Abstract
Sleep-wake control is dependent upon multiple brain areas widely distributed throughout the neural axis. Historically, the monoaminergic and cholinergic neurons of the ascending arousal system were the first to be discovered, and it was only relatively recently that GABAergic and glutamatergic wake- and sleep-promoting populations have been identified. Contemporary advances in molecular-genetic tools have revealed both the complexity and heterogeneity of GABAergic NREM sleep-promoting neurons as well as REM sleep-regulating populations in the brainstem such as glutamatergic neurons in the sublaterodorsal nucleus.
The sleep-wake cycle progresses from periods of wakefulness to non-rapid eye movement (NREM) sleep and subsequently rapid eye movement (REM) sleep. Each vigilance stage is controlled by multiple neuronal populations, via a complex regulation that is still incompletely understood. In recent years the field has seen a proliferation in the identification and characterization of new neuronal populations involved in sleep-wake control thanks to newer, more powerful molecular genetic tools that are able to reveal neurophysiological functions via selective activation, inhibition and lesion of neuroanatomically defined sub-types of neurons that are widespread in the brain, such as GABAergic and glutamatergic neurons.1,2
Keywords: sleep-wake circuitry, basal forebrain, dorsal raphe, laterodorsal and pedunculopontine tegmental nuclei, lateral hypothalamus, locus coeruleus, nucleus accumbens, parabrachial nucleus, parafacial zone, rostromedial tegmental nucleus, sublaterodorsal nucleus, tuberomamillary nucleus, ventrolateral periaqueductal gray, ventrolateral preoptic area, ventral medulla, ventral tegmental area, zona incerta
Wakefulness
In the wakeful state, the brain is highly active and the cerebral cortex electroencephalogram (EEG) is desynchronized. Cortical desynchronization results from excitatory pre-synaptic inputs from subcortical wake-promoting neuronal populations. In early experiments performed in the 1940s, Maruzzi and Magoun first discovered that stimulation of the area between the pons and the midbrain produced wakefulness. They named this the reticular activating system. Since then, most of these neuronal populations and their ascending projections as well as neurochemical signatures have been extensively studied and are reviewed in multiple review articles.3–7 They include the cholinergic basal forebrain (BF),8 the histaminergic tuberomammillary nucleus (TMN),9 the orexinergic lateral hypothalamus,10 the dopaminergic ventrolateral tegmental nucleus (VTA),11 the cholinergic laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT),12 the glutamatergic parabrachial nucleus (PB)13 and the noradrenergic locus coeruleus (LC).14 Generally, the glutamatergic, cholinergic, noradrenergic and dopaminergic neurons of the reticular formation project via two main pathways. One is a dorsal pathway to the thalamus, which then relays the arousal signals through thalamocortical neurons. The second is ventral pathway, via the basal forebrain (BF), posterior and lateral hypothalamus (LH), and the medial forebrain bundle15 which contain neurons projecting to the cortex as well (Figure 1).
Figure 1: Wake-promoting nuclei and projections. Sagittal plane of a mouse brain.
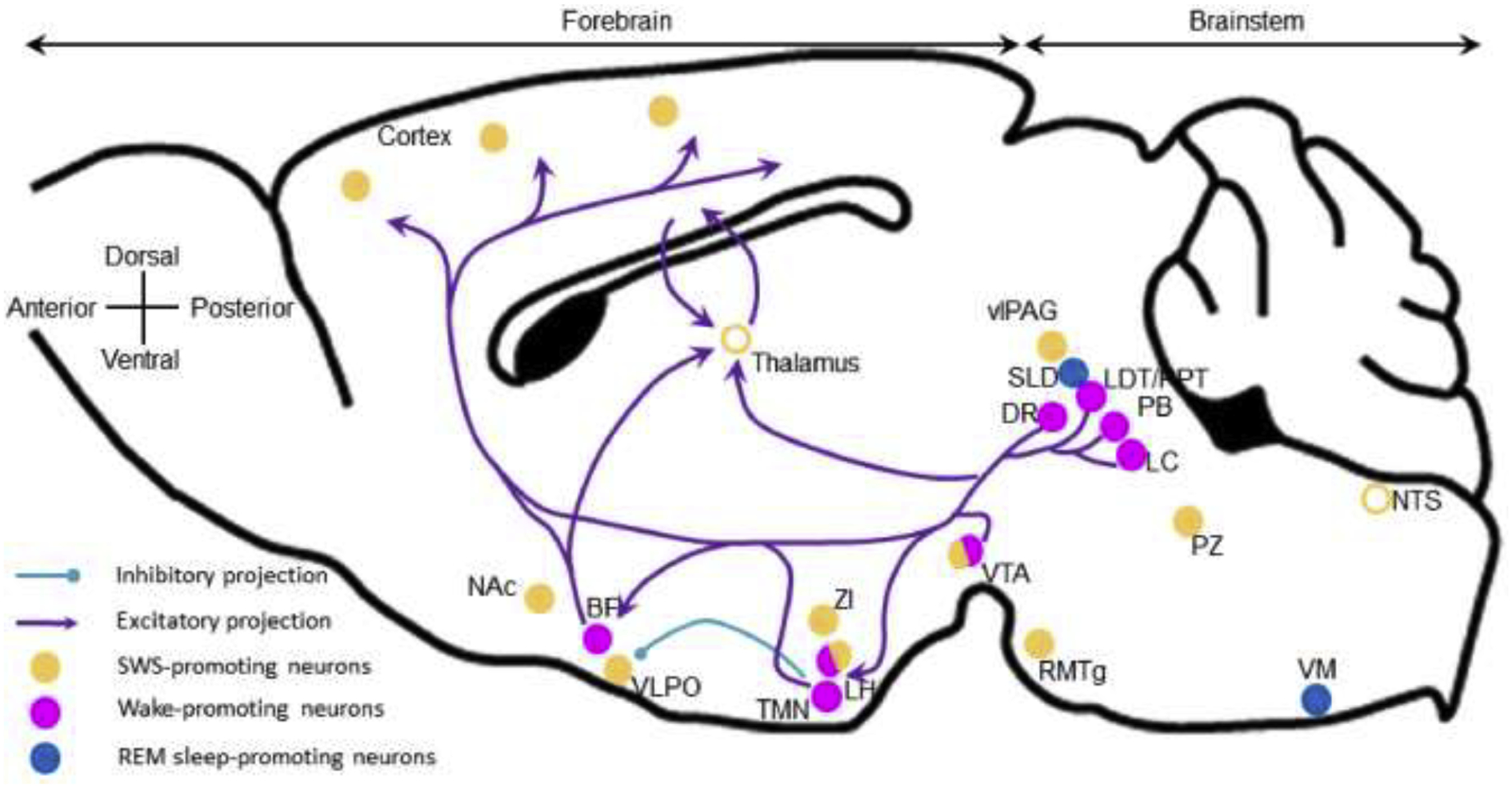
Wake-promoting neurons are located in the brainstem and in the forebrain, and project to the cortex to actively promote cortical activation and wakefulness. Histaminergic neurons located in the TMN actively inhibit VLPO sleep-promoting neurons. Open circles, brain areas contributing to sleep control but not sleep-promoting per se. BF, basal forebrain; DR, dorsal raphe; LDT/PPT, laterodorsal and pedunculopontine tegmental nuclei; LH, lateral hypothalamus; LC, locus coeruleus; NAc, nucleus accumbens; NTS, nucleus of the solitary tract; PB, parabrachial nucleus; PZ, parafacial zone; RMTg, rostromedial tegmental nucleus; SLD, sublaterodorsal nucleus; TMN, tuberomamillary nucleus; vlPAG, ventrolateral periaqueductal gray; VLPO, ventrolateral preoptic area; VM, ventral medulla; VTA, ventral tegmental area; ZI, zona incerta.
Different wake-promoting neurotransmitters seem to be specialized in promoting different aspects of wakefulness. For instance, acetylcholine is involved in cognitive functions, while noradrenaline is involved in salient experiences such as novelty and stress, as well as being involved in cognitive functions.16,17 Similarly, orexin is responsible for both consolidated wakefulness—narcolepsy being the result of the absence of this neurotransmitter—while it is also involved in motivated behaviors such as eating18 and drug seeking.19 And dopamine, which is also involved in a large number of motivated behaviors, has wake-regulatory activity, particularly during presentation of salient stimuli.20,21
Recent studies have identified GABAergic and glutamatergic wake-promoting systems. Within the BF, it was recently shown that cholinergic neurons are not wake-promoting per se but inhibit cortical synchronization.22,23 Instead, GABAergic BF neurons are responsible for cortical desynchronization and wakefulness.22,24 Similarly, it has recently been shown that in the LDT/PPT, cholinergic neurons inhibit cortical slow oscillations during NREM sleep and glutamatergic neurons are primarily responsible for wakefulness.12 An elegant series of studies has revealed that arousal from hypercapnia, as during apneic events, is accomplished via glutamatergic projections to the BF, central nucleus of the amygdala (CeA) and LH, from neurons in the lateral PB expressing calcitonin gene-related peptide.13 The posterior hypothalamus also contains GABAergic wake-promoting neurons.25,26 Within the LC, GABAergic interneurons could actively inhibit the wake-promoting noradrenergic neurons, when activated by prefrontal cortex neurons.27 Yet the cortex also promotes wakefulness through descending, likely glutamatergic, projections to the LC,28 which is similar to what is found in the neighboring PB.29 The reciprocal projections between the cortex and these, and possibly other wake-promoting areas, suggest that the cortex itself can exert executive control over wakefulness.
GABAergic projections to the VTA, from the nucleus accumbens (NAc)30 and the superior colliculus,31 play a role in the wake-promoting action of VTA dopaminergic neurons. The VTA also contains glutamatergic neurons that promote wakefulness via projections to the LH and the NAc.32 And finally, GABAergic neurons located in the bed nucleus of the stria terminalis promote wakefulness via orexinergic mechanism.33 Since these circuits all ultimately converge on the dopaminergic reward system, they provide a pathway for both wake-promoting drugs of abuse such as methamphetamine as well as the narcoleptic treatment modafinil.
The role of the thalamus in wakefulness is controversial. Large cell body lesions of the thalamus do not affect sleep-wake quantity.34 However, high frequency optogenetic stimulation of dorsomedial thalamus (DMT) calretinin-expressing neurons, paraventricular thalamic glutamatergic neurons and ventromedial thalamic neurons induces wakefulness and locomotor activity.35–37 Nonetheless, most common general anesthetics suppress cortico-thalamo-cortical activity,38 and this circuit plays a role in emergence from anesthesia.39
NREM sleep
In the sequential order of sleep-wake cycles, the first to appear following wakefulness is NREM sleep, a state of cortical EEG synchronization and low sub-cortical activity.40,41 Originally, it was believed that NREM sleep results in the cessation of sub-cortical excitatory inputs to the cortex, yet the recent discovery of many sub-cortical systems promoting NREM sleep, defined as being necessary for normal NREM sleep amount and sufficient to enhance NREM sleep, has changed our understanding of sleep control (Figure 2).
Figure 2: NREM sleep-promoting nuclei and projections.
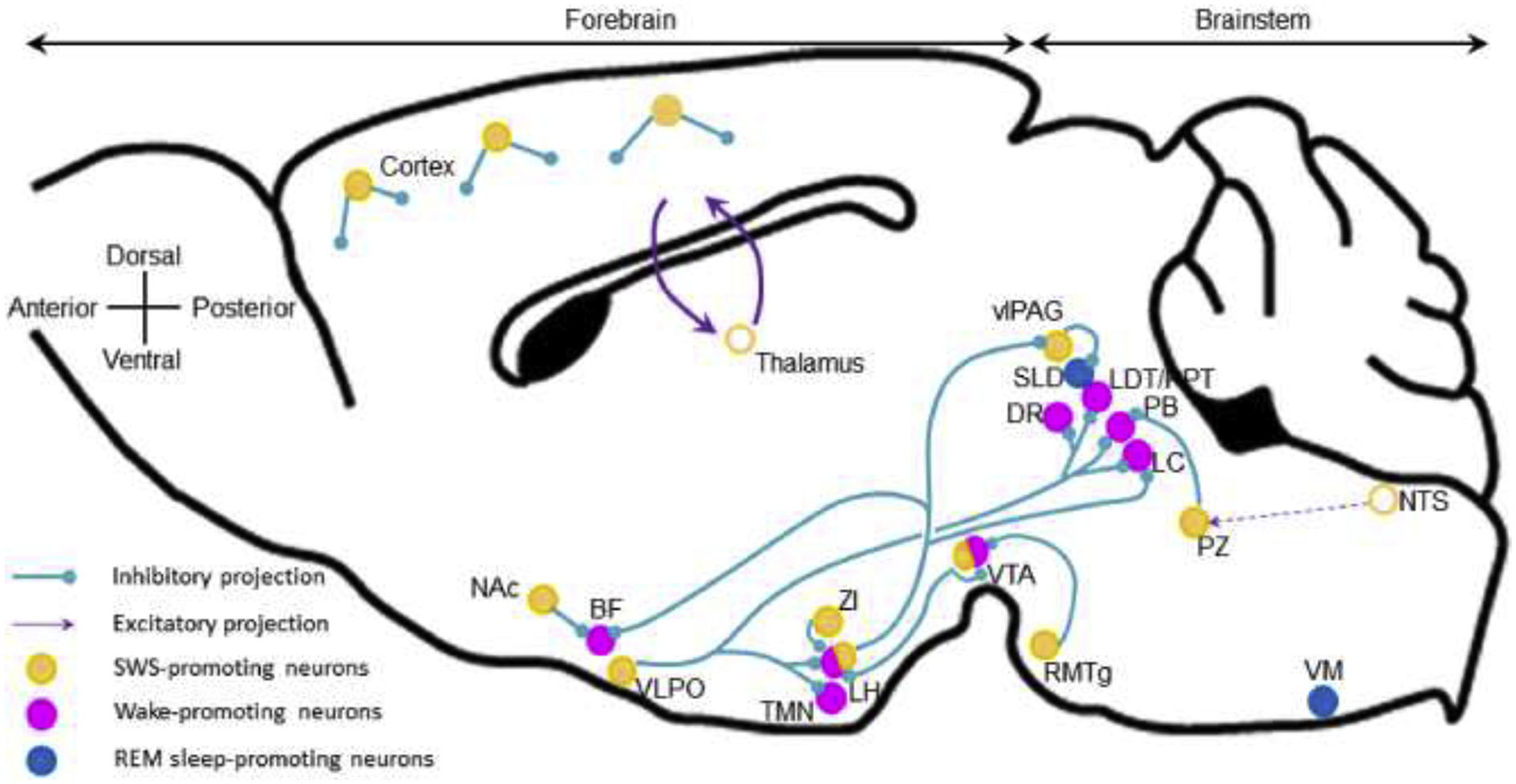
NREM-promoting neurons are distributed along the neural axis and also in the cortex. They are mainly GABAergic and inhibit wake-promoting systems. Cortical interneurons and the thalamo-cortico-thalamic feedback loop actively promotes cortical synchronization. Open circles, brain areas contributing to sleep control but not sleep-promoting per se. BF, basal forebrain; DR, dorsal raphe; LDT/PPT, laterodorsal and pedunculopontine tegmental nuclei; LH, lateral hypothalamus; LC, locus coeruleus; Nac, nucleus accumbens; NTS, nucleus of the solitary tract; PB, parabrachial nucleus; PZ, parafacial zone; RMTg, rostromedial tegmental nucleus; SLD, sublaterodorsal nucleus; TMN, tuberomamillary nucleus; vlPAG, ventrolateral periaqueductal gray; VLPO, ventrolateral preoptic area; VM, ventral medulla; VTA, ventral tegmental area; ZI, zona incerta.
Most of the sub-cortical sleep-promoting systems are GABAergic, and like the wake-promoting systems they too are distributed throughout the brain. The ventrolateral preoptic area (VLPO), located in the anterior hypothalamus, was the first known NREM sleep-promoting node. It contains inhibitory GABAergic/galaninergic neurons that are active during sleep,42,43 and project to and inhibit wake-promoting systems.44 Chemogenetic activation of VLPO galaninergic neurons increases NREM sleep amount and decreases REM sleep amount, while also significantly attenuating body temperature.45 The intersection of body temperature and sleep promotion is not uncommon for GABAergic neurons of the preoptic area in general,46 which suggests that these neurons may underlie the decrease in body temperature associated with NREM sleep.
Cortical EEG activity characteristic for each vigilance stage has long been believed to be driven by sub-cortical inputs. However, recent findings indicate that the cortex itself could also actively contribute to sleep regulation. Cortical interneurons expressing neuronal nitric oxide synthase (nNOS) control both NREM amount and slow wave activity (SWA, a marker of NREM sleep depth). Interestingly, parvalbumin-and somatostatin-expressing cortical interneurons have recently been shown to be responsible for the propagation of slow waves in the cortex.47,48 Finally, pyramidal neurons project to and regulate sub-cortical sleep-wake systems,27 including the thalamus to promote cortical synchronization.49 Thus, the cortex may also exert executive control over sleep, similar to that described above for wakefulness.
The parafacial zone (PZ) was the second sub-cortical system discovered to be involved in NREM sleep control.50 GABAergic PZ neurons (PZGABA) are both necessary and sufficient to induce deep NREM sleep, so called slow-wave-sleep (SWS) which is characterized by high amplitude cortical delta activity (Fig. 3B), a marker of cortical synchronization and sleep depth. Chemogenetic activation of PZGABA rapidly induces SWS at the expense of both wakefulness and REM sleep.51 Importantly, with SWS episodes being significantly longer in the 3–5 hour period after chemogenetic activation than during control conditions, the significant increase in SWS amounts can be attributed to the substantial increase in SWS consolidation (Figure 3). Moreover, the evoked SWS resembles SWS under control conditions in two key aspects. Firstly, it is possible to awaken the animals (Figure 3C). Secondly, unlike chemogenetic activation of VLPO galaninergic neurons,45 animals exhibit body temperature that one would normally find during SWS (Figure 3A–B). Thus, PZGABA activation induces a state that is neither akin to anesthesia nor torpor. Beyond that, increased SWS amount is associated with dramatically enhanced SWA, indicating a higher SWS quality,51 and PZGABA are so powerfully SWS-promoting that they can counteract the wake-promoting action of psychostimulants.52 Finally, intermingled glutamatergic neurons, the only other known cell type in this region thus far, seem to not play any role in sleep control.53
Figure 3: PZ chemogenetic activation of PZ GABAergic neurons promotes physiologic NREM sleep.
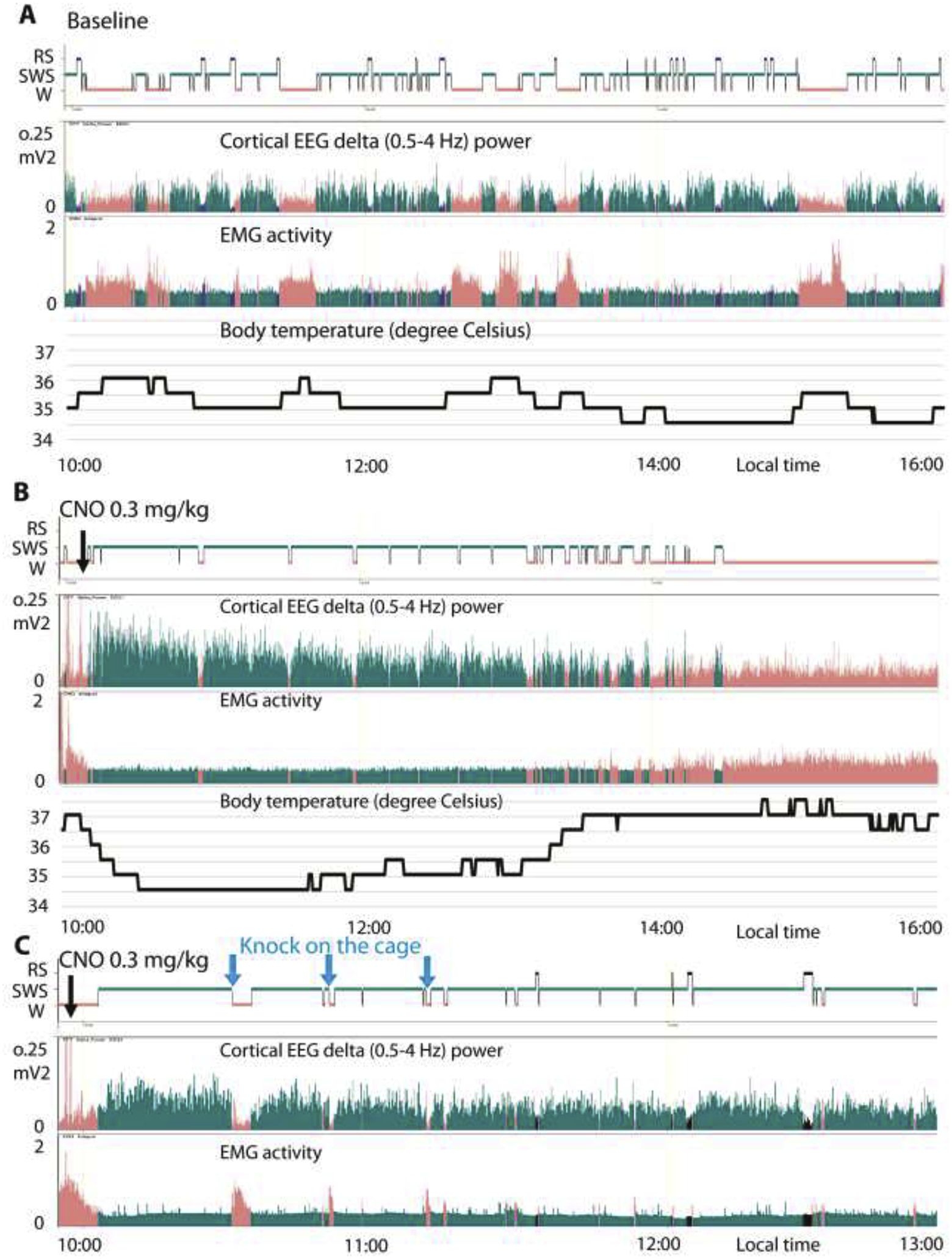
(A-B) Example hypnogram, fast Fourier transform (FFT)-derived delta (0.5–4 Hz) power, EMG activity and body temperature over 6 h of spontaneous sleep-wake cycles (A) or following clozapine N-oxide (CNO, designer drug, 0.3 mg per kg, intraperitoneal, 10 A.M.) administration (B) in a mouse with bilateral hM3Dq (excitatory designer receptor) expression in PZGABA neurons. Note that, as compared with spontaneous sleep, CNO injection rapidly induced consolidated slow-wave sleep, characterized by increased slow-wave activity (delta power), minimum muscle tone and a progressive decrease of body temperature down to the minimum body temperature recorded during spontaneous sleep. (C) Another mouse with bilateral hM3Dq was injected with CNO and woken up by knocking on the cage (blue arrows) three times, at 30, 50 and 70 min following CNO administration, during PZGABA induced SWS. This example confirms the reversibility of the state, a criteria for sleep. Salmon = wakefulness (W); cyan = slow-wave-sleep (SWS); and black = REM sleep (RS).
As mentioned above, the VTA was classically believed to be a wake-promoting center via its dopaminergic projections. However, a series of recent studies have revealed a more complex role of VTA in sleep-wake control. VTA dopaminergic projections to the dorsal striatum seem to be specialized in sleep promotion whereas VTA dopaminergic projections to the ventral striatum promote wakefulness.54 The VTA also contains GABAergic neurons that are both necessary and sufficient for sleep. Lesion and chemogenetic inhibition of VTA GABAergic neurons result in insomnia, while chemogenetic and optogenetic activation of these neurons promotes NREM sleep. The sleep-promoting action of VTA GABAergic neurons is mediated by local projections inhibiting VTA wake-promoting neurons and projections to the LH.32,55,56
Adenosine is a sleep factor which promotes NREM sleep via inhibition of wake-active neurons and also via activation of a subpopulation of VLPO sleep-promoting neurons.57 More recently, a key neuronal population mediating adenosine’s sleep-promoting action has been identified in the core region of the NAc. These neurons are inhibitory and express the adenosine receptor A2A (A2AR). They are not only necessary, but also sufficient to promote NREM sleep.58 These neurons enhance NREM sleep via inhibitory projections to the ventral pallidum (VP).58 A2AR-expressing neurons located in the rostral striatum are also NREM sleep-promoting via inhibition of parvalbumin-expressing neurons located in the external globus palidus.59
The posterior hypothalamus not only contains wake-promoting GABAergic neurons (described above) but also neighboring NREM sleep-promoting GABAergic neurons, in the zona incerta (ZI).60 ZI GABAergic neurons expressing the transcription factor Lhx6 are both sufficient and necessary for normal NREM sleep and REM sleep amount. They could control sleep by inhibiting the wake-promoting orexinergic neurons.
Known for its REM sleep inhibitory action,61,62 ventrolateral periaqueductal gray region (vlPAG) GABAergic neurons likely stabilize NREM sleep. The vlPAG contains neurons specifically active during NREM sleep.63 Optogenetic activation of vlPAG GABAergic neurons increases NREM sleep episode durations and decreases REM sleep episode durations.64 It has recently been shown that rostromedial tegmental nucleus (RMTg) GABAergic neurons are involved in NREM sleep control.65 Lesioning or inhibition of RMTg neurons decreases NREM sleep amount whereas their activation increases NREM sleep amount. This effect could be mediated by inhibition of dopaminergic neurons located in the substantia nigra compacta (SNc) and in the VTA.
Interestingly, though most of the NREM sleep-promoting neuronal populations are GABAergic/inhibitory, including a recently discovered population of GABAergic neurons co-expressing neurotensin in the CeA,66 two new glutamatergic NREM sleep-promoting neuronal populations were recently described. First, mainly REM sleep- or NREM/REM sleep-active neurons were identified in the deep mesencephalic nucleus (DpMe).63 Specific chemogenetic activation of DpMe glutamatergic neurons increases NREM sleep amounts and decreases REM sleep amounts.67 Second, perioculomotor glutamatergic neurons promote NREM sleep via projections to the preoptic area and to the ventromedial medulla.68
Finally, the rostro-ventral medullary reticular formation contains neurons that are specifically active during NREM sleep and could be involved in wakefulness to NREM sleep and NREM sleep to REM sleep transitions.69 The neurochemical identity, however, remains to be determined and necessity and sufficiency need to be confirmed using lesion/inhibition and activation of these neurons.
REM sleep
REM sleep was first described in humans as a stage of sleep associated with rapid eye movements (REM).70 Soon after, REM sleep was characterized in cat as the stage in which one observes both an active cortical EEG and muscle atonia and was therefore also named “paradoxical sleep” (PS).71 Most of the neurons involved in REM sleep control are located in the brainstem and comprise both REM-on (PS-on) and REM-off (PS-off) neurons (Figure 4).61,72 REM-on neurons are located in the sublaterodorsal nucleus (SLD; also called peri-LCα in cat). The SLD contains glutamatergic neurons that actively promote all features of REM sleep53 and can be divided into two populations, one projecting rostral to the forebrain and involved in cortical activation and hippocampal theta activity, the other projecting caudally to the ventromedial medulla (VM) and spinal motoneurons to actively promote muscle atonia. The SLD seems to also contain GABAergic neurons projecting to and inhibiting REM-off neurons.73 REM-off neurons are located in the vlPAG (GABA), lateropontine tegmentum (GABA), LDT/PPT (acethylcholine), LC (noradrenaline) and dorsal raphe (DR; serotonin), and each of these populations project to and actively inhibit REM-on neurons. These findings were recently confirmed using more advanced molecular genetic tools.62 Further, a hypothalamic regulation of REM sleep has recently been suggested. It involves GABAergic/galaninergic neurons from the dorsomedial hypothalamus74 and melanin-concentrating hormone (MCH) from the lateral hypothalamus.75 Finally, the VM seems to actively control REM sleep but the findings point to open questions to be investigated. For instance, in the rat, complete lesioning of VM neurons or chronic inactivation of inhibitory neurotransmission specifically results in REM sleep without atonia.76,77 Whereas in mice, the VM contains REM-on neurons and optogenetic activation of GABAergic neurons induces REM sleep.78
Figure 4: REM sleep-promoting nuclei and projections.
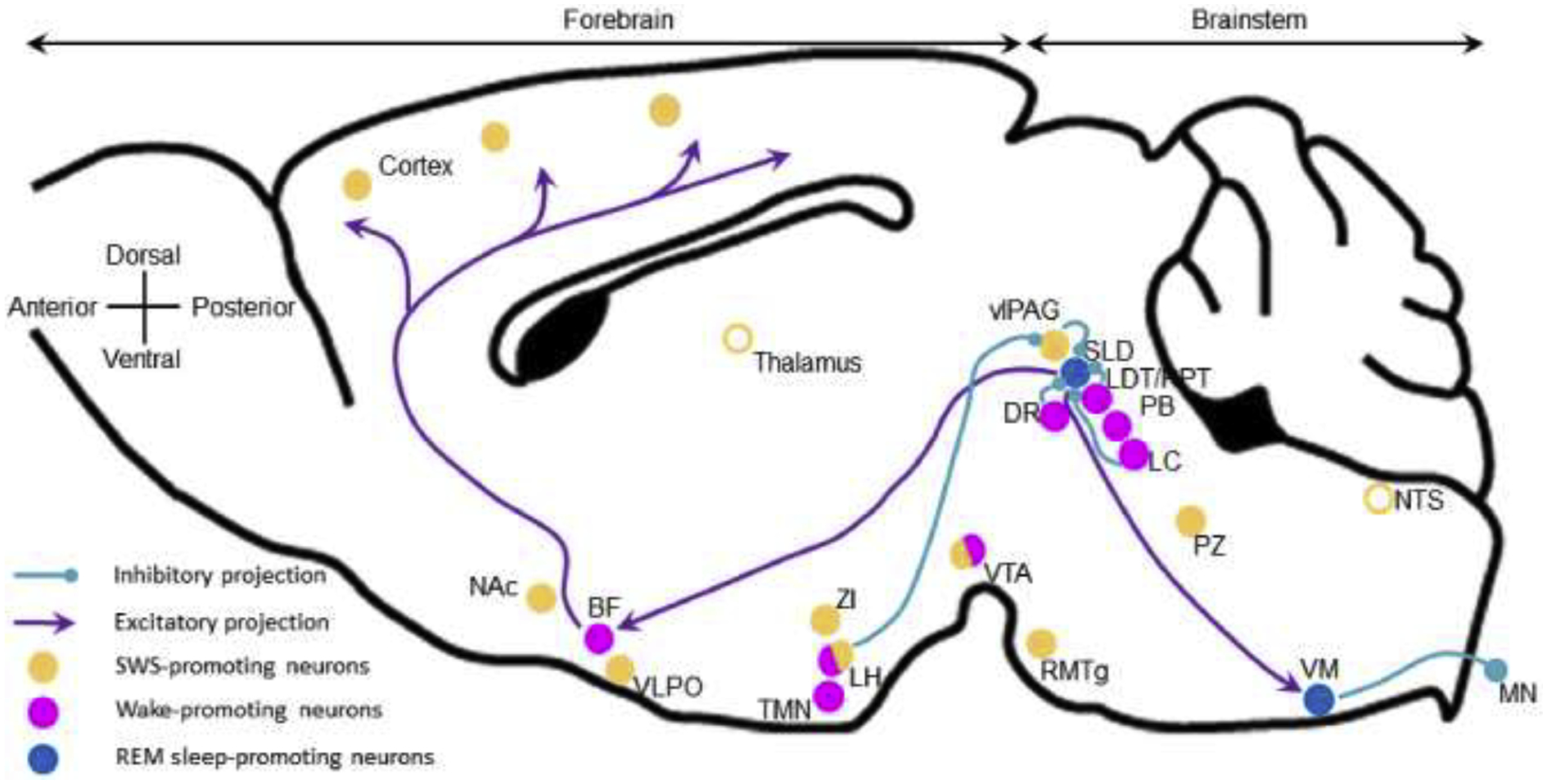
REM sleep is driven by the SLD which projects rostrally to promote cortical activation and caudally to actively drive muscle atonia. SLD REM sleep-promoting neurons are glutamatergic and receive inhibitory inputs from the pontine wake-promoting neurons. The LH contributes to REM sleep by inhibiting the vlPAG REM-off neurons. Open circles, brain areas contributing to sleep control but not sleep-promoting per se. BF, basal forebrain; DR, dorsal raphe; LDT/PPT, laterodorsal and pedunculopontine tegmental nuclei; LH, lateral hypothalamus; LC, locus coeruleus; MN, motoneurons; Nac, nucleus accumbens; NTS, nucleus of the solitary tract; PB, parabrachial nucleus; PZ, parafacial zone; RMTg, rostromedial tegmental nucleus; SLD, sublaterodorsal nucleus; TMN, tuberomamillary nucleus; vlPAG, ventrolateral periaqueductal gray; VLPO, ventrolateral preoptic area; VM, ventral medulla; VTA, ventral tegmental area; ZI, zona incerta.
Conclusion
Sleep-wake control is extremely complex, involving neuronal populations spread throughout the brain. Moreover, each vigilance stage is regulated by multiple brain areas and distinct neurochemical populations. The challenge for sleep research is now to understand how all of these neuronal populations interact and are synchronized to control sleep-wake cycle, under the homeostatic and circadian processes.3
Abbreviations list
- A2AR
adenosine receptor A2A
- BF
basal forebrain
- CeA
central nucleus of the amygdala
- CNO
clozapine N-oxide
- DMT
dorsomedial thalamus
- DpMe
deep mesencephalic nucleus
- DR
dorsal raphe
- EEG
electroencephalogram
- LC
locus coeruleus
- LDT
laterodorsal tegmental nucleus
- LH
lateral hypothalamus
- MCH
melanin-concentrating hormone
- MN
motoneuron
- NAc
core region of the nucleus accumbens
- nNos
neuronal nitric synthase
- NREM
non rapid eye movements sleep
- NTS
nucleus of the solitary tract
- PB
parabrachial nucleus
- PPT
pedunculopontine tegmental nucleus
- PS
paradoxical sleep
- PZ
parafacial zone
- PZGABA
parafacial zone GABAergic neurons
- REM
rapid eye movements sleep
- RMTg
rostromedial tegmental nucleus
- SNc
substantia nigra compacta
- SWA
slow wave activity
- SWS
slow-wave-sleep
- TMN
tuberomamillary nucleus
- vlPAG
ventrolateral periaqueductal gray region
- VLPO
ventrolateral preoptic area
- VM
ventromedial medulla
- VTA
ventral tegmental area
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
National Institutes of Health grants R00MH103399, R21NS106345, Coin for Alzheimer’s Research Trust (CART) and Citizens United for Research in Epilepsy (CURE) supported this work. The authors declare no competing financial interests.
References
- 1.Fuller PM, Yamanaka A & Lazarus M How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake. Temperature (Austin) 2, 406–417, doi: 10.1080/23328940.2015.1075095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drew VJ, Lee JM & Kim T Optogenetics: Solving the Enigma of Sleep. Sleep Medicine Research 9, 1–10, doi: 10.17241/smr.2018.00178 (2018). [DOI] [Google Scholar]
- 3.Scammell TE, Arrigoni E & Lipton JO Neural Circuitry of Wakefulness and Sleep. Neuron 93, 747–765, doi: 10.1016/j.neuron.2017.01.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eban-Rothschild A, Appelbaum L & de Lecea L Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 43, 937–952, doi: 10.1038/npp.2017.294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BE Arousal and sleep circuits. Neuropsychopharmacology, doi: 10.1038/s41386-019-0444-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saper CB & Fuller PM Wake-sleep circuitry: an overview. Curr Opin Neurobiol 44, 186–192, doi: 10.1016/j.conb.2017.03.021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RE, Basheer R, McKenna JT, Strecker RE & McCarley RW Control of sleep and wakefulness. Physiol Rev 92, 1087–1187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaborszky L et al. Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations. J Neurosci 38, 9446–9458, doi: 10.1523/JNEUROSCI.1676-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin D et al. Glutamate Activates the Histaminergic Tuberomammillary Nucleus and Increases Wakefulness in Rats. Neuroscience 413, 86–98, doi: 10.1016/j.neuroscience.2019.05.032 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Mahoney CE, Cogswell A, Koralnik IJ & Scammell TE The neurobiological basis of narcolepsy. Nat Rev Neurosci 20, 83–93, doi: 10.1038/s41583-018-0097-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oishi Y et al. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct Funct 222, 2907–2915, doi: 10.1007/s00429-017-1365-7 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Kroeger D et al. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J Neurosci 37, 1352–1366, doi: 10.1523/JNEUROSCI.1405-16.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur S et al. A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron 96, 1153–1167 e1155, doi: 10.1016/j.neuron.2017.10.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi H, Hopf FW, Li SB & de Lecea L In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness. Nat Commun 9, 5211, doi: 10.1038/s41467-018-07566-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones BE & Yang TZ The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242, 56–92 (1985). [DOI] [PubMed] [Google Scholar]
- 16.Ballinger EC, Ananth M, Talmage DA & Role LW Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 91, 1199–1218, doi: 10.1016/j.neuron.2016.09.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borodovitsyna O, Flamini M & Chandler D Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast 2017, 6031478, doi: 10.1155/2017/6031478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrigoni E, Chee MJS & Fuller PM To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology 154, 34–49, doi: 10.1016/j.neuropharm.2018.11.017 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Nevarez N & de Lecea L Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Res 7, doi: 10.12688/f1000research.15097.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oishi Y & Lazarus M The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci Res 118, 66–73, doi: 10.1016/j.neures.2017.04.008 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Cho JR et al. Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 94, 1205–1219 e1208, doi: 10.1016/j.neuron.2017.05.020 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Anaclet C et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun 6, 8744, doi: 10.1038/ncomms9744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study is the first showing that wakefulness can be promoted by GABAergic neurons. This study also showed that despite the traditionally held belief to the contrary, basal forebrain cholinergic neurons do not actively promote wakefulness.
- 23.Chen L et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology 41, 2133–2146, doi: 10.1038/npp.2016.13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim T et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A 112, 3535–3540, doi: 10.1073/pnas.1413625112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venner A, Anaclet C, Broadhurst RY, Saper CB & Fuller PM A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr Biol 26, 2137–2143, doi: 10.1016/j.cub.2016.05.078 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen NP et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun 8, 1405, doi: 10.1038/s41467-017-01004-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breton-Provencher V & Sur M Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 22, 218–228, doi: 10.1038/s41593-018-0305-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gompf HS et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci 30, 14543–14551, doi: 10.1523/JNEUROSCI.3037-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saper CB Reciprocal parabrachial-cortical connections in the rat. Brain Res 242, 33–40 (1982). [DOI] [PubMed] [Google Scholar]
- 30.Luo YJ et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat Commun 9, 1576, doi: 10.1038/s41467-018-03889-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z et al. Superior Colliculus GABAergic Neurons Are Essential for Acute Dark Induction of Wakefulness in Mice. Curr Biol 29, 637–644 e633, doi: 10.1016/j.cub.2018.12.031 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Yu X et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22, 106–119, doi: 10.1038/s41593-018-0288-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodani S, Soya S & Sakurai T Excitation of GABAergic Neurons in the Bed Nucleus of the Stria Terminalis Triggers Immediate Transition from Non-Rapid Eye Movement Sleep to Wakefulness in Mice. J Neurosci 37, 7164–7176, doi: 10.1523/JNEUROSCI.0245-17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller P, Sherman D, Pedersen NP, Saper CB & Lu J Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519, 933–956 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matyas F et al. A highly collateralized thalamic cell type with arousal-predicting activity serves as a key hub for graded state transitions in the forebrain. Nat Neurosci 21, 1551–1562, doi: 10.1038/s41593-018-0251-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren S et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362, 429–434, doi: 10.1126/science.aat2512 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Honjoh S et al. Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nat Commun 9, 2100, doi: 10.1038/s41467-018-04497-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudetz AG General anesthesia and human brain connectivity. Brain Connect 2, 291–302, doi: 10.1089/brain.2012.0107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ries CR & Puil E Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol 81, 1795–1801, doi: 10.1152/jn.1999.81.4.1795 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Cirelli C, Pompeiano M & Tononi G Fos-like immunoreactivity in the rat brain in spontaneous wakefulness and sleep. Arch Ital Biol 131, 327–330 (1993). [PubMed] [Google Scholar]
- 41.Sherin JE, Shiromani PJ, McCarley RW & Saper CB Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Lin JS & Sakai K Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161, 269–292 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Szymusiak R, Alam N, Steininger TL & McGinty D Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res 803, 178–188 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Sherin JE, Elmquist JK, Torrealba F & Saper CB Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18, 4705–4721 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroeger D et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun 9, 4129, doi: 10.1038/s41467-018-06590-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida K, Li X, Cano G, Lazarus M & Saper CB Parallel preoptic pathways for thermoregulation. J Neurosci 29, 11954–11964, doi: 10.1523/JNEUROSCI.2643-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funk CM et al. Role of Somatostatin-Positive Cortical Interneurons in the Generation of Sleep Slow Waves. J Neurosci 37, 9132–9148, doi: 10.1523/JNEUROSCI.1303-17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neske GT & Connors BW Distinct Roles of SOM and VIP Interneurons during Cortical Up States. Front Neural Circuits 10, 52, doi: 10.3389/fncir.2016.00052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gent TC, Bassetti C & Adamantidis AR Sleep-wake control and the thalamus. Curr Opin Neurobiol 52, 188–197, doi: 10.1016/j.conb.2018.08.002 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Anaclet C et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci 32, 17970–17976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anaclet C et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 17, 1217–1224, doi: 10.1038/nn.3789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study described the medullary sleep-promoting system that had been suspected for decades, beginning with the early transection experiments in animals. More importantly, parafacial zone GABAergic neurons are critically involved in NREM sleep promotion and maintenance because it is one of the two neuronal populations able to induce consolidated NREM sleep and enhance cortical slow-wave activity. This study also provides a powerful model of slow-wave-sleep enhancement.
- 52.Anaclet C, Griffith K & Fuller PM Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine. Neuropsychopharmacology 43, 415–425, doi: 10.1038/npp.2017.152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erickson ETM, Ferrari LL, Gompf HS & Anaclet C Differential Role of Pontomedullary Glutamatergic Neuronal Populations in Sleep-Wake Control. Frontiers in Neuroscience 13, doi: 10.3389/fnins.2019.00755 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study is the first showing that all features of REM sleep can by induced by specific activation of sublaterodorsal glutamatergic neurons. This study also provides a unique model of REM sleep enhancement.
- 54.Qiu MH, Zhong ZG, Chen MC & Lu J Nigrostriatal and mesolimbic control of sleep-wake behavior in rat. Brain Struct Funct 224, 2525–2535, doi: 10.1007/s00429-019-01921-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury S et al. GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 8, doi: 10.7554/eLife.44928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study showed for the first time that the ventral tegmental area (VTA) actively and powerfully promotes NREM sleep. VTA GABAergic neurons show similar NREM sleep promoting effects as the parafacial zone GABAergic neurons, including long lasting NREM sleep maintenance and enhancement of cortical slow-wave activity. This study also provides a powerful model of slow-wave-sleep enhancement.
- 56.Takata Y et al. Sleep and Wakefulness Are Controlled by Ventral Medial Midbrain/Pons GABAergic Neurons in Mice. J Neurosci 38, 10080–10092, doi: 10.1523/JNEUROSCI.0598-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazarus M, Oishi Y, Bjorness TE & Greene RW Gating and the Need for Sleep: Dissociable Effects of Adenosine A1 and A2A Receptors. Front Neurosci 13, 740, doi: 10.3389/fnins.2019.00740 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oishi Y et al. Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun 8, 734, doi: 10.1038/s41467-017-00781-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan XS et al. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. Elife 6, doi: 10.7554/eLife.29055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu K et al. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548, 582–587, doi: 10.1038/nature23663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luppi PH, Peyron C & Fort P Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med Rev 32, 85–94, doi: 10.1016/j.smrv.2016.03.002 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Peever J & Fuller PM The Biology of REM Sleep. Curr Biol 27, R1237–R1248, doi: 10.1016/j.cub.2017.10.026 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Sakai K Single unit activity of periaqueductal gray and deep mesencephalic nucleus neurons involved in sleep stage switching in the mouse. Eur J Neurosci, doi: 10.1111/ejn.13888 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Weber F et al. Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nat Commun 9, 354, doi: 10.1038/s41467-017-02765-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang SR et al. The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol 16, e2002909, doi: 10.1371/journal.pbio.2002909 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma C et al. Sleep Regulation by Neurotensinergic Neurons in a Thalamo-Amygdala Circuit. Neuron 103, 323–334 e327, doi: 10.1016/j.neuron.2019.05.015 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Hayashi Y et al. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science 350, 957–961, doi: 10.1126/science.aad1023 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z et al. An Excitatory Circuit in the Perioculomotor Midbrain for Non-REM Sleep Control. Cell 177, 1293–1307 e1216, doi: 10.1016/j.cell.2019.03.041 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Sakai K Behavioural state-specific neurons in the mouse medulla involved in sleep-wake switching. Eur J Neurosci 47, 1482–1503, doi: 10.1111/ejn.13963 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Aserinsky E & Kleitman N Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274 (1953). [DOI] [PubMed] [Google Scholar]
- 71.Jouvet M & Michel F [Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat.]. C R Seances Soc Biol Fil 153, 422–425 (1959). [PubMed] [Google Scholar]
- 72.Sakai K, Crochet S & Onoe H Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Arch Ital Biol 139, 93–107 (2001). [PubMed] [Google Scholar]
- 73.Lu J, Sherman D, Devor M & Saper CB A putative flip-flop switch for control of REM sleep. Nature 441, 589–594 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Chen KS et al. A Hypothalamic Switch for REM and Non-REM Sleep. Neuron 97, 1168–1176 e1164, doi: 10.1016/j.neuron.2018.02.005 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Kroeger D, Bandaru SS, Madara JC & Vetrivelan R Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience 406, 314–324, doi: 10.1016/j.neuroscience.2019.03.020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study esthabishes an anatomical link between forebrain and brainstem REM sleep promoting systems.
- 76.Chen MC et al. Ventral medullary control of rapid eye movement sleep and atonia. Exp Neurol 290, 53–62, doi: 10.1016/j.expneurol.2017.01.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valencia Garcia S et al. Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat Commun 9, 504, doi: 10.1038/s41467-017-02761-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber F et al. Control of REM sleep by ventral medulla GABAergic neurons. Nature 526, 435–438, doi: 10.1038/nature14979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


