Abstract
Maternal behavior is a defining characteristic of mammals, which is regulated by a core, conserved neural circuit. However, mothering behavior is not always a default response to infant conspecifics. For example, initial fearful, fragmented or aggressive responses toward infants in laboratory rats and mice can give way to highly motivated and organized caregiving behaviors following appropriate hormone exposure or repeated experience with infants. Therefore hormonal and/or experiential factors must be involved in determining the extent to which infants access central approach and avoidance neural systems. In this review we describe evidence supporting the idea that infant conspecifics are capable of activating distinct neural pathways to elicit avoidant, aggressive and parental responses from adult rodents. Additionally, we discuss the hypothesis that alterations in transcriptional regulation within the medial preoptic area of the hypothalamus may be a key mechanism of neural plasticity involved in programming the differential sensitivity of these neural pathways.
Keywords: parental care, infanticide, neglect, approach-avoidance model, epigenetics, experience-dependent plasticity
I. Introduction
Extensive work over the past four decades has explored mechanisms mediating caregiving behavior in a wide variety of model organisms (1,2). An understanding of the neurobiological basis of caregiving behavior, however, has emerged from the study of maternal behavior in female rats and there is good evidence for the conservation of a core maternal circuit spanning across several species and even sex (3). One consistent finding is that the capacity for caregiving behavior is innate, but the expression of care is not. In rodent models, both intrinsic (i.e. estrogens, progestins, placental lactogens) and extrinsic (i.e. context, experience) factors play important roles in triggering maternal motivation around the time of birth. This motivation to interact with infants is augmented relative to the typical initial response of sexually naïve rodents to young conspecifics, which might be aggressive, indifferent, exploratory or more rarely spontaneous parental (4). Thus, an identification of the neurobiological adaptions that initiate and sustain caregiving behavior may involve not only an understanding of how caregiving behaviors are switched on, but also how fear/defensive behaviors are switched off. In what follows, we describe the approach-avoidance model of the onset of maternal care in female rats and the neurobiological systems that have been found to mediate these responses. Next, we consider the extent to which conserved central approach and aversion systems mediate appetitive and aversive responses to pups in mouse models of mothering. Further, we describe the idea that experience-dependent plasticity, potentially mediated by transcriptional programming within these systems, may stabilize caregiving behavior across time and context. Finally, we discuss the possibility that variability in the initial response toward infants depends on the extent to which neurons within these systems are transcriptionally poised for parenting and we consider the implications of this work for understanding the neurobiology of infant abuse and neglect.
II. Models of mothering
Laboratory bred Rattus norvegicus (rats) and Mus musculus (mice) are common model organisms used to investigate the neurobiology of mothering behavior. They breed well in the laboratory, have a relatively short gestation (18-23 days) and do not form selective attachments with their biological offspring (3). This last point is important because it means that caregiving behavior can be assayed independently from an individual’s reproductive status through the use of foster pups. Thus, experiments can address the extent to which organisms have the capacity to care for infants, regardless of whether the display of caregiving behavior would be appropriate. Mothering has been modeled in the absence of gestation in females and (of course) males and this work has important implications for understanding the evolution of parenting. For example, the study of the non-hormonal basis of mothering in female rats and mice may elucidate how caregiving behavior has evolved to become emancipated from hormonal control in some species. Because rats and mice are uni-parental species, males do not take part in pup rearing and in fact when pups are encountered males tend to attack them in order to mate and reproduce themselves. Thus rats and mice are not particularly useful models of fathering behavior, however understanding how infants come to elicit caregiving responses from male rodents may tell us something about the evolution of fathering behavior in mammals.
Maternal behaviors are characterized as those behaviors that aim to ensure infant survival (3). For example, mothers care for altricial pups by building a nest and crouching over pups to keep them warm, nursing pups to provide nutrition, and retrieving pups back to the nest should they become displaced. Mothers also engage in defensive aggression to protect their young from predators and male conspecifics. With the exception of maternal aggression (5), pups can elicit these same caregiving behaviors from virgin female rats and mice as well as male rats/mice. In these cases, although lactation does not occur, males and virgin females are capable of adopting some of the same nursing postures over pups (6,7).
The three commonly used models to study the neurobiology of caregiving behavior (female rats, female mice and male mice) have dramatically different default responses to infants. Female rats avoid pups, female mice are relatively interested and male mice are aggressive. Further, rats and mice differ in the sensory inputs required to respond to infants with caregiving behavior. However, all three organisms are capable of caring for infants and therefore are useful models for understanding the neural circuits underlying both pro and antisocial responses toward infants and the critical changes that occur within these circuits to allow animals to transition between the two. Seminal work investigating the neurobiological basis of maternal care was conducted using female rats as a model organism. This work was instrumental in our understanding of the neurobiological mechanisms that mediate the transition from fearful/defensive responses to infants to highly motivated maternal behavior at birth and how mother-pup interactions at this time function to increase maternal responsiveness long-term. One goal of this review is to examine whether the more recent work that has investigated the neurobiological basis of caregiving behaviors using mice (male and female) (8–10) fits into this theoretical framework. However, in order to understand the extent to which the neural mechanisms that mediate caregiving behavior are conserved, we must first describe some critical differences between these three model organisms.
Multiple sensory systems are involved in recognition of infant stimuli (olfactory, vomeronasal, auditory, visual and tactile), however female rats and mice differ in the relative importance of these inputs to elicit caregiving responses. In female rats, no single sensory system appears to be critical for the initiation of caregiving behavior; rather multiple sensory systems must be eliminated to produce deficits in maternal responding (11). In contrast, male and female mice require olfactory information about pups to care for them because removal of the olfactory bulbs results in deficits in maternal behavior, such as decreased nest building, less time spent nursing, and an increased incidence of cannibalism (12–14). In addition to the main olfactory system, the vomeronasal organ of the accessory olfactory system responds to pup odors (15,16). However, in contrast to the main olfactory bulb, processing of pup odors via vomeronasal organ is not required for caregiving behavior. Instead vomeronasal inputs critically mediate pup-directed aggression (15,16).
As noted above, laboratory female rats and mice differ dramatically in their basal response to pups. For example, naïve virgin female rats initially respond to pups by actively avoiding them. If foster pups are placed directly in their nest, they will move their nest to the farthest location away from pups and continue to do so for several days with maternal responses emerging only after 6-12 consecutive days of pup exposure (17). In contrast, when naïve virgin female mice are presented with pups they actively investigate (touch, smell and even lick) infants and will subsequently display maternal behaviors within 15-45 minutes of this first exposure (4,7,18–20). Thus, an immediate maternal care response relies on the hormonal events of birth in both species, however in the absence of hormone stimulation the amount of pup exposure required to trigger maternal care is substantially different between these two species.
The default response of adult male mice toward pups is aggression (14,21). When presented with pups, virgin male mice rapidly approach, investigate and kill pups. Interestingly, following sexual experience this response is inhibited in a time-dependent manner (15,22–24). Once infanticidal tendencies are blocked, proximal pup cues elicit caregiving behaviors. Note that whereas this transition occurs artificially in the laboratory in uni-parental mice, evidence indicates that naturally bi-parental species (California mice, Mongolian gerbils) males also transition from a state of aggression to paternal care following sexual experience (25–27).
Although mice are variable in their response to pups, none of the responses described above involve active pup avoidance, as is the case in female rats. Thus, a relevant question is whether antisocial responses to pups can or should be understood in the context of a neural model derived from female rats in which an initial fearful response to pups must be overcome before caregiving behavior is displayed? Certainly the transition to motherhood may require distinct neurobiological modifications depending on the baseline level of maternal responsiveness of animals within a given species. However, if rats are unique in their active avoidance of pups what can be gained by reviewing the neurobiological mechanisms involved in suppressing a fear response to infants at birth if in fact this fear response is not conserved across mammalian species (or even commonly used model organisms!)? In what follows, we propose that the neurobiological basis of pup avoidance in rats has implications for understanding the neural basis of infant neglect more broadly. In support of this idea, we discuss evidence that a pup-induced activation of a central fear/defensive system is not unique to virgin female rats but that activation of this system is associated with neglectful and/or abusive responses to infants more generally. In other words, the ability of pup stimuli to activate a fear/defensive neural system is conserved even if a default fearful or avoidant response to pups is not.
III. Extrinsic and intrinsic triggers for caregiving behavior
Whereas the default response to pups is highly variable across sex and species, none of the model organisms described above are immediately maternal by default. If rats and mice are not immediately responsive to infant conspecifics, how then are maternal responses triggered? The best understood intrinsic trigger for caregiving behavior is the hormonal experience of gestation and birth, which induces an immediate onset of maternal care in female rats and mice. Although male mice are not exposed to these hormonal changes, the reproductive experience of mating, ejaculation and cohabitation with a gestating female alters subsequent responses toward pups in a time-dependent way (22).
Female rats avoid pups because they find their odor aversive and therefore one possibility is that gestational hormone exposure induces caregiving behavior by altering the perception of infant cues (17,28). Thus, the hormonal events of late pregnancy and birth, which consist of a rise in estradiol and a decline in progesterone, function to trigger an immediate maternal response at birth (29,30) at least in part by changing the way infant odors are perceived (31). In support of this idea in the day-to-hours before birth, the perception of infant odor changes such that female rats find infant odors attractive (32) and readily respond to pups (33). At this time, motivation to interact with pups is high in lactating dams. Mothers will learn to lever press for pup contact (34–36), form a place preference for a chamber that formerly contained pups (37), traverse a novel T-maze to retrieve pups back to the nest (38–40) and even cross an electrified grid to contact pups (41). Thus, pups have an increased incentive value for mothers (42).
Although female mice do not find pup odors aversive, the hormonal events of late birth and gestation do increase attraction toward and motivation for pups. For example, postpartum mice readily retrieve pups from a novel environment whereas naïve virgin female mice do not (39). Therefore, although virgin female mice readily care for pups in the familiar home cage environment, they are not motivated enough to retrieve pups in a novel context unless they have had previous maternal experience (7). Although the hormonal events of birth do not seem to alter the perception of infant odors (39), there is some evidence that postpartum mice are more sensitive to auditory cues from pups (43). Thus, the hormonal events of birth increase attraction to pup vocalizations and increase the likelihood that a female will respond with the appropriate maternal behavior (retrieval, nursing, etc) (44–46).
Male mice clearly do not experience hormonal changes associated with gestation, although a time-dependent change following ejaculation inhibits infanticidal tendencies roughly 3 weeks later, around the time when pups would be born (47,48). It’s presently unclear how this reproductive experience alters responsiveness in this time-dependent way. However, given that the vomeronasal organ of paternal males is unresponsive to pup cues (15), one possibility is that this process may involve a change in the way pup cues (odor and tactile) are processed.
Extrinsic factors are also capable of triggering maternal care, even in the complete absence of hormonal stimulation. For instance, gonadectomized virgin female rats will care for foster pups if they are continuously exposed to infants for several days (49). The tendency to actively avoid pups is reduced following several consecutive days of constant pup exposure. Eventually females begin to tolerate pup proximity and after 6-8 days of pup exposure, virgin rats approach pups and display caregiving behaviors (17). This process, termed sensitization, demonstrates that experience with infants in the absence of hormones ultimately results in the initiation of maternal care, although on a much slower time scale (49). Recall that the experience of interacting with pups can trigger caregiving behavior in less than 15 minutes in many laboratory strains of virgin female mice (18,19). Further, this experience-induced onset of caregiving behavior in female mice occurs in the complete absence of estradiol (7). Finally, sensitization can occur in gonadectomized virgin male mice, although to our knowledge there is no evidence of an experience-induced transition from pup-directed aggression to pup care in intact virgin male mice (20). However, it should be noted that whereas mating and ejaculation suppress infanticidal behavior (23), cohabitation with a gestating female is required for this effect in most strains of mice tested (22,50,51).
As noted above the role of intrinsic factors, such as the hormonal events of late pregnancy and birth, in promoting an immediate attraction toward and motivation to interact with pups has been extensively studied in rats. However, the facilitatory effect of late gestational hormone exposure alone is not stable. For example, if female rats do not interact with pups for at least 30 minutes during this critical window of hormonal stimulation then they will return to their default level of responding (52). In other words, hormonal stimulation alone is not capable of producing a long-term change in caregiving behavior, but rather it functions to potentiate the effects of pup exposure on the initiation and maintenance of caregiving behavior. For this reason, gestational hormones are sometimes described as “priming” the maternal brain. Importantly, hormonal priming not only facilitates the onset of maternal care, it also induces a high level of maternal motivation in dams. In the absence of hormonal priming, significantly more time interacting with pups is required to induce the same level of maternal motivation (53–55) and this is true even of virgin females who don’t find pups aversive (39). For example, although virgin female mice are significantly less responsive to pups than postpartum female mice when tested in a novel environment, experienced virgin female mice are ultimately capable of showing maternal responses that are not significantly different from lactating dams (39) and much like postpartum animals, caregiving responses are displayed long-term (for at least a month after experience with infants) (56).
IV. An approach-avoidance model for the onset of maternal care
Rosenblatt and Mayer first proposed the approach-avoidance model for the onset of maternal behavior based on the finding that the onset of maternal behavior in rats is related to the combination of increased attraction to and reduced aversion of novel infant stimuli (57). As described above, in female rats aversion to pups is mediated by a fear of pup odors. However, removal of the aversive odor of pups alone is not sufficient to produce an immediate onset of caregiving behavior in rats (58–60). In other words, the onset of caregiving behavior occurs when the tendency to approach infants is greater than the tendency to avoid infants. Hormones and experience with infants are thought to act on these neural systems to decrease infant activation of a central aversion system and increase infant activation of a conserved neural circuit for caregiving behavior.
4.1. Competing neural systems regulate pup approach and pup avoidance
The neural systems that are known and hypothesized to regulate pup approach and pup avoidance have been well described (61–63). In order to understand how plasticity in these systems may mediate the continued selection of caregiving behavior in response to pup presentation, it is first necessary to carefully review the neural basis of the approach-avoidance model, which has been described in female rats. The increase in pup approach and reduction in pup avoidance, whether through intrinsic or extrinsic factors, is coordinated by the medial preoptic area of the rostral hypothalamus (MPOA). Lesions of the MPOA, particularly the central MPOA, disrupt the onset of maternal behavior in both sensitized virgin and postpartum rats and mice (64,65). The MPOA is also a key site for the hormonal induction of maternal behavior by estradiol (66,67), prolactin and placental lactogens (68,69). Peripheral oxytocin hormone release plays an important role in labor induction, whereas the central action of oxytocin as a neuropeptide plays a role in the onset of maternal behavior in rats via its action on either the MPOA or the VTA (70). Importantly, whereas hormonal and oxytocinergic stimulation of the MPOA promotes an immediate onset of maternal behavior in female rats, the maintenance of maternal care is not dependent on hormone stimulation (71).
In response to hormonal priming, the MPOA initiates and maintains maternal responding via its interactions with central approach and aversion neural systems (61–63), although the precise role of the MPOA in the maintenance of caregiving behavior may vary by species. For example, lesions of the MPOA abolish ongoing maternal behavior (retrieval, nestbuilding and nursing) in primiparous (66,72) and experienced rats (73). In contrast, discrete lesions of the central region of the MPOA in female mice not only disrupt maternal behavior but also induce pup-directed aggression in female mice, suggesting that MPOA output may actively inhibit pup-directed aggression in this species (65). Interestingly, although female mice responded to pups with caregiving behavior before surgery, postpartum mice with central MPOA lesions showed infanticide for six consecutive test days. Similarly, lactating hamsters also rely on an intact MPOA to promote caregiving behavior and prevent infanticide (74). Thus, MPOA output may maintain maternal behavior, at least in part, by continually inhibiting infanticide in some species. Seminal work in rats has indicated that the major projection sites of maternally activated cells in the MPOA and adjoining ventral bed nucleus of the stria terminalis (vBNST) are the ventromedial nucleus of the hypothalamus (VMN) and ventral tegmental area (VTA), respectively (75), and more recent work indicates that these connections are conserved in male and female mice (9,10). Importantly, the VMN is part of a hypothalamic circuit involved in regulating defensive and attack behaviors; both the VMN and the region just rostral to it, the anterior hypothalamic nucleus (AHN), comprise the hypothalamic aggressive area in rat (76). In contrast, the VTA contains cell bodies that produce and release dopamine into the nucleus accumbens (NA) and is a critical component of the mesolimbic dopamine system. Therefore, the output of the MPOA is directed at two critical neural regions involved in behavioral avoidance and approach responses (Figure 1).
Figure 1.
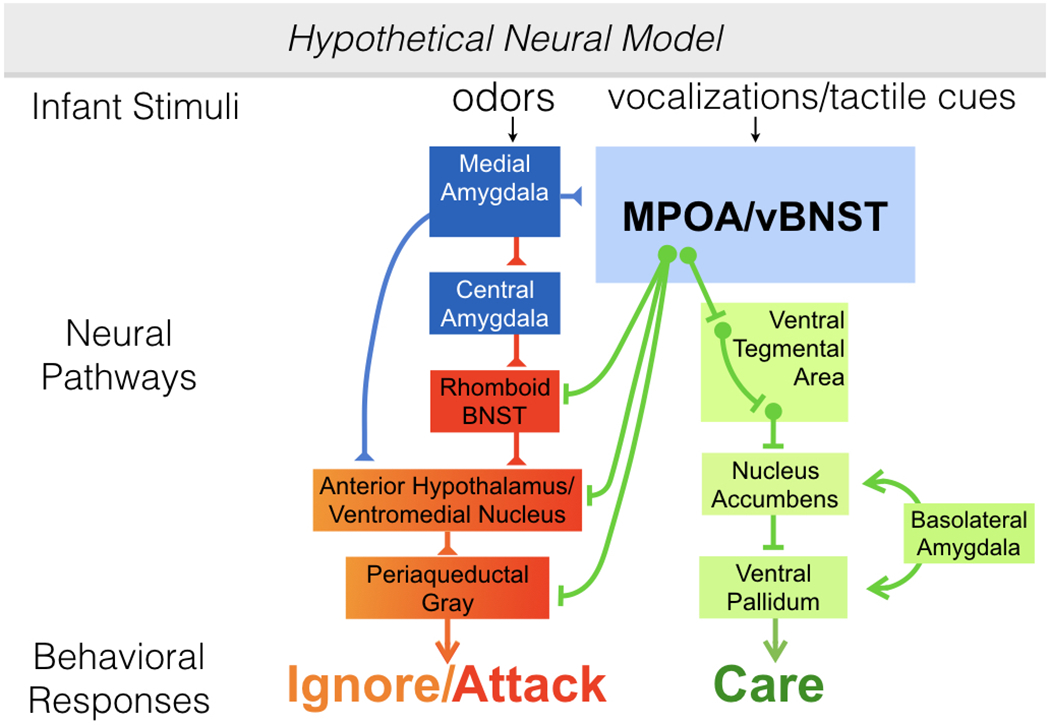
A neural model illustrating the regulation of prosocial and antisocial responses to infant conspecifics. The MPOA/vBNST coordinates caregiving behavior through GABAergic efferent projections to several regions of the central aversion system as well as GABAergic efferent projections to the VTA. MPOA projections to the central aversion system are hypothesized to directly inhibit these regions, disrupting their ability to respond to olfactory cues from pups with the activation of avoidant, fearful or aggressive behaviors. Note that reciprocal inhibitory connections exist between the MPOA and the central aversion system (not drawn). Therefore, the activation of aggressive or avoidant responses to pups is also related to a direct inhibition of caregiving behaviors. MPOA projections to the VTA function to elicit the release of dopamine into the NA, possibly through a disinhibition of these cells. Dopamine release into the NA disinhibits the VP so that pup inputs from the basolateral amygdala can be responded to with parental care. Evidence for this model is presented in the text. Abbreviations: AHN- anterior hypothalamic nucleus; BNST- bed nucleus of the stria terminalis; MPOA- medial preoptic area; vBNST- ventral bed nucleus of the stria terminalis
4.2. A central aversion system in the regulation of fear responses to novel infant odors
The idea that a central aversion system might be involved in directly inhibiting maternal care was based on the finding that virgin rats do not simply fail to care for pups, but rather actively avoid and withdraw from them (61). Further, because removal of olfactory input (from either the vomeronasal organ or the primary olfactory system) was capable of facilitating the onset of maternal behavior in virgin rats, early work explored the hypothesis that destruction of nuclei within the amygdaloid complex, which are target sites for these olfactory systems, could also facilitate the onset of care (77). Excitotoxic lesions of the medial amygdala (meA) or destruction of its efferent projections via the stria terminalis reduced the amount of pup exposure required to induce the onset of caregiving behavior in virgin female rats. However, note that destruction of the MPOA and the meA prevents any facilitatory effects of meA lesions alone, thus the meA likely inhibits MPOA in naïve rats (78). Finally, the main target for the meA is the VMN (79), a region that was first implicated in the inhibition of maternal care when Bridges and Mann (1994) investigated whether prolactin infusions into the VMN would promote maternal responsiveness. Although prolactin infusions did promote the onset of maternal care in sub-optimally hormone-primed female rats, vehicle infusions alone produced an identical effect, suggesting that damage to the VMN as a result of the cannula track might be ultimately responsible (80). On the basis of these data, Numan and Sheehan (1997) proposed that pups activate a central aversion system in virgin female rats, which must be inhibited by the hormonal changes of late pregnancy and birth for the onset of maternal care to occur. They investigated regions of the brain that may be involved in the inhibition of maternal care by exposing maternal (optimally hormone primed) and non-maternal (sub-optimally hormone primed) female rats to foster pups and thoroughly investigating expression of the immediate early gene cFos, which is expressed following cellular activation, in a number of hypothalamic and midbrain nuclei (81). This work identified several regions that showed significantly higher cFos expression when non-maternal (compared to maternal) rats were exposed to pups, including the posterior dorsal meA, AHN, principle nucleus of the bed nucleus of the stria terminalis (BNST), and dorsal, medial and central parts of the VMN (VMNdm,c). In support of the idea that these regions cause pup avoidance, excitotoxic lesions of the AHN/VMN also promote the onset of maternal care in non-maternal female rats (82) and disconnection of the meA and AHN/VMN facilitates the onset of caregiving behavior in non-maternal rats (83). This last finding emphasizes the role for a direct pathway from the meA to the VMN in the inhibition of maternal behavior. A major projection site of the AHN/VMN involved in the regulation of pup avoidance is the periaqueductal gray (PAG). The extent to which PAG lesions would also promote the onset of maternal behavior in hormone-primed rats has not been examined, however lesions of the dorsal PAG have been found to reinstate maternal behavior in stressed female rats (84).
4.3. Does a central aversion system mediate aggressive or indifferent responses to pups?
An important series of studies has investigated the role of a central aversion system in pup-directed aggression as well as how the ability of pups to activate this system may change across time as males transition from attack to care. This work has compared cFos expression within some nodes of the central aversion system between aggressive virgin males and parental, sexually experienced fathers and investigated how the activation or inhibition of these sites affect the behavioral transition from aggression to parental care (15,85). In male mice, pup odors processed by the vomeronasal system also drive the activation of a central aversion system, although the behavioral outcome is pup-directed aggression rather than active avoidance. Recall that male mice find pup odors aversive and detection of pup odors by the vomeronasal organ plays a critical role in pup attack. For example, pup exposure induced cFos expression in the vomeronasal organ of sexually naïve male mice, but not fathers and ablation of the vomeronasal organ in these virgin male mice eliminated infanticide and promoted caregiving behaviors (15). Recent work has identified the precise vomeronasal receptors that respond to pup chemosensory signals to mediate pup-directed aggression in male mice (16). The vomeronasal organ projects to the meA, the dorsal bed nucleus of the stria terminalis (dBNST) and the AHN/VMN (86). Exposure to sensory cues from pups activates cFos expression in the AHN as well as the central and ventrolateral regions of the VMN, whereas parental fathers show no pup-induced activation of cFos in these regions (15). Pup cues also activate the medial, intermediate, and lateral regions of the medio-posterior BNST in both nonaggressive virgin and paternal male mice, although cFos expression is significantly higher in aggressive males (15). Thus far, the regions described are nearly identical to those activated in avoidant female rats, therefore a relevant question is how this pathway mediates avoidance versus pup-directed aggression. Tsuneoka and colleagues (2015) identified a uniquely activated site in aggressive males, the rhomboid part of the dorsal BNST (rhBNST), which was highly predictive of aggressive responses. Using cFos immunoreactivity they measured neuronal activation across the MPOA to BNST extent during a number of social behaviors such as paternal care, mating, and inter-male aggression and were able to retro-actively discriminate which behavioral activity a male was engaged in based on the pattern of cFos expression. For example, if cFos expression in the MPOA was below a certain threshold activity and cFos expression in the rhBNST was above a certain threshold of activity, the authors could predict with over 90% accuracy that the male had been engaged in infanticidal behavior. The rhBNST receives most of its input from the central nucleus of the amygdala and therefore olfactory information about pups could be routed to the rhBNST by way of a medial to central amygdala pathway (87,88). However, the finding that rhBNST lesions delayed, but did not fully inhibit, pup-directed aggression suggests that the rhBNST may regulate infanticide through its projection to a downstream aspect of this circuit that is capable acting in the absence of rhBNST input (87). Together these data implicate the rhBNST, meA, and AHN/VMN sites in the regulation of aversive responses toward pups, however the exact pathway mediating pup-directed aggression remains unclear.
The work described above supports the idea that a central aversion system mediates at least two aversive responses toward pups: active pup avoidance and pup-directed aggression. This idea is intuitive given that these aversive responses can be considered fearful and defensive, which is consistent with the types of responses mediated by a central aversion system. However, a relevant question is whether this system could also mediate pup indifference or a lack of any response to pups. Importantly both pup avoidance and pup indifference result in infant neglect, however pup avoidance may represent a unique state of virgin female rats and therefore a relevant question is whether the absence of caregiving behavior (rather than a fear of pup odors) is associated with a pup-induced activation of the central aversion system. This question is a particularly interesting one to address in female mice because unlike the female rat and male mouse models of mothering, female mice do not need to overcome an initial aversion to infants to care for them. Thus, an important question is whether pups would ever be able to activate a central aversion system in these animals. For example, given that naïve virgin mice are unresponsive and indifferent to pups in a novel environment, would this context-specific occurrence of infant neglect (pup indifference) in otherwise maternal animals be associated with an activation of this central aversion system? Note that this failure to respond to offspring is not related to a greater fear response to a novel context in general; non-responsive mice are not more fearful than pup-responsive mice (89). Further, naïve virgin female mice readily approach and investigate pups in the novel T-maze, but subsequently fail to retrieve them back to the nest. Our laboratory has recently investigated the extent to which cfos transcription differed within the central aversion system between mice that are maternally responsive across familiar and novel contexts and those that are not (39). Our data indicate that virgin female mice that are maternally responsive in a familiar context but not in the novel context show significantly higher expression of two immediate early genes, cfos and egr1, in the AHN/VMN compared with pup-responsive groups in the novel context. These data support the idea that context-specific pup neglect is associated with the activation of at least one node in the central aversion system.
Finally, with respect to delineating aspects of the central aversion system that regulate avoidance, indifference or aggression specifically in male and female rodents, future research will need to gain a better understanding of how specific cell populations within the AHN-VMN continuum mediate distinct aspects of behavioral avoidance and conspecific aggression. This question has recently been investigated with respect to behavioral responses in male mice to adult male or female conspecifics. Optogenetic stimulation of the dorsal medial part of the VMN (VMNdm) elicits escape and hiding behavior in male mice, whereas the ventrolateral region of the VMN (VMNvl) regulates attack behavior (90). Note that although the AHN and both dorsomedial and ventrolateral aspects of VMN have been examined with respect to pup avoidance and aggression, at present it is difficult to know whether these discrete regions of the hypothalamus link to particular aspects of aversive responses toward pups. For example, whereas pups induce cFos exclusively in the AHN and VMNdmc of non-maternal rats, cFos is induced in response to pup cues in the VMNvl of both maternal and non-maternal rats (and note that none of these animals engaged in pup-directed aggression) (81).
4.4. A central approach system in the regulation of the onset of maternal behavior in rats
The MPOA and adjacent vBNST coordinate proactive and prosocial responses toward pups through an interaction with the mesolimbic dopamine system. The hormonal events of pregnancy, which promote the onset of maternal care, are by themselves capable of increasing cFos expression in these regions (91). Further, unilateral inactivation of the MPOA, which alone is not capable of impairing maternal care, reduced cFOS expression in regions of the central approach pathway on the side of the brain ipsilateral, but not contralateral, to the lesion (92). The most convincing causal evidence for an interaction between the MPOA and the VTA in the regulation of maternal care comes from work conducted by Numan and colleagues. First, bilateral, but not unilateral, damage to the MPOA or the VTA (or reversible pharmacological depression of the MPOA or VTA) produces severe impairments in maternal behavior (93,94). Second, bilateral disconnection of this system through unilateral lesion of the MPOA on one side of the brain paired with a contralateral lesion (or reversible depression) of the VTA dramatically impairs maternal care in female rats compared to females that have experienced unilateral disruption of these regions on the ipsilateral side of the brain (93,94).
In response to MPOA input, the VTA elicits dopamine release into the NA (95). Direct interference with dopamine action at the level of the NA blocks maternal responding in female rats (96–98), and notably in non-maternal females, exposure to pups does not induce dopamine release into this region above baseline (99). On the other hand, microinjection of a dopamine D1-type receptor agonist into the NA promotes the onset of care in sub-optimally hormone primed rats (100). The NA is primarily comprised of GABAergic neurons, which project to the ventral pallidum (VP) (101–103). The finding that VP lesions, temporary inactivation of the VP, or bilateral disconnection of the MPOA and the VP severely impair maternal behavior led to the hypothesis that the pup-induced release of dopamine into the NA functions to disinhibit the VP (104).
4.5. Does a central approach system mediate caregiving behavior in non-avoidant mice?
Recall that male and female mice readily approach pups regardless of whether or not they care for them. Thus, a relevant question is whether activation of the central approach system is related to the approach of pups in general or whether a pup-induced activation of this system is related specifically to the onset of parental behaviors (retrieval of displaced pups to the nest, licking, crouching) in mice. Another possibility is that pups have some access to this central approach system by default, but that the activity of this system is upregulated by hormonal or experiential factors, which promote parental motivation.
A series of experiments conducted in male mice of the ICR strain provide some support for the idea that an activation of the central approach system is related to caregiving behavior (rather than pup approach in general). Although sexually experienced male ICR mice readily approach and sniff pups, these males do not show spontaneous caregiving behavior (or pup-directed aggression) under standard test conditions. However, following cohabitation with a female, pup retrieval and other aspects of maternal care can be induced in these males by auditory and pheromone signals from their female pair-mate (the female they were cohoused with) (105). There is some evidence that the induction of paternal care in these males depends upon an MPOA induced activation of a central approach system. Electrolytic lesions of either the MPOA or the VP disrupt retrieval behavior in males (106) and retrieving males show elevated cFos expression in the MPOA, VTA, NA and VP in response to pair-mate signals (107). Interestingly, communicative signals from the female pair-mate are capable of inducing local estradiol synthesis in the brain as aromatase (the enzyme that synthesizes estradiol) is elevated in the MPOA, VTA, NA, VP, and VMN of retrieving males (108).
In contrast, work conducted in C57BL6/J mice supports the idea that an MPOA induced activation of the mesolimbic dopamine system mediates a motivation to approach pups in general, regardless of whether aggressive or caregiving responses occur upon pup contact (9). For example, optogenetic activation of neurons in the MPOA that project to the VTA increased the effort males engaged in to approach pups (climbing over a barrier wall), although they tended to kill pups once they made contact. These data are consistent with recent work from our laboratory that found a similar level of cfos expression in the VTA in response to infants in both aggressive and pup-responsive male C57BL/6J mice (109). Certainly one possibility is that the central approach system is not responsive to pups by default in laboratory strains of mice, but that motivated responses to pups (whether to care or kill) are processed by separate populations of cells within this pathway (110).
With respect to the data presented for male mice, perhaps it should be noted that female mice, unlike males, do not typically display aggression. In female mice, caregiving behavior has been linked to a pup-induced activation of the mesolimbic dopamine system. Immediate early gene activation in response to pups is higher in maternal compared to indifferent, non-responsive female mice, despite the fact that these mice do approach and sniff pups (39). Further, direct optogenetic stimulation of neurons in the MPOA that project to the VTA can induce motivated responses to care for pups in maternal female mice (9,10).
Together the work described above indicates that interference with an MPOA-induced activation of the mesolimbic dopamine system interrupts ongoing maternal behavior. These findings support the idea that this central approach circuit is required for continual maternal performance (not just the onset of maternal behavior). In addition, several lines of work in rats and mice support the idea that variation in maternal responsiveness is associated with the relative activity of dopamine in the NA. For example, dopamine release into the NA is higher in rat dams that spend more time licking and grooming pups (111). Further experience with pups potentiates the amount of dopamine released into the NA, possibly through a strengthening of mPOA-to-VTA connectivity (95,112).
V. The MPOA coordinates caregiving behavior through its interactions with central approach and avoidance systems
Despite its critical role in the onset and maintenance of caregiving behavior, it should be noted that the MPOA is comprised of a highly heterogenous population of cells, some of which regulate other behaviors such as sexual behavior, thermoregulation, and thirst (113–115). A number of papers have sought to identify the phenotype of pup-activated (cFos expressing) cells in the MPOA (8,65,116). A consistent finding across research groups is that the majority of pup-activated cells in parental mice and female rats are GABAergic (8,10,65,85,117–120). Thus, the efferent GABAergic projections of the MPOA to nodes of the avoidance pathway (85,118) likely function to directly inhibit aversive responses to pups, whereas the GABAergic projections to the VTA may function to disinhibit dopamine neurons involved in the activation of pup approach responses (10).
Given that estradiol action on the MPOA is both necessary and sufficient for the immediate induction of maternal care, it is not surprising that several laboratories have determined that cFos+ cells in the MPOA overlap with cells expressing estrogen receptor alpha or its transcript (Esr1) (10,65,116,119). Further, Esr1+ MPOA cells project to both VTA and AHN/VMN (10,121). Recent work in mice suggests that the projection from MPOA Esr1+ cells to VTA is involved in motivated responses to pups in female mice (10). For example, optogenetic stimulation of Esr1+ cells projecting to the VTA induced pup retrieval in virgin female mice tested in a novel environment. Although the projection of Esr1+ cells to the VMNvl was not further explored, it is interesting to speculate that this projection might play a role in the suppression of pup avoidance.
Other work has identified the neuropeptide galanin as a potential marker for a parenting specific population of cells in the MPOA (8,9). About 30-40% of the cells expressing cfos during parental care also express the galanin transcript (Gal). Importantly this overlap occurs in parental (virgin females, postpartum females, and sexually experienced, paternal male mice), but not aggressive virgin male mice. In a series of experiments, Wu et al (2014) provide support for the idea that Gal+ cells are both necessary and sufficient for parental care. Selective ablation of Gal+ cells in the MPOA inhibits parental care in both virgin and postpartum females as well as sexually experienced paternal males. Interestingly, virgin females with more than 50% of Gal+ cell ablation not only fail to retrieve pups, but also show pup-directed aggression. Furthermore, Gal/Esr1+ cells that are activated in the MPOA also co-express Gad1, suggesting that some cells active during parental care in virgin mice function to directly suppress infanticidal tendencies (8,119). In support of this idea, stimulation of Gal+ cells in virgin male mice was capable of switching the behavioral response of male mice from pup-directed aggression to caregiving behavior. These data beg the question of what unique Gal+ cell projections might exist in sexually naïve mice and whether these pathways function to directly inhibit the central aversion system. A follow up study partly addressed this question and identified a projection from the MPOA to PAG that is significantly more active in virgin mice (males and females) during pup grooming. However, optogenetic stimulation of MPOA Gal+ cells that project to the PAG reduced pup-directed aggression in virgin males, but it had no effect on caregiving behavior in virgin females. An inhibition of pup-directed aggression following activation of this MPOA-to-PAG pathway in virgin male mice is consistent with the idea that one function of an MPOA-to-PAG pathway might be to inhibit pup aversion. The fact that activation of an MPOA-to-PAG had no effect on the behavioral response to pups in virgin female mice could reflect that the fact that virgin female mice do not find pups aversive in a familiar environment and thus an activation of the MPOA-to-PAG pathway would not produce a observable effect. Further, although the caudal PAG is involved in the regulation of nursing behavior in lactating dams (122), virgin female mice are of course not lactating, thus perhaps even artificial activation of this pathway would not stimulate a “nursing” response. Together, these data suggest that the MPOA(Gal+)-to-PAG pathway is involved in the inhibition of pup-directed aggression in males, but is not involved in inhibiting pup aversion in females (at least under conditions in which female mice do not find pups aversive). However, note that total Gal+ cell ablation within the MPOA is capable of inducing infanticide in virgin females (but not parental male and female mice), thus Gal+ cells in the MPOA probably do play a role in the inhibition of infanticide in female mice, but perhaps through a different projection (8). On a final note, it is important to consider that whereas Gal+ cells were manipulated in these experiments, Gal itself was not, thus the precise role of Gal is unclear. Along this line it should be emphasized that Gal expression largely overlaps with Esr1 expression within the MPOA (119) and therefore it’s ultimately unclear which transcript within these cells is responsible for behavioral effects reported.
VI. Stabilization of response selection: A reorganized MPOA
Thus far we have examined evidence for the role of central approach and avoidance systems in mediating appetitive and aversive responses to infant conspecifics and described pathways through which efferent projections of pup-responsive cells in the MPOA might coordinate the response of these systems to pups. Together these sections have emphasized a role for the MPOA in gating pup access to these two competing neural systems. Since intrinsic and extrinsic triggers for parenting function to permit plasticity within these systems in order to maintain or stabilize the selection of caregiving responses over time (52,54,56,123–125) it would stand to reason that these mechanisms act directly on the MPOA, which may serve as a gatekeeper. Along this line note that while the MPOA might gate infant access to downstream approach/avoidance pathways, the MPOA is highly responsive to pups regardless of the behavioral response that is selected (15,43,81,126,127). Therefore, the MPOA may undergo reorganization in response to infant cues although at present the mechanisms that act to alter MPOA output remain to be elucidated (Figure 2). One possibility is that hormonal and/or experiential factors impact gene expression programs in the MPOA, which ultimately coordinate the output of MPOA neurons. In support of this idea, a large number of differentially expressed genes between postpartum and naïve virgin mice have been identified in the MPOA (128). Given that both hormonal and/or experiential factors are capable of sustaining caregiving behavior long-term, a relevant question is whether a common epigenetic mechanism is involved in regulating a transcriptional response to pup exposure within the MPOA (129).
Figure 2.
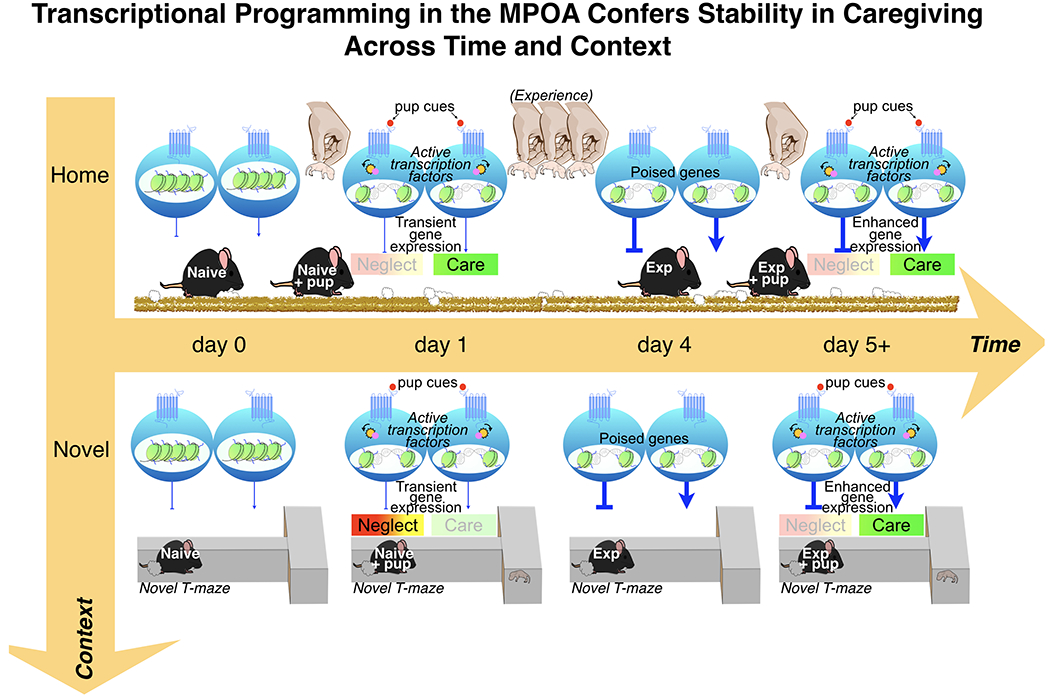
Proposed neural model for the regulation of experience-dependent changes in caregiving behavior in female mice. A naïve female’s reaction to pups depends on context. In the familiar home cage, caregiving behaviors typically emerge within 15 minutes of pup exposure. Pup cues stimulate medial preoptic area (MPOA) neurons that project to both approach (care) and avoidance (neglect) pathways. In the absence of competing cues, the output of these pathways is sufficient to block pup aversion and induce pup care. However, in a novel context, the initial output of these pathways is not sufficient to induce caregiving behavior. During this initial experience, pup cues transiently induce the expression of relevant genes within MPOA neurons. Following repeated experience with pups, chromatin alterations confer a transcriptional memory within these projection neurons that allows for a stabilization of caregiving responses across time and context. Hypothesized mechanisms underlying transcriptional memory include alterations in DNA methylation and histone post-translational modifications at relevant gene promoters and enhancers (155). These changes strengthen the output of MPOA neurons (as indicated by the thicker lines) and poise genes for subsequent pup stimulus-driven transcription. Note that for ease of illustration, MPOA outputs are drawn to indicate the functional consequences on behavior rather than the neurotransmitter released. As described in the text, both pathways likely release GABA.
Several lines of research support the idea that epigenetic mechanisms might mediate experience-dependent plasticity in maternal neural circuits. First, epigenetic programming of Esr1 transcription in the MPOA mediates natural variation in maternal care in rat dams, at least in part through an effect on the mesolimbic dopamine system (130–132). Importantly, the receipt of increased tactile contact (licking) in the early life period reduces DNA methylation in the Esr1 promoter, which allows for an augmentation of estradiol-induced Esr1 expression as well as subsequent oxytocin receptor expression when adult female offspring become mothers themselves. Importantly, differences in the transcriptional regulation and expression of Esr1 cause the social transmission of mothering style (high versus low pup licking) from mother-to-daughter because overexpression of Esr1 in the early life period causes offspring of low licking mothers to become high licking mothers in adulthood (132). In addition to the alterations in estradiol sensitivity within the MPOA, increased early maternal contact is also linked to an increased sensitivity of the mesolimbic dopamine system toward infants and infant related stimuli in adult females (131).
Together these findings support the idea that estradiol stimulation at birth functions to increase attraction to and motivation for pups through potentiating the output of MPOA neurons, particularly those containing estrogen receptors that project to the VTA. However, thus far we have emphasized a role for estradiol stimulation exclusively in the immediate onset of maternal behavior; lactation is characterized by a suppression of estrous cyclicity. Thus, a relevant question is whether the transcriptional programming of Esr1 would be expected to play a role in maintaining maternal care? The answer to this question is related to the issue of whether effects of estrogen receptors may be separable from the effects of their cognate ligand. For example, whereas estradiol is not required for the maintenance of maternal care following its onset, recent evidence indicates that estrogen receptor alpha may still be necessary. Viral mediated knockdown of Esr1 in the MPOA of female mice disrupts pup retrieval, pup licking and nursing behavior across postpartum days 4-7 (67). This finding suggests that estrogen receptor alpha may be activated in a ligand-independent manner (133). The mechanisms through which Esr1 transcription initiates and sustains maternal care have not been elucidated. Given that estrogen receptor stimulation can induce genomic effects at estrogen response elements and beyond, and that nongenomic effects of estrogen receptor signaling are also now clear, multiple possibilities exist. The effects of Esr1 silencing on maternal care in virgin mice have not been investigated, however, if estradiol is not required for estrogen receptor signaling an interesting possibility is that experiential factors are capable of activating estrogen receptors. The fact that Esr1 expressing cells are necessary for the expression of maternal care in virgin mice supports this idea (10). Thus, a common molecular mechanism for the activation of caregiving behavior by extrinsic and intrinsic factors might involve estrogen receptor signaling.
How might experiential factors access estrogen receptor signaling pathways in the MPOA? One possibility is that repeated exposure to pups activates intracellular signaling cascades, which in turn activate the transcription factor cyclic AMP response element binding protein (CREB). In support of this idea, interaction with infants increases calcium influx in Esr1+ and Gal+ neurons within the MPOA of male and female mice (9,10). Exposure to pups also activates MAP kinase signaling pathways, which are capable of activating CREB (127). Finally, virgin and postpartum female mice interacting with pups show increased CREB phosphorylation in the MPOA and genetic deletion of CREB interferes with maternal care (134).
There are multiple conceivable mechanisms through which CREB and estrogen receptor signaling could interact. First, CREB is capable of activating estrogen receptor alpha in a ligand-independent way (135). Second, CREB and estrogen receptor alpha are capable of regulating the transcription of some of the same genes, particularly those candidates involved in the regulation of maternal care (136). Third, nongenomic actions of estrogen receptor stimulation are capable of activating CREB (137). At present it is unclear how or if these two signaling systems interact within the MPOA to regulate caregiving behavior. Interestingly, both CREB and estrogen receptor alpha recruit the same coactivator and histone acetyltransferase (HAT) enzyme, CREB binding protein (CBP) to gene regulatory regions. Thus, one possibility is that alterations in histone acetylation may be involved in experience-dependent plasticity within the MPOA. In support of this idea, CBP-mediated histone acetylation has been implicated in estradiol facilitation of learning and memory within the hippocampus (138).
Although many enzymes possess HAT capabilities in the central nervous system, CBP stands out as a potentially critical mediator of experience-dependent plasticity. Interference with the HAT activity of CBP interferes with memory consolidation and this effect is rescued by treatment with the histone deactylase (HDAC) inhibitor, sodium butyrate (139), however note that in the complete absence of CBP activity sodium butyrate has no effect on memory (140). Together these data suggest that CBP is an important regulator of memory formation and that HDAC inhibitor treatments may affect experience-dependent plasticity through effects on CBP. This last point is surprising given the non-specific mechanism of HDAC inhibitor action. For example, sodium butyrate blocks 8 of the 18 known HDAC enzymes (HDACs 1,2,3,4,5,7,8,9). Further, despite the fact that HDAC inhibitor drugs are frequently administered systemically, the reported behavioral effects are highly dependent on context and gene expression changes are surprisingly limited (89,141,142). The apparent specificity of HDAC inhibitor effects may be related to the organization of HDAC binding across the mouse genome. For example, the distribution of HDACs 1,2,3 and 6 tend to overlap with CBP at actively transcribed genes (143). Thus, HDACs may function as a braking mechanism for activity-dependent gene transcription. In support of this idea, HDAC2 and HDAC3 have been identified as negative regulators of memory formation in the hippocampus via their direct interfering with CREB-mediate gene transcription (144,145). Along this line, it’s conceivable that HDAC inhibitor treatment effects are relatively specific because they function to remove the brakes from CREB signaling pathways, which would be activated in a context-dependent way. In support of this idea, global histone acetylation is elevated in the amygdala following fear conditioning in all rats, but those treated with an HDACi have a longer period of histone acetylation (146).
With respect to the idea that histone acetylation may regulate experience-dependent plasticity in maternal neural circuits, systemic administration of sodium butyrate reduced the amount of pup experience required to sustain caregiving behavior in a novel environment (39,56,89). Whereas C57BL6/J virgin female mice typically require 4 consecutive 2-hour exposures to pups in the home cage environment in order to sustain caregiving behaviors across time and context, HDAC inhibitor-treated females required half the experience. These data were the first to suggest that epigenetic mechanisms contribute to the experience-induced onset and maintenance of caregiving behavior. Importantly, females treated with an HDAC inhibitor during 2 brief experiences with pups remain responsive to pups, even in a novel environment, 30 days later (56). Further, HDAC inhibitor treatment increased the expression of several genes that are typically upregulated by the hormonal events of birth, such as estrogen receptor beta (Esr2) and oxytocin (Oxt) in the MPOA (89). However, in support of the idea that HDAC inhibitor treatment affects the expression of genes that may already be transcriptionally poised, HDAC inhibitor treatment had no effect on several other candidate genes including Avp and Avp1ar. Given that brief exposure to pups does not typically sustain caregiving behavior in a novel context, we recently examined whether HDAC inhibitor treatment in virgin female mice increased the likelihood that regions of the maternal neural circuit, rather than regions of the fear/avoidance circuit, were activated during the challenging task of pup retrieval in a novel T-maze (39). The results of this study indicate that HDAC inhibitor treatment reduced immediate early gene expression in the AHN/VMN, but increased immediate early gene expression in the VTA. These data suggest that the elimination of caregiving behavior under stressful circumstances was associated with the activation of an otherwise latent fear/defense/avoidance circuit in a stressful context. This finding is the first evidence to suggest that a stress-induced reduction in maternal behavior is associated with a pup-induced activation of fear/defensive neural pathways and it implies that aberrant activation of this pathway could be a neural substrate for maternal neglect. Further, repeated experience with pups may stabilize caregiving responses by not only increasing the likelihood that pup stimuli activate an approach neural circuit, but also by reducing the likelihood that pup stimuli activate a fear/defensive neural pathway in a novel environment. Thus, the prolonged interest in pups following maternal experience may not only be related to an upregulation of maternal circuits, but may also be related to a downregulation of a pup avoidance neural pathway.
Finally, while we have emphasized a role for MPOA efferent projections in the regulation of caregiving behavior, it should be noted that plasticity in cortical sites that project to the MPOA might also play a critical role in sustaining caregiving behavior. For example, there is good evidence that both hormonal and experiential factors act at the level of auditory cortex to fine tune behavioral responses to pup calls (147). Depending on the frequency, pup calls can signal a dam to engage in retrieval, pup grooming or a nursing bout (44). Plasticity in auditory cortex likely contributes to the initiation of appropriate maternal responses to pup calls and recent work shows that the processing of isolation calls from pups facilitates induction of pup retrieval (45). The DNA methyl binding protein, MECP2, is a critical modulator of plasticity at this site (148). Mice with a heterozygous deletion of MECP2 in neurons of auditory cortex never learn to retrieve pups. The finding that mice with this deletion are able to hear and that auditory neurons respond electrophysiologically to stimuli suggest that MECP2 mutation specifically affects plasticity within these cells rather than processing of pup cues more generally.
Other evidence indicates that sensory regions in the cortex aside from the auditory field undergo plasticity as a result of sensory input from pups to sustain maternal care. For example, lactating female rats show increases in cortical representation in the S1 region as a result of tactile stimulation of the ventrum during nursing behavior (149). Furthermore, different sensory modalities may even integrate with each other to alter cortical representation and how mothers respond to their infants. Exposure to pup odors, for example, in lactating and pup-experienced virgin female mice altered sensitivity to auditory stimuli (150).
6.1. Can HDAC inhibitors reveal genes that are transcriptionally poised for parenting?
An implication of the work described above is that virgin female mice respond to HDAC inhibitor treatment with augmented maternal care responses because the circuits that regulate care and inhibit pup avoidance are transcriptionally primed. For example, HDAC inhibitor treatment has been associated with a dynamic change in DNA accessibility, but this change depends on the pattern of transcription factor placement prior to HDAC inhibitor treatment (151). Specifically, the pattern of transcription factor placement can predict the extent to which gene expression is altered by HDAC inhibitor treatment. If HDAC inhibitor treatment affects the transcription of poised or actively transcribed genes, rather than transcriptionally silenced genes, then this treatment should have no effect on animals in which the relevant transcriptional pathways for caregiving behavior are not active. In support of this idea, we recently found that HDAC inhibition had no effect on caregiving behavior in virgin male mice that respond to pups aggressively (109). Thus, HDAC inhibitor treatment may be capable of revealing important information about which genomic sites are accessible in mice that are responsive, indifferent or aggressive toward infants.
VII. Conclusions and future directions
The data reviewed herein support the idea that conserved neural circuits regulate pro and antisocial responses toward infants. Female rats and male mice must overcome an initial aversion of infants in order to care them. This transition from anti- to pro-maternal state depends on alterations within both central aversion and central approach pathways. In other words, interference with a central aversion system alone is not capable of inducing caregiving behavior, and similarly activation of a central approach system alone is not sufficient to produce caregiving responses in otherwise fearful or aggressive animals. Further, the ability of infants to activate a central aversion system is not unique to animals that must overcome a default aversive reaction to infants. Instead, when examined, anti-social responses to pups have been found to be associated with an aberrant activation of this central aversion system. The alterations within approach and avoidance systems that bias a reproductively experienced animal toward caregiving responses largely remain to be determined, but likely involve experience-dependent plasticity mediated by transcriptional programming within these systems. An identification of these transcriptional changes will elucidate the neural mechanisms that prevent neglect and promote care, and will be an important area of future investigation. Further, an understanding of how particular transcriptional programs are involved in setting default reactions to infants within these systems is missing from the literature. This issue is particularly important given that early social experience has known effects on adult caregiving behavior. There is good evidence that early social experience can impact adult social behavior through effects on the developing mesolimbic dopamine system (152–154), however the extent to which alterations in the neural system regulating fearful/defensive responses underlie social aversion has not been described. Thus, future work will need to uncover the extent to which the occurrence of maternal abuse/neglect is related to an aberrant activation of a central aversion system in response to infants.
Acknowledgments
Funding: This work was supported by the National Institute of Child Health and Human Development [1R01HD087709-01A1] and the University of California, Davis Provost’s Fellowship for first year graduate students.
Footnotes
Disclosure of Conflicts of Interest:
The authors declare no conflicts of interest.
References:
- 1.Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345(6198):765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Royle NJ, Alonzo SH, Moore AJ. Co-evolution, conflict and complexity: what have we learned about the evolution of parental care behaviours? Current opinion in behavioral sciences. 2016;12:30–6. [Google Scholar]
- 3.Numan M, Insel TR. The neurobiology of parental behavior. Vol. 1. Springer-Verlag; 2003. [Google Scholar]
- 4.Noirot E The onset of maternal behavior in rats, hamsters and mice. Advances in the Study of Behavior. 1972;4:106–45. [Google Scholar]
- 5.Svare B, Gandelman R. Suckling stimulation induces aggression in virgin female mice. Nature. 1976;260(5552):606. [DOI] [PubMed] [Google Scholar]
- 6.Stern JM. Nursing posture is elicited rapidly in maternally naive, haloperidol-treated female and male rats in response to ventral trunk stimulation from active pups. Hormones and behavior. 1991;25(4):504–17. [DOI] [PubMed] [Google Scholar]
- 7.Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. Journal of neuroendocrinology. 2011;23(4):345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014. May 15;509(7500):325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, et al. Functional circuit architecture underlying parental behaviour. Nature. 2018;556(7701):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y-Y, Yamaguchi T, Song SC, Tritsch NX, Lin D. A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron. 2018. April 4;98(1):192–207.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach FA, Jaynes J. Studies of maternal retrieving in rats. III. Sensory cues involved in the lactating female’s response to her young. Behaviour. 1956; [Google Scholar]
- 12.Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T. Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behavioural brain research. 2010;215(1):141–5. [DOI] [PubMed] [Google Scholar]
- 13.Gandelman R, Zarrow MX, Denenberg VH, Myers M. Olfactory bulb removal eliminates maternal behavior in mouse. Science. 1971; [DOI] [PubMed] [Google Scholar]
- 14.Gandelman R The development of cannibalism in male Rockland-Swiss mice and the influence of olfactory bulb removal. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 1973;6(2):159–64. [DOI] [PubMed] [Google Scholar]
- 15.Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. Journal of Neuroscience. 2013;33(12):5120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isogai Y, Wu Z, Love MI, Ahn MH-Y, Bambah-Mukku D, Hua V, et al. Multisensory logic of infant-directed aggression by males. Cell. 2018;175(7):1827–1841.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiology & Behavior. 1981;27(5):863–8. [DOI] [PubMed] [Google Scholar]
- 18.Gandelman R The ontogeny of maternal responsiveness in female Rockland-Swiss albino mice. Hormones and behavior. 1973;4(3):257–68. [DOI] [PubMed] [Google Scholar]
- 19.Leussis MP, Bond TL, Hawken CM, Brown RE. Attenuation of maternal behavior in virgin CD-1 mice by methylphenidate hydrochloride. Physiology & behavior. 2008;95(3):395–9. [DOI] [PubMed] [Google Scholar]
- 20.Gandelman R, vom Saal FS. Pup-killing in mice: the effects of gonadectomy and testosterone administration. Physiology & behavior. 1975;15(6):647–51. [DOI] [PubMed] [Google Scholar]
- 21.Svare B, Mann M. Infanticide: genetic, developmental and hormonal influences in mice. Physiology & Behavior. 1981;27(5):921–7. [DOI] [PubMed] [Google Scholar]
- 22.Elwood RW, Ostermeyer MC. Does copulation inhibit infanticide in male rodents? Animal Behaviour. 1984;32(1):293–4. [Google Scholar]
- 23.Vom Saal FS. Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol Behav. 1985;34(7):15. [DOI] [PubMed] [Google Scholar]
- 24.Brooks RJ, Schwarzkopf L. Factors affecting incidence of infanticide and discrimination of related and unrelated neonates in male Mus musculus. Behavioral and neural biology. 1983;37(1):149–61. [DOI] [PubMed] [Google Scholar]
- 25.de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus). Hormones and behavior. 2009;56(2):220–31. [DOI] [PubMed] [Google Scholar]
- 26.Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus). Hormones and behavior. 2001;40(1):32–42. [DOI] [PubMed] [Google Scholar]
- 27.Elwood RW. Changes in the responses of male and female gerbils (Meriones unguiculatus) towards test pups during the pregnancy of the female. Animal Behaviour. 1977;25:46–51. [Google Scholar]
- 28.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats: I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. Journal of comparative and physiological psychology. 1974;86(2):221. [DOI] [PubMed] [Google Scholar]
- 29.Siegel HI, Rosenblatt JS. Progesterone inhibition of estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Hormones and behavior. 1975;6(3):223–30. [DOI] [PubMed] [Google Scholar]
- 30.Doerr HK, Siegel HI, Rosenblatt JS. Effects of progesterone withdrawal and estrogen on maternal behavior in nulliparous rats. Behavioral and neural biology. 1981;32(1):35–44. [DOI] [PubMed] [Google Scholar]
- 31.Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on ‘timidity’and attraction to pup-related odors in female rats. Physiology & behavior. 1989;46(3):449–53. [DOI] [PubMed] [Google Scholar]
- 32.Kinsley CH, Bridges RS. Morphine treatment and reproductive condition alter olfactory preferences for pup and adult male odors in female rats. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 1990;23(4):331–47. [DOI] [PubMed] [Google Scholar]
- 33.Mayer AD, Rosenblatt JS. Prepartum changes in maternal responsiveness and nest defense in Rattus norvegicus.. Journal of Comparative Psychology. 1984;98(2):177. [PubMed] [Google Scholar]
- 34.Wilsoncroft WE. Babies by bar-press: maternal behavior in the rat. Behavior Research Methods & Instrumentation. 1968;1(6):229–30. [Google Scholar]
- 35.Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Hormones and behavior. 1985;19(4):454–68. [DOI] [PubMed] [Google Scholar]
- 36.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behavioural Brain Research. 1999;100(1–2):15–31. [DOI] [PubMed] [Google Scholar]
- 37.Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine-or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology. 2003;167(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridges RS, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 1972;5(2):123–7. [DOI] [PubMed] [Google Scholar]
- 39.Mayer HS, Helton J, Torres LY, Cortina I, Brown WM, Stolzenberg DS. Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Hormones and behavior. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Hormones and Behavior. 1976;7(3):305–16. [DOI] [PubMed] [Google Scholar]
- 41.Nissen HW. A study of maternal behavior in the white rat by means of the obstruction method. The Pedagogical Seminary and Journal of Genetic Psychology. 1930;37(3):377–92. [Google Scholar]
- 42.Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behavioural brain research. 2006;175(1):139–48. [DOI] [PubMed] [Google Scholar]
- 43.Geissler DB, Schmidt HS, Ehret G. Limbic brain activation for maternal acoustic perception and responding is different in mothers and virgin female mice. Journal of Physiology-Paris. 2013;107(1–2):62–71. [DOI] [PubMed] [Google Scholar]
- 44.Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Animal Behaviour. 1986;34(3):821–30. [Google Scholar]
- 45.Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. European Journal of Neuroscience. 2006;23(11):3087–97. [DOI] [PubMed] [Google Scholar]
- 46.Lin FG, Galindo-Leon EE, Ivanova TN, Mappus RC, Liu RC. A role for maternal physiological state in preserving auditory cortical plasticity for salient infant calls. Neuroscience. 2013;247:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrigo G, Bryant WC, vom Saal FS. A unique neural timing system prevents male mice from harming their own offspring. Animal Behaviour. 1990;39(3):535–9. [Google Scholar]
- 48.Perrigo G, Belvin L, vom Saal FS. Time and sex in the male mouse: Temporal regulation of infanticide and parental behavior. Chronobiology international. 1992;9(6):421–33. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156(3781):1512–3. [DOI] [PubMed] [Google Scholar]
- 50.Elwood RW. Inhibition of infanticide and onset of paternal care in male mice (Mus musculus). Journal of Comparative Psychology. 1985;99(4):457. [Google Scholar]
- 51.Kennedy HF, Elwood RW. Strain differences in the inhibition of infanticide in male mice (Mus musculus). Behavioral & Neural Biology. 1988; [DOI] [PubMed] [Google Scholar]
- 52.Orpen BG, Fleming AS. Experience with pups sustains maternal responding in postpartum rats. Physiology & Behavior. 1987;40(1):47–54. [DOI] [PubMed] [Google Scholar]
- 53.Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: Effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22(1):44–53. [Google Scholar]
- 54.Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behavioral neuroscience. 2006;120(3):676. [DOI] [PubMed] [Google Scholar]
- 55.Seip KM, Morrell JI. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiology & behavior. 2008;95(4):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stolzenberg DS, Stevens JS, Rissman EF. Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology. 2014;155(9):3674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenblatt JS, Mayer AD. An analysis of approach/withdrawal processes in the initiation of maternal behavior in the laboratory rat. Behavioral development: Concepts of approach/withdrawal and integrative levels. 1995;177–230. [Google Scholar]
- 58.Benuck I, Rowe FA. Centrally and peripherally induced anosmia: Influences on maternal behavior in lactating female rats. Physiology & Behavior. 1975;14(4):439–47. [DOI] [PubMed] [Google Scholar]
- 59.Jirik-Babb P, Manaker S, Tucker AM, Hofer MA. The role of the accessory and main olfactory systems in maternal behavior of the primiparous rat. Behavioral and neural biology. 1984;40(2):170–8. [DOI] [PubMed] [Google Scholar]
- 60.Kolunie JM, Stern JM. Maternal aggression in rats: effects of olfactory bulbectomy, ZnSO4-induced anosmia, and vomeronasal organ removal. Hormones and Behavior. 1995;29(4):492–518. [DOI] [PubMed] [Google Scholar]
- 61.Numan M, Sheehan TP. Neuroanatomical Circuitry for Mammalian Maternal Behavior a. Annals of the New York Academy of Sciences. 1997;807(1):101–25. [DOI] [PubMed] [Google Scholar]
- 62.Numan M Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews. 2006;5(4):163–90. [DOI] [PubMed] [Google Scholar]
- 63.Numan M Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 2007;49(1):12–21. [DOI] [PubMed] [Google Scholar]
- 64.Numan M Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol. 1974. October;87(4):746–59. [DOI] [PubMed] [Google Scholar]
- 65.Tsuneoka Y, Maruyama T, Yoshida S, Nishimori K, Kato T, Numan M, et al. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J Comp Neurol. 2013. May 1;521(7):1633–63. [DOI] [PubMed] [Google Scholar]
- 66.Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977. February;91(1):146–64. [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, et al. siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proceedings of the National Academy of Sciences. 2012;201214094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM, Mann PE. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology. 1996;64(1):57–64. [DOI] [PubMed] [Google Scholar]
- 69.Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology. 1997;138(2):756–63. [DOI] [PubMed] [Google Scholar]
- 70.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral neuroscience. 1994;108(6):1163. [DOI] [PubMed] [Google Scholar]
- 71.Numan M, Corodimas KP. The effects of paraventricular hypothalamic lesions on maternal behavior in rats. Physiology & behavior. 1985;35(3):417–25. [DOI] [PubMed] [Google Scholar]
- 72.Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD. Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behavioral neuroscience. 1988;102(3):381. [DOI] [PubMed] [Google Scholar]
- 73.Franz JR, Leo RJ, Steuer MA, Kristal MB. Effects of hypothalamic knife cuts and experience on maternal behavior in the rat. Physiology & behavior. 1986;38(5):629–40. [DOI] [PubMed] [Google Scholar]
- 74.Miceli MO, Malsbury CW. Sagittal knife cuts in the near and far lateral preoptic area-hypothalamus disrupt maternal behaviour in female hamsters. Physiology & behavior. 1982;28(5):857–67. [PubMed] [Google Scholar]
- 75.Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. Journal of neuroendocrinology. 1997;9(5):369–84. [DOI] [PubMed] [Google Scholar]
- 76.Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. NeuroScience & Biobehavioral Reviews. 1991;15(4):527–38. [DOI] [PubMed] [Google Scholar]
- 77.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiology & behavior. 1980;25(5):731–43. [DOI] [PubMed] [Google Scholar]
- 78.Fleming AS, Miceli M, Moretto D. Lesions of the medial preoptic area prevent the facilitation of maternal behavior produced by amygdala lesions. Physiology & behavior. 1983;31(4):503–10. [DOI] [PubMed] [Google Scholar]
- 79.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: A PHAL study in the rat. Journal of Comparative Neurology. 1995. September 18;360(2):213–45. [DOI] [PubMed] [Google Scholar]
- 80.Bridges RS, Mann PE. Prolactin-brain interactions in the induction of maternal behavior in rats. Psychoneuroendocrinology. 1994;19(5):611–22. [DOI] [PubMed] [Google Scholar]
- 81.Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behavioral neuroscience. 2000;114(2):337. [DOI] [PubMed] [Google Scholar]
- 82.Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. Journal of neuroendocrinology. 1999; [DOI] [PubMed] [Google Scholar]
- 83.Sheehan TP, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106(2):341–56. [DOI] [PubMed] [Google Scholar]
- 84.Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behavioural brain research. 2010;209(2):226–33. [DOI] [PubMed] [Google Scholar]
- 85.Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, et al. Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. The EMBO journal. 2015;34(21):2652–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutiérrez-Castellanos N, Martínez-Marcos A, Martínez-García F, Lanuza E. Chemosensory function of the amygdala In: Vitamins & Hormones. Elsevier; 2010. p. 165–96. [DOI] [PubMed] [Google Scholar]
- 87.Dong H-W, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. Journal of Comparative Neurology. 2003;463(4):434–72. [DOI] [PubMed] [Google Scholar]
- 88.Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain research bulletin. 1991;27(5):651–62. [DOI] [PubMed] [Google Scholar]
- 89.Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Hormones and behavior. 2012;62(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Chen IZ, Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron. 2015;85(6):1344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheehan TP, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75(1):12–23. [DOI] [PubMed] [Google Scholar]
- 92.Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behavioural brain research. 2002;131(1–2):17–36. [DOI] [PubMed] [Google Scholar]
- 93.Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav Neurosci. 2009. August;123(4):740–51. [DOI] [PubMed] [Google Scholar]
- 94.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984. August;98(4):712–27. [DOI] [PubMed] [Google Scholar]
- 95.Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiology & behavior. 1999;67(5):659–69. [DOI] [PubMed] [Google Scholar]
- 97.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005. December;119(6):1588–604. [DOI] [PubMed] [Google Scholar]
- 98.Silva MRP, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacology Biochemistry and Behavior. 2001;68(3):461–8. [DOI] [PubMed] [Google Scholar]
- 99.Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup-and food-stimuli in the female rat. Hormones and Behavior. 2009;56(1):11–23. [DOI] [PubMed] [Google Scholar]
- 100.Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D₁ receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behavioral neuroscience. 2007;121(5):907. [DOI] [PubMed] [Google Scholar]
- 101.Pennartz CM, Groenewegen HJ, da Silva FHL. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in neurobiology. 1994;42(6):719–61. [DOI] [PubMed] [Google Scholar]
- 102.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. Journal of Comparative Neurology. 1993;327(2):220–32. [DOI] [PubMed] [Google Scholar]
- 103.Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120(3):783–98. [DOI] [PubMed] [Google Scholar]
- 104.Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005. March 7;158(1):53–68. [DOI] [PubMed] [Google Scholar]
- 105.Liu H-X, Lopatina O, Higashida C, Fujimoto H, Akther S, Inzhutova A, et al. Displays of paternal mouse pup retrieval following communicative interaction with maternal mates. Nature communications. 2013;4:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akther S, Fakhrul AA, Higashida H. Effects of electrical lesions of the medial preoptic area and the ventral pallidum on mate-dependent paternal behavior in mice. Neuroscience letters. 2014;570:21–5. [DOI] [PubMed] [Google Scholar]
- 107.Zhong J, Liang M, Akther S, Higashida C, Tsuji T, Higashida H. c-Fos expression in the paternal mouse brain induced by communicative interaction with maternal mates. Molecular brain. 2014;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akther S, Huang Z, Liang M, Zhong J, Fakhrul AA, Yuhi T, et al. Paternal retrieval behavior regulated by brain estrogen synthetase (aromatase) in mouse sires that engage in communicative interactions with pairmates. Frontiers in neuroscience. 2015;9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayer HS, Marc Crepeau, Duque-Wilckens N, Torres LY, Trainor BC, Stolzenberg DS. Histone deacetylase inhibitor treatment promotes spontaneous caregiving behavior in C57BL/6J male mice. BioRxiv. 2018; 10.1101/433995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Numan M Neurobiology of social behavior: toward an understanding of the prosocial and antisocial brain. Academic Press; 2014. [Google Scholar]
- 111.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience. 2004;24(17):4113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Afonso VM, King SJ, Novakov M, Burton CL, Fleming AS. Accumbal dopamine function in postpartum rats that were raised without their mothers. Hormones and Behavior. 2011;60(5):632–43. [DOI] [PubMed] [Google Scholar]
- 113.Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science. 2017;357(6356):1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiology & behavior. 2005;86(3):356–68. [DOI] [PubMed] [Google Scholar]
- 115.Tan CL, Cooke EK, Leib DE, Lin Y-C, Daly GE, Zimmerman CA, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lonstein JS, Gréco B, De Vries GJ, Stern JM, Blaustein JD. Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology. 2000;72(2):91–101. [DOI] [PubMed] [Google Scholar]
- 117.Lonstein JS, De Vries GJ. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neuroscience. 2000;100(3):557–68. [DOI] [PubMed] [Google Scholar]
- 118.Matsushita N, Muroi Y, Kinoshita K, Ishii T. Comparison of c-Fos expression in brain regions involved in maternal behavior of virgin and lactating female mice. Neuroscience letters. 2015;590:166–71. [DOI] [PubMed] [Google Scholar]
- 119.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, et al. Molecular, spatial and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao C, Eisinger B, Gammie SC. Characterization of GABAergic neurons in the mouse lateral septum: a double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PloS one. 2013;8(8):e73750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fahrbach SE, Morrell JI, Pfaff DW. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. Journal of Comparative Neurology. 1986;247(3):364–82. [DOI] [PubMed] [Google Scholar]
- 122.Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behavioral neuroscience. 1998;112(6):1502. [DOI] [PubMed] [Google Scholar]
- 123.Bridges RS. Retention of rapid onset of maternal behavior during pregnancy in primiparous rats. Behavioral Biology. 1978;24(1):113–7. [DOI] [PubMed] [Google Scholar]
- 124.Bridges RS. Parturition: Its role in the long term retention of maternal behavior in the rat. Physiology & Behavior. 1977;18(3):487–90. [Google Scholar]
- 125.Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology & Behavior. 1975;14(3):245–9. [DOI] [PubMed] [Google Scholar]
- 126.Calamandrei G, Keverne EB. Differential expression of Fos protein in the brain of female mice dependent on pup sensory cues and maternal experience. Behavioral neuroscience. 1994;108(1):113. [DOI] [PubMed] [Google Scholar]
- 127.Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Molecular and Cellular Neuroscience. 2007;36(2):121–31. [DOI] [PubMed] [Google Scholar]
- 128.Driessen TM, Eisinger BE, Zhao C, Stevenson SA, Saul MC, Gammie SC. Genes showing altered expression in the medial preoptic area in the highly social maternal phenotype are related to autism and other disorders with social deficits. BMC neuroscience. 2014;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: the role of experience and epigenetic mechanisms. Hormones and behavior. 2016;77:204–10. [DOI] [PubMed] [Google Scholar]
- 130.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–15. [DOI] [PubMed] [Google Scholar]
- 131.Peña CJ, Neugut YD, Calarco CA, Champagne FA. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. European Journal of Neuroscience. 2014;39(6):946–56. [DOI] [PubMed] [Google Scholar]
- 132.Peña CJ, Champagne FA. Neonatal overexpression of estrogen receptor-α alters midbrain dopamine neuron development and reverses the effects of low maternal care in female offspring. Developmental neurobiology. 2015;75(10):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Molecular endocrinology. 2005;19(4):833–42. [DOI] [PubMed] [Google Scholar]
- 134.Jin S-H, Blendy JA, Thomas SA. Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience. 2005;133(3):647–55. [DOI] [PubMed] [Google Scholar]
- 135.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Molecular endocrinology. 2003;17(7):1296–314. [DOI] [PubMed] [Google Scholar]
- 136.Bale TL, Dorsa DM. Transcriptional regulation of the oxytocin receptor gene In: Vasopressin and Oxytocin. Springer; 1998. p. 307–15. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137(5):2163–6. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proceedings of the National Academy of Sciences. 2010;107(12):5605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36(8):1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. Journal of Biological Chemistry. 2004; [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide Mapping of HATs and HDACs Reveals Distinct Functions in Active and Inactive Genes. Cell. 2009. September 4;138(5):1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. Journal of Neuroscience. 2011;31(2):764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455(7211):411. [DOI] [PubMed] [Google Scholar]
- 146.Yeh S-H, Lin C-H, Gean P-W. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology. 2004;65(5):1286–92. [DOI] [PubMed] [Google Scholar]
- 147.Banerjee SB, Liu RC. Storing maternal memories: hypothesizing an interaction of experience and estrogen on sensory cortical plasticity to learn infant cues. Frontiers in neuroendocrinology. 2013;34(4):300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krishnan K, Lau BY, Ewall G, Huang ZJ, Shea SD. MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nature communications. 2017;8:14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. Journal of Neuroscience. 1994;14(3):1710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cohen L, Rothschild G, Mizrahi A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron. 2011;72(2):357–69. [DOI] [PubMed] [Google Scholar]
- 151.Qu K, Zaba LC, Satpathy AT, Giresi PG, Li R, Jin Y, et al. Chromatin accessibility landscape of cutaneous T cell lymphoma and dynamic response to HDAC inhibitors. Cancer Cell. 2017;32(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Champagne FA. Early adversity and developmental outcomes: Interaction between genetics, epigenetics, and social experiences across the life span. Perspectives on Psychological Science. 2010;5(5):564–74. [DOI] [PubMed] [Google Scholar]
- 153.Curley JP, Champagne FA. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in neuroendocrinology. 2016;40:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Palombo DJ, Nowoslawski M, Fleming AS. Motherless rats show deficits in maternal behavior towards fostered pups. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 2010;52(2):142–8. [DOI] [PubMed] [Google Scholar]
- 155.D’Urso A, Brickner JH. Epigenetic transcriptional memory. Current genetics. 2017;63(3):435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]


