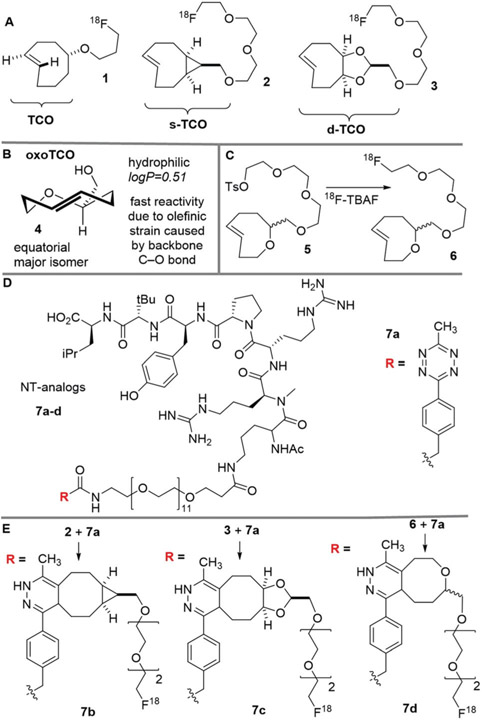
Abstract
An 18F-labeled trans-5-oxocene (oxoTCO) that is used to construct a PET probe for neurotensin receptor (NTR) imaging through tetrazine ligation is described here. PET probe construction proceeds with 70% RCY based on 18F-oxoTCO and is completed within seconds. The in vivo behaviour of the oxoTCO based PET probe was compared with those of analogous probes that were prepared from 18F-labeled s-TCO and d-TCO tracers. The hydrophilic 18F-oxoTCO probe showed a significantly higher tumor-to-background ratio while displaying comparable tumor uptake relative to the 18F-dTCO and 18F-sTCO derived probes.
Bioorthogonal reactions are unnatural reactions that can proceed in a biological context with minimal interference from biological functionalities.1-4 Tetrazine ligation—the inverse electron Diels–Alder reaction between s-tetrazines and alkenes—is a rapid biorthogonal reaction involving trans-cyclooctenes (TCOs), norbornene, cyclopropenes and α-olefins dienophiles.5-9 The fast kinetics of the tetrazine ligation with TCO has enabled a range of biomedical applications, including applications in nuclear medicine.10-13 We have shown that the rate of tetrazine ligation can be further accelerated through the use of conformationally strained trans-cyclooctene derivatives s-TCO and more hydrophilic d-TCO with rates as fast as 3.3 × 106 M−1 s−1 and 3.7 × 105 M−1 s−1, respectively (Scheme 1A).14,15
Scheme 1.
(A) 18F-labeled trans-cyclooctene radiotracers based on TCO, s-TCO and d-TCO. (B) The recently developed hetero-trans-cyclooctene oxoTCO displays fast reactivity and higher hydrophilicity due to the oxygen in the cyclic backbone. (C) Radio synthesis of 18F-labeled oxoTCO. (D) An analog of neurotensin (NT) with a conjugated tetrazine (7a) is a precursor to the 18F-labeled analogs for cancer imaging. (E) 18F-labeled NT analogs 7b–d from s-TCO, d-TCO, and oxoTCO, respectively. Only one isomer is shown for the Diels–Alder adducts.
Positron emission tomography (PET) is a non-invasive imaging modality that allows non-invasive monitoring of diverse biological processes in vivo. The most commonly used PET isotope, 18F (half-life ~ 110 min), has become widely used for the attachment of radiolabels to biological macromolecules. In order to overcome the limitations imposed by the short half-life and the low concentration of F-18, our labs have developed fast and efficient labelling methods to generate 18F-labeled PET probes with an optimized lesion-to-background contrast.16-19 In 2010, 18F-labeled TCO 1 (Scheme 1A) was described and Diels–Alder conjugates were subsequently used in a range of imaging applications.20 More recently, we introduced 18F-labeled s-TCO 2 (Scheme 1A) as the most reactive dienophile for 18F probe construction, and subsequently a similar design was used by Bormans and co-workers to prepare 18F-labeled s-TCO and d-TCO probes.21-23 While the 18F-attachment with these strained TCO probes is very efficient, the acquired images can have a relatively high background, leading to a modest target-to-background ratio. We hypothesized that the background is caused by the hydrophobicity of the probe, leading to a high background signal from both renal and hepatic pathways.24
Several approaches have been explored to improve the physiochemical properties of tetrazine ligation reaction partners. Smaller dienophiles, including cyclopropenes and cyclobutenes, have been developed as lower molecular weight alternatives to trans-cyclooctenes, but with a compromise of the reaction rate.25,26 18F-labeled dialkyl-s-tetrazines have also been developed to increase the hydrophilicity of Diels–Alder conjugates for PET imaging applications.27 Recently, oxygen-containing TCO derivatives with improved solubility properties have been described.28 A trans-5-oxocene (oxoTCO, 4) was shown to display enhanced reactivity compared to 5-hydroxy-trans-cyclooctene, and enhanced hydrophilicity (log P 0.51) relative to 5-hydroxy-trans-cyclooctene (log P 1.11) and d-TCO (log P 0.91). Here, we describe the preparation of labelling precursor 5 and 18F-labeled oxoTCO 6, and compare the in vivo imaging results for a series of probes 7b–d that target the neurotensin (NT) receptor, which is upregulated in prostate, pancreatic, lung, and colorectal cancers.29-32 Probes 7b–d were prepared by combining a tetrazine–peptide conjugate 7a with 18F-s-TCO (2), 18F-d-TCO (3) and 18F-oxoTCO (6). A significant improvement in the tumor-to-background ratio was realized by using the oxoTCO-based probe 7d in place of the more hydrophobic probes 7b and 7c.
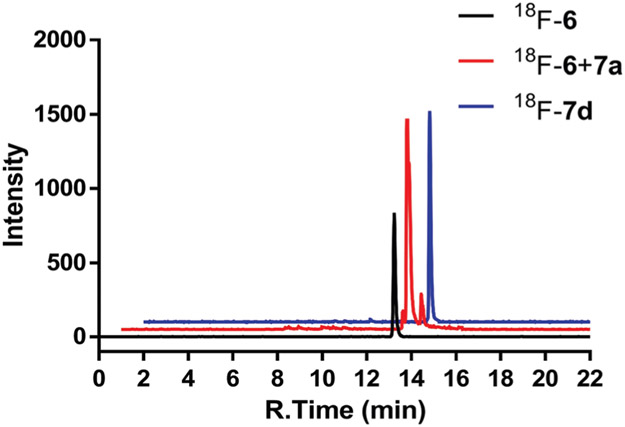
Radiochemical synthesis was modeled after our previously described procedure for preparing 18F-s-TCO 2.23 oxoTCO was prepared as a 3.4 : 1 mixture of equatorial : axial diastereomers as described previously.28 The 18F-labeling precursor 5 was prepared by treating diastereomers of oxoTCO (4) with triethylene glycol di(p-toluenesulfonate), followed by treatment with 18F-TBAF to provide 6 in 15.2 ± 1.9% radiochemical yield. Efforts to improve the radiochemical yield of 6 by increasing the concentration of 5 or prolonging the reaction time were unsuccessful. While the radiochemical yield for tosylate displacement was moderate, it is high enough to be useful and is in alignment with yields obtained in many procedures for F-18 probe construction.33 Radiolabeled s-TCO and d-TCO were prepared in a similar fashion, and cold standards were prepared using 19F-TBAF. For 18F-6, the radiochemical purity was >99% after initial purification (Fig. 1). After incubation for 1 h in PBS, the radiochemical purity of 6 was 85.2%, indicating a level of stability that was good but not as high as a cold oxoTCO compound stored under similar conditions.28 In a previous study, we found that oxoTCO decomposes more rapidly under conditions conducive to radical formation, and it may be that the radiolysis contributes to the decomposition of 6. The oxoTCO derived probe 7d was prepared by mixing 6 with 7a. As shown in Fig. 1, there is only one major peak which aligns with that of 19F-7d. No 18F-6 was left indicating a complete consumption of the 18F labeled oxoTCO. The reactions to prepare 7b and 7c were similarly efficient, and all three reactions can be completed in seconds with comparable conversion efficiency.
Fig. 1.
Radio-HPLC profile of freshly prepared 18F-6, crude reaction of 18F-6 and 7a and freshly prepared 18F-7d.
The log P values were evaluated for each of the 18F-radiolabeled TCO tracers and their derived NT-probes. The log P value for 18F-oxoTCO 6 was 0.57 ± 0.02, which was lower than those for18F-s-TCO 2 (log P 0.95 ± 0.02) and 18F-d-TCO 3 (log P 0.91 ± 0.02). Similar to the corresponding dienophiles, oxoTCO-derived probe 7d is the most hydrophilic probe with a log P of −2.47 ± 0.05, while s-TCO derived 7b and d-TCO derived 7c showed log P values of −1.10 ± 0.04 and −1.59 ± 0.01, respectively.
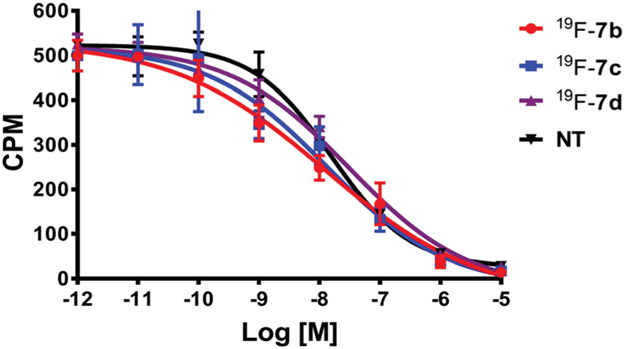
The 19F-labeled NT-probe compounds were subjected to an in vitro competitive cell binding assay to ensure that the prosthetic linker does not compromise the binding affinity of the targeting moiety. As shown in Fig. 2, 19F-labeled probes 7b–d showed comparable binding affinity with the unmodified NT peptide. The IC50 values for NT, and 19F-labeled probes 7b, 7c and 7d are 16.2 ± 2.7 nM, 20.5 ± 14.1 nM, 15.4 ± 3.4 nM and 31.6 ± 7.1 nM, respectively.
Fig. 2.
Competitive cell binding assays of 7b–d and the original NT peptide.
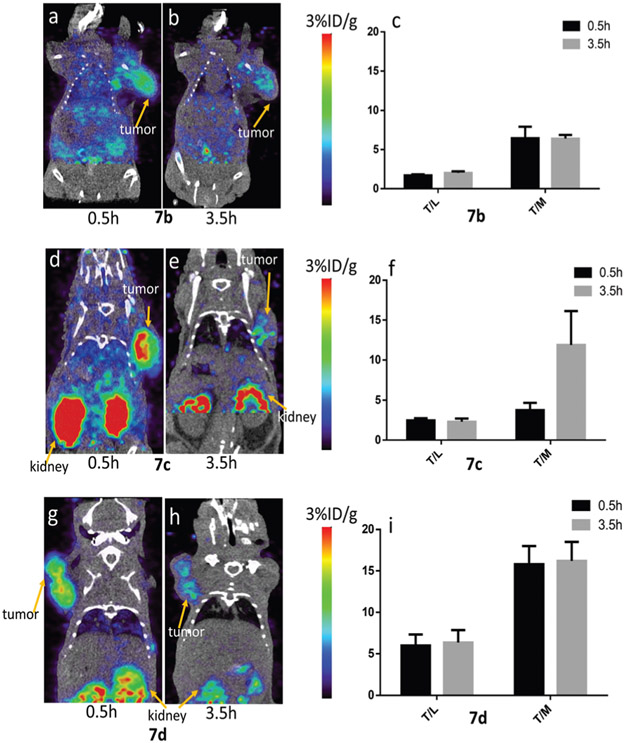
We evaluated the in vivo behavior and targeting efficiency of all three PET tracers. 3.7 MBq (100 μCi) doses of 7b–d were injected into NTR positive PC-3 tumor-bearing mice. Static PET/CT scans were acquired at 0.5 and 3.5 hours post injection and the images are shown in Fig. 3. As can be seen, tumors were clearly visualized in all the groups at both time points, indicating that all tracers have reasonable targeting efficiency in vivo. d-TCO derived tracer 7c showed the highest tumor uptake of 2.1 ± 1.0%ID/g at 0.5 h post injection. Slightly lower uptake was observed for oxoTCO derived 7d (1.7 ± 0.1%ID/g) and s-TCO derived 7b (1.5 ± 0.1%ID/g). Though d-TCO derived tracer 7c showed higher uptake than the others, there is no significant difference between any two groups. At 3.5 h post injection, the tumor uptake in all three groups decreased significantly and comparable values around 1.1%ID/g were observed.
Fig. 3.
Tumor-to-background ratio is improved by using oxoTCO derived 7d. Representative PET/CT images of the PC-3 tumor-bearing mice at 0.5 h and 3.5 h post-injection with (a) and (b) 7b, (d) and (e) 7c and (g) and (h) 7d. Tumor-to-liver and tumor-to-muscle ratios of (c) 7b, (f) 7c and (i) 7d in the mice bearing PC-3 xenografts at 0.5 and 3.5 h post-injection.
Fig. 3 shows the representative PET/CT images of the PC-3 tumor-bearing mice after injecting probes 7b–d and the corresponding tumor to liver and tumor to muscle ratios. Although 18F-d-TCO derived 7c showed the highest tumor uptake among the three tracers, it also showed relatively high background. High background was also observed with 7b. The more hydrophilic 18F-oxoTCO derived probe 7d showed the highest tumor- to-liver ratio and the best tumor-to-muscle ratio among the three probes. As shown in Table 1, at 0.5 h post injection, the tumor-to-muscle ratio was 6.5 ± 1.5 and 3.8 ± 0.9 for 7b and 7c, while more hydrophilic 7d showed a greatly improved tumor-to-muscle ratio of 15.8 ± 2.2. Given that the tumor uptake of 7d is not the highest among the three compounds, it can be concluded that the high tumor-to-background ratio is due to low uptake in the muscle and the liver. At 3.5 h post injection, the high tumor-to-muscle ratio of 7d was maintained at 16.2 ± 2.3, indicating a faster clearance rate at non-specific binding regions than at tumor sites. The tumor to muscle ratio of 7b remained at 6.4 ± 0.5, whereas that of 7c increased to 11.9 ± 4.3%, an effect that is possibly related to the reduced lipophilicity of the dTCO-derived probe relative to the sTCO-derived probe. As expected, in all images, the kidneys showed the highest uptake for all PET agents, which could be attributed to the relativity small size and hydrophilicity of the three probes.
Table 1.
Tumor to muscle uptake ratio of MePhTz-NT with different TCOs in the PC-3 xenograft at 0.5 and 3.5 h post-injection
| Tumor/muscle | 0.5 h | 3.5 h |
|---|---|---|
| 7b | 6.5 ± 1.5 | 6.4 ± 0.5 |
| 7c | 3.8 ± 0.9 | 11.9 ± 4.3 |
| 7d | 15.8 ± 2.2 | 16.2 ± 2.3 |
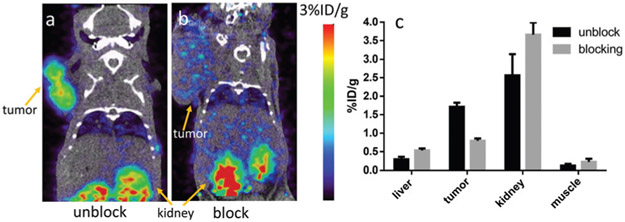
The targeting specificity of 7d was further confirmed by a blocking experiment, in which 100 μg of NT peptide was co-injected with 7d into PC-3 tumor-bearing mice and imaged at 0.5 h post injection (Fig. 4). The tumor uptake significantly decreased in the blocking group from 1.7 ± 0.1%ID/g to 0.8 ± 0.1%ID/g (P < 0.05).
Fig. 4.
Representative PET/CT images of the PC-3 tumor-bearing mice at 0.5 h post-injection of 7d (a) without and (b) with a blocking dose. (c) Quantitative uptake of the major organs determined from the PET images.
In summary, an 18F-labeled trans-5-oxocene (18F-oxoTCO, 6) for the rapid construction of PET probes via tetrazine ligation is described. The tracer showed comparable tumor uptake with the previously described s-TCO and d-TCO based method. However, the increased hydrophilicity of the oxoTCO enabled a faster clearance rate of the tracer from non-target organs, which led to a significantly higher tumor to background ratio compared with those of the s-TCO and d-TCO counterparts. This newly developed 18F-oxoTCO dienophile holds great potential for PET probe construction for in vivo applications.
Supplementary Material
Acknowledgments
This work was supported by the NIBIB (5R01EB014354-03), P30-CA016086-35-37 from the National Cancer Institute, and the UNC Radiology Department and the BRIC. Spectra were obtained using instrumentation supported by NIH grants P20GM104316, P30GM110758, S10RR026962, S10OD016267, and 1S10OD023611 and NSF grants CHE-0840401 and CHE-1229234.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc09747j
Conflicts of interest
There are no conflicts to declare.
References
- 1.Lang K and Chin JW, ACS Chem. Biol, 2014, 9, 16–20. [DOI] [PubMed] [Google Scholar]
- 2.Meyer JP, Adumeau P, Lewis JS and Zeglis BM, Bioconjugate Chem., 2016, 27, 2791–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson DM, Nazarova LA and Prescher JA, ACS Chem. Biol, 2014, 9, 592–605. [DOI] [PubMed] [Google Scholar]
- 4.Ramil CP and Lin Q, Chem. Commun, 2013, 49, 11007–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman ML, Royzen M and Fox JM, J. Am. Chem. Soc, 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaraj NK, Weissleder R and Hilderbrand SA, Bioconjugate Chem., 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YJ, Kurra Y, Yang Y, Torres-Kolbus J, Deiters A and Liu WR, Chem. Commun, 2014, 50, 13085–13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederwieser A, Spate AK, Nguyen LD, Jungst C, Reutter W and Wittmann V, Angew. Chem., Int. Ed, 2013, 52, 4265–4268. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira BL, Guo Z, Boutureira O, Guerreiro A, Jimenez-Oses G and Bernardes GJ, Angew. Chem., Int. Ed. Engl, 2016, 55, 14683–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA and Weissleder R, Angew. Chem., Int. Ed, 2009, 48, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiner T and Zeglis BM, J. Labelled Compd. Radiopharm, 2014, 57, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossin R, Läppchen T, van den Bosch SM, Laforest R and Robillard MS, J. Nucl. Med, 2013, 54, 1989–1995. [DOI] [PubMed] [Google Scholar]
- 13.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R and Lewis JS, J. Nucl. Med, 2013, 54, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darko A, Wallace S, Dmitrenko O, Machovina MM, Mehl RA, Chin JW and Fox JM, Chem. Sci, 2014, 5, 3770–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor MT, Blackman ML, Dmitrenko O and Fox JM, J. Am. Chem. Soc, 2011, 133, 9646–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, Wu Z, Lu H and Li Z, Theranostics, 2017, 7, 1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Li L, Liu S, Yakushijin F, Yakushijin K, Horne D, Conti PS, Li Z, Kandeel F and Shively JE, J. Nucl. Med, 2014, 55, 1178–1184. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, Conti PS and Kandeel F, J. Nucl. Med, 2013, 54, 244–251. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Mao C, Wang H, Ling X, Wu Z, Li Z and Ming X, Mol. Pharmaceutics, 2017, 14, 3391–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Cai H, Hassink M, Blackman ML, Brown RC, Conti PS and Fox JM, Chem. Commun, 2010, 46, 8043–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billaud EMF, Belderbos S, Cleeren F, Maes W, Van de Wouwer M, Koole M, Verbruggen A, Himmelreich U, Geukens N and Bormans G, Bioconjugate Chem., 2017, 28, 2915–2920. [DOI] [PubMed] [Google Scholar]
- 22.Billaud EMF, Shahbazali E, Ahamed M, Cleeren F, Noel T, Koole M, Verbruggen A, Hessel V and Bormans G, Chem. Sci, 2017, 8, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Svatunek D, Rohlfing K, Liu Y, Wang H, Giglio B, Yuan H, Wu Z, Li Z and Fox J, Theranostics, 2016, 6, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozma E, Nikic I, Varga BR, Aramburu IV, Kang JH, Fackler OT, Lemke EA and Kele P, ChemBioChem, 2016, 17, 1518–1524. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Seckute J, Cole CM and Devaraj NK, Angew. Chem., Int. Ed, 2012, 51, 7476–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, Enns B, Evans B, Wang N, Shang X, Sittiwong W, Dussault PH and Guo J, Chem. Commun, 2017, 53, 10604–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denk C, Svatunek D, Filip T, Wanek T, Lumpi D, Frohlich J, Kuntner C and Mikula H, Angew. Chem., Int. Ed, 2014, 53, 9655–9659. [DOI] [PubMed] [Google Scholar]
- 28.Lambert WD, Scinto SL, Dmitrenko O, Boyd SJ, Magboo R, Mehl RA, Chin JW, Fox JM and Wallace S, Org. Biomol. Chem, 2017, 15, 6640–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgat C, Mishra AK, Varshney R, Allard M, Fernandez P and Hindie E, J. Nucl. Med, 2014, 55, 1650–1657. [DOI] [PubMed] [Google Scholar]
- 30.Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP and Dziegielewski J, Cancer Res., 2011, 71, 6817–6826. [DOI] [PubMed] [Google Scholar]
- 31.Souaze F, Dupouy S, Viardot-Foucault V, Bruyneel E, Attoub S, Gespach C, Gompel A and Forgez P, Cancer Res., 2006, 66, 6243–6249. [DOI] [PubMed] [Google Scholar]
- 32.Alifano M, Souaze F, Dupouy S, Camilleri-Broet S, Younes M, Ahmed-Zaid SM, Takahashi T, Cancellieri A, Damiani S, Boaron M, Broet P, Miller LD, Gespach C, Regnard JF and Forgez P, Clin. Cancer Res, 2010, 16, 4401–4410. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson O, Kiesewetter DO and Chen X, Bioconjugate Chem., 2015, 26, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.