Abstract
We have previously described new pathways of vitamin D3 activation by CYP11A1 to produce a variety of metabolites including 20(OH)D3 and 20,23(OH)2D3. These can be further hydroxylated by CYP27B1 to produce their C1α-hydroxyderivatives. CYP11A1 similarly initiates the metabolism of lumisterol (L3) through sequential hydroxylation of the side chain to produce 20(OH)L3, 22(OH)L3, 20,22(OH)2L3 and 24(OH)L3. CYP11A1 also acts on 7-dehydrocholesterol (7DHC) producing 22(OH)7DHC, 20,22(OH)27DHC and 7-dehydropregnenolone (7DHP) which can be converted to the D3 and L3 configurations following exposure to UVB. These CYP11A1-derived compounds are produced in vivo and are biologically active displaying anti-proliferative, anti-inflammatory, anti-cancer and pro-differentiation properties. Since the protective role of the classical form of vitamin D3 (1,25(OH)2D3) against UVB induced damage is recognized, we recently tested whether novel CYP11A1-derived D3- and L3-hydroxyderivatives protect against UVB-induced damage in epidermal human keratinocytes and melanocytes. We found that along with 1,25(OH)2D3, CYP11A1-derived D3-hydroxyderivatives and L3 and its hydroxyderivatives exert photoprotective effects. These included induction of intracellular free radical scavenging and attenuation and repair of DNA damage. The protection of human keratinocytes against DNA damage included the activation of the NRF2-regulated antioxidant response, p53-phosphorylation and its translocation to the nucleus, and DNA repair induction. These data indicate that novel derivatives of vitamin D3 and lumisterol are promising photoprotective agents. However, detailed mechanisms of action, and the involvement of specific nuclear receptors, other vitamin D binding proteins or mitochondria, remain to be established.
Keywords: Ultraviolet B, vitamin D, lumisterol, skin, oxidative stress, DNA damage, NRF2, NFκB, VDR, RORs, AhR, mitochondrion
Introduction to vitamin D biochemistry
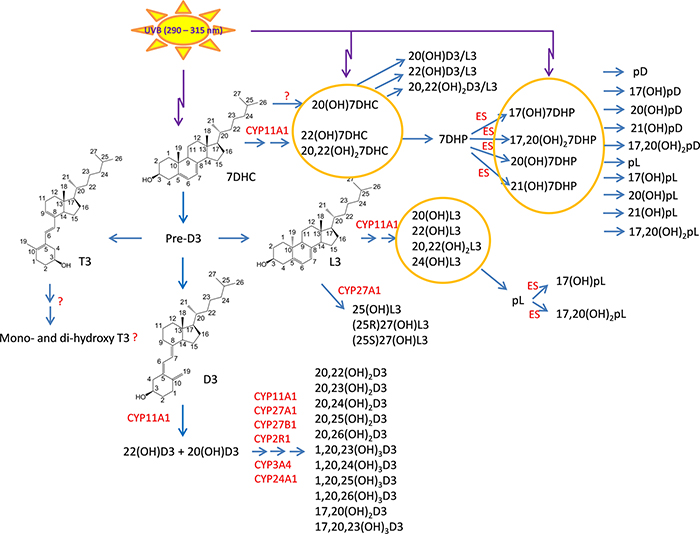
7-Dehydrocholestrol (7DHC), the immediate precursor to cholesterol in the Kandutsch-Russel pathway, can absorb ultraviolet B radiation (UVB) producing pre-vitamin D3 which isomerizes to vitamin D3 (D3) or, after prolonged exposure to UVB, to lumisterol 3 (L3) and tachysterol 3 (T3)1–3(Fig. 1). In the canonical pathway, D3 is activated by a 25-hydroxylase (CYP2R1 or CYP27A1) and 1a-hydroxylase (CYP27B1) to produce 1,25(OH)2D3. Both 25(OH)D3 and 1,25(OH)2D3 are degraded by sequential oxidations of the side chain catalyzed by CYP24A1, in the latter case producing calcitroic acid 4,5. A similar sequence of reactions is involved in the activation of vitamin D2 (D2), a product of UVB induced transformation of fungi- or phytoplankton-derived ergosterol 2,3,5 A novel, non-canonical pathway of D3 activation is initiated by CYP11A1 5,6 with initial hydroxylation at C20 7,8 or C22 9 and subsequent hydroxylations at C20, C22, C23 and/or C17 7,8,10. The resulting products can be selectively hydroxylated by CYP27A1, CYP24A1, CYP2R1 and/or CYP3A4, with additional hydroxylation at C1a occurring for all intermediates except those possessing a hydroxyl group at C17 5,11–16(Fig. 1). Many of the above intermediates/products can be synthesized ex vivo in skin cells, placenta and adrenal glands and some are detectable in human serum and/or epidermis and in the pig adrenal gland 17–20. Vitamin D2 is also hydroxylated by CYP11A1 producing 20(OH)D2, 17,20(OH)2D2 and 17,20,24(OH)3D2 21,22, with 20(OH)D3 being hydroxylated by CYP27B1 to 1,20(OH)2D2 23. These D2 metabolites are also produced by human placenta, skin cells and a pig adrenal glands 24.
Figure 1.
Noncanonical pathways of vitamin D3 and lumisterol (L3) activation. D3, L3 and 7DHC are substrates for CYP11A1 activity that by itself or in cooperation with other CYPs enzymes produces the corresponding hydroxyderivatives. In the case of L3 and 7HDC, the side chain can be cleaved by CYP11A1 to produce 7DHP or pL that can be further metabolized by steroidogenic enzymes (ES). In the skin UVB acting on 5,7-dienes can lead to production of D3, L3 and T3 derivatives with a full-length side chain, and pD, pL and pT derivatives with a shortened side chain. While the cut-off for UVC/UVB is 280 nm, we show the range 290–315 nm because wavelengths below 290 nm are filtered by the ozone layer and no additional pre-D3 is produced above 315 nm 143.
7DHC and L3 are also substrates for CYP11A1. 7DHC is converted to 22(OH)7DHC, 20,22(OH)27DHC and 7-deydropregnenolone (7DHP) 7,25–27, while L3 produces 20(OH)L3, 22(OH)L3, 20,22(OH)2L3, 24(OH)L3 and pregnalumisterol (pL) 28,29. 7DHP and pL can be further metabolized to hydroxy-7DHP or 7-dehydroprogesterone, and hydroxy-pL compounds, respectively, by steroidogenic enzymes26,27,29. These pathways have been observed to operate in cultured skin cells, placenta and adrenal glands incubated ex-vivo, and the products have been identified in the human epidermis, serum, placenta and in pig adrenal glands 26,27,29. Most recently, we have also shown that CYP27A1 can efficiently hydroxylate L3 to three major products, 25(OH)L3, (25R)27(OH)L3 and (25S)27(OH)L3 30. Finally, in the skin the 5,7-dienal compounds with a full-length or shortened side chain can undergo UVB-induced photoisomerization to give the corresponding D3, L3 or T3 products. The short side chain products include pregnacalciferol (pD), pL and pregnatachysterol (pT) 25,31–34.
Mechanism of action of vitamin D derivatives
Receptor mediated mechanisms
The major hormonally active form of vitamin D3, 1,25(OH)2D3, not only regulates calcium homeostasis and the musculoskeletal system, but also exerts a vast spectrum of activities including stimulation of differentiation and inhibition of proliferation of cells of different lineage, anti-cancerogenic effects and stimulation of innate and inhibition of adaptive immunity and inflammation. It also regulates endocrine and central nervous systems and plays an important role in development 2,35–38. In the skin it is involved in the formation of the epidermal barrier, regulates the functions of adnexal structures including hair follicles, and has a wide variety of ameliorating effects on skin cancer and proliferative and inflammatory cutaneous diseases 2,37,39–42. The traditional view is that most, if not all of these pleiotropic effects are mediated through the interaction with the nuclear vitamin D receptor (VDR). After binding to 1,25(OH)2D3 the receptor heterodimerizes with RXR and is translocated to the nucleus where it binds to the VDR responsive element (VDRE) in target genes to influence gene expression 36,39,43. In addition, it has been proposed that 1,25(OH)2D3 actions involve non-genomic activities associated with its binding to 1,25D3-MARRS (ERp57 or PDIA3), the endoplasmic membrane-associated protein that acts as thiol-disulfide oxidoreductase and also participates in the folding and quality control of newly synthesized glycoproteins 44–49.
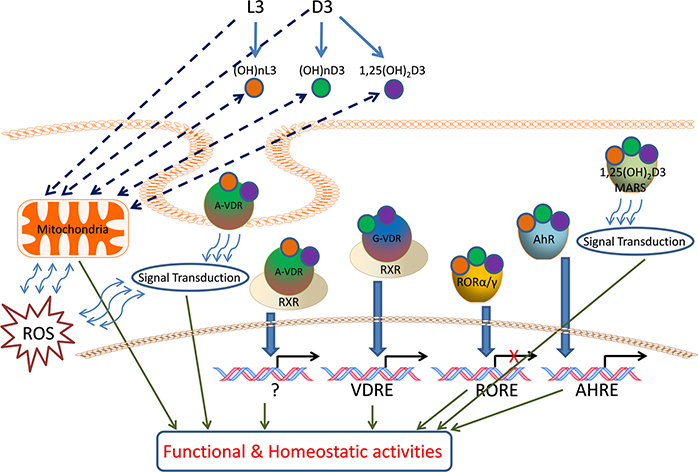
Novel CYP11A1-derived D3-hydroxyderivatives also exert anti-proliferative, pro-differentiation, and anti-inflammatory effects in vitro that are comparable or better than those of 1,25(OH)2D3 9,50–60. They also show antifbrotic activities both in vitro 54–56 and in vivo 56. In addition, they demonstrate anti-melanoma and anti-tumor properties that are cell-type lineage dependent 9,55,57,61–66. Importantly, CYP11A1-derived hydroxymetabolites of D3 that have not been acted on by CYP27B1 and therefore lack a C1α hydroxyl group, are non-calcemic 56,61,63,67. Thus, hydroxylation at C1α determines the mode of signal transduction, e.g., CYP11A1-derived ligands without a C1α hydroxyl group act as biased agonists on the VDR, producing some but not all responses, while the D3-derivatives with a C1α (OH) act as full agonists on this receptor 58,68–70. Importantly, alternative nuclear receptors for CYP11A1-derived D3-hydroxyderivatives (and also likely for classical 1,25(OH)2D3 to some extent) have been discovered. These include the retinoid-related orphan receptors (ROR)α and γ and the arylhydrocarbon receptor (AhR) on which they act as inverse agonists and agonists, respectively 60,68,71. The interaction of different D3-derivatives with genomic and non-genomic binding sites of the VDR, ROR α and γ and AhR, and possibly 1,25D3-MARRS, is dependent on the structural localization of hydroxyl groups which defines their specificity and affinity (Fig. 2).
Figure 2.
Vitamin D3 and L3 target receptors and mitochondria for regulation of the cell phenotype and homeostatic activities.
D3 and L3 precursors are hydroxylated by either microsomal or mitochondrial CYPs to generate (OH)nD3, (OH)nL3 and classical 1,25(OH)2D3, which can bind to the A or G site of VDR, or to RORs, AhR or 1,25D3 MARRS to activate genomic or non-genomic signal transduction pathways. The mitochondrion is also a target for these hydroxyderivatives.
It has recently been demonstrated that lumisterol hydroxyderivatives are produced and exert biological activity in epidermal and dermal skin cells 29. Their mechanism of action appears to include functioning as inverse agonists of RORs and acting on the non-genomic pocket of the VDR 29. Based on their structural similarities they are also likely to interact with other nuclear receptors that interact with sterols.
Recently, Holick and coworkers, 2019 presented the microarray data from “buffy coat” of volunteers supplemented with 600, 4000 or 10,000 IU of vitamin D for 6 months 72. This treatment resulted in modulation of expression of 162, 320 and 1289 genes, respectively. It this paper the authors postulated that supplementation with a high dose of vitamin D (10,000 IU) upregulates pathways involved in histone and chromatin modifications and functions as a key factor of transcriptional regulation at the epigenomic level. This finding partially explains the pleiotropic effects of vitamin D and is consistent with our hypothesis that these effects are secondary to production of multiple D3-hydroxyderivatives that act on the VDR and alternative nuclear receptors 6,34,71.
Mitochondria as a switchboard for vitamin D and lumisterol actions
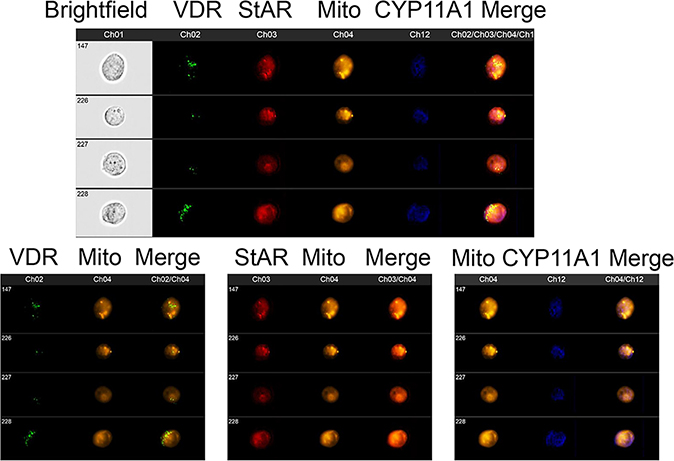
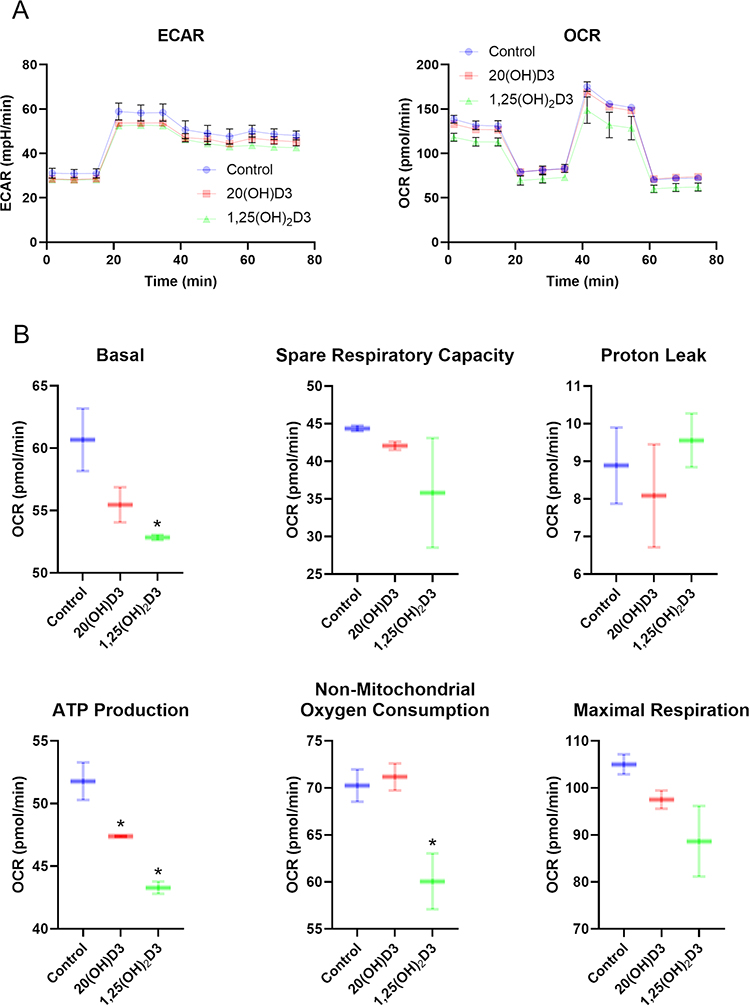
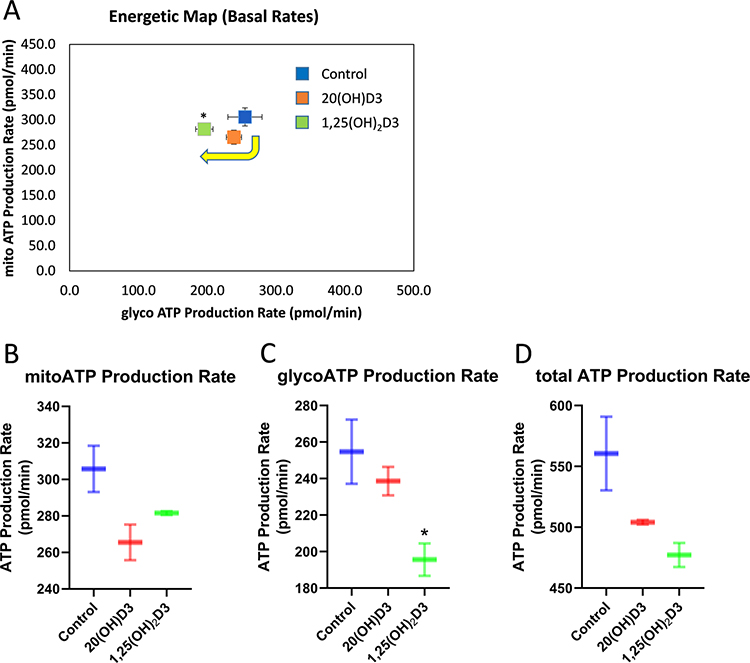
Crucial enzymes involved in the activation (CYP11A1, CYP27A1, CYP27B1) and inactivation (CYP24A1) of vitamin D are located within the mitochondrion and require NADPH as an energy source. CYP27A1 and CYP11A1 also hydroxylates L3 and in studies currently underway also appear to hydroxylate T3 (Figs. 1, 2). Therefore, reactions catalyzed by them may affect mitochondrial functions and their hydroxy-D3, L3 and T3-derivatives could act in the mitochondrial environment as regulators (Fig. 2). In support of this hypothesis there are reports showing the presence of the VDR in mitochondria 73 as well as on the inhibitory effect of 1,25(OH)2D3 on energy yielding metabolism and electron transport 74–77 and the role of the VDR in this process 78. We have data to support these findings, identifying co-localization of the VDR, StAR and CYP11A1 within mitochondria of keratinocytes (Fig. 3) and showing inhibition of mitochondrial respiration and ATP production by 1,25(OH)2D3, and to a lesser degree by 20(OH)D3 (Figs 4, 5).
Figure 3.
Co-localization of VDR, StAR and CYP11A1 with mitochondria in human keratinocytes.
Fixed and permeabilized HaCaT cells (human keratinocytes) were stained for expression of VDR (Ch2:green), StAR (Ch3:red), mitochondria (Ch4:Orange) and CYP11A1 (Ch12:blue) and analyzed using an ImageStream II (Amms, Seattle, WA, USA) cytometer as described previously144. The composite image of four different cells (upper panel) shows that all of the StAR colocalizes with mitochondria. VDR and CYP11A1 have different subcellular distributions but both of them are partially found colocalized with mitochondria. This is observed with greater clarity when the same four cells are analyzed for VDR and mitochondria (lower left panel), StAR and mitochondria (lower middle panel) and CYP11A1 and mitochondria (lower right panel). HaCaT cells were detached and processed as previously described52. The cells were fixed, permeabilized and stained with antibodies to VDR (Santa Cruz; Dallas, TX, USA), CYP11A1 (Cell signaling technology; Danvers, MA, USA), StAR (Santa Cruz; Dallas, TX, USA), and Mitotracker Red (10 nM - CMX Ros Invitrogen; Carlsbad, CA, USA) as described previously144. Data were analyzed using IDEAS software (Amms, Seattle, WA, USA).
Figure 4.
The effects of vitamin D3 derivatives on mitochondrial function. A, Representative traces of mitochondrial oxygen consumption rates (OCR) and, extracellular acidification rates (ECAR) in control (vehicle), 100n M 20(OH)D3 or 1,25(OH)2D3-treated HaCaT cells. B, Indices of mitochondrial function include basal, ATP-linked, maximal, reserve capacity, proton leak, and nonmitochondrial oxygen consumption rates. Data are presented as mean ± SD, n = 2. *P < 0.05 (Student’s t-test).
A Seahorse XFe24 Analyzer (Agilent Technologies, Inc., Santa Clara, CA) was used to determine ATP production rates, extracellular acidification rates (ECAR) and oxygen consumption rates (OCR). An XF Real-Time ATP Rate Assay Kit and an XF Cell Mito Stress Test Kits were used according to the manufacture’s protocol. HaCaT cells were cultured on a XF Cell Culture Microplate in DMEM media containing 5% charcoal-stripped FBS for 24 h followed by treatment with vitamin D3 derivatives, as indicated, for 24 h. Next, cells were washed with assay media and incubated for 60 min prior to OCR and ECAR assays.
Figure 5.
The effects of vitamin D3 derivatives on OXPHOS and glycolytic flux. A, Energetic map indicates that there is a shift from mitochondrial to glycolytic metabolism after treatment HaCaT cells with 100 nM 20(OH)D3 or 1,25(OH)2D3 for 24 h. The effects of vitamin D3 derivatives on ATP production by mitochondria (B), glycolysis (C), and total amounts (cytoplasm) (D) are shown. Data are means ± SD, n = 2). *P < 0.05 (Student’s t-test).
We have previously reported Ingenuity’s toxicity pathway analyses of microarray data deposited by us at NCBI GEO (GSE11735171) Additional bioinformatics analysis (ingenuity canonical pathway analysis (IPA) with cut-off 2.0 and gene set enrichment analysis (GSEA)) of this microarray data shows that 20,23(OH)2D3 downregulates the citric acid (TCA) cycle and respiratory electron transport, glucose metabolism, fatty acid metabolism, ATP synthesis, glycolysis, mitochondrial fatty acid beta-oxidation, fatty acyl-CoA biosynthesis, pyruvate metabolism, mitochondrial calcium ion transport, and mitochondrial biogenesis with increased mitochondrial protein import (Table 1). Similar effects on glucose and fatty acid metabolism were seen for 1,25(OH)2D3 with some differences 71(Table 1). The GSEA analysis shows downregulation of fatty acyl-CoA biosynthesis, glycolysis and glucose metabolism. In contrast to 20,23(OH)2D3, 1,25(OH)2D3 upregulated several pathways, including mitochondrial fatty acid beta-oxidation and pyruvate metabolism. This suggests a protective role for 1,25(OH)2D3 against diseases associated with lipid storage. Similar to 20,23(OH)2D3, 1,25(OH)2D3 decreased the activity of respiratory electron transport and mitochondrial calcium ion transport pathways. These findings indicate genetic alterations that have long-term impact on mitochondrial bioenergetics.
Table 1.
Gene Set Enrichment Analysis for signalling associated with mitochondrial metabolism based on microarray data obtained after 24 h of in vitro incubation of primary human epidermal keratinocytes with 1,25(OH)2D3 or 20,23(OH)2D3.
| Gene Set Enrichment Analysis for 1,25(OH)2D3 | Gene Set Enrichment Analysis for 20,23(OH)2D3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Reactome Pathway | Normalized Enriched Score | P-value | FDR | Direction | Normalized Enriched Score | P-value | FDR | Direction |
| Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins | −1.368 | 0.068 | 0.258 | Down | −3.195 | 0.00 | 3.496 | Down |
| Respiratory electron transport | −1.352 | 0.091 | 0.270 | Down | −3.095 | 0.00 | 6.049 | Down |
| Fatty acyl-CoA biosynthesis | −0.720 | 0.745 | 0.871 | Down | −1.980 | 0.035 | 0.029 | Down |
| Glucose metabolism | −1.842 | 0.00 | 0.037 | Down | −3.081 | 0.00 | 6.189 | Down |
| Glycolysis | −2.000 | 0.00 | 0.016 | Down | −2.726 | 0.016 | 0.012 | Down |
| Mitochondrial calcium ion transport | −1.343 | 0.077 | 0.277 | Down | −2.527 | 0.00 | 0.014 | Down |
| Glucagon signaling in metabolic regulation | −1.837 | 0.00 | 0.038 | Down | ||||
| Digestion and absorption | −0.676 | 0.886 | 0.904 | Down | ||||
| Mitochondrial Fatty Acid Beta-Oxidation | 1.207 | 0.224 | 0.455 | Up | −2.051 | 0.00 | 0.024 | Down |
| Citric acid cycle (TCA cycle) | 0.948 | 0.480 | 0.693 | Up | −2.354 | 0.00 | 0.015 | Down |
| Pyruvate metabolism and Citric Acid (TCA) cycle | 1.044 | 0.333 | 0.597 | Up | −2.441 | 0.00 | 0.014 | Down |
| Pyruvate metabolism | 0.621 | 0.913 | 0.927 | Up | −1.916 | 0.039 | 0.035 | Down |
| Glycogen breakdown (glycogenolysis) | 2.290 | 0.00 | 0.146 | Up | ||||
| Glycogen metabolism | −2.271 | 0.020 | 0.017 | Down | ||||
| Glycogen synthesis | −2.335 | 0.00 | 0.015 | Down | ||||
| Mitochondrial protein import | 1.559 | 0.071 | 0.158 | Up | ||||
| Mitochondrial biogenesis | −1.462 | 0.089 | 0.139 | Down | ||||
| Fatty acid metabolism | −2.817 | 0.00 | 0.012 | Down | ||||
| Peroxisomal lipid metabolism | −2.432 | 0.00 | 0.014 | Down | ||||
Interestingly, detailed analyses of upregulated genes from the microarray data published by Holick and coworkers, 2019 (supplementary data Table 272 ), showed vitamin D3-mediated upregulation of the expression of several genes involved in mitochondrial transcription and translation. These included genes encoding transcription factor B1 (TFB1M), mitochondrial RNA processing (RMRP), mitochondrial ribosomal proteins (MRPS15, MRPL55, MRPL22, MRPL51), and mitochondrial import and protein folding proteins (TIMM17A, TOMM20, TIMM23, GRPEL1). In addition, vitamin D3 increased the expression of genes coding for several enzymes involved in fatty acid metabolism including peroxisomal acyl-coenzyme A oxidase 1 (ACOX1), mitochondrial 2,4-dienoyl-CoA reductase 1 (DECR1), mitochondrial uncoupling protein 2, the mitochondrial complex III component ubiquinol-cytochrome c reductase core protein II (UQCRC2), and cytochrome c oxidase assembly factor 3 (COA3). Although the microarray data requires further confirmation by other methods, it can be postulated that vitamin D supplementation affects mitochondrial fonctions which is already validated by the analysis performed above.
Based on the results above, we conclude that mitochondria are involved in the regulation of the cellular phenotype in response to the actions of vitamin D3 and L3 hydroxyderivatives, secondary to their involvement in the synthesis and metabolism of these compounds. We suggest that the intramitochondrial actions of D3-, L3- and possibly T3-hydroxyderivatives may be through receptor independent mechanisms or involve nuclear receptor(s) in a non-genomic manner, localized to mitochondria (Fig. 2). Notably, they also can alter mitochondrilal function related to oxphos and TCA cycle activities with expected inhibitory effects.
Photoprotective properties of canonical vitamin D3 derivatives
UVR is a key physical agent inducing photocarcinogenesis and skin damage through mechanisms that depend on its wavelength, for example, chromophore-based absorption of UVB energy and/or generation of reactive oxygen and nitrosyl species (ROS and RNS) by UVA 59,79–86. UVB directly induces DNA damage predominantly via the formation of mutagenic and cytotoxic cyclobutane-pyrimidine dimers (CPDs) and 6–4 pyrimidine photoproducts (6–4PP), with some contribution from the generation of ROS 79–81,85,87–89 CPDs and 6–4PP play a crucial role in cutaneous carcinogenesis and contribute to mutations in the p53 gene, an important tumor suppressor 90–92. The nucleotide excision repair (NER) system, nuclear factor E2-related factor 2 (NRF2) with its downstream antioxidant elements, and p53 signaling are crucial mechanisms protecting against mutations and photocarcinogenesis 91,93–98.
The photoprotective functions of vitamin D3 and its active forms are well documented 99–110. In addition, high doses of vitamin D3 given orally can reverse UVB-induced skin damage with attenuation of inflammation and induction of barrier repair mechanisms 110. In this context, the work from Dr Mason’s group was fundamental in establishing the protective functions of natural and synthetic hydroxyderivatives of vitamin D3, including 1,25(OH)2D3, against UVR 49,77,99,104,111. In addition to 1,25(OH)2D3, a synthetic L3 hydroxyderivative, 1,25(OH)2L3, protected against UVR-induced DNA damage and reduced proinflammatory responses in human and mouse skin and human keratinocytes. These effects were connected with increased expression of p53 and accompanied by attenuation of UVB-induced immunosuppression 104,112. Because 1,25(OH)2L3 binds to the A-pocket of the VDR, a nongenomic mechanism of action was suggested 112. This is consistent with a mechanism proposed by others 113–115, and the interaction with the A-pocket by 25(OH)D3, 1,25(OH)2D3, 1,25(OH)2L3 112,114 and some CYP11A1-derived hydroxylumisterol derivatives 29, but not CYP11A1-derived vitamin D3 hydroxyderivatives 68. In addition, the same group proposed that other receptor-dependent pathways can counteract UVB damage 49,105,106,116. Interestingly, they proposed that UVB effects on epidermal keratinocytes are directly linked to 1,25(OH)2D3 action on mitochondria to enhance glycolysis and energy-conserving processes such as autophagy and mitophagy, which would help in the repair of CPDs and would decrease oxidative DNA damage 77.
Through studies of cutaneous carcinogenesis in VDR−/− and RXR−/− mice, several groups indicated the importance of the VDR and its partner, RXR, in the mechanism of attenuation of photocarcinogenesis 39,42,112,117–122. This led to the proposal that that the VDR acts as a tumor suppressor in epidermal phocarcinogenesis123,124,125. This concept also appears to be valid for melanoma, since a decrease or loss of the VDR is associated with a negative outcome for the disease as measured by tumor stage and overall and disease-free survival 126. However, 1,25(OH)2D3 can inhibit BCC in an animal model acting through both the VDR and by inhibition of Hedgehog (Hh)-signaling which is independent on the VDR 127. Moreover, vitamin D3 by itself inhibited the growth of BCC in vitro and in vivo through inhibition of Hh-signaling acting independently of the VDR 128. Recent studies on UVB-induced BCC (basal cell carcinoma) carcinogenesis in mice show that inhibition of UVB-induced transformation of 7DHC to D3 in the skin accelerates BCC carcinogenesis. Topical application of vitamin D3 inhibited BCC photocancerogenesis, with orally delivered vitamin D3 having no effect 109. Thus, it is possible that the sterol binding site in Smoothened 129,130 can be a target for non-canonical D3-derivatives and L3-derivatives (Fig. 1) in cancer therapy 131.
Thus, vitamin D3 as the prohormone, its active form 1,25(OH)2D3 and low calcemic-chemically synthesized derivatives of D3 and 1,25(OH)2L3, promise to serve as excellent protectors of the skin against the damaging effect of UVR and cutaneous cancerogenesis.
Photoprotective properties of novel (non-cannonical) vitamin D3 derivatives and L3 photoproducts
Previously, we showed that 20(OH)D3, 20,23(OH)2D3 and 1,25(OH)2D3, and to some extent 20(OH)7DHC and 25(OH)D3, can protect cultured human keratinocytes and melanocytes against UVB damage 18. The effects included attenuation of ROS, H2O2 and NO production and upregulation of the expression of genes encoding enzymes responsible for defense against oxidative stress 18. 20(OH)D3, 20,23(OH)2D3 and 1,25(OH)2D3 showed strong protection against UVR-induced DNA damage with activation of p53 by 20(OH)D3 and 1,25(OH)2D3 18. Interestingly, these effects were seen at a concentration of 10−7 M which is 100–1,000 fold lower that of melatonin and its metabolites that produce similar effects 59,83,132. Melatonin and metabolites are recognized photoprotectors 133–135. Importantly, topical application of 20(OH)D3, the first major metabolite ofCYP11A1 action on vitamin D3, protected mouse skin against UVB-induced DNA damage, reduced sunburn edema and protected against UVR-induced immunosuppression to a similar degree to 1,25(OH)2D3 136. We are currently testing 20(OH)D3 for inhibition of UVB induced cancerogenesis in a Ptch1+/−/SKH-1 hairless mouse model 137 with very promising results.
Most recently, we tested several CYP11A1-derived vitamin D3 (20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, 1,20,23(OH)3D3) and L3 (20(OH)L3, 22(OH)L3, 20,22(OH)2L3, and 24(OH)L3) hydroxyderivatives and compared the photoprotective effects to those of 1,25(OH)2D3 and L3 (precursor) in human epidermal keratinocytes 138. They attenuated UVB-induced oxidative stress and DNA damage as measured by CPD levels and the tail moment of comets, and induced DNA repair measured from 6–4PP production. These effects correlated with enhanced expression of antioxidant response genes downstream of NRF-2 (GR, HO-1, CAT, SOD1, and SOD2), and the expression of HO-1, CAT, and MnSOD proteins 138. Hydroxyderivatives of D3 and L3 not only stimulated the phosphorylation of p53 at Ser-15, but also induced p53 and NRF2 translocation into the nucleus. Importantly, treatment of keratinocytes with D3 and L3 hydroxyderivatives after UVB-treatment was able to reverse the radiation-induced damage 138, similar to what was reported for melatonin and its metabolites 83,132 Ofparticular note is the observation that UVB can upregulate CYPP11A1 expression in human and murine skin. 139,140,141
Of great interest is that the above effects by D3 and L3 hydroxyderivatives correlated with stimulation of the NRF2- and p53- dependent responses71 and stimulation of the DNA repair system. This is consistent with microarray analyses reported previously showing a highly significant effect of 20,23(OH)2D3 on p53 signaling, GADD45 signaling, mismatch repairs in eukaryotes, the protein folding response and the NRF2-mediated oxidative stress response 71 (supplemental figure 1). These responses were less pronounced than those of 1,25(OH)2D3 71. The GSEA analysis of these data (GSE117351) 71 re-emphasized the induction of protective mechanisms by 20,23(OH)2D3. This analysis showed upregulation of p53 transcriptional activity and downregulation of p53 activity through methylation, and downregulation of oxidative stress induced senescence and of the SUMOylation of proteins involved in the repair of damaged DNA (Table 2). Similarly, 1,25(OH)2D3 downregulated oxidative stress-induced senescence and of SUMOylation of proteins involved in the DNA damage response, while upregulating detoxification of ROS (Table 2).
Table 2.
Gene Set Enrichment Analysis (GSEA) for signalling connected with DNA repair and oxidative stress based on microarray data obtained after 24 h of in vitro incubation of primary human epidermal keratinocytes with 1,25(OH)2D3 or 20,23(OH)2D3.
| Gene Set Enrichment Analysis for 1,25(OH)2D3 | Gene Set Enrichment Analysis for 20,23(OH)2D3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Reactome Pathway | Normalized Enriched Score | P-value | FDR | Direction | Normalized Enriched Score | P-value | FDR | Direction |
| Oxidative Stress Induced Senescence | −1.931 | 0.00 | 0.024 | Down | −2.509 | 0.00 | 0.013 | Down |
| SUMOylation of DNA damage response and repair proteins | −3.236 | 0.00 | 0.00 | Down | −2.213 | 0.00 | 0.018 | Down |
| Cellular response to heat stress | −2.889 | 0.00 | 1.616 | Down | ||||
| DNA Damage/Telomere Stress Induced Senescence | −1.382 | 0.140 | 0.249 | Down | ||||
| TNF receptor superfamily (TNFSF) members mediating noncanonical NF-kB pathway | −1.231 | 0.245 | 0.356 | Down | ||||
| TNFR2 noncanonical NF-kB pathway | −1.999 | 0.020 | 0.028 | Down | ||||
| TRAF6 mediated NF-kB activation | −1.362 | 0.174 | 0.262 | Down | ||||
| FCERI mediated NF-kB activation | −2.604 | 0.00 | 0.014 | Down | ||||
| NIK-noncanonical NF-kB signaling | −2.761 | 0.00 | 0.012 | Down | ||||
| Interferon gamma signaling | −1.890 | 0.00 | 0.030 | Down | −2.706 | 0.00 | 0.013 | Down |
| Interferon Signaling | −3.851 | 0.00 | 0.00 | Down | −2.706 | 0.00 | 0.013 | Down |
| Interleukin-20 family signaling | −1.591 | 0.022 | 0.118 | Down | ||||
| Interleukin-1 signaling | −1.888 | 0.016 | 0.038 | Down | ||||
| Interleukin-1 family signaling | −1.796 | 0.035 | 0.050 | Down | ||||
| Inflammasomes | −1.593 | 0.057 | 0.117 | Down | ||||
| Interleukin-12 signaling | −1.773 | 0.043 | 0.054 | Down | ||||
| Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha | −2.626 | 0.00 | 0.013 | Down | ||||
| AURKA Activation by TPX2 | −2.409 | 0.00 | 1.876 | Down | ||||
| Regulation of Hypoxia-inducible Factor (HIF) by oxygen | −2.275 | 0.021 | 0.017 | Down | ||||
| Arachidonic acid metabolism | −2.245 | 0.00 | 0.018 | Down | ||||
| TP53 Regulates Transcription of Genes Involved in Cytochrome C Release | 0.838 | 0.686 | 0.768 | Up | 2.115 | 0.00 | 0.062 | Up |
| TP53 Regulates Metabolic Genes | 1.392 | 0.102 | 0.307 | Up | ||||
| TP53 Regulates Transcription of Cell Cycle Genes | 1.982 | 0.020 | 0.074 | Up | ||||
| Regulation of TP53 Activity through Methylation | −1.796 | 0.00 | 0.050 | Down | ||||
| Detoxification of Reactive Oxygen Species | 1.522 | 0.041 | 0.280 | Up | ||||
| Interleukin-4 and Interleukin-13 signaling | 2.109 | 0.024 | 0.152 | Up | 2.859 | 0.00 | 9.922 | Up |
| Interleukin-10 signaling | 1.748 | 0.036 | 0.201 | Up | ||||
Most recently, we tested the protective effects of 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3 and 1,20,23(OH)3D3 in comparison to 1,25(OH)2D3, against UVB-induced keratinocyte inflammatory responses. We observed that there is modulation of the expression of IL-17, NFκBp65, IκB-α and other genes. There was also the reduction of NFκB-p65 activity and attenuation of proinflammatory mediators IL-17, TNF-α and IFN-γ that were stimulated by UVB 142. In addition, 1,25(OH)2D3, 20(OH)D3, 1,20(OH)2D3, and 20,23(OH)2D3 stimulated the expression of the major markers of epidermal differentiation in UVB-irradiated cells. This led to the proposal that D3-hydroxyderivatives protect the epidermis from UVB-induced damage via activation of IκB-α expression and suppression of NFκB-p65 activity and its downstream cytokines including TNF-α, and IFN-γ, inhibition of IL-17 production, and stimulation of keratinocyte differentiation 142. This concept is further supported by previous data showing that both calcemic and non-calcemic D3-derivatives cause downregulation of NFκB activity and production of Th1 and Th17 cytokines and inverse agonist activity of RORs51,52,58–60,62,68,69. The anti-inflammatory activities of 20,23(OH)2D3 and 1,25(OH)2D3 also had this signature in the microarray data (GSE117351) 71. Again, the IPA and GSEA of the data showed downregulation of interferon (INF) signaling, TNFR2 non-canonical and FCER1-activated and NIK-noncanonical NFκB pathways, downregulation of IL-1 and IL-12 pathways, downregulation of arachidonic acid metabolism and upregulation of IL-4 and IL-13 signaling (Table 2). Similarly, 1,25(OH)2D3 downregulated INF signaling, TNSFF non-canonical and TRAF-activated NFκβ pathways, downregulated inflammasomes and upregulated IL-4, IL-10 and IL-13 signaling.
In summary, it has been demonstrated by a variety of methods that calcemic and non-calcemic D3-hydroxyderivatives induce several protective pathways against UVB damage, including p53, NRF2 and DNA damage response systems, and downregulate proinflammatory responses via downregulation of NFκB signaling. The future challenge is to determine which pathways are affected by L3-hydroxyderivatives in addition to their stimulation of p53, NRF2 and DNA damage responses systems.
Conclusions and future directions
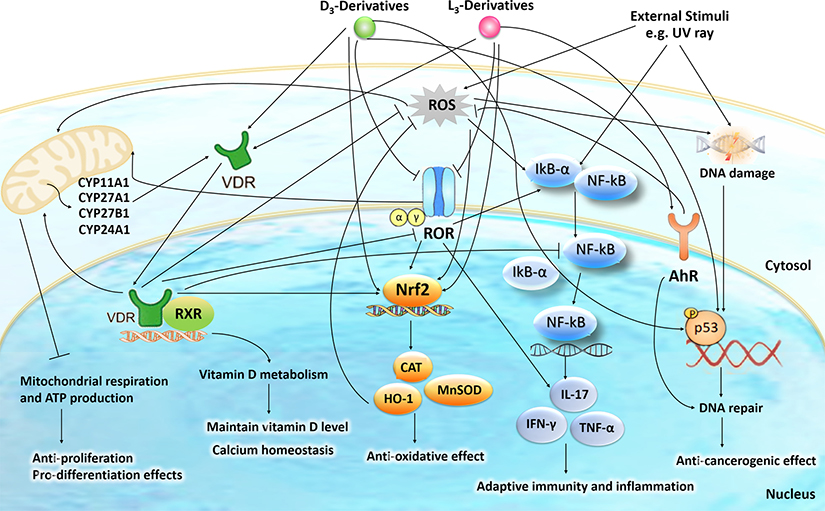
Based on the information above it is clear that the phenotypic effects of products of the canonical and non-canonical pathways of D3 activation will depend on their chemical structure (location of hydroxyl group and presence of C1α(OH), subcellular localization (plasma membrane, mitochondrion cytoplasm and nucleus), concentration and ability to interact with various regulatory proteins (nuclear receptors, transporters, CYP enzymes).The list of nuclear receptors these compounds may interact with include the VDR, RORs and AhR with each particular compound potentially displaying different affinities (Fig. 2). Although 1,25(OH)2D3 is believed to have the highest affinity for VDR, involvement of these other receptors in photoprotection is expected because they are expressed in the epidermis, where D3 is activated and thus the local concentrations of active products should be high, allowing them to act on multiple receptors at once. Hence the next challenge is how to connect the particular receptor activity with other transcriptional master regulators such as NRF2, p53 and NFκB and others mentioned above, to coordinate anti-oxidative, DNA repair and anti-inflammatory mechanisms to attenuate the UVB induced damage (Fig. 6). Another challenge is to identify other receptors in addition to RORs and A pocket of the VDR mentioned above, that may interact with L3- and putative T3-hydroxyderivatives (Figs. 1, 2). For the AhR our recent molecular modeling predicts that vitamin D3 derivatives share the same ligand binding pocket with the corresponding native ligand in the LBDs for AhR (Fig. 7).
Figure 6.
The intracellular action of vitamin D3 (D3)- and lumisterol (L3)-hydroxyderivatives in photoprotection against UVR.
Signal transduction includes activation of nuclear receptors including the VDR, RORα/γ, and AhR and the action of D3- and L3-hydroxyderivatives on mitochondrial processes. The nuclear receptors activities are linked with the transcriptional master regulators Nrf2, p53 and NFκB to coordinate anti-oxidative, DNA repair, anti-inflammatory, and anti-proliferative as well as anti-carcinogenesis mechanisms.
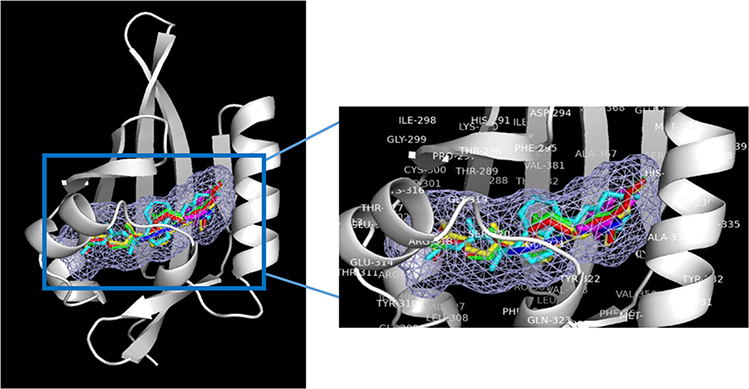
Figure 7.
Binding modes for 1α, 20S(OH)2D3(red), 1α,25(OH)2D3 (yellow), 20S(OH)D3 (green) and 20S,23R(OH)2D3 (cyan), indirubin (native ligand, blue) and indole acetic acid (native ligand, magenta) to the ligand binding domain (LBD) of AhR (in white, Homology model from previous study145). The light blue meshing area shown in the figure is the hydrophobic binding pocket in AhR. Vitamin D3 derivatives share the same ligand binding pocket with the corresponding native ligand in the LBDs for AhR.
Emerging from these studies is the concept of mitochondria as the hub for D3 and L3 activation and metabolism, with products that may directly or indirectly affect the mitochondrion and cellular bioenergetics through biochemical (non-receptor) and receptor-depend mechanisms of action that regulate homeostasis at the cellular (keratinocytes) and organ (epidermis, skin with adnexa) levels (Figs. 1–6). The role of mitochondria in such process could be crucial 77, as already proposed for another endogenous molecule, melatonin 135.
In summary, there are multiple signaling pathways activated by different D3 and L3-hydroxyderivatives acting in concert to protect the skin from or reverse UVB-induced damage. Thus, the epidermal ecosystem involves many different but related molecules, with different receptors, which surprisingly produce a similar phenotypic effect, photoprotection.
Supplementary Material
Acknowledgement
The study was supported by NIH grants 1R01AR073004-01A1 and R01AR071189-01A1 and by a VA merit grant (no. 1I01BX004293-01A1) to ATS, internal (UAB) funds to ATS and CR and by the Intramural Research Program of the NIEHS, NIH Z01-ES-101585 (to AMJ).
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Holick MF & Clark MB The photobiogenesis and metabolism of vitamin D. Fed Proceed 37, 2567–2574 (1978). [PubMed] [Google Scholar]
- 2.Holick MF Vitamin D: A millenium perspective. J Cell Biochem 88, 296–307, doi: 10.1002/jcb.10338 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD Vitamin D: an ancient hormone. Exp Dermatol 20, 7–13, doi: 10.1111/j.1600-0625.2010.01202.x (2011). [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson C The vitamin D metabolome: An update on analysis and function. Cell Biochem Funct, doi: 10.1002/cbf.3421 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Tuckey RC, Cheng CYS & Slominski AT The serum vitamin D metabolome: What we know and what is still to discover. J Steroid Biochem Mol Biol 186, 4–21, doi: 10.1016/j.jsbmb.2018.09.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski AT et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol 151, 25–37, doi: 10.1016/j.jsbmb.2014.11.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guryev O, Carvalho RA, Usanov S, Gilep A & Estabrook RW A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA 100, 14754–14759, doi: 10.1073/pnas.2336107100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. The FEBS journal 272, 4080–4090, doi: 10.1111/j.1742-4658.2005.04819.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuckey RC et al. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. DMD 39, 1577–1588, doi: 10.1124/dmd.111.040071 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuckey RC et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. The FEBS J 275, 2585–2596, doi: 10.1111/j.1742-4658.2008.06406.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang EK et al. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. DMD 38, 1553–1559, doi:dmd.110.034389 [pii] 10.1124/dmd.110.034389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tieu EW et al. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol 129, 163–171, doi: 10.1016/j.jsbmb.2011.11.012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang EK et al. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. DMD 41, 1112–1124, doi: 10.1124/dmd.113.050955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z et al. Chemical Synthesis and Biological Activities of 20S,24S/R-Dihydroxyvitamin D3 Epimers and Their 1alpha-Hydroxyl Derivatives. J Med Chem 58, 7881–7887, doi: 10.1021/acs.jmedchem.5b00881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tieu EW et al. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol 149, 153–165, doi: 10.1016/j.jsbmb.2015.02.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CY, Slominski AT & Tuckey RC Hydroxylation of 20-hydroxyvitamin D3 by human CYP3A4. J Steroid Biochem Mol Biol 159, 131–141, doi: 10.1016/j.jsbmb.2016.03.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski AT et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep 5, 14875, doi: 10.1038/srep14875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT et al. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol 148, 52–63, doi: 10.1016/j.jsbmb.2015.01.014 (2015).1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski AT et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J 26, 3901–3915, doi: 10.1096/fj.12-208975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski AT, Kim TK, Li W & Tuckey RC Classical and non-lassical metabolic transformation of vitamin D in dermal fibroblasts. Exp Dermatol 25, 231–232, doi: 10.1111/exd.12872 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slominski A et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. The FEBS journal 273, 2891–2901, doi:EJB5302 [pii] 10.1111/j.1742-4658.2006.05302.x (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen MN, Slominski A, Li W, Ng YR & Tuckey RC Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1). DMD 37, 761–767, doi: 10.1124/dmd.108.025619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski AT et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300, C526–541, doi:ajpcell.00203.2010 [pii] 10.1152/ajpcell.00203.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski AT et al. In vivo production of novel vitamin D2 hydroxyderivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol 383, 181–192, doi:S0303–7207(13)00527–3 [pii] 10.1016/j.mce.2013.12.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slominski A et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271, 4178–4188, doi: 10.1111/j.1432-1033.2004.04356.x (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski AT et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS one 4, e4309, doi: 10.1371/journal.pone.0004309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski AT et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol 44, 2003–2018, doi: 10.1016/j.biocel.2012.07.027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuckey RC et al. Lumisterol is metabolized by CYP11A1: discovery of a new pathway. Int J Biochem Cell Biol 55, 24–34, doi: 10.1016/j.biocel.2014.08.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski AT et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci Rep 7, 11434, doi: 10.1038/s41598-017-10202-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuckey RC et al. CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J Steroid Biochem Mol Biol 181, 1–10, doi: 10.1016/j.jsbmb.2018.02.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zmijewski MA et al. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci 7, 1570–1576, doi: 10.1039/b809005j (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zmijewski MA et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids 74, 218–228, doi: 10.1016/j.steroids.2008.10.017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zmijewski MA et al. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids 76, 193–203, doi:S0039–128X(10)00261–8 [pii] 10.1016/j.steroids.2010.10.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slominski AT et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J Steroid Biochem Mol Biol 177, 159–170, doi: 10.1016/j.jsbmb.2017.06.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle DD Vitamin D and the skin. J Bone Miner Metab 28, 117–130, doi: 10.1007/s00774-009-0153-8 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Bikle DD Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 347, 80–89, doi:S0303–7207(11)00259–0 [pii] 10.1016/j.mce.2011.05.017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF Vitamin D deficiency. N Engl J Med 357, 266–281, doi:357/3/266 [pii] 10.1056/NEJMra070553 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Plum LA & DeLuca HF Vitamin D, disease and therapeutic opportunities. Nature Rev Drug Discover 9, 941–955, doi: 10.1038/nrd3318 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Bikle DD Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J Invest Dermatol 128, 2357–2361, 10.1038/jid.2008.249 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elias PM Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol 132, 2131–2133, doi: 10.1038/jid.2012.246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bikle DD Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab 21, 375–384, doi:S1043–2760(10)00005–6 [pii] 10.1016/j.tem.2010.01.003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Indra AK et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol 127, 1250–1260, doi: 10.1038/sj.jid.5700672 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Carlberg C & Molnar F Current status of vitamin d signaling and its therapeutic applications. Cur Top Med Chem 12, 528–547 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Khanal RC & Nemere I The ERp57/GRp58/1,25D3-MARRS Receptor: Multiple Functional Roles in Diverse Cell Systems. Curr Med Chem 14, 1087–1093, Doi: 10.2174/092986707780362871 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Nemere I, Garbi N, Hammerling G & Hintze KJ Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids 77, 897–902, doi: 10.1016/j.steroids.2012.04.002 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Nemere I, Garbi N & Winger Q The 1,25D3-MARRS receptor/PDIA3/ERp57 and lifespan. J Cell Biochem 116, 380–385, doi: 10.1002/jcb.24986 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Tohda C, Urano T, Umezaki M, Nemere I & Kuboyama T Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Scientific Reports 2, 535, doi: 10.1038/srep00535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J et al. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1alpha,25(OH)2D3. Cell Signal 25, 2362–2373, doi: 10.1016/j.cellsig.2013.07.020 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Sequeira VB et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol Endocrinol 26, 574–582, doi: 10.1210/me.2011-1161 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zbytek B et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol 128, 2271–2280, doi: 10.1038/jid.2008.62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janjetovic Z et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PloS one 4, e5988, doi: 10.1371/journal.pone.0005988 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr. & Slominski AT 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. Journal of cellular physiology 223, 36–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 75, 926–935, doi: 10.1016/j.steroids.2010.05.021 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slominski AT et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifbrotic activity. J Invest Dermatol 131, 1167–1169, doi: 10.1038/jid.2010.425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski A et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol 5, 7–19, doi: 10.4161/derm.23938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slominski A et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifbrogenic activity in vivo. J Clin Endo Metab 98, E298–303, doi: 10.1210/jc.2012-3074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slominski AT et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res 32, 3733–3742 (2012). [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Z et al. Investigation of 20S-hydroxyvitamin D3 analogs and their 1alpha-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci Rep 8, 1478, doi: 10.1038/s41598-018-19183-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janjetovic Z et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res 57, 90–102, doi: 10.1111/jpi.12146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski AT et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 28, 2775–2789, doi: 10.1096/fj.13-242040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J et al. 20-hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res 32, 739–746 (2012). [PMC free article] [PubMed] [Google Scholar]
- 62.Janjetovic Z et al. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer 105, 1874–1884, doi: 10.1038/bjc.2011.458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slominski AT et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PloS one 5, e9907, doi: 10.1371/journal.pone.0009907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasiewicz T et al. Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int J Mol Sci 16, 6645–6667, doi: 10.3390/ijms16046645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wierzbicka JM et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol 47, 1084–1096, doi: 10.3892/ijo.2015.3088 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skobowiat C et al. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget, doi: 10.18632/oncotarget.14193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J et al. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res 34, 2153–2163 (2014). [PMC free article] [PubMed] [Google Scholar]
- 68.Slominski AT et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J Steroid Biochem Mol Biol 173, 42–56, doi: 10.1016/jjsbmb.2016.09.024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin Z et al. 1alpha,20S-Dihydroxyvitamin D3 Interacts with Vitamin D Receptor: Crystal Structure and Route of Chemical Synthesis. Sci Rep 7, 10193, doi: 10.1038/s41598-017-10917-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim T-K et al. Correlation between secosteroid induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol 361, 143–152, doi: 10.1016/j.mce.2012.04.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski AT et al. Differential and Overlapping Effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)2D3. Int J Mol Sci 19, doi: 10.3390/ijms19103072 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirvani A, Kalajian TA, Song A & Holick MF Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double Blind Clinical Trial. Sci Rep 9, 17685, doi: 10.1038/s41598-019-53864-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silvagno F, Consiglio M, Foglizzo V, Destefanis M & Pescarmona G Mitochondrial translocation of vitamin D receptor is mediated by the permeability transition pore in human keratinocyte cell line. PloS one 8, e54716, doi: 10.1371/journal.pone.0054716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abu el Maaty MA, Alborzinia H, Khan SJ, Büttner M & Wölfl S 1,25(OH)2D3 disrupts glucose metabolism in prostate cancer cells leading to a truncation of the TCA cycle and inhibition of TXNIP expression. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1864, 1618–1630, doi: 10.1016/j.bbamcr.2017.06.019 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Zhang P et al. Vitamin D and testosterone co-ordinately modulate intracellular zinc levels and energy metabolism in prostate cancer cells. J Steroid Biochem Mol Biol 189, 248–258, doi: 10.1016/j.jsbmb.2019.01.006 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Ricca C et al. Vitamin D inhibits the epithelial-mesenchymal transition by a negative feedback regulation of TGF-beta activity. J Steroid Biochem Mol Biol 187, 97–105, doi: 10.1016/j.jsbmb.2018.1L006 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Rybchyn MS et al. Enhanced Repair of UV-Induced DNA Damage by 1,25-Dihydroxyvitamin D3 in Skin Is Linked to Pathways that Control Cellular Energy. J Invest Dermatol 138, 1146–1156, doi: 10.1016/j.jid.2017.11.037 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Ricca C et al. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int J Mol Sci 19, doi: 10.3390/ijms19061672 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slominski AT, Zmijewski MA, Plonka PM, Szafarski JP & Paus R How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 159, 1992–2007, doi: 10.1210/en.2017-03230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wondrak GT, Jacobson MK & Jacobson EL Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci 5, 215–237, doi: 10.1039/b504573h (2006). [DOI] [PubMed] [Google Scholar]
- 81.Pfeifer GP & Besaratinia A UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 11, 90–97, doi: 10.1039/c1pp05144j (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brash DE UV signature mutations. Photochem Photobiol 91, 15–26, doi: 10.1111/php.12377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janjetovic Z et al. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep 7, 1274, doi: 10.1038/s41598-017-01305-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kleszczynski K et al. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J Pineal Res 58, 117–126, doi: 10.1111/jpi.12197 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Kleszczynski K, Zillikens D & Fischer TW Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NhEK). J Pineal Res 61, 187–197, doi: 10.1111/jpi.12338 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Kleszczynski K et al. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int JMol Sci 19, doi: 10.3390/ijms19123786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panich U, Sittithumcharee G, Rathviboon N & Jirawatnotai S Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int 2016, 7370642, doi: 10.1155/2016/7370642 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heck DE, Vetrano AM, Mariano TM & Laskin JD UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem 278, 22432–22436, doi: 10.1074/jbc.C300048200 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Drigeard Desgarnier MC & Rochette PJ Enhancement of UVB-induced DNA damage repair after a chronic low-dose UVB prestimulation. DNA Repair (Amst) 63, 56–62, doi: 10.1016/j.dnarep.2018.01.008 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Raad H et al. NADPH Oxidase-1 Plays a Key Role in Keratinocyte Responses to UV Radiation and UVB-Induced Skin Carcinogenesis. J Invest Dermatol 137, 1311–1321, doi: 10.1016/jjid.2016.12.027 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Jeayeng S et al. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med 108, 918–928, doi: 10.1016/j.freeradbiomed.2017.05.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roy S, Deep G, Agarwal C & Agarwal R Silibinin prevents ultraviolet B radiation-induced epidermal damages in JB6 cells and mouse skin in a p53-GADD45alpha-dependent manner. Carcinogenesis 33, 629–636, doi: 10.1093/carcin/bgr299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lo HL et al. Differential biologic effects of CPD and 6–4PP UV-induced ^DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer 5, 135, doi: 10.1186/1471-2407-5-135 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ikehata H & Yamamoto M Roles of the KEAP1-NRF2 system in mammalian skin exposed to UV radiation. Toxicol Appl Pharmacol 360, 69–77, doi: 10.1016/j.taap.2018.09.038 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Ma Q Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53, 401–426, doi: 10.1146/annurev-pharmtox-011112-140320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X et al. DNA damage strength modulates a bimodal switch of p53 dynamics for cell-fate control. BMC Biol 11, 73, doi: 10.1186/1741-7007-11-73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oda K et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862, doi: 10.1016/s0092-8674(00)00073-8 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Steegenga WT, van der Eb AJ & Jochemsen AG How Phosphorylation Regulates the Activity of p53. J Mol Biol 263, 103–113, doi: 10.1006/imbi.1996.0560 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Gupta R et al. Photoprotection by 1,25 dihydroxyvitamin D3 is associate with an increase in p53 and adecrease in nitric oxide products. J Invest Dermatol 127, 707–715, doi: 10.1038/sj.jid.5700597 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Lee J & Youn JI The photoprotective effect of 1,25- dihydroxyvtamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci 18, 11–18 (1998). [DOI] [PubMed] [Google Scholar]
- 101.Wong G et al. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol 89–90, 567–570, doi: 10.1016/j.jsbmb.2004.03.072 (2004). [DOI] [PubMed] [Google Scholar]
- 102.De Haes P et al. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B 78, 141–148, doi: 10.1016/j.jphotobiol.2004.09.010 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Dixon KM et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol 103, 451–456, doi: 10.1016/j.jsbmb.2006.11.016 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Mason RS et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol 121, 164–168, doi: 10.1016/j.jsbmb.2010.03.082 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Gordon-Thomson C et al. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv Exp Med Biol 810, 303–328, doi: 10.1007/978-1-4939-0437-2_17 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Tongkao-On W et al. Novel vitamin D compounds and skin cancer prevention. Dermatoendocrinol 5, 20–33, doi: 10.4161/derm.23939 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dixon KM et al. Vitamin D and death by sunshine. Int J Mol Sci 14, 1964–1977, doi: 10.3390/ijms14011964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dixon KM et al. Differential photoprotective effects of 1,25-dihydroxyvitamin D3 and a low calcaemic deltanoid. Photochem Photobiol Sci 11, 1825–1830, doi: 10.1039/c2pp25208b (2012). [DOI] [PubMed] [Google Scholar]
- 109.Makarova A et al. Vitamin D3 Produced by Skin Exposure to UVR Inhibits Murine Basal Cell Carcinoma Carcinogenesis. J Invest Dermatol 137, 2613–2619, doi: 10.1016/j.jid.2017.05.037 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Scott JF et al. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J Invest Dermatol 137, 2078–2086, doi: 10.1016/j.jid.2017.04.040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dixon KM et al. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol 97, 137–143, doi: 10.1016/j.jsbmb.2005.06.006 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Dixon KM et al. 1alpha,25(OH)(2)-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila) 4, 1485–1494, doi: 10.1158/1940-6207.CAPR-11-0165 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Haussler MR, Jurutka PW, Mizwicki M & Norman AW Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab 25, 543–559, doi: 10.1016/j.beem.2011.05.010 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Mizwicki MT & Norman AW The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2, re4, doi: 10.1126/scisignal.275re4 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Mizwicki MT et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A 101, 12876–12881, doi: 10.1073/pnas.0403606101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sequeira VB et al. Opening of chloride channels by 1alpha,25-dihydroxyvitamin D3 contributes to photoprotection against UVR-induced thymine dimers in keratinocytes. J Invest Dermatol 133, 776–782, doi: 10.1038/jid.2012.343 (2013). [DOI] [PubMed] [Google Scholar]
- 117.Hu L, Bikle DD & Oda Y Reciprocal role of vitamin D receptor on beta-catenin regulated keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 144 Pt A, 237–241, doi:S0960–0760(13)00232-X [pii] 10.1016/j.jsbmb.2013.11.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bikle DD et al. Protective role of vitamin D signaling in skin cancer formation. J Steroid Biochem Mol Biol 136, 271–279, doi:S0960–0760(12)00187–2 [pii] 10.1016/j.jsbmb.2012.09.021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bikle DD, Oda Y, Tu CL & Jiang Y Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol 148, 47–51, doi:S0960–0760(14)00252–0 [pii] 10.1016/j.jsbmb.2014.10.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bikle DD, Jiang Y, Nguyen T, Oda Y & Tu CL Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front Physiol 7, 296, doi: 10.3389/fphys.2016.00296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chagani S et al. In Vivo Role of Vitamin D Receptor Signaling in UVB-Induced DNA Damage and Melanocyte Homeostasis. J Invest Dermatol 136, 2108–2111, doi: 10.1016/j.jid.2016.06.004 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Wang Z et al. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol 131, 177–187, doi: 10.1038/jid.2010.290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slominski AT et al. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest 97, 706–724, doi: 10.1038/labinvest.2017.3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bikle DD Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharmacol 93, 349–354, doi: 10.1139/cjpp-2014-0367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bikle DD The vitamin D receptor: a tumor suppressor in skin. Adv Exp Med Biol 810, 282–302, doi: 10.21236/ada614241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brozyna AA, Hoffman RM & Slominski AT Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer research 40, 473–489, doi: 10.21873/anticanres.13976 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uhmann A et al. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther 10, 2179–2188, doi:1535–7163.MCT-11–0422 [pii] 10.1158/1535-7163.MCT-11-0422 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Tang JY et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila) 4, 744–751, doi: 10.1158/1940-6207.CAPR-10-0285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang P et al. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell 166, 1176–1187 e1114, doi: 10.1016/j.cell.2016.08.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nachtergaele S et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol 8, 211–220, doi: 10.1038/nchembio.765 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Slominski AT et al. The role of classical and novel forms of vitamin D in the pathogenesis and progression of non-melanoma skin cancers. Advances in Experimental Medicine and Biology in press (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skobowiat C et al. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J Pineal Res 65, e12501, doi: 10.1111/jpi.12501 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ & Paus R Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol 17, 713–730, doi: 10.1111/j.1600-0625.2008.00767.x (2008). [DOI] [PubMed] [Google Scholar]
- 134.Fischer TW, Kleszczynski K, Hardkop LH, Kruse N & Zillikens D Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J Pineal Res 54, 303–312, doi: 10.1111/jpi.12018 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Slominski AT et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci 74, 3913–3925, doi: 10.1007/s00018-017-2617-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tongkao-On W et al. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol 148, 72–78, doi: 10.1016/j.jsbmb.2014.11.015 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Chaudhary SC et al. Shh and p50/Bcl3 signaling crosstalk drives pathogenesis of BCCs in Gorlin syndrome. Oncotarget 6, 36789–36814, doi: 10.18632/oncotarget.5103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaiprasongsuk A et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol 24, 101206, doi: 10.1016/j.redox.2019.101206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skobowiat C, Nejati R, Lu L, Williams RW & Slominski AT Genetic variation of the cutaneous HPA axis: an analysis of UVB- induced differential responses. Gene 530, 1–7, doi: 10.1016/j.gene.2013.08.035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC & Slominski A Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Amer J Physiol. Endo Metab 301, E484–493, doi: 10.1152/ajpendo.00217.2011. 10.1152/ajpendo.00217.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Skobowiat C & Slominski AT UVB Activates Hypothalamic- Pituitary-Adrenal Axis in C57BL/6 Mice. J Invest Dermatol 135, 16380–1648, doi: 10.1038/jid.2014.450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaiprasongsuk A JZ, Kim TK, Tuckey RC, Li W, Raman C, Panich U, Slominski AT. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. J Invest Dermatol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.MacLaughlin JA, Anderson RR & Holick MF Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 216, 1001–1003, doi: 10.1126/science.6281884 (1982). [DOI] [PubMed] [Google Scholar]
- 144.Mier-Aguilar CA, Cashman KS, Raman C & Soldevila G CD5-CK2 Signaling Modulates Erk Activation and Thymocyte Survival. PloS one 11, e0168155, doi: 10.1371/jouraal.pone.0168155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Slominski A et al. Differential and overlapping effects of 20, 23(OH)2D3 and 1, 25 (OH)2D3 on gene expression in human epidermal keratinocytes: identification of AhR as an alternative receptor for 20, 23 (OH) 2D3. Int J Mol Sci 19, 3072 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.