Abstract
There is increasing awareness that self-reported sleep abnormalities are negatively associated with brain structure and function in older adults. Less is known, however, about how objectively measured sleep associates with brain structure. We objectively measured at-home sleep to investigate how sleep architecture and sleep quality related to white matter microstructure in older adults. 43 cognitively normal, older adults underwent diffusion tensor imaging (DTI) and a sleep assessment within a six-month period. Participants completed the PSQI, a subjective measure of sleep quality, and used an at-home sleep recorder (Zeo, Inc.) to measure total sleep time (TST), sleep efficiency (SE), and percent time in light sleep (LS), deep sleep (DS), and REM sleep (RS). Multiple regressions predicted fractional anisotropy (FA) and mean diffusivity (MD) of the corpus callosum as a function of total PSQI score, TST, SE, and percent of time spent in each sleep stage, controlling for age and sex. Greater percent time spent in RS was significantly associated with higher FA (β = 0.41, p = 0.007) and lower MD (β = -0.30, p = 0.03). Total PSQI score, TST, SE, and time spent in LS or DS were not significantly associated with FA or MD (p>0.13). Percent time spent in REM sleep, but not quantity of light and deep sleep or subjective/objective measures of sleep quality, positively predicted white matter microstructure integrity. Our results highlight an important link between REM sleep and brain health that has the potential to improve sleep interventions in the elderly.
1. Introduction
The prevalence of neurodegenerative diseases is increasing as the world’s population continues to age [1]. The number of older adults living with age-related neurodegenerative diseases is predicted to increase three-fold over the next 40 years [2]. Modifiable lifestyle factors, such as sleep, may be low-cost, highly scalable approaches to delay the onset of or even prevent age-related cognitive decline.
More than half of the older adult population experiences chronic sleep disturbances including increased sleep fragmentation, difficulty falling asleep, and decreased total sleep duration [1,3,4]. Moreover, older adults spend less time in rapid eye movement (REM) and deep sleep, more time in light sleep, and have shorter and fewer sleep cycles than younger adults [4–6]. These age-related changes in sleep and sleep architecture are hypothesized to enhance the risk of cognitive decline later in life [4,7–12]. In a prospective cohort study consisting of 1,245 cognitively intact older women, lower sleep efficiency increased the chance of developing mild cognitive impairment or dementia by 150 percent [1]. Furthermore, studies have shown that adults with a shorter duration of REM and deep sleep had poorer cognitive and memory performance the next day [10,13], and those with less REM sleep showed steeper longitudinal declines in global cognition [14]. Although the association between sleep and cognitive decline is bidirectional, the above studies indicate that poor sleep may significantly contribute to the onset of age-related neurological dysfunction.
Sleep also appears to be directly linked to age-related changes in brain structure. In functionally intact, older adults, self-reported poorer sleep quality and shorter sleep duration on the Pittsburg Sleep Quality Index (PSQI) are associated with greater reduction in total cerebral gray matter, ventricular expansion, as well as reduced hippocampal and thalamic volume on structural T1 imaging [9,15]. Additionally, fragmentation of sleep-wake rhythms has been shown to account for more than 19 percent of the variance in medial temporal lobe volumes [16]. These studies show that age-related changes in sleep may affect brain structure volumes, including regions that are important for memory.
Although many studies have reported on the relationship between sleep and grey matter, less is known about sleep’s relationship with white matter in older adults. White matter is frequently injured in sleep disorders such as obstructive sleep apnea or REM sleep behavior disorder [17,18], and reduced white matter integrity can result in slower processing speeds and declined executive function [19]. Over the past five years, studies have begun investigating the relationship between white matter microstructure assessed by diffusion tensor imaging (DTI) and subjective sleep quality measures [3,20]. In a study by Sexton et al. which followed 448 community-dwelling older adults, individuals reporting poorer current sleep quality measured by the global PSQI score demonstrated reduced global FA and increased global axial diffusivity and radial diffusivity, indicating poorer white matter microstructure integrity [3]. As one of the first imaging studies to investigate the association between sleep and white matter microstructure in community dwelling older adults, the results indicate that improving sleep quality may help to maintain white matter microstructure in aging.
While the findings reported by Sexton et al. are compelling, current sleep research has been limited by the sole use of subjective, or self-reported, sleep measures such as the PSQI or sleep diaries. Poor to moderate correlations have been measured between self-reported and objective sleep measures [21–23]. In a community-based study of 969 older adults, 34% of participants under- or over-estimated their sleep duration by more than one hour of their total sleep duration as measured by actigraphy [21]. The discrepancy between self-reported sleep duration and objective sleep duration highlights the potential bias of subjective sleep measures such as the PSQI, which incorporates self-reported sleep duration into the global PSQI score. Sleep studies reporting on associations between white matter and subjective sleep measures may be influenced by participants’ inaccurately estimating their sleep duration. To further elucidate the relationship between sleep and brain white matter metrics, it is necessary to utilize objective and reliable sleep measures.
Objective sleep measures quantify participants’ overall sleep quality (i.e. total sleep time, wake time after sleep onset, and sleep efficiency) while providing nightly information on sleep architecture and fragmentation [24]. Polysomnography (PSG), actigraphy, and at-home electroencephalographic (EEG) devices are common methods for objectively measuring sleep. Unlike PSG and actigraphy, at-home EEG sleep devices allow researchers the unique opportunity to comprehensively measure typical, habitual sleep over a series of nights under ecological validity [25]. Using an at-home, simplified EEG device, we investigated how sleep architecture relates to white matter microstructure in cognitively healthy, older adults. Given previous research showing the importance of sleep quality and duration of deep sleep and REM sleep on cognition, we hypothesized that greater sleep efficiency, total sleep time, and longer time spent in deep sleep and REM sleep would be associated with higher white matter microstructure integrity.
2. Material and methods
2.1. Participants
43 community-dwelling older adults were recruited from the longitudinal Hillblom Healthy Aging Network at the University of California, San Francisco (UCSF) Memory and Aging Center. Study exclusion criteria followed protocols described by Staffaroni et al. [26]. Exclusion from the study included diagnosis of memory impairment (dementia or MCI) or other neurological and psychiatric disorders that may impact sleep and cognition (e.g. Parkinson’s disease, epilepsy, bipolar disorder, schizophrenia). Individuals with substance use disorders or serious medical conditions such as cancer, were excluded from the study. Participants who completed an at-home sleep study, had an MRI scan within 6 months of their sleep study (either before or after), and were determined to be clinically normal by a formal committee comprised of a neurologist and board-certified neuropsychologist were included in the study. Each participant provided written, informed consent. The consent form and study protocol were approved by the UCSF Committee on Human Research.
2.2. General research visit
All participants completed a general research visit through ongoing observational studies in which cognitively healthy older adults participate in at the UCSF Memory and Aging Center (Hillblom Healthy Aging Network and Mechanisms of Executive Decline studies). These visits included neuropsychological testing, neurological examination, and Clinical Dementia Rating (CDR, completed via interview with study partner). A “clinically normal” diagnosis was assigned by a formal committee if at least 2 of the following criteria were met: no cognitive concerns during the neurological examination, a participant performed within expected ranges for their age and education on neuropsychological assessments, and the study informant / partner did not raise any concerns over the participant’s cognition during the CDR interview. Blood samples were processed to obtain measures of cholesterol levels (total cholesterol:high-density lipoprotein (HDL) ratio), HOMA-IR, and Apolipoprotein E (ApoE) genotype. In addition to a full medical history and general neurological examination, the neurologists measured participants’ weight, heart rate, and systolic blood pressure. Due to collection procedures, weight, heart rate, and blood pressure were not measured on all participants. A diagnosis of hyperlipidemia (HLD) was determined by chart review (self-reported diagnosis of HLD and/or cholesterol-lowering medications listed) and by cholesterol:HDL value (>3.5).
2.3. Lifestyle questionnaires
To assess risk of sleep apnea, participants completed the Berlin Sleep Apnea Questionnaire (BQ) [27]. The BQ is a 10-item questionnaire that classifies participants as “high risk” or “low risk” for sleep apnea. Participants completed the PSQI to better understand subjective sleep quality [28]. The PSQI is a nine-item assessment that asks participants about their sleep habits over the last month. To assess physical activity, participants completed the Physical Activity Scale for the Elderly (PASE) [29]. The PASE is an 11-item questionnaire that asks participants about their leisure time, and household and work-related activity levels. Participants reported all medications they took concurrently with the sleep study.
2.4. At-home sleep assessment
Participants were asked to wear a wireless sleep-monitoring device (Zeo, Inc.) in their habitual sleeping environment for up to 10 nights. Participants were instructed to place the headband on only when they were about to start trying to fall asleep. They were also instructed to keep the headband on throughout the night, and only remove it when they were ready to get up to start their day. The headband is lightweight with three dry electrodes at approximately Fp1, Fpz and Fp2. Signals are emitted from the headband to a small bedside device, which processes the electrophysiological signals in 30 second epochs using a proprietary neural network (Zeo, Inc.) to assess latency to sleep onset, duration of wake after sleep onset, light sleep (stages 1 and 2 Non-REM sleep), deep sleep (stage 3 Non-REM sleep) and REM sleep. Previous papers have shown relatively high levels of agreement between REM sleep identified by the Zeo autoscoring algorithm versus polysomnography scorers [30,31].
The first night of data collection was removed from the dataset to account for habituation to wearing the headband device. To be included in analyses, participants needed at least three additional nights of usable data, where the amount of unscored data was less than 45 minutes across the night. The unscored data may have included periods when the participant went to the restroom where signal transmission to the device may have been lost.
Light sleep, deep sleep and REM sleep were analyzed as a percent of total sleep. Sleep efficiency was calculated as the percentage of time spent asleep divided by the total time in bed trying to sleep.
2.5. MRI acquisition
All participants completed an MRI scan within 6 months of their sleep study (either before or after). Participants were scanned at the UCSF Neuroscience Imaging Center on a Siemens Trio 3T scanner equipped with a 64-channel head coil. Volumetric MPRAGE sequences were used to acquire T1-weighted images of the entire brain (coronal slice orientation; slice thickness = 1.0 mm; in-plane resolution = 1.0 × 1.0 mm; matrix = 240 × 256; TR = 2,300 ms; TE = 3 ms; TI = 900 ms; flip angle = 9°). Diffusion-weighted images were acquired using a single-short spin-echo sequence with the following parameters: repetition time = 5300 ms; echo time = 88 ms; inversion time = 2500 ms; flip angle = 90; field of view = 256*256 mm; 2 diffusion values of b = 0 and 1000 s/mm; 12 diffusion directions; 4 repeats; 40 slices; matrix size = 128*128;voxel size = 2 mm*2 mm; slice thickness = 3 mm; and generalized auto-calibrating partial parallel acquisition = 2.
2.6. DTI processing
DTI processing began with denoising [32]. The b = 0 image was co-registered with the diffusion direction images, followed by gradient direction, eddy current and distortion correction using FSL [33,34]. Diffusion tensors were calculated using a non-linear least-squares algorithm in Dipy [35]. Registration of diffusion data was accomplished through the DTI-TK software package (http://dti-tk.sourceforge.net) based on previously published methods [36]. DTI-TK implements a tensor-based registration paradigm, maximizing the alignment of white matter structures and minimizing interpolation of DTI images. An inter-subject template was created through iterative linear and non-linear registration of diffusion tensor images. Diffusion tensor images in the group space were diagonalized into eigenvectors from which FA, MD, AD, and RD maps were calculated. FA, MD, AD, and RD maps were not skeletonized, instead the mean of each metric was taken across all voxels in each tract ROI as a whole [26,37]. Global FA, MD, AD, and RD were extracted from scalar images masked with whole brain white matter by averaging across all white matter voxels.
The corpus callosum (CC) region of interest (ROI) was extracted from the ICBM-DTI-81 white matter labels and tract atlas [38]. CC FA, MD, AD, and RD values were calculated by averaging the FA, MD, AD, or RD values of three sub-regions of the CC (genu, body, and splenium). The CC, a large white matter tract in the brain, was used as an outcome measure because this region is susceptible to injury during neurodegenerative processes, white matter microstructure is reliably measured in this region, and previous sleep studies have found significant associations between sleep quality and DTI measures of the CC [3,39–45]. We conducted sensitivity analyses investigating the relationship between REM sleep and global FA/MD, covarying for age and sex, and the same pattern of results were observed (global FA: β = 0.35, p = 0.04, global MD: β = -0.38, p = 0.01). Full models analyzing the relationship between REM sleep and global FA/MD are found in S1 Table.
2.7. Statistical analyses
All statistical analyses were conducted using Stata Statistical Software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Participants with less than three nights of sleep data were excluded from the analyses. To look for outliers in the sleep data, DTI data, and vascular risk measures, the range of values were assessed to ensure all values were biologically possible. Next, datapoints were visualized with histograms and outliers were identified as datapoints over 3 standard deviations from the mean. Based on this method, one outlier was identified. On the PSQI measure, one participant had a score of 17 (0.3 above the 3 standard deviation cut-off). As the PSQI represents a total score from a self-reported survey where 17 is a reasonable response, it was determined appropriate to retain this score in our analyses. No other outliers were found. Thus, all available data points were included in analyses. Due to study protocols, some participants had missing data. Participants with missing data were only excluded from the analysis directly affected by the missing data point and retained in all other analyses.
To understand the relationship between sleep and DTI measures, we performed multiple regressions controlling for age and sex (Model 1). For Model 1, separate regressions were conducted with each of the sleep metrics (PSQI (continuous measure), sleep efficiency, total sleep time, and percent time spent in light sleep, deep sleep, and REM sleep) individually as the predictor, and with the dependent variables being FA, MD, AD, or RD of the CC, as well as global FA or MD in a separate sensitivity analysis. For any of these relationships that were statistically significant, we fitted two additional regression models (Model 2 and Model 3). For Model 2, we considered several potential vascular health confounds including HOMA-IR, evidence of hyperlipidemia, systolic blood pressure, physical activity (PASE), and sleep apnea risk as measured by the BQ. To ensure that we accounted for potential collinearity of these measures, we assessed the variable inflation factor (VIF) for all covariates under consideration. HOMA-IR and hyperlipidemia were highly collinear (VIFs>8.7). As hyperlipidemia is used more often in clinical contexts, HOMA-IR was removed as a covariate. After this step, all VIFs were below 2, which, based on Craney et al. [46], enabled us to confidently proceed with our analyses. In Model 3, we controlled for ApoE gene status, in addition to age and sex, to determine if our results were independent of the ε4 genotype, which is associated with sleep disturbances [47,48]. We also performed follow-up analyses to determine if sleep-modifying medications contributed to the significant relationship between CC FA/MD and REM sleep.
3. Results
3.1. Demographics
Participants had a mean age of 75 ± 4.51 years (66–84 years), mean education of 18 ± 2.1 years (13–20 years), and 27 participants identified as female. All participants were cognitively normal with a mean mini-mental state examination (MMSE) of 29 ± 1.19 (26–30) and CDR box score of 0. A total of 7 participants took sleep modifying medications (sleep enhancement medication, anxiolytics, and/or antidepressants). Additional data describing DTI values and vascular risk factors are presented in Table 1.
Table 1. Diffusion tensor imaging and vascular risk factor data.
| N | Mean | Std. Dev. | Range | |
|---|---|---|---|---|
| Corpus Callosum DTI | ||||
| Fractional Anisotropy (FA) of Corpus Callosum (CC) | 43 | 0.54 | 0.04 | 0.46–0.64 |
| Mean Diffusivity (MD) of Corpus Callosum (CC) | 43 | 0.75 | 0.06 | 0.63–0.87 |
| Vascular Risk Factors | ||||
| Systolic Blood Pressure | 35 | 134.8 | 19.1 | 104–186 |
| PASE | 32 | 116.6 | 38.4 | 47–233 |
| Diagnosis of Hyperlipidemia (HLD) (N, % with HLD) | 43, 56% | - | - | - |
| Berlin Questionnaire (N, % at High Risk for OSA) | 41, 14.6% | - | - | - |
DTI and vascular risk factor data for subject cohort of 43 cognitively normal, older adults. All subjects participated in an MRI scan on a Siemens Trio 3T scanner, fasting blood draw, and comprehensive neurological assessment. DTI = diffusion tensor imaging, FA = fractional anisotropy, MD = mean diffusivity, HDL = high-density lipoprotein, bpm = beats per minute, PASE = Physical Activity Scale for the Elderly.
3.2. Sleep parameters
Participants included in our study had an average PSQI of 5.31 ± 3.79 (1–17). 13 out of 42 participants with PSQI data had a PSQI score greater than 5 and were classified as “poor” sleepers. PSQI data, however, were analyzed as a continuous variable. 6 out of 41 participants were classified as “high risk” for sleep apnea based on the BQ. Across the nights, participants spent an average of 52.2% ± 8.73% (28.2–68.7%) in light sleep, 7.75% ± 4.01 (0.73–17.9%) in deep sleep, and 26.6% ± 7.79% (11.0–47.0%) in REM sleep. Additionally, participants had a mean total sleep time of 6.45 hrs ± 0.81 hrs (4.27–7.83 hrs) and mean sleep efficiency of 84.2% ± 32.3% (64.3–97.25%) (Table 2).
Table 2. Sleep architecture and sleep quality data.
| N | Mean | Std. Dev. | Range | |
|---|---|---|---|---|
| Sleep efficiency (%) | 43 | 84.2 | 8.15 | 64.3–97.3 |
| Total sleep time (hrs) | 43 | 6.45 | 0.81 | 4.27–7.83 |
| Light Sleep (%) | 43 | 52.2 | 8.73 | 28.2–68.7 |
| Deep Sleep (%) | 43 | 7.75 | 4.01 | 0.73–17.9 |
| REM Sleep (%) | 43 | 26.6 | 7.79 | 11.0–47.0 |
| PSQI | 42 | 5.31 | 3.79 | 1.0–17.0 |
Descriptive statistics for sleep architecture and sleep quality data for subject cohort of 43 cognitively normal, older adults. PSQI = Pittsburg Sleep Quality Index, REM = rapid eye movement.
3.3. Sleep architecture and white matter microstructure
We present associations with callosal DTI as a proxy for global white matter integrity due to its susceptibility in various neurodegenerative disease processes [49]. We performed a sensitivity analysis comparing global DTI metrics to sleep measures, which showed a similar pattern of results as CC DTI metrics and sleep measures.
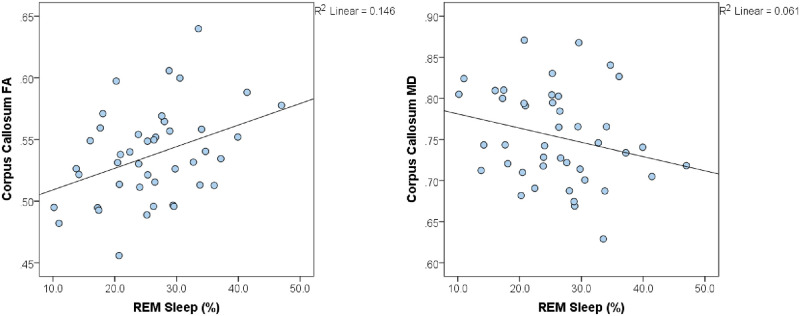
Adjusting for age and sex (Table 3, Model 1), the percent of time spent in REM sleep was significantly associated with CC FA (β = 0.41, p = 0.007) and CC MD (β = -0.30, p = 0.03) (Fig 1). Of note, REM sleep was also significantly associated with CC RD (β = -0.37, p = 0.008), but not CC AD (β = -0.15, p = 0.32) (Table 4, Model 1). Similar results were observed when comparing global FA (β = 0.35, p = 0.04), MD (β = -0.38, p = 0.01), AD (β = -0.24, p = 0.10), and RD (β = -0.25, p = 0.10 to percent of time spent in REM sleep (S1 Table, Model 1; S2 Table, Model 1). Percent time spent in light sleep and deep sleep did not significantly relate to the FA or MD of the corpus callosum (Table 5).
Table 3. Regression models of REM sleep and DTI of corpus callosum FA and MD.
| beta | t | 95% CI | p | Partial η2 | beta | t | 95% CI | P | Partial η2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1: REM Sleep | A. FA of Corpus Callosum (N = 43), R2 Adj = 0.233197 | B. MD of Corpus Callosum (N = 43), R2 Adj = 0.35023 | ||||||||
| Age | -0.37 | -2.75 | -0.005, -0.0008 | 0.009** | 0.159 | 0.56 | 4.46 | 0.004, 0.010 | <0.0001** | 0.352 |
| Sex | -0.13 | -0.89 | -0.016, 0.006 | 0.38 | 0.018 | 0.12 | 0.89 | -0.009, 0.022 | 0.38 | 0.020 |
| REM Sleep (%) | 0.41 | 2.85 | 0.0006, 0.003 | 0.007** | 0.196 | -0.30 | -2.29 | -0.004, -0.0003 | 0.03* | 0.133 |
| Model 2: REM Sleep and Vascular Risk | A. FA of Corpus Callosum (N = 33), R2 Adj = 0.092024) | B. MD of Corpus Callosum (N = 33), R2 Adj = 0.334091 | ||||||||
| Age | -0.40 | -2.00 | -0.006, 8.8e-5 | 0.05* | 0.138 | 0.60 | 3.52 | 0.003, 0.012 | 0.0004** | 0.332 |
| Sex | 0.09 | 0.44 | -0.02, 0.03 | 0.66 | 0.008 | -0.13 | -0.79 | -0.06, 0.025 | 0.0499* | 0.025 |
| Cholesterol Risk | -0.17 | -0.91 | -0.02, 0.007 | 0.37 | 0.032 | 0.13 | 0.84 | -0.011, 0.028 | 0.28 | 0.027 |
| Systolic Blood Pressure | -0.02 | -0.13 | -0.0007, 0.0006 | 0.89 | 0.001 | 0.09 | 0.56 | -0.0006, 0.0011 | 0.99 | 0.013 |
| Berlin Risk | -0.04 | -0.22 | -0.04, 0.03 | 0.82 | 0.002 | 0.12 | 0.83 | -0.03, 0.07 | 0.62 | 0.027 |
| PASE | -0.02 | -0.15 | -0.0004, 0.0003 | 0.88 | 0.001 | -0.14 | -0.94 | -0.0007, 0.0002 | 0.19 | 0.034 |
| REM Sleep (%) | 0.47 | 2.24 | 0.0002, 0.004 | 0.03* | 0.167 | -0.40 | -2.21 | -0.006, -0.0002 | 0.037* | 0.163 |
| Model 3: REM Sleep and ApoE | A. FA of Corpus Callosum (N = 42), R2 Adj = 0.222068 | B. MD of Corpus Callosum (N = 42), R2 Adj = 0.360989 | ||||||||
| Age | -0.34 | -2.42 | -0.005, -0.0005 | 0.021* | 0.134 | 0.60 | 4.65 | 0.004, 0.011 | <0.0001** | 0.383 |
| Sex | -0.15 | -1.06 | -0.02, 0.005 | 0.29 | 0.028 | 0.07 | 0.51 | -0.012, 0.02 | 0.45 | 0.007 |
| ApoE Polymorphism | 0.028 | 0.20 | -0.011, 0.028 | 0.85 | 0.0003 | 0.002 | 0.02 | -0.017, 0.017 | 0.72 | 0.0001 |
| REM Sleep (%) | 0.43 | 2.82 | 0.0006, 0.004 | 0.008** | 0.206 | -0.26 | -1.87 | -0.004, -0.0002 | 0.07 | 0.101 |
DTI = diffusion tensor imaging, REM = rapid eye movement, FA = fractional anisotropy, MD = mean diffusivity, PSQI = Pittsburg Sleep Quality Index, ApoE = Apolipoprotein E, BQ = Berlin Sleep Apnea Questionnaire, BP = blood pressure, PASE = Physical Activity Scale for the Elderly.
* = p<0.05,
** = p<0.01.
Fig 1. Percent of time spent in REM sleep is significantly associated with FA and MD of the corpus callosum.
REM sleep percentage was significantly associated with FA and MD of the corpus callosum (FA: p = 0.007, MD: p = 0.03). Each data point represents one subject, with the solid line representing the line of best fit. REM = rapid eye movement, FA = fractional anisotropy, MD = mean diffusivity.
Table 4. Regression models of REM sleep and DTI of corpus callosum AD and RD.
| beta | t | 95% CI | p | Partial η2 | beta | t | 95% CI | p | Partial η2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1: REM Sleep | A. AD of Corpus Callosum (N = 43), R2 Adj = 0.219278 | B. RD of Corpus Callosum (N = 43), R2 Adj = 0.360796 | ||||||||
| Age | 0.50 | 3.67 | 0.003, 0.012 | 0.0007** | 0.275 | 0.53 | 4.29 | 0.003, 0.010 | <0.0001** | 0.329 |
| Sex | 0.07 | 0.45 | -0.016, 0.025 | 0.65 | 0.006 | 0.14 | 1.04 | -0.007, 0.023 | 0.30 | 0.027 |
| REM Sleep (%) | -0.15 | -1.00 | -0.004, 0.001 | 0.32 | 0.027 | -0.37 | -2.81 | -0.005, -0.0007 | 0.008** | 0.19 |
| Model 2: REM Sleep and Vascular Risk | A. AD of Corpus Callosum (N = 33), R2 Adj = 0.269165 | B. RD of Corpus Callosum (N = 33), R2 Adj = 0.299156 | ||||||||
| Age | 0.54 | 3.07 | 0.003, 0.015 | 0.005** | 0.274 | 0.57 | 3.31 | 0.003, 0.012 | 0.003** | 0.304 |
| Sex | -0.1 | -0.57 | -0.068, 0.039 | 0.58 | 0.013 | -0.14 | -0.83 | -0.056, 0.024 | 0.41 | 0.027 |
| Cholesterol Risk | 0.07 | 0.43 | -0.021, 0.032 | 0.67 | 0.007 | 0.16 | 0.99 | -0.01, 0.03 | 0.33 | 0.038 |
| Systolic Blood Pressure | 0.11 | 0.65 | -0.0008, 0.002 | 0.52 | 0.017 | 0.07 | 0.43 | -0.0007, 0.001 | 0.67 | 0.007 |
| Berlin Risk | 0.14 | 0.89 | -0.039, 0.099 | 0.38 | 0.031 | 0.11 | 0.66 | -0.034, 0.068 | 0.51 | 0.017 |
| PASE | -0.21 | -1.29 | -0.001, 0.0002 | 0.21 | 0.063 | -0.09 | -0.57 | -0.0006, 0.0003 | 0.57 | 0.013 |
| REM Sleep (%) | -0.25 | -1.31 | -0.006, 0.001 | 0.20 | 0.064 | -0.47 | -2.48 | -0.006, -0.0005 | 0.02* | 0.198 |
| Model 3: REM Sleep and ApoE | A. AD of Corpus Callosum (N = 42), R2 Adj = 0.26721 | B. RD of Corpus Callosum (N = 42), R2 Adj = 0.350845 | ||||||||
| Age | 0.59 | 4.26 | 0.005, 0.013 | <0.0001** | 0.34659 | 0.54 | 4.18 | 0.010, 0.544 | 0.0002** | 0.331 |
| Sex | -0.01 | -0.10 | -0.021, 0.019 | 0.92 | 0.00011 | 0.11 | 0.81 | -0.009, 0.021 | 0.42 | 0.017 |
| ApoE Polymorphism | 0.05 | 0.35 | -0.018, 0.025 | 0.72 | 0.00296 | -0.03 | -0.19 | -0.019, 0.015 | 0.85 | 0.001 |
| REM Sleep (%) | -0.08 | -0.57 | -0.003, 0.002 | 0.57 | 0.00878 | -0.33 | -2.42 | -0.004, -0.0004 | 0.021* | 0.160 |
DTI = diffusion tensor imaging, REM = rapid eye movement, AD = axial diffusivity, RD = radial diffusivity, PSQI = Pittsburg Sleep Quality Index, ApoE = Apolipoprotein E, BQ = Berlin Sleep Apnea Questionnaire, BP = blood pressure, PASE = Physical Activity Scale for the Elderly.
* = p<0.05,
** = p<0.01.
Table 5. Associations between sleep data and DTI.
| Beta | t | p-value | |
|---|---|---|---|
| Outcome Variable: CC FA | |||
| PSQI | -0.08 | -0.49 | 0.63 |
| Sleep efficiency (%) | -0.13 | -0.85 | 0.4 |
| Total sleep time (min) | 0.11 | 0.73 | 0.47 |
| Light Sleep (%) | -0.23 | -1.56 | 0.13 |
| Deep Sleep (%) | 0.03 | 0.18 | 0.861 |
| REM Sleep (%) | 0.41 | 2.85 | 0.007** |
| Outcome Variable: CC MD | |||
| PSQI | 0.18 | 1.28 | 0.21 |
| Sleep efficiency (%) | -0.06 | -0.44 | 0.67 |
| Total sleep time (min) | -0.15 | -1.13 | 0.26 |
| Light Sleep (%) | 0.14 | 1.03 | 0.31 |
| Deep Sleep (%) | 0.02 | 0.12 | 0.91 |
| REM Sleep (%) | -0.30 | -2.29 | 0.027* |
CC FA = corpus callosum fractional anisotropy, CC MD = corpus callosum mean diffusivity, PSQI = Pittsburg Sleep Quality Index, REM = rapid eye movement.
* = p<0.05,
** = p<0.01.
The relationships between REM sleep and CC FA/MD/RD were independent of vascular risk factors and ApoE status (Table 3, Models 2A, 2B, and 3A; Table 4, Models 2B and 3B), with the exception of the relationship between CC MD and REM sleep when controlling for ApoE (Table 3, Model 3B). CC AD was not significantly related to REM sleep when controlling for ApoE gene status or controlling for vascular risk factors (p’s > 0.20) (Table 4, Models 2A and 3A).
There were no group differences in percent of REM sleep between those taking sleep modifying medications and those who do not (p = 0.277). Further, the relationship between REM sleep and CC FA/MD remained significant after removing the 7 individuals taking sleep modifying medication (CC FA: β = 0.36, p = 0.046, CC MD: β = -0.35, p = 0.030).
3.4. Sleep quality and white matter microstructure
Objectively measured total sleep time (CC FA: β = 0.11, p = 0.47, CC MD: β = -0.152, p = 0.26) and sleep efficiency (CC FA: β = -0.13, p = 0.40; CC MD: β = -0.06, p = 0.67) were not significantly associated with CC FA or CC MD (Table 5). Subjective sleep quality determined by the PSQI did not significantly relate to white matter microstructure (CC FA: β = -0.09, p = 0.61; CC MD: β = 0.18, p = 0.23).
4. Discussion
The goal of our study was to further investigate the relationship between objective sleep measures and white matter microstructure using an at-home EEG sleep device and DTI. We aimed to evaluate how sleep efficiency, total sleep time, and time spent (percent) in light sleep, deep sleep, and REM sleep, related to white matter microstructure. Surprisingly, we found that greater time spent in REM sleep (percent) was significantly related to higher CC FA, lower CC MD values, and lower CC RD values. Our results indicate that increased duration of REM sleep relates to healthier white matter microstructure in cognitively normal older adults, independent of sleep apnea, vascular, or genetic risk factors.
4.1. Measures of sleep quality
Notably, total PSQI score, a subjective measure of sleep quality, did not relate to CC FA or CC MD in our cohort of cognitively normal, older adults. These results are inconsistent with previous research findings that report a significant relationship between total PSQI score and DTI in healthy older adults and individuals with insomnia [3,40]. Our cohort of healthy older adults had a mean age of 75 years and was older than the cohort in Sexton et al., which had a mean age of 69 years. Both sleep disturbances and white matter injury increase with age, and this age difference may be contributing to the differing study outcomes [3,4,7,50]. Additionally, our results may have been inconsistent with Sexton et al. because we compared total PSQI score to a mean measure of CC FA and MD, as a proxy for whole brain white matter, while they used various DTI ROIs and CC sub-regions. Finally, our cohort consisted of 43 individuals, compared to 348 in Sexton et al. Although the percentages of individuals reporting “poor” sleep quality were similar in both studies, the differing sample sizes may also contribute to our contradicting results. The PSQI may have a small effect size, and our cohort of 43 participants may not have been sufficient to find a significant result.
Our results are consistent with recent studies investigating the relationship between measures of sleep efficiency and sleep duration with brain DTI measures [3,51]. In our study, objectively measured sleep efficiency and total sleep time, both measures of sleep quality, did not significantly relate to CC FA or CC MD. Spiegelhalder et al. found a similar result in their cohort of 24 participants with insomnia. All 24 participants completed an MRI scan and two consecutive nights of PSG sleep recording. Spiegelhalder et al. found no significant relationship between objectively measured total sleep time and FA outcome measures [51]. Furthermore, a similar result was found when comparing subjectively measured sleep duration and sleep efficiency to DTI metrics. Sexton et al. derived measures of sleep duration and sleep efficiency from the PSQI on 448 healthy older adults. Sexton et al. did not find a significant relationship between self-reported sleep duration or sleep efficiency with DTI metrics. Our results support previous studies that indicate no relationship between sleep duration, sleep efficiency and brain white matter microstructure.
Although previous research indicates an association between subjective measures of sleep quality (e.g. PSQI or sleep diaries) and white matter microstructure, it is unsurprising that few studies have found similar results when objectively measuring sleep quality. Several studies have reported a high-level of disagreement between objective and subjective sleep quality measures in older adults [21–23]. Subjective sleep measures are influenced by personal characteristics such as sex, age, degree of cognitive impairment, and mood and may introduce subject bias when evaluating sleep quality [21]. The unreliability of subjective sleep measures supports the need for future sleep studies to include both subjective and objective sleep measures.
4.2. REM sleep and neural white matter
There are several factors that could have contributed to our finding that the percent time spent in REM sleep was associated with white matter FA and MD in cognitively normal healthy older adults. We included many of these in our analyses to determine if the finding may be specific to REM sleep itself or to other contributing factors such as vascular health, obstructive sleep apnea, and ApoE status. These are discussed below.
4.3. Vascular health, obstructive sleep apnea, and neural white matter health
Research has shown that white matter microstructure reduces with age and is sensitive to vascular risk modifiers such as hypertension, high body mass index (BMI), high cholesterol, and low exercise [52–54]. Notably, the relationship between the percent time spent in REM sleep and CC FA/MD remained significant after co-varying for vascular risk factors. Therefore, the specific association between the percent time in REM sleep and CC FA and CC MD was not explained by variation in vascular health in this cohort.
Furthermore, moderate to severe obstructive sleep apnea (OSA) contributes to cerebral white matter change [55]. OSA is a sleep disorder characterized by obstruction to the upper airway throughout the night [17]. During OSA, it is hypothesized that the brain enters a state of hypoxia, altering cerebral blood flow, and resulting in cerebrovascular shearing [17]. Individuals with OSA spend less time in REM sleep because OSA episodes occur more frequently and last longer during REM sleep compared to non-REM sleep [56,57]. In our cohort, 6 participants were at high risk for sleep apnea as determined by the BQ. After co-varying for sleep apnea risk and other vascular risk factors (systolic blood pressure, cholesterol risk, and exercise) the relationship between REM sleep and CC FA/MD remained significant. Further research is required to determine the role of sleep apnea in this relationship using an objective, physiologic measure of sleep apnea; however, in our current data set using the BQ, we demonstrated that self-reported OSA did not strongly impact our findings.
4.5. ApoE gene status and neural white matter health
ApoE-ε4 carriers are at higher risk for cognitive decline than their non-ε4 counterparts [47]. Cognitive impairment is associated with reduced REM sleep and increased slow-wave sleep disturbances [48]. Additionally, ApoE-ε4 carriers with mild cognitive impairment (MCI) are more likely to have decreased REM sleep duration than non-carriers with MCI [48]. We therefore assessed the contribution of Apo-ε4 gene status to our findings. The association between REM sleep and CC FA/MD was independent of ApoE polymorphism. It would be of interest in future studies to incorporate amyloid and tau measures to identify how they are associated with the REM sleep and white matter relationship we are describing here.
4.6. REM sleep and neural white matter health
The potential mechanisms underlying the relationship between REM sleep and brain white matter microstructure remain unclear mechanistically. Oligodendrocyte precursor cells (OPCs) develop oligodendrocytes, which are the cells that produce myelin. In studies of OPCs and sleep, REM sleep alone was positively correlated with OPC proliferation [58]. Though OPC proliferation decreases with age and may not be occurring in our older adult cohort, this previous study supports a link between REM sleep and the promotion of white matter structural health. In further support of this potential link, our data suggests that the relationship between FA/MD and REM sleep was being driven by RD, not AD. This result could be evidence that the DTI association with REM sleep is driven by myelin loss, rather than axonal loss, however, not all research supports the validity of drawing such inferences from the AD vs RD distinction [59,60]. Future research using more sophisticated models such as neurite orientation dispersion and density imaging (NODDI) could help elucidate the biological underpinnings of this finding.
REM sleep has also been associated with regulating the chemical and physical properties of the blood brain barrier (BBB). One study found that mice deprived of REM sleep had increased BBB permeability [61]. This increase in BBB permeability could have negative consequences on overall brain health. BBB dysfunction has been associated with increased levels of inflammation and subsequent damage to brain white matter microstructure [62–65]. These studies suggest that individuals with lower amounts of REM sleep in our cohort may be at greater risk for BBB dysfunction that contributes to downstream white matter injury.
High amplitude-theta wave bursts are characteristic of REM sleep [66], and a recent study indicated that the theta waves may have a restorative effect on white matter injury [67]. Using a rat stroke model, Segal et al. divided 18 injured rats into three treatment groups. Injured rats exposed to an electromagnetic field with the frequency of theta waves for four weeks showed greater white matter integrity on brain MRI compared to controls and had histological evidence for neuronal regeneration and plasticity [68]. This preliminary study suggests that theta waves may promote recovery of brain white matter.
4.7. Limitations
This study was limited by its relatively small sample size (n = 43) and lack of longitudinal data. In some cases, it was further limited with missing variables for some participants. Our results highlight an important link between REM sleep and brain health that has the potential to improve sleep interventions in the elderly; yet, our study sample was highly educated, which may limit the generalizability of our findings to more socio-economically diverse populations. Sleep quantity and quality greatly varies with age and neurodegenerative disease processes [67]. Although we were able to collect 3–10 nights of sleep data, we were unable to obtain longitudinal sleep measures to investigate how changes in sleep habits over time influence brain white matter integrity. Furthermore, our study did not assess objective measures of sleep apnea. Quantifying sleep apneic events will help elucidate the relationship between REM sleep and white matter microstructure. Finally, additional measurements of white matter injury such as inflammation, may give insight into how REM sleep and brain white matter relate mechanistically. Future studies with larger sample sizes, longitudinal sleep data, objective measures of sleep apnea, and molecular assessments of brain injury, such as inflammation, are needed to further investigate the relationship between REM sleep and brain white matter.
5. Conclusions
Overall, our study found that subjective (total PSQI score) and objective measures (sleep efficiency and total sleep time) of sleep quality did not relate to CC FA/MD. Our results, however, do indicate that duration of REM sleep may be a greater contributor to brain white matter integrity than overall sleep quality. It is imperative that future research objectively measures sleep architecture in addition to sleep quality to elucidate the relationship between sleep and brain white matter.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
A special thank you to Alaisa Emery, Samantha Walters, Sophia Weiner-Light, Nina Djukic, Kathleen Walker, and Jonathan Varbel for their help on this research study.
Abbreviations
- ApoE
Apolipoprotein
- BBB
Blood Brain Barrier
- BMI
Body Mass Index
- BQ
Berlin Sleep Apnea Questionnaire
- CC
Corpus Callosum
- CDR
Clinical Dementia Rating
- DS
Deep Sleep
- DTI
Diffusion Tensor Imaging
- EEG
Electroencephalography
- FA
Fractional Anisotropy
- HDL
High Density Lipoprotein
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- LS
Light Sleep
- MCI
Mild Cognitive Impairment
- MD
Mean Diffusivity
- MMSE
Mini-Mental State Examination
- OSA
Obstructive Sleep Apnea
- PSG
Polysomnography
- PSQI
Pittsburg Sleep Quality Index
- REM
Rapid Eye Movement
- ROI
Region of Interest
- RS
REM Sleep
- SE
Sleep Efficiency
- TST
Total Sleep Time
- WM
White Matter
Data Availability
Data cannot be shared publicly because of participant approval limited to sharing data with collaborators. Data are available from the UCSF Institutional Data Access / Ethics Committee (contact via IRB@ucsf.edu) for researchers who meet the criteria for access to confidential data.
Funding Statement
This study was supported by the NIH-NIA grant 5R01AG032289-07 (CMW). We also received support from the Larry L. Hillblom Network grant 2014-A-004-NET (JHK) and the Rainwater Foundation (CMW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Diem SJ, Blackwell TL, Stone KL, Yaffe K, Tranah G, Cauley JA, et al. Measures of Sleep-Wake Patterns and Risk of Mild Cognitive Impairment or Dementia in Older Women. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2016. March;24(3):248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011. September;10(9):819–28. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton CE, Zsoldos E, Filippini N, Griffanti L, Winkler A, Mahmood A, et al. Associations between self-reported sleep quality and white matter in community-dwelling older adults: A prospective cohort study. Hum Brain Mapp. 2017. November;38(11):5465–73. 10.1002/hbm.23739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron. 2017. April;94(1):19–36. 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooneratne NS, Vitiello M V. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014. August;30(3):591–627. 10.1016/j.cger.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello M V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004. November;27(7):1255–73. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 7.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014. November;27(6):478–83. 10.1097/YCO.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011. October;34(10):1347–56. 10.5665/SLEEP.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo JC, Loh KK, Zheng H, Sim SKY, Chee MWL. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014. July;37(7):1171–8. 10.5665/sleep.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafortune M, Gagnon J-F, Martin N, Latreille V, Dubé J, Bouchard M, et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res. 2014. April;23(2):159–67. 10.1111/jsr.12108 [DOI] [PubMed] [Google Scholar]
- 11.Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006. April;61(4):405–10. 10.1093/gerona/61.4.405 [DOI] [PubMed] [Google Scholar]
- 12.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014. April;37(4):655–63. 10.5665/sleep.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh A-Y, Pressler SJ, Giordani BJ, Pozehl BJ, Berger AM. Integrative Review of the Relationship Between Sleep Disturbances and Episodic Memory in Older Adults. Biol Res Nurs. 2018. July;20(4):440–51. 10.1177/1099800418768070 [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep. 2015. March;38(3):411–21. 10.5665/sleep.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y-R, Fan D-Q, Gui W-J, Long Z-L, Lei X, Yu J. Sleep-related brain atrophy and disrupted functional connectivity in older adults. Behav Brain Res. 2018. July;347:292–9. 10.1016/j.bbr.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 16.Van Someren EJW, Oosterman JM, Van Harten B, Vogels RL, Gouw AA, Weinstein HC, et al. Medial temporal lobe atrophy relates more strongly to sleep-wake rhythm fragmentation than to age or any other known risk. Neurobiol Learn Mem. 2019. April;160:132–8. 10.1016/j.nlm.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Yun C-H, Thomas RJ, Lee SH, Seo HS, Cho ER, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013. May;36(5):709–715B. 10.5665/sleep.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger MM, Belke M, Menzler K, Heverhagen JT, Keil B, Stiasny-Kolster K, et al. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. Sleep. 2010. June;33(6):767–73. 10.1093/sleep/33.6.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993. August;50(8):818–24. 10.1001/archneur.1993.00540080029009 [DOI] [PubMed] [Google Scholar]
- 20.Raikes AC, Bajaj S, Dailey NS, Smith RS, Alkozei A, Satterfield BC, et al. Diffusion Tensor Imaging (DTI) Correlates of Self-Reported Sleep Quality and Depression Following Mild Traumatic Brain Injury. Front Neurol. 2018;9:468 10.3389/fneur.2018.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Berg JF, Van Rooij FJA, Vos H, Tulen JHM, Hofman A, Miedema HME, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008. September;17(3):295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- 22.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008. November;19(6):838–45. 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, et al. Comparison of Self-Reported Sleep Duration With Actigraphy: Results From the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. Am J Epidemiol. 2016. March;183(6):561–73. 10.1093/aje/kwv251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008. September;9 Suppl 1:S10–7. [DOI] [PubMed] [Google Scholar]
- 25.Lucey BP, Mcleland JS, Toedebusch CD, Boyd J, Morris JC, Landsness EC, et al. Comparison of a single-channel EEG sleep study to polysomnography. J Sleep Res. 2016. December;25(6):625–35. 10.1111/jsr.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staffaroni AM, Ljubenkov PA, Kornak J, Cobigo Y, Datta S, Marx G, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019. February;142(2):443–59. 10.1093/brain/awy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netzer N, Stoohs R, Netzer C, Clark K, Strohl K. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991. August;14(4):331–8. [PubMed] [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993. February;46(2):153–62. 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 30.Griessenberger H, Heib DPJ, Kunz AB, Hoedlmoser K, Schabus M. Assessment of a wireless headband for automatic sleep scoring. Sleep Breath. 2013. May;17(2):747–52. 10.1007/s11325-012-0757-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res. 2012. April;21(2):221–30. 10.1111/j.1365-2869.2011.00944.x [DOI] [PubMed] [Google Scholar]
- 32.Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016. November;142:394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016. January;125:1063–78. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012. August;62(2):782–90. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 35.Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keihaninejad S, Zhang H, Ryan NS, Malone IB, Modat M, Cardoso MJ, et al. An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer’s disease. Neuroimage. 2013. May;72:153–63. 10.1016/j.neuroimage.2013.01.044 [DOI] [PubMed] [Google Scholar]
- 37.Elahi FM, Marx G, Cobigo Y, Staffaroni AM, Kornak J, Tosun D, et al. Longitudinal white matter change in frontotemporal dementia subtypes and sporadic late onset Alzheimer’s disease. NeuroImage Clin. 2017;16:595–603. 10.1016/j.nicl.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori S, Wakana S, Van Zijl P, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier; 2005; [DOI] [PubMed] [Google Scholar]
- 39.Bettcher BM, Walsh CM, Watson C, Miller JW, Green R, Patel N, et al. Body mass and white matter integrity: the influence of vascular and inflammatory markers. PLoS One. 2013;8(10):e77741 10.1371/journal.pone.0077741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Tian J, Bauer A, Huang R, Wen H, Li M, et al. Reduced Integrity of Right Lateralized White Matter in Patients with Primary Insomnia: A Diffusion-Tensor Imaging Study. Radiology. 2016. August;280(2):520–8. 10.1148/radiol.2016152038 [DOI] [PubMed] [Google Scholar]
- 41.Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini AR, Sancesario G, et al. When, where, and how the corpus callosum changes in MCI and AD: a multimodal MRI study. Neurology. 2010. April;74(14):1136–42. 10.1212/WNL.0b013e3181d7d8cb [DOI] [PubMed] [Google Scholar]
- 42.Bettcher BM, Watson CL, Walsh CM, Lobach I V, Neuhaus J, Miller JW, et al. Interleukin-6, age, and corpus callosum integrity. PLoS One. 2014;9(9):e106521 10.1371/journal.pone.0106521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, et al. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiol Aging. 2006. November;27(11):1613–7. 10.1016/j.neurobiolaging.2005.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danielian LE, Iwata NK, Thomasson DM, Floeter MK. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage. 2010. January;49(2):1572–80. 10.1016/j.neuroimage.2009.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JY, Abdi H, Bakhadirov K, Diaz-Arrastia R, Devous MDS. A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage. 2012. April;60(2):1127–38. 10.1016/j.neuroimage.2011.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craney TA, Surles JG. Model-Dependent Variance Inflation Factor Cutoff Values. Qual Eng [Internet]. 2002. March 25;14(3):391–403. 10.1081/QEN-120001878 [DOI] [Google Scholar]
- 47.Spira AP, An Y, Peng Y, Wu MN, Simonsick EM, Ferrucci L, et al. APOE Genotype and Nonrespiratory Sleep Parameters in Cognitively Intact Older Adults. Sleep. 2017. August;40(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE ε4 genotype. Curr Alzheimer Res. 2012. March;9(3):290–7. 10.2174/156720512800107609 [DOI] [PubMed] [Google Scholar]
- 49.Di Paola M, Luders E, Cherubini A, Sanchez-Castaneda C, Thompson PM, Toga AW, et al. Multimodal MRI Analysis of the Corpus Callosum Reveals White Matter Differences in Presymptomatic and Early Huntington’s Disease. Cereb Cortex [Internet]. 2012. January 5;22(12):2858–66. 10.1093/cercor/bhr360 [DOI] [PubMed] [Google Scholar]
- 50.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009. December;19(4):415–35. 10.1007/s11065-009-9113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiegelhalder K, Regen W, Prem M, Baglioni C, Nissen C, Feige B, et al. Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp. 2014. July;35(7):3431–8. 10.1002/hbm.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams VJ, Leritz EC, Shepel J, McGlinchey RE, Milberg WP, Rudolph JL, et al. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp. 2013. August;34(8):1826–41. 10.1002/hbm.22030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, et al. White matter integrity in physically fit older adults. Neuroimage. 2013. November;82:510–6. 10.1016/j.neuroimage.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs HIL, Leritz EC, Williams VJ, Van Boxtel MPJ, van der Elst W, Jolles J, et al. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013. January;34(1):77–95. 10.1002/hbm.21412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H, Yun C-H, Thomas RJ, Lee SH, Seo HS, Cho ER, et al. Obstructive Sleep Apnea as a Risk Factor for Cerebral White Matter Change in a Middle-Aged and Older General Population. Sleep. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofauer B, Philip P, Wirth M, Knopf A, Heiser C. Effects of upper-airway stimulation on sleep architecture in patients with obstructive sleep apnea. Sleep Breath. 2017;21(4):901–8. 10.1007/s11325-017-1519-0 [DOI] [PubMed] [Google Scholar]
- 57.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985. April;87(4):432–6. 10.1378/chest.87.4.432 [DOI] [PubMed] [Google Scholar]
- 58.Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013. September;33(36):14288–300. 10.1523/JNEUROSCI.5102-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009. May;61(5):1255–60. 10.1002/mrm.21965 [DOI] [PubMed] [Google Scholar]
- 60.Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002. November;17(3):1429–36. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- 61.Gómez-González B, Hurtado-Alvarado G, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano JÁ, Velázquez-Moctezuma J. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res. 2013. August;10(3):197–207. 10.2174/15672026113109990002 [DOI] [PubMed] [Google Scholar]
- 62.Brück W, Bitsch A, Kolenda H, Brück Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997. November;42(5):783–93. 10.1002/ana.410420515 [DOI] [PubMed] [Google Scholar]
- 63.Wang L-Y, Tu Y-F, Lin Y-C, Huang C-C. CXCL5 signaling is a shared pathway of neuroinflammation and blood-brain barrier injury contributing to white matter injury in the immature brain. J Neuroinflammation. 2016. January;13:6 10.1186/s12974-015-0474-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain. 2015. June;138(Pt 6):1548–67. 10.1093/brain/awv077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, et al. Aging of cerebral white matter. Ageing Res Rev. 2017. March;34:64–76. 10.1016/j.arr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bastianini S, Silvani A, Berteotti C, Lo Martire V, Zoccoli G. High-amplitude theta wave bursts during REM sleep and cataplexy in hypocretin-deficient narcoleptic mice. J Sleep Res. 2012. April;21(2):185–8. 10.1111/j.1365-2869.2011.00945.x [DOI] [PubMed] [Google Scholar]
- 67.Segal Y, Segal L, Blumenfeld-Katzir T, Sasson E, Poliansky V, Loeb E, et al. The Effect of Electromagnetic Field Treatment on Recovery from Ischemic Stroke in a Rat Stroke Model: Clinical, Imaging, and Pathological Findings. Stroke Res Treat. 2016;2016:6941946 10.1155/2016/6941946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011. March;21(1):41–53. 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data cannot be shared publicly because of participant approval limited to sharing data with collaborators. Data are available from the UCSF Institutional Data Access / Ethics Committee (contact via IRB@ucsf.edu) for researchers who meet the criteria for access to confidential data.