Abstract
Genetic studies including chromosome analysis, telomere reduction and telomere activity, DNA microsatellites and loss of heterozygosity (LOH) studies have been performed on giant cell tumor (GCT) of bone however whether this primary skeletal neoplasm represents a monoclonal or polyclonal proliferation is unknown. Utilizing a new assay to study the polymorphic human androgen receptor locus (HUMARA), the ratio of maternal inactive X-chromosome to the paternal inactive X (Lyon hypothesis) is determined via a methylation - specific polymerase chain reaction (PCR) technique to detect X-chromosome polymorphisms. Characterization of the genetic tumorigenesis of this unpredictable neoplasm may lend insight into its biological behavior and offer improvements in therapeutic intervention, as new information emerges regarding osteoclastic bone resorption. Seventeen female patients with giant cell tumor of bone had their DNA harvested and their X-chromosome inactivation pattern and polymorphisms determined and compared to control. A polyclonal proliferation pattern was identified in all informative samples studied.
Keywords: HUMARA, Giant cell tumor, PCR, X-chromosome
Introduction
Giant cell tumor (GCT) of bone is a solid, primary skeletal neoplasm composed of stromal mononuclear cells and large multinucleated cells, which resemble osteoclasts. It represents 5% of primary bone tumors and has an unpredictable pattern of biological aggressiveness. This manifests as a high local recurrence rate and the infrequent occurrence of benign pulmonary metastasis. Cytogenetic studies have demonstrated telomeric associations and other chromosome abnormalities, specifically rings and markers, in as many as 50% of tumor cells in the majority of patients. Telomerase activity and telomere reduction have been demonstrated in tumor cells from GCT patients. In addition, microsatellite instability and loss of heterozygosity (LOH) studies have been performed on cells from GCT patients using several polymorphic DNA markers with no instability or LOH found [3–5].
The cells of origin and tumorigenesis of GCT remain debatable. Does it originate from hematopoietic stem cells? If so, it may represent a monoclonal genetic neoplasm such as other hematopoietic tumors (e.g., lymphoma and myeloma). Does it represent a solid tumor similar to other mesenchymal neoplasms? Most solid tumors and skeletal neoplasms are polyclonal with most tumor cells having different genetic makeup.
The purpose of this study is to determine whether GCT is monoclonal or polyclonal in origin by using a new molecular genetic assay of the X-chromosome.
Materials and methods
To assess whether the population of GCT cells is monoclonal or polyclonal in proliferation, DNA was isolated from fresh-frozen tumor and normal tissue of GCT individuals and purified using the wizard DNA clean up system (Promega, Madison, WI). Only females with giant cell tumor of bone were included in this study. Fresh-frozen tumor was obtained intraoperatively and stored in a −70 °C freezer. Control leukocytes were obtained from the individual’s peripheral blood and served as a control. A polymerase chain reaction (PCR)-based assay for methylation of the polymorphic X-linked human androgen receptor gene (HUMARA) was used to assess clonality following established protocols [1,2]. The assay involves the chemical modification of DNA with sodium bisulfite and subsequent PCR amplification. By using the assay with specific primers for the methylated allele, we obtained an X-chromosome inactivation pattern based upon the ratio of the maternal derived inactive X-chromosome to the paternal inactive X-chromo-some. The assay involves two steps: (1) chemical treatment of DNA with sodium bisulfite to convert all active unmethylated (but not the methylated) cytosines to uracil and (2) PCR amplification with primers specific for methylated versus unmethylated DNA.
DNA was treated with sodium bisulfite. DNA (1 pg) was denatured with sodium hydroxide and incubated at 55 °C overnight with hydroquinone (Sigma) and sodium bisulfite (Sigma). The DNA was purified and precipitated with ethanol. The treated DNA was then re-suspended in 50 μl TE (10 mM TRIS, 1 mM EDTA, pH 8.0). The rapid PCR methylation assay has been developed for polymorphic androgen receptor (HUMARA) gene locus where methylation of this region correlates with X-inactivation. The AR-M (methylated) primer sequence was designed for exon 1 of the HUMARA gene (Genbank accession number M35844) against the unmodified DNA sequence of the methylated inactive X-chromosome copy. The AR-U (unmethylated) primer set was designed for the amplification of the modified (sodium bisulfate) unmethylated alleles on the active X-chromosome. (The assumption being that the exon 1 nucleotides are methylated on the inactive X-chromosome and unmethylated on the active X-chromosome.) PCR was carried out at 95 °C for 10 min. The DNA was amplified for 35 cycles at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s. This was followed by a final extension at 72 °C for 10 min. The PCR products were mixed with markers and loading dye. The mixed products were separated on a 10% acrylamide gel and displayed. Stained with SYBR Gold (Molecular Probes Eugene, OR), gels were scanned and analyzed using a Kodak image station (Kodak, Rochester, NY) and densitometry data recorded for each DNA allele. If the HUMARA gene was polymorphic (two distinct bands present representing two different X-alleles) in normal tissue then clonality studies could be performed in tumor cells. If only one X-chromosome band was seen in normal cells, the subject was considered uninformative and no additional testing was done. In each case, the tumor was compared to its internal control (i.e., peripheral blood).
Results
Seventeen female GCT subjects ranging in age from 12–63 years were examined (Tables 1 and 2). In each of 16 informative cases the androgen receptor gene was polymorphic and the tumor cells studied supported polyclonal proliferation (Fig. 1). One case was uninformative and thus no information was obtained.
Table 1.
Sex, age and tumor location of patients with GCT of bone
| Subject & number | Sex | Age (years) | Location of GCT |
|---|---|---|---|
| RC 1 |
F | 43 | Right tibia |
| BC 2 |
F | 41 | Right proximal tibia |
| RE 3 |
F | 26 | Right proximal tibia |
| BG 4 |
F | 30 | Left distal femur |
| MH 5 |
F | 26 | Left posterior iliac wing |
| DJ 6 |
F | 43 | Left distal radius |
| VK 7 |
F | 40 | Right distal femur |
| BL 8 |
F | 33 | Right distal femur |
| NM 9 |
F | 63 | Right distal femur |
| HP 10 |
F | 26 | Left medial femoral condyle |
| BP 11 |
F | 12 | Sacrum |
| CR 12 |
F | 34 | Left distal femur lateral condyle |
| AS 13 |
F | 22 | Right radius |
| SS 14 |
F | 26 | Right tibia |
| MS 15 |
F | 25 | Left scapula |
| GT 16 |
F | 19 | Right distal radius |
| RW 17 |
F | 23 | Distal tibia |
Table 2.
Sex, age, informativeness and clonality status of patients with GCT of’ bone determined by androgen receptor methylation specific PCR
| Subject & Number | Sex | Age (years) | Informativenessa | Polyclonalityb |
|---|---|---|---|---|
| RC 1 |
F | 43 | − | ? |
| BC 2 |
F | 41 | + | Yes |
| RE 3 |
F | 26 | + | Yes |
| BG 4 |
F | 30 | + | Yes |
| MH 5 |
F | 26 | + | Yes |
| DJ 6 |
F | 43 | + | Yes |
| VK 7 |
F | 40 | + | Yes |
| BL 8 |
F | 33 | + | Yes |
| NM 9 |
F | 63 | + | Yes |
| HP 10 |
F | 26 | + | Yes |
| BP 11 |
F | 12 | + | Yes |
| CR 12 |
F | 34 | + | Yes |
| AS 13 |
F | 22 | + | Yes |
| SS 14 |
F | 26 | + | Yes |
| MS 15 |
F | 25 | + | Yes |
| GT 16 |
F | 19 | + | Yes |
| RW 17 |
F | 23 | + | Yes |
Indicates heterozygosity for two alleles in normal tissue.
Two different X-chromosome alleles present in the tumor cells indicating polyclonal cellular proliferation. Standardization of human androgen receptor gene methylation specific PCR using densitometry ratio (expected) = 1.0.
Fig 1.
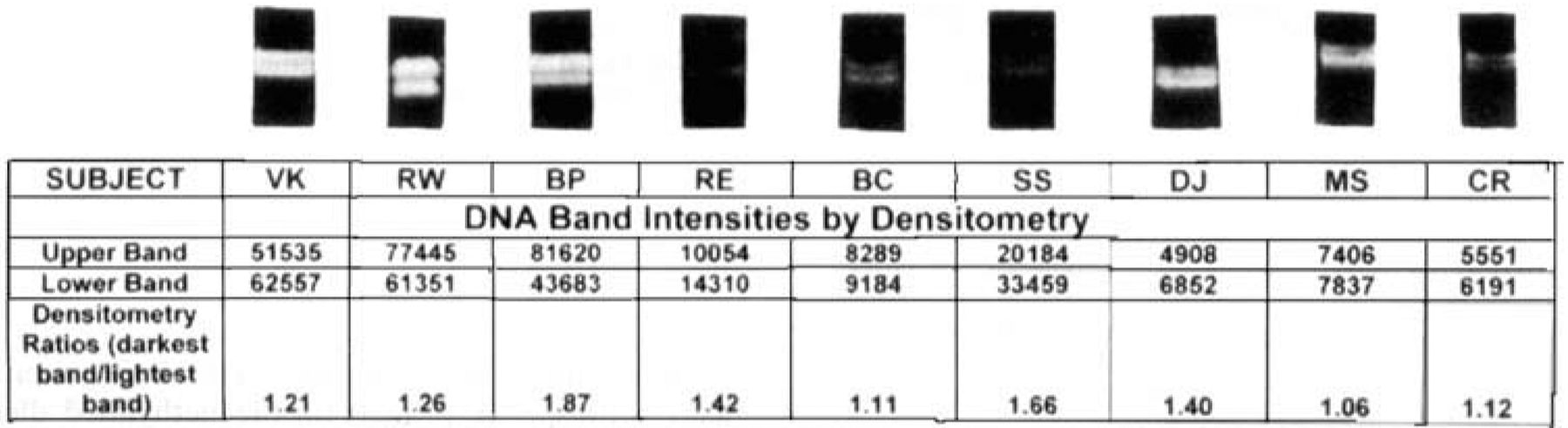
Genomic DNA extracted from GCT in females determined to have two HUMARA alleles in their peripheral blood controls. Equal densitometry ratios (~1:1 ) indicate polyclonality via random X-chromosome inactivation Clonal proliferation would manifest as chromosome skewing with different sized alleles of the HUMARA gene expressing ratios of 3 to 9:1
Sixteen informative female GCT patients were identified who were heterozygous for the HUMARA allele. This was determined by methylation specific PCR studies performed on control (peripheral blood) DNA. Similar analyses on their tumor DNA revealed dual banded gel displays. The densitometry ratios of maternally and paternally derived X-inactivation HUMARA gene alleles approximated 1: 1. This indicates inactivation of GCT X-chromosomes, as proposed in the Lyon hypothesis.
Discussion
In each female cell, a choice is made independently, with an equal probability of the maternally or paternally derived X-chromosome becoming inactive. Random inactivation occurs in the majority of females (Lyon hypothesis). However, preferential selection of X-chromosome inactivation occurs occasionally. Asymptomatic female carriers of X-linked disease can demonstrate preferential X-chromosome inactivation. In these cases, the non-mutated X-chromosome occurs in target cells representing nature’s attempt to pass on the normal genotype. Similarly, preferential selection of X-chromosome inactivation occurs in neoplasms, which proliferate in a clonal manner. Due to the polymorphic nature of the HUMARA gene and by using the rapid PCR methylation assay, 16 of the 17 GCT patients showed two different alleles of this gene in normal cells allowing for clonality studies in tumor cells. In 16 of the 16 informative patients, two different isointense bands were seen representing two different X-chromosomes in the tumor cells.
Methylation specific PCR assays offer powerful tools for studying X-inactivation studies accurately depict which X-chromosome is deleted in a (tumor) cell. When the methylated primer amplified exon 1 HUMARA unmodified methylated DNA is electrophoresed against the unmethylated primer modified DNA, a 2 × 2 gel image is created. The two columns of methylated and unmethylated DNA are created in addition to two rows representing each allele. The densitometric ratios displayed yield the ratio of X-chromosome inactivation. Typically ratios of greater than 3:l indicate monoclonality.
The therapeutic significance of understanding tumor cell biology lies in the osteolysis mechanism. Monoclonal tumors, like lymphomas, are more likely to behave in an identical pattern from cell-to-cell. Polyclonal tumors are the opposite. As osteoclast biology evolves, and mechanistic fundamentals of osteolysis emerge, it becomes that single forms of treatment may be most therapeutic for a monoclonal tumor. For example, the natural competition between osteoclastogenesis mediators, osteoprotegerin (OPG), and RANK (receptor for n-Kappa B) ligand, can be therapeutically manipulated if the majority of tumor cells utilize a similar mechanism of bone resorption. In contrast, polyclonal solid tumors offer surgical/medical oncologists their greatest challenges as no single mechanism of osteolysis predominates.
This study indicates that GCT of bone is polyclonal in its manner of proliferation. Therefore our data suggest GCTs origin is more comparable to a mesenchymal solid tumor rather than a hematopoietic neoplasm. In addition, polyclonal proliferation has also been found in giant cell tumor of tendon sheath, which is a common soft tissue tumor of synovial cell origin [6].
Acknowledgements
The authors wish to gratefully acknowledge the financial support received from Howmedica to enable this study to be performed. No benefits have been or will be received by the authors related to the subject of this article.
References
- [1].Kubota T, Das S, Christian SL, Baylin SB, Herman JG, Ledbetter DH. Methylation specific PCR simplifies imprinting analysis. Nat Genet 1997;16:16–7. [DOI] [PubMed] [Google Scholar]
- [2].Kubota T, Nonoyama S, Tonoki H, Masuno M, Imaizumi K, Kojima M, et al. A new assay for the analysis of X-chromosome inactivation based on methylation-specific PCR. Hum Genet 1999:104(1):W 55. [DOI] [PubMed] [Google Scholar]
- [3].Scheiner M, Schwartz HS, Hedges LK, Butler MG. Lack of microsatellite instability in giant cell tumor of bone. Cancer Genet Cytogenet 1996;88:35–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwartz HS, Dahir GA, Butler MG. Telomere reduction in giant cell tumor of bone and with aging. Cancer Genet Cytogenet 1993; 7 1 : 1 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz HS, Juliao SF, Sciadini MF, Miller LK, Butler MG. Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer 1995;75: 1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vogrincic GS, O’Connell JX, Gilks CB. Giant cell tumor of tendon sheath is a polyclonal cellular proliferation. Hum Pathol 1997:815–9. [DOI] [PubMed] [Google Scholar]


