluation of individuals >40 years old who have enrolled in a randomized clinical trial, and the results may not be applicable to broader populations with HIV. The study population has a well-preserved CD4 cell count (median, 620/ µ L) and other immunologic parameters that are similar to those in other large cohorts. Nonetheless, we did recruit a relatively healthy population interested in pursuing CVD prevention. Some of the results in this study may reflect the known evolution in changes of the immune system over time in response to ART and differences in the severity of illness between study participants before enrollment and may not be related to the use of specific antiretroviral regimens. The absence of longitudinal clinical information before enrollment makes it difficult to know why specific prior ART regimens or agents were chosen or changed. However, the patterns track with the availability of different antiretrovirals and evolving practice guidelines [45]. We do not have information on within-individual changes in specific parameters, such as BMI, CD4 cell count, and CD4/CD8 ratio, that might influence choice of antiretrovirals; firm conclusions are thus challenging to make.
In summary, we have demonstrated differences in the use of ART regimens and specific antiretrovirals over time and region among a global cohort of REPRIEVE trial participants. A number of associations between demographic and health related factors were observed with CD4 cell counts and CD4/CD8 ratio. The most important implications of this analysis are that the study population recruited is reflective of PWH with suppressed viral replication and preserved CD4 cell counts, representing the ideal group to study CVD prevention. Furthermore, knowing the ART history and entry immune profile sets the stage for future analyses that can correlate with prospective clinical outcomes with the REPRIEVE trial. These observations should also help inform future prospective analyses of the relationship between ART, the immune system, other specific health characteristics and outcomes within other global studies of PWH.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation and contributions to the trial. They also thank the AIDS Clinical Trial Group (ACTG) for clinical site support; ACTG Clinical Trials Specialists for regulatory support; the data management center, Frontier Science Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support.
REPRIEVE Investigators – Author List: Adrian Curran, MD, PhD, Adrienne Baranauskas, RN, BSN, Aimee Wilkin, MD, MPH, Alexandra J. Abrams-Downey, MD, Allison Ross Eckard, MD, Alysse G. Wurcel, MD MS, Ana González-Cordón, MD, PhD, Armando Paez, MD, Beverly E. Sha, MD, Breno Santos, MD, Carina Beppu Yoshida, RN, Charlotte-Paige Rolle, MD, MPH, Charurut Somboonwit, MD, Connie Funk, RN, MPH, Cristina Gómez-Ayerbe, MD, PhD, Cynthia Frank, PhD, RN, Daniel E. Nixon, DO, PhD, Daniel J. Skiest, MD, David Choi, MPH, David M. Mushatt, MD, MPH&TM, David Rial-Crestelo, Deborah K. Perez, RN, CCRP, Deirdre J. Burke, MPH, Desirée V. G. dos Santos, MD, Dushyantha T. Jayaweera, MD, Edward M. Gardner, MD, Edwin DeJesus, MD, FACP, FIDSA, Elizabeth Connick, M.D., Emerline G. Lam, BS, Emily J. Hecker, RN, MSN, Esper G. Kallas, MD, PhD, Esteban Martinez, MD, PhD, Evan Waters, RN, BSN, BSH, Frank Rhame, MD, Fred R. Sattler, MD, Gary P. Wang, MD, PhD, Helen May Enrile Seedhom, RN BSN, Isabel C. F. Tavares, MD, MSc, Jack T. Stapleton, M.D., Jaclyn Bennet, MBBCH (Wits), James B. Brock, MD, MSCI, James Scott, BSN, Janet Forcht, RN, Javier R. Lama, MD, MPH, Javier Valencia, MD, Jennifer K. Brumfield, RN, CCRC, MHS, Jennifer K. Brumfield, RN, CCRC, MHS, Joaquin Portilla, MD, Jonathan Kumar, BS, Jordi Navarro, MD, Jorge A. Pinto, MD, DSc, Jose G. Castro, MD, Jose I. Bernardino, MD, Jose L. Casado, José Valdez Madruga, MD, Josu Baraiaetxaburu, Juan Berenguer, MD, PhD, Judith A. Aberg, MD, Judith S. Currier, MD, MSc, Karen T. Tashima, MD, Ken Ho, MD, MPH, Laura V. L. Costa, Leire Perez Latorre, MD, PhD, Lerato Mohapi, MBBCh, López-González L., MD, Lori E. Fantry, MD, MPH, Lourdes Domínguez-Domínguez, Luz Martín-Carbonero, Lynne M. Cornelissen, MBChB, DipHIVMan, Mamta K. Jain, MD, MPH, Mar Masiá, Marcus V. G Lacerda, PhD, Maria Saumoy, PhD, Marije Van Schalkwyk, MD, GH&TM, Marina Villalobos-Hernández, MD, Mark Mall, RN, Maureen E. Kubat, RN, BSN, Melissa Carreres, Michael Frank, MD, Michael J. Kozal, MD, Mireia De la Peña, Nadim Salomon, MD, Nagalingeswaran Kumarasamy, MBBS,FRCP,PhD, Natapol Kosashunhanan, MD, Ntebo D. Mogashoa, MBChB, PDM, Nwora Lance Okeke, MD, MPH, Pamela G. Mukwekwerere, MBChB, Patcharaphan Sugandhavesa, MD, Pilar Vizcarra, Princy N. Kumar, MD, FIDSA, MACP, Renee Weinman, MPPM, Richard M. Novak, MD, Rina Chaudhary, PhD, EDS, Rodney Dawson, MD, Roger Bedimo, MD, Romina Chinchay, MSc, MBA, Sandy Pillay, MD, Sara H. Bares, MD, Sharlaa Badal-Faesen, MBBCH, Sharon L. Walmsley, CM, MD, Sigrid Perez-Frontera, MD, AAHIVS, Sondra Middleton, MHS, PA-C, Stockton Mayer, DO, Suzanne L. Adams, RN, BSN, Umesh G. Lalloo, MBChB, FCP, MD, Vicente Estrada, Vicky Watson, RN, Victor Chiang.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI) or the National Institute of Allergy and Infectious Diseases; the National Institutes of Health (NIH); or the US Department of Health and Human Services
Financial support. This work was supported by the NHLBI (grants U01 HL123336 and U01 HL123339) and the National Institute of Allergy and Infectious Diseases (grants UM1 AI068636, UM1 AI106701, and UMI AI069501).
Supplement sponsorship. This supplement is sponsored by Massachusetts General Hospital, through NIH funding including U01HL123336.
Potential conflicts of interest. C. J. F. reports grants from Gilead Sciences, ViiV Healthcare, Janssen, Merck, Amgen, and Cytodyn, as well as personal fees from Clinical Care Options and PointofCare, outside the submitted work. H. J. R. reports grants from NIH/NHLBI and Kowa Pharmaceuticals America, during the conduct of the study. J. L. C. reports grants from NIH/NHLBI and Kowa Pharmaceuticals America, during the conduct of the study. E. T. O. reports grants from NIH during the conduct of the study; grant support through his institution from ViiV Healthcare, Gilead Sciences, Janssen, and Bavarian Nordic, outside the submitted work; and personal fees from Gilead Sciences, Theratechnologies, ViiV Healthcare, and Merck, outside the submitted work. M. V. Z. reports grant support through her institution from Gilead Sciences, during the conduct of the study. J. A. A. reports grants from Massachusetts General Hospital during the conduct of the study; and grant support from Frontier Technologies and Gilead Sciences, grant support and personal fees from Janssen, Merck, GlaxoSmithKline-ViiV Healthcare, and personal fees from Medicure and Theratechnologies, outside the submitted work. K. V. F. reports an educational grant from Gilead Sciences, outside the submitted work. E. M. reports grants from Gilead Sciences Janssen, ViiV Healthcare, and MSD, outside the submitted work. J. L. reports grants from NIH and personal fees from ViiV Healthcare and Gilead Sciences, outside the submitted work. K. M. is an employee of Gilead Sciences. C. A. S. is an employee of Kowa Pharmaceuticals America. J. S. C. reports personal fees from Merck, outside the submitted work. U. H. reports grant support through his institution from Kowa Pharmaceuticals America, for the conduct of the study, and grants from MedImmune/AstraZeneca, HeartFlow, and Duke University (Abbott), and other support from Duke University (NIH) and Recor Medical, outside the submitted work. P. S. D. reports grant support from Kowa Pharmaceuticals America and Gilead Sciences, for the conduct of the study. S. K. G. reports grant support through his institution from Kowa Pharmaceuticals America and Gilead Sciences, for the conduct of the study, and from Theratechnologies, Navidea, and ViiV Healthcare, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
REPRIEVE Investigators:
Adrian Curran, Adrienne Baranauskas, Aimee Wilkin, Alexandra J Abrams-Downey, Allison Ross Eckard, Alysse G Wurcel, Ana González-Cordón, Armando Paez, Beverly E Sha, Breno Santos, Carina Beppu Yoshida, Charlotte-Paige Rolle, Charurut Somboonwit, Connie Funk, Cristina Gómez-Ayerbe, Cynthia Frank, Daniel E Nixon, Daniel J Skiest, David Choi, David M Mushatt, David Rial-Crestelo, Deborah K Perez, Deirdre J Burke, Desirée V G dos Santos, Dushyantha T Jayaweera, Edward M Gardner, Edwin DeJesus, Elizabeth Connick, Emerline G Lam, Emily J Hecker, Esper G Kallas, Esteban Martinez, Evan Waters, Frank Rhame, Fred R Sattler, Gary P Wang, Helen May Enrile Seedhom, Isabel C F Tavares, Jack T Stapleton, Jaclyn Bennet, James B Brock, James Scott, Janet Forcht, Javier R Lama, Javier Valencia, Jennifer K Brumfield, Jennifer K Brumfield, Joaquin Portilla, Jonathan Kumar, Jordi Navarro, Jorge A Pinto, Jose G Castro, Jose I Bernardino, Jose L Casado, José Valdez Madruga, Josu Baraiaetxaburu, Juan Berenguer, Judith A Aberg, Judith S Currier, Karen T Tashima, Ken Ho, Laura V L Costa, Leire Perez Latorre, Lerato Mohapi, L López-González, Lori E Fantry, Lourdes Domínguez-Domínguez, Luz Martín-Carbonero, Lynne M Cornelissen, Mamta K Jain, Mar Masiá, Marcus V G Lacerda, Maria Saumoy, Marije Van Schalkwyk, Marina Villalobos-Hernández, Mark Mall, Maureen E Kubat, Melissa Carreres, Michael Frank, Michael J Kozal, Mireia De la Peña, Nadim Salomon, Nagalingeswaran Kumarasamy, Natapol Kosashunhanan, Ntebo D Mogashoa, Nwora Lance Okeke, Pamela G Mukwekwerere, Patcharaphan Sugandhavesa, Pilar Vizcarra, Princy N Kumar, Renee Weinman, Richard M Novak, Rina Chaudhary, Rodney Dawson, Roger Bedimo, Romina Chinchay, Sandy Pillay, Sara H Bares, Sharlaa Badal-Faesen, Sharon L Walmsley, Sigrid Perez-Frontera, Sondra Middleton, Stockton Mayer, Suzanne L Adams, Umesh G Lalloo, Vicente Estrada, Vicky Watson, and Victor Chiang
References
- 1. Joint United Nations Programme on HIV and AIDS (UNAIDS). Fact sheet—latest statistics on the status of the AIDS epidemic: UNAIDS. 2017. http://www.unaids.org/en/resources/fact-sheet. Accessed 4 January 2020.
- 2. Danforth K, Granich R, Wiedeman D, Baxi S, Padian N. Global mortality and morbidity of HIV/AIDS. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, eds. Major infectious diseases. Washington, DC: The International Bank for Reconstruction and Development/The World Bank, 2017. [PubMed] [Google Scholar]
- 3. Joint United Nations Programme on HIV and AIDS (UNAIDS). Ending AIDS: progress towards the 90-90-90 targets. Global AIDS update 2019 https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data. Accessed 29 January 2020.
- 4. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann U, Lu MT, Olalere D, et al. ; REPRIEVE Investigators Rationale and design of the mechanistic substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016; 117:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grinspoon SK, Ribaudo HJ, Hoffman U, Douglas PS. Leveraging a landmark trial of primary cardiovascular disease prevention in HIV: introduction from the REPRIEVE co-principal investigators. J Infect Dis 2020; 222(Suppl 1):S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laut KG, Shepherd L, Gottfredsson M, et al. ; EuroSIDA study group Variation in antiretroviral treatment coverage and virological suppression among three HIV key populations. AIDS 2018; 32:2807–19. [DOI] [PubMed] [Google Scholar]
- 12. Gumede SB, Fischer A, Venter WDF, Lalla-Edward ST. Descriptive analysis of World Health Organization-recommended second-line antiretroviral treatment: a retrospective cohort data analysis. S Afr Med J 2019; 109:919–26. [DOI] [PubMed] [Google Scholar]
- 13. Haas AD, Keiser O, Balestre E, et al. ; IeDEA southern Africa, east Africa, and west Africa Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV 2015; 2:e271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freedman DS, Flanders WD, Barboriak JJ, Malarcher AM, Gates L. Cigarette smoking and leukocyte subpopulations in men. Ann Epidemiol 1996; 6:299–306. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen KM, Çolak Y, Ellervik C, Hasselbalch HC, Bojesen SE, Nordestgaard BG. Smoking and increased white and red blood cells. Arterioscler Thromb Vasc Biol 2019; 39:965–77. [DOI] [PubMed] [Google Scholar]
- 16. Andreoli C, Bassi A, Gregg EO, Nunziata A, Puntoni R, Corsini E. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin Chem Lab Med 2015; 53:57–64. [DOI] [PubMed] [Google Scholar]
- 17. Kroeze S, Ondoa P, Kityo CM, et al. Suboptimal immune recovery during antiretroviral therapy with sustained HIV suppression in sub-Saharan Africa. AIDS 2018; 32:1043–51. [DOI] [PubMed] [Google Scholar]
- 18. Mutoh Y, Nishijima T, Inaba Y, et al. Incomplete recovery of CD4 cell count, CD4 percentage, and CD4/CD8 ratio in patients with human immunodeficiency virus infection and suppressed viremia during long-term antiretroviral therapy. Clin Infect Dis 2018; 67:927–33. [DOI] [PubMed] [Google Scholar]
- 19. Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006; 6:280–7. [DOI] [PubMed] [Google Scholar]
- 20. Pantazis N, Papastamopoulos V, Paparizos V, et al. ; AMACS Long-term evolution of CD4+ cell count in patients under combined antiretroviral therapy. AIDS 2019; 33:1645–55. [DOI] [PubMed] [Google Scholar]
- 21. Roul H, Mary-Krause M, Ghosn J, et al. ; FHDH-ANRS CO4 CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS 2018; 32:2605–14. [DOI] [PubMed] [Google Scholar]
- 22. Giles ML, Achhra AC, Abraham AG, et al. Sex-based differences in antiretroviral therapy initiation, switching and treatment interruptions: global overview from the International Epidemiologic Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc 2018; 21:e25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gandhi RT, Spritzler J, Chan E, et al. ; ACTG 384 Team Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr 2006; 42:426–34. [DOI] [PubMed] [Google Scholar]
- 24. Boatman JA, Baker JV, Emery S, et al. ; INSIGHT START Study Group Risk factors for low CD4+ count recovery despite viral suppression among participants initiating antiretroviral treatment with CD4+ counts > 500 cells/mm3: findings from the Strategic Timing of AntiRetroviral Therapy (START) trial. J Acquir Immune Defic Syndr 2019; 81:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. ; Icona Foundation Study Group CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 27. Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010; 55:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lang S, Mary-Krause M, Simon A, et al. ; French Hospital Database on HIV (FHDH)–ANRS CO4 HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis 2012; 55:600–7. [DOI] [PubMed] [Google Scholar]
- 29. Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J Infect Dis 2015; 211:1726–34. [DOI] [PubMed] [Google Scholar]
- 30. Petoumenos K, Choi JY, Hoy J, et al. ; Australian HIV Observational Database; TREATAsia HIV Observational Database CD4:CD8 ratio comparison between cohorts of HIV-positive Asians and Caucasians upon commencement of antiretroviral therapy. Antivir Ther 2017; 22:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis 2020; 33:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerchberger AM, Sheth AN, Angert CD, et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2019:1–8. doi: 10.1093/cid/ciz853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS 2020; 34:109–14. [DOI] [PubMed] [Google Scholar]
- 35. Calza L, Colangeli V, Borderi M, et al. Weight gain in antiretroviral therapy-naive HIV-1-infected patients starting a regimen including an integrase strand transfer inhibitor or darunavir/ritonavir. Infection 2020; 48:213–21. [DOI] [PubMed] [Google Scholar]
- 36. Palermo B, Bosch RJ, Bennett K, Jacobson JM. Body mass index and CD4+ T-lymphocyte recovery in HIV-infected men with viral suppression on antiretroviral therapy. HIV Clin Trials 2011; 12:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Body mass index and early CD4 T-cell recovery among adults initiating antiretroviral therapy in North America, 1998–2010. HIV Med 2015; 16:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roth J. Evolutionary speculation about tuberculosis and the metabolic and inflammatory processes of obesity. JAMA 2009; 301:2586–8. [DOI] [PubMed] [Google Scholar]
- 39. Lin HH, Wu CY, Wang CH, et al. Association of obesity, diabetes, and risk of tuberculosis: two population-based cohorts. Clin Infect Dis 2018; 66:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yen YF, Hu HY, Lee YL, et al. Obesity/overweight reduces the risk of active tuberculosis: a nationwide population-based cohort study in Taiwan. Int J Obes (Lond) 2017; 41:971–5. [DOI] [PubMed] [Google Scholar]
- 41. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15:914–20. [DOI] [PubMed] [Google Scholar]
- 42. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998; 394:897–901. [DOI] [PubMed] [Google Scholar]
- 43. Maurya R, Bhattacharya P, Dey R, Nakhasi HL. Leptin functions in infectious diseases. Front Immunol 2018; 9:2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackey-Lawrence NM, Petri WA Jr. Leptin and mucosal immunity. Mucosal Immunol 2012; 5:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edition Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.