Abstract
Organisms must learn novel strategies to adapt to changing environments. Activity in different neurons often exhibits synchronization that can dynamically enhance their communication and might create flexible brain states that facilitate changes in behavior. We studied the role of gamma-frequency (~40 Hz) synchrony between prefrontal parvalbumin interneurons, in mice learning multiple new cue-reward associations. Voltage indicators revealed cell type-specific increases of cross-hemispheric gamma synchrony between parvalbumin interneurons, when mice received feedback that previously learned associations were no longer valid. Disrupting this synchronization by delivering out-of-phase optogenetic stimulation caused mice to perseverate on outdated associations, an effect not reproduced by in-phase stimulation or out-of-phase stimulation at other frequencies. Gamma synchrony was specifically required when new associations utilized familiar cues that were previously irrelevant to behavioral outcomes, not when associations involved novel cues, or for reversing previously learned associations. Thus, gamma synchrony is indispensable for reappraising the behavioral salience of external cues.
Reporting Summary
Further information on research design is available in the Life Sciences Reporting Summary linked to this article.
Introduction
Adapting to a changing environment requires detecting when behavioral strategies become outdated, then suppressing them and learning new ones. Deficits in this ability are hallmarks of prefrontal dysfunction in schizophrenia, classically measured by the Wisconsin Card Sorting Task (WCST)1,2. Like the WCST, many natural behaviors also involve rapidly learning novel strategies that use external cues which were previously unimportant. Mechanisms underlying this kind of adaptation remain unknown. Synchrony between activity in different neurons may regulate how those neurons interact with each other and their downstream targets3–13. Thus, by transiently enhancing interactions between specific neurons, synchrony could generate dynamic brain states that facilitate behavioral adaptation. Synchronized gamma-frequency (~30–80 Hz) activity occurs in the medial prefrontal cortex (mPFC) when rodents change behavior14–16. Interneurons, particularly those expressing parvalbumin (PV), generate synchronized gamma-frequency activity. However, it remains deeply controversial whether gamma synchrony between activity in different regions contributes to behavior or simply reflects increased PV interneuron recruitment17,18.
To address this, we studied a task involving the type of behavioral adaptation outlined above14,15,19–22. Each trial, mice choose between two bowls to find hidden food rewards (Fig. 1a,c). Each bowl contains different odor and texture cues. Mice first form an initial association between one cue and reward, then learn a “rule shift” from odor to texture (or vice versa). By contrast, during a “rule reversal” the type of rule (odor or texture) does not change, but the previously unrewarded cue now becomes rewarded (Fig. 1b). Unlike tasks requiring well-trained mice to switch between previously learned behaviors, in this task mice form new associations using familiar cues that were previously either irrelevant (rule shift) or predictive (rule reversal) with respect to behavioral outcomes.
Fig. 1: Prefrontal PV interneurons are recruited after errors during rule shifts.
a, Rule shift task schematic. On each trial, a mouse chooses one of two bowls, each scented with a different odor (O1 or O2) and filled with a different textured digging medium (TA or TB), to find a food reward. Mice first learn an initial association (IA) between one of these cues (e.g., odor O1) and food reward (the cue associated with reward is indicated in orange). Once mice reach the learning criterion (8/10 consecutive trials correct), this association undergoes an extra-dimensional rule shift (RS; e.g., from O1 to TA). b, Rule reversal task schematic. Mice learn an initial association (IA) between one cue (e.g., odor O1) and food reward (the rewarded cue is indicated in orange). Once mice reach the learning criterion, this association undergoes an intra-dimensional rule reversal (RR), e.g., from O1 to O2. c, Trial timeline. A mouse begins each trial by entering the home cage, then makes a decision, indicated by digging in one bowl. If the mouse is correct, food reward is consumed. The mouse is then transferred to the holding cage until the next trial. The intertrial interval is longer after incorrect choices. d, Representative image showing mPFC FLEX-GCaMP6f expression in a PV-Cre mouse (scale bar, 100 μm). e, Averaged PV interneuron photometry signal (dF/F), aligned to the time of dig, which indicates a decision, for correct (white line) vs. incorrect trials (black line; n = 8 mice). f, Peak dF/F during the 4 sec following the decision. Signals are significantly higher on incorrect than correct trials (n = 8 mice; two-tailed, paired t-test; t(7) = 3.93, **P = 0.006). Data are shown as means (e); shading (e) denotes s.e.m.
Several observations indicate that rule shifts, but not rule reversals, depend on interneuron-generated rhythmic mPFC activity. mPFC lesions disrupt rule shifts but not rule reversals19. Inhibiting prefrontal GABAergic interneurons similarly disrupts rule shifts but not initial associations or rule reversals14, and mutant mice with abnormal PV interneurons and deficient task-evoked mPFC gamma power have the same pattern of impairments14. In these mutant mice, stimulating prefrontal interneurons at gamma-frequencies (40–60 Hz) normalizes learning during rule shifts14. Thus, synchronized gamma-frequency mPFC activity can improve pathological behavior, although its role in normal behavior remains unclear. In fact, gamma-frequency stimulation of prefrontal interneurons improves sensory detection, attention, and social behavior22–24. However, none of these studies have directly addressed two fundamental questions: first, do these behavioral effects require synchronization across brain regions, or is enhancing rhythmic inhibition within local circuits sufficient? Second, even if artificially increasing synchrony improves behavior, is naturally-occurring synchrony necessary for normal behavior?
Here we explored how cross-hemispheric gamma synchrony between prefrontal PV interneurons contributes to learning during rule shifts. Gamma-frequency synchrony between PV interneurons in the left and right mPFC increases during rule shifts but not initial associations or rule reversals. Delivering weak gamma-frequency optogenetic stimulation out-of-phase between the left and right mPFC disrupts rule shifts, whereas in-phase stimulation has no effect. This disambiguates the role of cross-hemispheric synchrony from that of rhythmic inhibition within a local circuit. Furthermore, perturbing cross-hemispheric synchrony does not affect initial associations or rule reversals. Thus, there is a 1:1 correspondence between whether a type of learning normally elicits increased gamma synchrony, and whether it is disrupted when that synchrony is perturbed.
Results
PV interneurons are recruited after rule shift errors.
We used bulk calcium imaging25,26 (fiber photometry) to explore how PV interneurons are normally recruited during rule shifts (Fig. 1d–f; Extended Data Fig. 1). We injected AAV1-Syn-FLEX-GCaMP6f27 into the mPFC of PV-Cre mice and implanted an optical fiber to measure fluorescence (Fig. 1d). We examined activity time-locked to trial start, decisions (indicated by digging in one bowl), trial end (cessation of digging), and intertrial intervals (Fig. 1c). Supplementary videos 1–4 show task mechanics and time-locked photometry traces. On error trials, PV interneuron activity increased after decisions, i.e., when animals failed to receive rewards that would have been expected based on the previously learned association (Fig. 1e,f). PV activity did not increase following correct decisions (Fig. 1e,f). Because rule shifts are uncued, the absence of expected rewards signals that previously learned associations are no longer valid.
Cross-hemispheric gamma synchrony between PV interneurons increases after rule shift errors.
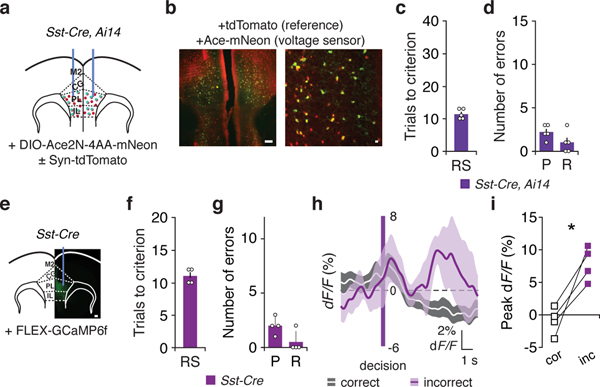
We examined whether this PV activity at critical behavioral timepoints was associated with gamma-frequency synchronization across sites using TEMPO (Trans-membrane Electrical Measurements Performed Optically). TEMPO utilizes bulk fluorescence from the voltage indicator Ace2N-4AA-mNeon (‘Ace-mNeon’) to monitor specific cell types28. We injected AAV into mPFC bilaterally to drive Cre-dependent Ace-mNeon expression in PV-Cre, Ai14 mice and implanted optical fibers to measure Ace-mNeon and tdTomato fluorescence from PV interneurons in the left and right mPFC (Fig. 2a,b). Some mice were additionally injected with AAV1-Synapsin-tdTomato. tdTomato provides non-voltage-dependent reference signals. We measured signals from both prefrontal cortices (Fig. 2c) while mice learned an initial association and rule shift (Fig. 2d).
Fig. 2: Cross-hemispheric gamma synchrony of PV interneurons increases after errors during rule shifts.
a, PV-Cre, Ai14 mice had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. b, Representative images of tdTomato (red) and Ace-mNeon (green) fluorescence in a coronal section of mPFC (left), alongside a high-power image (right). Scale bars: 100 μm and 25 μm, respectively. c, Schematic for dual-site TEMPO measurements. Each fiber-optic implant, for delivering illumination and collecting fluorescence, connects to a mini-cube coupled to two LEDs and two photoreceivers (PR) to separately excite and collect emitted fluorescence from Ace-mNeon and tdTomato. Two lock-in amplifiers modulate LED output and demodulate PR signals, which are then acquired by a multichannel real-time signal processor. d, Initial association (IA) and rule shift (RS) performance in this cohort (n = 12 mice). e, Overview of dual-site TEMPO analysis: tdTomato and Ace-mNeon fluorescence signals from each hemisphere are filtered around a frequency of interest, then both tdTomato signals and one Ace-mNeon signal are used to model the second Ace-mNeon signal. Performance is compared to models based on shuffled versions of the first Ace-mNeon signal. f, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric PV interneuron synchrony, during the last 3 IA trials and the first 5 RS trials in one mouse. g, Synchrony was not different after correct decisions vs. during the baseline period (n = 12 mice; two-way ANOVA; condition X frequency interaction: F2,33 = 1.05, P = 0.36). h–i, 30–50 Hz synchronization was specifically higher after RS errors than during the baseline period (n = 12 mice; two-way ANOVA; main effect of condition: F1,33 = 10.51, **P = 0.003; frequency X condition interaction: F2,33 = 8.23, **P = 0.001; 15–25 Hz: post hoc t(33) = 0.43, P > 0.99; 30–50 Hz: post hoc t(33) = 5.18, ****P = 0.00003; 50–70 Hz: post hoc t(33) = 0.007, P > 0.99) or after RS correct decisions (n = 12 mice; two-way ANOVA; condition X frequency interaction: F2,33 = 7.32, **P = 0.002; post hoc t(33) = 4.36, ***P = 0.0004). j, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric Sst interneuron synchrony during the last 3 IA trials and the first 5 RS trials in one mouse. k–m, Cross-hemispheric Sst synchrony (n = 5 mice) was not different between the baseline period and RS correct trials (two-way ANOVA; main effect of condition: F1,12 = 0.07, P = 0.79; main effect of frequency: F2,12 = 6.07, *P = 0.015; condition X frequency interaction F2,12 = 0.10, P = 0.90), baseline period and RS incorrect trials (two-way ANOVA; main effect of condition: F1,12 = 0.47, P = 0.51; main effect of frequency: F2,12 = 7.34, **P = 0.008; condition X frequency interaction: F2,12 = 0.26, P = 0.78), nor correct and incorrect trials (two-way ANOVA; main effect of condition: F1,12 = 0.25, P = 0.63; main effect of frequency: F2,12 = 4.66, *P = 0.03; condition X frequency interaction: F2,12 = 0.034, P = 0.97). n, In a cohort of PV-Cre mice used for simultaneous dual-site TEMPO measurements and LFP recordings (n = 5 mice), 30–50 Hz synchronization between left and right PV interneuron TEMPO signals was specifically higher after RS errors than after RS correct decisions (two-way ANOVA; frequency X condition interaction: F2,12 = 7.13, **P = 0.009; post hoc t(12) = 2.83, *P = 0.045). o, Difference in zero-phase-lag LFP wavelet coherence following errors relative to after correct decisions (i.e., incorrect – correct; n = 5 mice). p, LFP wavelet coherence was higher at 20 and 40 Hz following RS errors than after correct decisions (n = 5 mice; two-way ANOVA; main effect of condition: F1,12 = 21.40, ***P = 0.0006; main effect of frequency: F2,12 = 1.86, P = 0.199; condition X frequency: F2,12 = 0.17, P = 0.85; 20 Hz post hoc t(12) = 2.85, *P = 0.04; 40 Hz post hoc t(12) = 2.96, *P = 0.04). Data are shown as means (d, o); error bars (d, o) denote s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons were used, unless otherwise noted. Comparisons were not significant, unless otherwise noted.
Fluorescence from genetically encoded voltage indicators is strongly contaminated by hemodynamic artifacts28,29 such that non-neuronal artifacts dominate conventional spectral analyses of these signals (power or coherence). Previous studies have addressed this using unsupervised methods to separate signal from noise in single-site recordings28,29. However, this fails to resolve gamma-frequency signals in freely-behaving mice. We overcame this barrier by leveraging dual-site recordings. To quantify zero-phase lag cross-hemispheric synchronization, we filtered all signals around a frequency of interest, then predicted the right Ace-mNeon signal using a linear model based on the left Ace-mNeon signal, left tdTomato signal, and right tdTomato signal (Fig. 2e). At every point in time, we compared this model’s performance to predictions from 100 models that used the actual tdTomato signals but time-shifted versions of the left Ace-mNeon signal. The tdTomato signals capture shared sources of noise (hemodynamic signals, movement artifacts, fiber bending, etc.) and the shuffled left Ace-mNeon signal matches the degrees of freedom. Thus, the degree to which the model based on actual signals outperforms those based on shuffled signals should reflect the amount of information the left Ace-mNeon signal carries about the right Ace-mNeon signal, i.e., the (zero-phase lag) cross-hemispheric synchronization between prefrontal PV interneurons. This metric measures synchrony within a band centered around a frequency of interest, e.g., 30–50 Hz, which reflects synchrony ~40 Hz. Using this method, we analyzed the first five rule shift trials (Fig. 2f–i). (We hypothesize that these trials are crucial for learning and hence most likely to exhibit learning-related signals). Cross-hemispheric PV interneuron synchronization at 30–50 Hz was significantly higher following errors than at baseline or following correct decisions (Fig. 2f–i). Notably, error-related synchrony was frequency-specific. For both lower (15–25 Hz) and higher (50–70 Hz) frequency bands, cross-hemispheric PV interneuron synchronization was similar at baseline, after correct decisions, and after errors.
Synchronization at arbitrary phase lags can be expressed as the sum of in-phase and 90 degree out-of-phase components. Therefore, to explore potential synchrony at non-zero phase lags, we measured synchronization using left mPFC PV interneuron Ace-mNeon signals that were phase-shifted 90 degrees (Extended Data Fig. 2). In this case, there were no differences in 30–50 Hz synchronization at baseline vs. after correct or incorrect decisions (Extended Data Fig. 2); it is specifically the zero-phase lag component of gamma-frequency synchronization that increases following rule shift errors. We also measured voltage dynamics from somatostatin (Sst) interneurons using Sst-Cre mice (Fig. 2j–m; Extended Data Fig. 3a–d). Cross-hemispheric synchrony between prefrontal Sst interneurons did not increase following errors, even though, similar to PV interneurons, Sst interneuron photometry signals increase after rule shift errors (Extended Data Fig. 3e–i). Thus, the synchronization we found is specific for trial outcome, frequency, phase lag, and cell type. These are controls for nonspecific artifacts related to movement, respiration, hemodynamics, etc.
Increased gamma synchrony also occurs in LFP recordings
To validate that TEMPO tracks aspects of network activity that can also be measured in other ways, we simultaneously recorded TEMPO signals and local field potentials (LFPs) from the left and right mPFC during rule shifts. Zero-phase lag synchronization computed from left vs. right mPFC LFPs reproduced our key TEMPO finding: ~40 Hz cross-hemispheric synchrony increases for errors during rule shifts, compared to correct trials (Fig. 2n–p). Notably, in LFP recordings, lower frequency synchrony also increased (Fig. 2p), possibly reflecting the fact that TEMPO is cell-type specific, whereas LFP recording is not. Thus, within PV interneurons, increases in ~40 Hz synchrony may be most prominent, whereas other cell types may generate LFP synchrony at lower frequencies.
Increased gamma synchrony is specific for rule shifts
Next we examined whether increased gamma synchrony is specific for rule shifts, or more generically reflects error detection or reinforcement. First, the increases in PV interneuron photometry signals that follow rule shift errors do not occur after initial association errors (Fig. 3a–d). Second, TEMPO showed no differences between cross-hemispheric PV interneuron gamma synchrony on correct vs. incorrect trials during initial associations (Fig. 3g) or rule reversals (Fig. 3h). Thus, 30–50 Hz synchrony was significantly higher after rule shift errors than following errors during initial associations (Fig. 3i) or rule reversals (Fig. 3j). Notably, this difference was specific for 30–50 Hz.
Fig. 3: Cross-hemispheric synchrony does not increase during initial associations and rule reversals.
a, Averaged PV interneuron photometry signal (dF/F), aligned to the time of dig, which indicates a decision, for correct (brown line) and incorrect trials (yellow line) during the initial association (IA) (n = 8 mice). b, Peak dF/F during the 4 sec following the decision. Signals are comparable during correct and incorrect IA trials (n = 8 mice; two-tailed, paired t-test; t(7) = 0.44, P = 0.67). c, Averaged PV interneuron photometry signal (dF/F), aligned to the time of dig, which indicates a decision, for incorrect trials during the IA (yellow line) or rule shift (RS) (black line; n = 8 mice). d, Peak dF/F during the 4 sec following the decision. Signals on incorrect trials are significantly lower during the IA than the RS (n = 8 mice; two-tailed, paired t-test; t(7) = 2.87, *P = 0.024). (Note: to be conservative and include all data, we did not exclude one datapoint which appeared to be an outlier; however, had this datapoint been excluded, this P value would have been 0.0096). e, PV-Cre, Ai14 mice had bilateral injections of AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato in mPFC and fiber-optic implants in mPFC. Experimental design: Day 1: IA followed by RS or RR; Day 2: IA followed by the task not performed on Day 1. f, PV-Cre, Ai14 mice performed rule shifts (RS) and rule reversals (RR) in a similar number of trials (n = 7 mice; two-tailed, paired t-test; t(6) = 0.92, P = 0.39). g, During the IA, synchrony did not differ following correct vs. incorrect trials (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.0007, P = 0.98; condition X frequency interaction: F2,18 = 0.068, P = 0.93). h, During the RR, synchrony did not differ following correct vs. incorrect trials (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.10, P = 0.76; condition X frequency interaction F2,18 = 4.55, *P = 0.025; 15–25 Hz: post hoc t(18) = 0.25, P > 0.99; 30–50 Hz: post hoc t(18) = 1.71, P = 0.32; 50–70 Hz: post hoc t(18) = 2.50, P = 0.07). i, Following errors, synchrony was specifically higher for the 30–50 Hz band during RS than IA (n = 7 mice; two-way ANOVA; condition X frequency interaction: F2,18 = 6.02, **P = 0.0099; 30–50 Hz post hoc t(18) = 3.42, **P = 0.009). j, Following errors, synchrony was specifically higher in the 30–50 Hz band RS than RR (two-way ANOVA; condition X frequency interaction F2,18 = 3.96, *P = 0.038; 30–50 Hz post hoc t(18) = 2.64, *P = 0.0499). Data are shown as means (a, c); shading (a, c) denotes s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons, unless otherwise noted.
Both rule shifts and rule reversals are uncued. Therefore, the nature of the rule change, and any associated differences in synchrony, should only become apparent over time. To confirm that this is the case, we analyzed trial-by-trial differences in gamma synchrony for incorrect vs. correct trials, during rule shifts vs. rule reversals. Indeed, the tendency for gamma synchrony to be higher on incorrect vs. correct trials during rule shifts but not rule reversals was difficult to discern during the first two trials of a rule change, then became significantly larger over the next three trials (Extended Data Fig. 4k).
Note: we quantified synchrony using the fraction of 1-second time windows for which the R2 between actual TEMPO signals exceeds that for 99% of time-shuffled signals. This bootstrapped metric is sparse in time. Therefore, we obtained a meaningful estimate of synchrony by averaging over many 1-second windows per trial and all trials of a given type, e.g., time windows following errors including the following intertrial intervals (ITIs). As a consequence, our estimate of synchrony has low temporal resolution and cannot pinpoint specific moments of high synchrony. Rather we can only say that synchrony tended to be higher during a set of time windows, e.g., those following errors. This makes it difficult to link moments of high synchrony to specific behaviors, e.g., digging or immobility. Nevertheless, when we restricted our analysis to time windows which fell during ITIs, cross-hemispheric PV interneuron gamma synchrony was higher during ITIs following rule shift errors than ITIs following initial association or rule reversal errors (Extended Data Fig. 4l). This indicates that increased gamma synchrony was not just a direct byproduct of specific movements or events occurring during the trial period, but rather reflects feedback from the prior trial.
Now, we cannot fully rule out that feedback from the prior trial alters ITI behavior in ways that mediate increased gamma synchrony. However, we consider this unlikely because behavior is not grossly different during ITIs following rule shift errors vs. those after initial association errors. We scored videos of ITI behavior. These were of limited quality, because the camera was focused on the test cage, not the holding cage, and the holding cage was partially covered by a translucent lid. Therefore, our analysis only assessed whether mice moved from one part of the cage to another. The fraction of timepoints spent moving was almost identical during ITIs following rule shift (RS) or initial association (IA) errors (RS: 68% moving vs. IA: 70% moving; Extended Data Fig. 4m–n).
We also analyzed PV photometry signals during ITIs (Extended Data Fig. 1f). During rule shifts, these were higher for ITIs following errors than correct trials. Notably, this was not the case during initial associations. Thus, increases in PV interneuron activity and gamma synchrony that occur after RS errors persist into the subsequent ITI, when mice are in holding cages and task-related cues are absent. Finally, while we could not pinpoint specific moments of high synchrony as outlined above, we did characterize behavior at the time of dF/F peaks, both during trials (Extended Data Fig. 1g), and ITIs (Extended Data Fig. 1h–i). During trials, peak PV interneuron activity was not consistently associated with any specific behavior, and could occur while mice were digging before removal of the non-chosen bowl, or after bowl removal while mice were digging, moving, or immobile. During ITIs, peak PV interneuron activity occurred during both movement and immobility, and the fraction of peaks associated with each condition did not differ between ITIs following IA vs. RS errors. Thus, peaks in PV activity are not driven by specific behaviors, and differences in PV activity during ITIs following RS vs. IA errors do not reflect gross differences in movement.
Perturbing gamma synchrony disrupts learning during rule shifts
To directly test the functional significance of increased synchrony following rule shift errors, we would like to artificially manipulate cross-hemispheric gamma synchronization between PV interneurons. We previously showed that 40 Hz stimulation of interneurons delivered in-phase across the left and right mPFC does not impair rule shifts in normal mice14. Critically, whereas others have examined behavioral effects of 40 Hz stimulation of PV interneurons using light powers ~5–7 mW22,24, we used ~1 mW. Light power is not the only determinant of optogenetic efficacy, as ChR2 expression varies. Nevertheless, another study that stimulated PV interneurons at 40 Hz using ~1 mW observed only slight changes in excitatory neuron firing rates23. Thus, one strategy would be using modest optogenetic stimulation to entrain PV interneurons in the left vs. right mPFC with different phase relationships, to determine whether these patterns, which should elicit similar changes in levels of inhibition but differentially affect cross-hemispheric synchronization, produce similar vs. distinct behavioral effects.
Based on this, we performed two experiments. First, stimulating prefrontal PV interneurons at 40 Hz but out-of-phase between hemispheres disrupts rule shift learning (Fig. 4a–e). Mice receiving out-of-phase stimulation took significantly longer to learn compared to either control (eYFP-expressing) mice, or to themselves on a different day with no stimulation. Because odor-texture pairings vary randomly from trial to trial, during rule shifts mice can make perseverative and random errors. Perseverative errors occur when the originally-rewarded cue and the newly-rewarded cue are in different bowls, and the mouse chooses the originally-rewarded one. Random errors occur when the originally-rewarded and newly-rewarded cues are in the same bowl, but the mouse chooses the other bowl. Out-of-phase 40 Hz stimulation specifically increased perseverative errors (Fig. 4b,c; Extended Data Fig. 5a–f). Next, a different cohort of PV-Cre mice underwent: a) out-of-phase 20 Hz stimulation, b) out-of-phase 40 Hz stimulation, and c) in-phase 40 Hz stimulation, delivered to PV interneurons in the left vs. right mPFC during rule shifts (Fig. 4d). Out-of-phase 20 Hz stimulation did not disrupt rule shifts (Fig. 4e; compare to Fig. 2d). Again, out-of-phase 40 Hz stimulation markedly disrupted rule shift learning and increased perseveration, but when we delivered in-phase 40 Hz stimulation the next day, the same mice again learned normally (Fig. 4e; Extended Data Fig. 5g–i). This phenomenon was specific to PV interneurons: stimulating Sst interneurons out-of-phase (at 20 or 40 Hz) did not affect rule shifts (Extended Data Fig. 6).
Fig. 4. Out-of-phase, but not in-phase, gamma-frequency stimulation of PV interneurons disrupts learning during rule shifts but not during initial associations or rule reversals.
a, PV-Cre, Ai14 mice had injections of AAV-DIO-eYFP or AAV-DIO-ChR2-eYFP in mPFC and fiber-optic implants bilaterally in mPFC. Experimental design: Day 1: out-of-phase 40 Hz stimulation during the rule shift (RS); Day 2: no stimulation. b–c, Out of phase 40 Hz stimulation impairs rule shift performance in ChR2-expressing mice compared to eYFP-expressing controls (n = 5 mice in each cohort; two-way ANOVA; main effect of day: F1,8 = 40.2, ***P = 0.0002; main effect of virus: F1,8 = 32.5, ***P = 0.0005; day X virus interaction: F1,8 = 47.3, ***P = 0.0001). b, Performance of eYFP-expressing controls did not change from Day 1 to 2 (n = 5 mice; post hoc t(8) = 0.38, P > 0.99). c, Out-of-phase 40 Hz stimulation of PV interneurons across hemispheres during the RS on Day 1 impaired rule shifts in ChR2-expressing mice, compared to no stimulation on Day 2 (n = 5 mice; post hoc t(8) = 9.34, ****P = 0.00003). d, PV-Cre mice had bilateral injections of AAV-DIO-ChR2-eYFP and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 20 Hz stimulation; Day 2: out-of-phase 40 Hz stimulation; Day 3: in-phase 40 Hz stimulation. e, Out-of-phase 40 Hz stimulation (Day 2) impairs rule shifts relative to out-of-phase 20 Hz stimulation (Day 1) or in-phase 40 Hz stimulation (Day 3) (n = 5 mice; one-way repeated measures ANOVA followed by Tukey’s multiple comparisons test, main effect of treatment: F1.294,5.175 = 25.3, **P = 0.003; Day 1 vs. Day 2: *P = 0.025, Day 2 vs. Day 3: **P = 0.006, Day 1 vs. Day 3: P = 0.47). f, PV-Cre, Ai14 mice had bilateral injections of AAV-DIO-eYFP or AAV-DIO-ChR2-eYFP and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 40 Hz stimulation during the initial association (IA); Day 2: no stimulation during the IA, followed by out-of-phase 40 Hz stimulation during the rule reversal (RR). g, Out-of-phase 40 Hz stimulation does not affect the ability of ChR2-expressing mice to learn an IA (n = 5 mice in each cohort; two-tailed, unpaired t-test; compared to control eYFP-expressing mice; t(8) = 0.69, P = 0.51). h, Out-of-phase 40 Hz stimulation does not affect the ability of ChR2-expressing mice to learn a RR (n = 5 mice in each cohort; two-tailed, unpaired t-test; compared to control eYFP-expressing mice; t(8) = 0.89, P = 0.40). Data are shown as means (g, h); error bars (g, h) denote s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons, unless otherwise noted.
To clarify how these patterns of optogenetic stimulation affect microcircuit activity, first we recorded from mPFC using silicon probes in head-fixed PV-Cre mice injected with virus for Cre-dependent ChR2 expression during 40 Hz PV interneuron stimulation (using the same light power as our behavioral experiments). Putative PV interneurons were single units with fast-spiking waveforms and reliable short latency responses to stimulation (8/10 fast-spiking units met our criteria)30. Stimulation strongly entrained “optogenetically-tagged” PV interneurons (Extended Data Fig. 7). Stimulation also entrained regular-spiking neurons, but only modestly suppressed their overall spike rate (4.87 ± 0.35 vs. 4.07 ± 0.33 spikes/sec before vs. during stimulation, respectively; n = 237 cells). A second experiment recorded LFPs from mPFC in freely moving PV-Cre mice injected with virus to drive Cre-dependent ChR2 expression, while delivering 40 Hz optogenetic stimulation, either in- or out-of-phase between hemispheres (Extended Data Fig. 8). (We also recorded while delivering light in control ChR2-negative mice). Both patterns produced a slight bump ~40 Hz and suppressed high frequency (60–200 Hz) activity (relative to baseline recordings without stimulation). This suppression, which likely reflects the ability of optogenetically-evoked inhibition to reduce overall mPFC activity, was most prominent >150 Hz and similar in magnitude for in- vs. out-of-phase stimulation.
Perturbing gamma synchrony does not affect initial associations or rule reversals
Given that gamma synchrony increased during rule shifts but not initial associations or rule reversals, we wondered whether it is specifically necessary for rule shifts, or whether perturbing gamma synchrony would also disrupt these other types of learning. To examine this, we stimulated prefrontal PV interneurons at 40 Hz, but out-of-phase across the two hemispheres during initial associations or rule reversals. In contrast to its ability to disrupt rule shifts, out-of-phase 40 Hz stimulation did not affect learning during initial associations or rule reversals (Fig. 4f–h). Unlike initial associations or rule reversals, rule shifts require mice to stop utilizing one set of cues, and to instead learn a new association which reappraises the behavioral significance of cues that were previously irrelevant to the outcome of each trial. In fact, once mice learned an initial association and rule shift, out-of-phase 40 Hz stimulation did not disrupt the ability of mice to revert to the original rule (Extended Data Fig. 9). For this, we switched back to the original rule (i.e., initial association) after mice learned a rule shift, and only delivered out-of-phase stimulation while switching back to the original rule. This provides additional confirmation that 40 Hz synchrony is specifically required for behavioral reappraisal, not generic forms of learning or switching.
Gamma synchrony fails to increase in mutant mice that exhibit perseveration
Finally, to evaluate how changes in gamma synchrony might contribute to, or be targeted to alleviate, pathological phenotypes, we exploited Dlx5/6+/− mice, which have abnormal PV interneurons, deficient task-evoked gamma oscillations, and perseveration during rule shifts14. The error-related photometry signals normally observed in wild-type (Dlx5/6+/+) PV interneurons during rule shifts were significantly attenuated in Dlx5/6 mutants (Fig. 5e,f). By contrast, PV interneuron photometry signals following correct decisions were unaltered (Fig. 5c,d). In contrast to normal mice, in mutants 30–50 Hz cross-hemispheric synchronization was not higher after rule shift errors vs. correct decisions (Fig. 5g–i; Extended Data Fig. 10b,i–l). Correspondingly, the normal increase in 30–50 Hz cross-hemispheric PV interneuron synchronization following rule shift errors (relative to correct trials) was significantly attenuated in Dlx5/6+/− mice compared to wild-types (Fig. 5j). This difference was specific for frequency (30–50 Hz vs. 15–25 or 50–70 Hz), task (rule shift vs. initial association or rule reversal), and cell type (PV vs. Sst interneurons) (Fig. 5j; Extended Data Fig. 10q–s; Fig. 5k–n; Extended Data Fig. 10c,d,m–p).
Fig. 5: Cross-hemispheric gamma synchrony fails to increase during rule shifts in mutant mice.
a, Representative FLEX-GCaMP6f expression in a Dlx5/6+/−, PV-Cre mouse (scale bar, 100 μm). b, Rule shift (RS) performance is impaired in mutant mice (blue; n = 7 mice) compared wild-type (Dlx5/6+/+) littermates (black; n = 8 mice; two-tailed, unpaired t-test; t(13) = 7.82, ****P = 0.000003). c, Averaged dF/F from PV interneurons in mutant (blue; n = 7 mice) vs. wild-type (black; n = 8 mice) mice, aligned to the time of correct decisions. d, Peak PV interneuron dF/F values during the 4 sec following correct decisions during rule shifts were similar in Dlx5/6+/− (blue; n = 7 mice) vs. wild-types (black; n = 8 mice; two-tailed, unpaired t-test; t(13) = 0.79, P = 0.44). e, Averaged dF/F from PV interneurons in mutant (blue; n = 7 mice) vs. wild-types (black; n = 8 mice), aligned to the time of incorrect decisions. f, Peak dF/F from PV interneurons during the 4 sec following incorrect decisions is significantly decreased in Dlx5/6+/− mice (blue; n = 7 mice) compared to wild-types (black; n = 8 mice) (two-tailed, unpaired t-test; t(8.085) = 3.18, *P = 0.01). g, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric interneuron synchronization between TEMPO signals from PV interneurons in mutant mice during the last 3 IA and first 5 RS trials in one Dlx5/6+/− mouse. h, In Dlx5/6+/− mice, cross-hemispheric PV interneuron synchronization was not different following errors vs. correct decisions (n = 8 mice; two-way ANOVA; main effect of condition: F1,21 = 0.09, P = 0.77; condition X frequency interaction: F2,21 = 0.29, P = 0.75). i, Rule shift performance is impaired in mutant mice (blue; n = 8 mice) compared to wild-type (black) littermates (n = 12 mice; two-tailed, unpaired t-test; t(8.071) = 7.40, ****P = 0.00007). j, Increases in PV interneuron synchrony following errors (relative to synchrony after correct decisions) are significantly attenuated in mutants (n = 8 mice) compared to wild-type littermates (n = 12 mice), specifically in the 30–50 Hz frequency band (two-way ANOVA; genotype X frequency interaction: F2,36 = 3.98, *P = 0.028; 15–25 Hz: post hoc t(54) = 1.15, P = 0.76; 30–50 Hz: post hoc t(54) = 2.67, *P = 0.03; 50–70 Hz: t(54) = 0.63, P > 0.99). k, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric Sst interneuron synchronization during the last 3 IA and first 5 RS trials in one Dlx5/6+/− mouse. l, In mutants (n = 5 mice), cross-hemispheric Sst interneuron synchrony is similar following correct vs. incorrect decisions (two-way ANOVA; main effect of condition: F1,12 = 0.70, P = 0.42; main effect of frequency: F2,12 = 2.16, P = 0.16; condition X frequency interaction: F2,12 = 0.71, P = 0.51). m, Rule shift performance is impaired in mutants (n = 5 mice) compared to wild-type littermates (n = 5 mice; two-tailed, unpaired t-test; t(8) = 8.64, ****P = 0.00003). n, Changes in Sst interneuron synchrony following errors (relative to synchrony after correct decisions) are not different in mutants (n = 5 mice) vs. wild-types (n = 5 mice; two-way ANOVA; main effect of genotype: F1,8 = 0.06, P = 0.82; genotype X frequency interaction: F2,16 = 0.54, P = 0.59). Data are shown as means (b–f, i–j, m–n); error bars (b, d, f, i–j, m–n) and shading (c, e) denote s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons were used, unless otherwise noted.
Only in-phase gamma-frequency PV interneuron stimulation rescues perseveration
We previously found that 40 Hz stimulation of mPFC interneurons rescues learning during rule shifts in Dlx5/6 mutant mice14. Our new results suggest that gamma synchrony between prefrontal PV interneurons during the early portions of a rule shift may be particularly critical for this effect. We tested this three ways. First, restricting optogenetic stimulation to only PV interneurons and just the first five rule shift trials was sufficient to rescue learning in Dlx5/6 mutants (Fig. 6a–c; Extended Data Fig. 10e,f). Second, 20 or 40 Hz stimulation of PV interneurons that was out-of-phase between hemispheres did not improve rule shift learning in mutants (Fig. 6d–f; Extended Data Fig. 10g,h). However, consistent with our earlier findings14, in-phase 40 Hz stimulation rescued their rule shift performance (Fig. 6f; Extended Data Fig. 10e–h). (Importantly, without optogenetic stimulation, rule shift performance in Dlx5/6 mutants does not improve over three consecutive days of testing14). These results show that gamma-frequency activity in prefrontal PV interneurons is not sufficient to facilitate rule shifts unless it is precisely synchronized across hemispheres. Finally, in-phase, 40 Hz stimulation bilateral stimulation of Sst interneurons did not improve rule shift performance in Dlx5/6 mutants (Extended Data Fig. 6l–p).
Fig. 6: Restoring cross-hemispheric PV interneuron gamma synchrony is required to rescue rule shift performance in Dlx5/6+/− mutant mice.
a, Dlx5/6+/−, PV-Cre mice had bilateral AAV-DIO-ChR2-eYFP injections and fiber-optic implants in mPFC. Experimental design: Day 1: no stimulation; Day 2: in-phase 40 Hz stimulation during the first 5 RS trials. b, Representative ChR2-eYFP expression in the mPFC of a Dlx5/6+/−, PV-Cre mouse (scale bar, 100 μm). c, In-phase 40 Hz stimulation on Day 2 normalizes rule shift performance in mutant mice (n = 6 mice; two-tailed, paired t-test; t(5) = 10.3, ***P = 0.0001). d, Dlx5/6+/−, PV-Cre mice had bilateral AAV-DIO-ChR2-eYFP injections and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 20 Hz stimulation; Day 2: out-of-phase 40 Hz stimulation; Day 3: in-phase 40 Hz stimulation. e, Representative ChR2-eYFP expression in the mPFC of a Dlx5/6+/−, PV-Cre mouse (scale bar, 100 μm). f, In mutants (n = 5 mice), in-phase 40 Hz stimulation (Day 3), but not out-of-phase 40 Hz stimulation (Day 2), rescues rule shift performance (one-way repeated measures ANOVA followed by Tukey’s multiple comparisons test, main effect of treatment: F1.451,5.806 = 12.98, **P = 0.009; Day 1 vs. Day 2: P = 0.98, Day 2 vs. 3: *P = 0.016, Day 1 vs. 3: *P = 0.01). Two-way ANOVA followed by Bonferroni post hoc comparisons were used, unless otherwise noted.
A pro-cognitive pharmacological intervention specifically increases gamma synchrony
Optogenetic stimulation might be viewed as ‘artificially’ altering gamma synchrony. Therefore, we explored whether manipulations engaging endogenous physiological mechanisms also increase gamma synchrony and elicit similar behavioral effects. For this, we used low (sub-anxiolytic and sub-sedative) doses of the benzodiazepine clonazepam. We previously14 showed that clonazepam (0.0625 mg/kg, I.P.), like 40 Hz optogenetic stimulation, normalizes rule shifts in Dlx5/6+/− mice (we also reproduced that here: Fig. 7a–c). Now, using TEMPO, we found that clonazepam increased cross-hemispheric gamma synchrony between prefrontal PV interneurons in Dlx5/6+/− mice (Fig. 7d,e,h). This specifically occurred after rule shift errors (not at baseline period or after correct decisions) (Fig. 7f,g) and for 30–50 Hz (Fig. 7h).
Fig. 7: Low-dose clonazepam increases cross-hemispheric gamma synchrony during rule shifts in mutant mice.
a, Dlx5/6+/−, PV-Cre, Ai14 mice (n = 5 mice) had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. Experimental design: all mice received vehicle only (veh) on Day 1. On Day 2, some mice received clonazepam (clz; n = 3 mice); others received vehicle. On Day 3, we administered clz to those mice that received veh on Day 2 (n = 2 mice). b, Low-dose clonazepam normalizes rule shift performance in mutants (n = 5 mice; two-tailed, paired t-test; t(4) = 8.07, **P = 0.0013). c, Low-dose clonazepam decreases perseverative and random errors (n = 5 mice; two-tailed, paired t-test; t(4) = 6.15, **P = 0.0036 for perseverative, t(4) = 6.53, **P = 0.0028 for random). d, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric interneuron synchronization between TEMPO signals from PV interneurons in one Dlx5/6+/− mouse, during the last 3 IA and first 5 RS trials, in the vehicle condition. e, R2 values, measuring zero-phase lag ~40 Hz cross-hemispheric interneuron synchronization between TEMPO signals from PV interneurons in the same mutant mouse, during the last 3 IA and first 5 RS trials in the clonazepam condition. f, During the baseline period, synchrony did not differ between the vehicle and clonazepam conditions (n = 5 mice; two-way ANOVA; main effect of treatment: F1,12 = 1.37, P = 0.26; frequency X treatment interaction: F2,12 = 3.22, P = 0.08). g, Synchrony did not differ following correct trials in the vehicle and clonazepam conditions (n = 5 mice; two-way ANOVA; main effect of treatment: F1,12 = 0.08, P = 0.78; interaction F2,12 = 1.60, P = 0.24; 15–25 Hz: post hoc t(12) = 0.19, P > 0.99; 30–50 Hz: post hoc t(12) = 1.57, P = 0.43; 50–70 Hz: post hoc t(12) = 0.88, P > 0.99). h, Following RS errors, synchrony was specifically higher in the clonazepam condition for the 30–50 Hz band (n = 5 mice; two-way ANOVA; treatment X frequency interaction: F2,12 = 8.63, **P = 0.005; 30–50 Hz post hoc t(12) = 3.73, **P = 0.009). Two-way ANOVA followed by Bonferroni post hoc comparisons were used, unless otherwise noted.
Discussion
These results directly address long-standing controversies about the functional significance of synchronization across neuronal structures31–33,7,8,11,18. We found a double dissociation between therapeutic or disruptive effects elicited by in-phase vs. out-of-phase stimulation. This confirms that certain aspects of behavior depend on cross-hemispheric gamma synchrony between PV interneurons, not just rhythmic inhibition in local circuits. Furthermore, in both stimulation and TEMPO experiments, gamma synchrony was not involved in generic aspects of learning, decision-making, or flexibility, but rather specifically contributed to behavioral reappraisal: the formation of new associations based on familiar cues that were previously irrelevant to behavioral outcomes. This study focuses on normal behavior, but experiments in Dlx5/6 mutant mice independently validate this relationship between gamma synchrony and behavioral reappraisal. Notably, this type of learning occurs without extensive prior training, differentiating it from the switching between well-learned behaviors that is commonly studied in mice. Learning that reappraises the salience of external cues is critical for adaptation to changing environments.
Extracting synchrony from genetically encoded voltage indicators
Several controls confirm that our method measures task-dependent changes in gamma-frequency synchronization between PV interneurons. Increased synchrony after errors is specific for: 1) the gamma band, 2) rule shifts, and 3) PV interneurons. If the increased synchrony we observed was driven by non-neuronal artifacts, kinetics of Ace-mNeon, etc., then it should have been present in both PV and Sst interneurons. If increased synchrony was driven by nonspecific aspects of PV neuron activity (as opposed to gamma-frequency activity), synchrony should have increased in other frequency bands. And if this increased synchrony was driven by nonspecific aspects of our task, e.g., generic reward or error signals, movements mice make after errors, etc., it should have been observed during rule reversals and initial associations (as well as rule shifts). Finally, increased gamma synchrony was only observed for in-phase Ace-mNeon signals, not when one Ace-mNeon signal was shifted 90 degrees out-of-phase (~6 ms). This confirms that this increased ability to predict one Ace-mNeon signal using the other reflects synchrony between these signals, not the fact that they have similar autocorrelations or higher-order statistics.
In addition to the negative controls described above, clonazepam enhanced gamma synchrony. This represents a positive control that our method is sensitive to manipulations known to enhance PV interneuron output and gamma oscillations.
LFPs and signals from genetically encoded voltage indicators (GEVIs) measure different things and are contaminated by distinct noise sources. Thus, there is not likely to be a 1:1 correspondence between them. Nevertheless, we used LFPs to validate the essence of our GEVI findings: cross-hemispheric LFPs exhibit increased gamma-frequency (~40 Hz) synchrony after incorrect trials, relative to correct ones, during rule shifts. The precise relationship between LFPs and GEVI signals remains an important topic for future studies.
Our current approach does not measure absolute levels of synchrony, because the variation in measurements across mice is high, making it necessary to perform some kind of within-mouse normalization. As a result, we cannot exclude the possibility that baseline gamma synchrony is elevated in Dlx5/6 mutant mice (relative to normal, Dlx5/6+/+ mice), such that the failure of these mice to increase gamma synchrony during rule shifts reflects a ceiling effect. Another limitation is that while GEVIs are ideal for measuring mesoscale patterns of activity within sparse cell types, our approach does not reveal how individual neurons might encode information via the phase of their firing. Finally, while it may be possible to detect action potentials by specifically imaging the soma of neurons, bulk measurements from voltage indicators should be dominated by subthreshold signals, for two reasons. First, spikes are very brief compared to subthreshold oscillations. Second, most neuronal surface area is located in the dendrites (~96% for PV interneurons34), and spike propagation into PV interneuron dendrites is poor35.
The significance of zero-phase lag synchronization
Increases in gamma synchrony occurred for simultaneously recorded Ace-mNeon signals, but not when one signal was shifted 90 degrees out-of-phase. This indicates that the population-level phase lag is near zero. This may seem puzzling, because synaptic communication between the hemispheres involves time delays that are commonly assumed to produce phase differences. In fact, zero-phase lag synchrony commonly emerges in bidirectionally coupled oscillators, even when they communicate with significant delays. Consider two phase oscillators, representing the hemispheres, which emit output (spikes) upon completing each cycle. Assume this output reaches the other oscillator after a quarter-cycle delay (~6 ms for 40 Hz), perturbing the phase of the post-synaptic oscillator proportional to the cosine of its current phase. (Such coupling is not hard to imagine: suppose that output arriving when excitatory neuron firing approaches its peak recruits more excitation, accelerating the next cycle, whereas output arriving later, when inhibitory neurons have been recruited, mainly increases inhibition, delaying the next cycle). For weak coupling, this system exhibits zero-phase lag synchrony, even though these two oscillators communicate with a quarter cycle delay.
Another potential concern is about the function of zero-phase lag synchrony. We have previously shown that inputs modulated at gamma frequency transmit greater information to downstream neurons than non-rhythmic inputs9. Suppose that the left and right mPFC converge on a common downstream target. Then, when they are synchronized with zero-phase lag, inputs from the left and right mPFC hemispheres will summate in downstream neurons in a manner preserving the gamma-frequency modulation within each individual signal. By contrast, when activity is out-of-phase between hemispheres, the rhythmic modulation of their summated input will be degraded, compromising information transmission to downstream targets. Thus, cross-hemispheric gamma synchrony may potentiate prefrontal outputs to other regions that serve to update the behavioral salience of external cues. Indeed, we observed that 40 Hz optogenetic stimulation of PV interneurons strongly entrained regular spiking (RS) units. An important future direction is determining whether specific classes of prefrontal pyramidal neurons, projecting to particular targets, exhibit increased gamma synchrony during rule shifts. This may be true for projections to dorsomedial striatum, nucleus accumbens, and/or mediodorsal thalamus, because these projections are important for cognitive flexibility15,36,37 and PV interneurons strongly inhibit mPFC neurons which project bilaterally to these structures38.
Whatever the function of cross-hemispheric synchrony is, it is specific for synchrony ~40 Hz, as optogenetically disrupting 20 Hz synchronization did not disrupt rule shift performance. The simplest explanation for this is that out-of-phase 20 Hz stimulation does not prevent PV interneurons from synchronizing at frequencies ~40 Hz. Indeed, the previously described coupled oscillators which normally synchronize at 40 Hz continue to do so even when receiving simulated out-of-phase stimulation at half their natural frequency (i.e., 10 ms pulses at 20 Hz).
Optogenetically perturbing synchrony
Optogenetic stimulation and inhibition are commonly used to test the causal significance of specific patterns of neural activity. However, optogenetic manipulations induce firing that is, by definition, ‘artificial.’ Several observations indicate that our optogenetic results inform normal circuit function, rather than simply inducing non-physiological states. First, we used modest optogenetic stimulation that we and others found did not markedly alter overall levels of circuit activity. Second, we delivered exactly the same pattern of stimulation to each PFC, either in- or out-of-phase, and behavior was completely normal during in-phase stimulation. Thus, the disruptive effects of out-of-phase stimulation cannot be attributed to excessive PV interneuron firing or hypersynchrony within one hemisphere. Rather, the disruption of rule shifts must reflect the induction of artificial (nonzero) phase differences.
Finally, in-phase stimulation, which does not affect behavior in normal mice, rescues rule shift performance in mutant mice. This same effect can be produced using sub-anxiolytic and sub-sedative doses of clonazepam14, which also restore increases in gamma synchrony normally seen in wild-type mice after rule shift errors. This suggests that in-phase stimulation is functionally similar to clonazepam, which acts by enhancing endogenous PV interneuron output. Thus in-phase stimulation may reproduce physiologically and therapeutically-relevant states, rather than creating aberrant ones. In this way, optogenetic experiments reveal how specific aspects of normally-occurring activity (zero-phase lag cross-hemispheric gamma synchrony between PV interneurons) contribute to behavior.
Clinical relevance
Disruptions in PV interneurons and gamma synchrony39–41 are hypothesized to contribute to cognitive deficits at the core of schizophrenia42–43. Deficits in PV interneurons and gamma synchrony may also contribute to cognitive deficits in Alzheimer’s disease44 and driving synchronized gamma oscillations may ameliorate behavioral and neuropathological aspects of this disorder45–46. Our findings suggest that interventions that restore gamma oscillations may treat cognitive deficits, but only when they involve the proper cell types and reproduce endogenous patterns of synchronization.
In individuals at high risk for psychosis, deficits in the ability to learn new associations based on previously irrelevant cues are strongly correlated with impairments in insight, the capacity to appraise and modify distorted beliefs about anomalous experiences47. Impaired insight plays a central role in the development and maintenance of psychosis48. This suggests that gamma synchrony may be relevant to psychosis itself (not just cognitive dysfunction) in schizophrenia.
METHODS
Further information and requests for resources and reagents should be directed to and will be fulfilled by Vikaas Sohal (vikaas.sohal@ucsf.edu).
Mice
All animal care, procedures, and experiments were conducted in accordance with the NIH guidelines and approved by the Administrative Panels on Laboratory Animal Care at the University of California, San Francisco. Mice were group housed (2–5 siblings) in a temperature-controlled environment (22–24°C), had ad libitum access to food and water, and reared in normal lighting conditions (12-h light-dark cycle), until rule shift experiments began. Dlx5/6 mice (Wang et al., 2010, Cho et al., 2015) were backcrossed to C57Bl/6 mice for at least 6 generations and then crossed to the Cre driver lines: PV-Cre (The Jackson Laboratory), Sst-Cre (The Jackson Laboratory), and Ai14 (The Jackson Laboratory). Both male and female adult mice (10–20 weeks at time of experiment) were used in the behavioral experiments. All experiments were done using Dlx5/6+/− mice and their age-matched Dlx5/6+/+ littermates (crossed to PV-Cre, Sst-Cre, and/or Ai14 lines). All experiments that contained different groups of mice, e.g., Dlx5/6+/+ and Dlx5/6+/− mice or ChR2-expressing and eYFP-expressing mice, were performed blind to genotype and/or virus injected. This was the case for all experiments except for the experiments shown in Figure 3 (in which all mice were Dlx5/6+/+, PV-Cre, Ai14) and Figures 6a–c (in which all mice were Dlx5/6+/−, PV-Cre and expressed ChR2). All subjects were randomly assigned to different experimental conditions used in this study. Animals included in each experiment are described in Supplementary Table 1.
When we initially began experiments, we were uncertain whether the Ai14-driven tdTomato fluorescence would be comparable in magnitude to the mNeon fluorescence, so as noted below, we also injected a subset of animals with virus to drive additional tdTomato expression. It turned out that the reference fluorophore signals were similar in magnitude / adequate in both cases, so in later cohorts, we no longer injected additional tdTomato virus.
Cloning of viral constructs
To produce AAV5-I12b-BG-DIO-eYFP (2.1E+ 13 vg/mL), we introduced MluI and BamHI compatible sticky ends to the DlxI12b-BG sequence with PCR. The pAAV-EF1α-DIO-eYFP (Addgene) was then cut with MluI/BamHI and ligated to the PCR insert to exchange the EF1α promoter for DlxI12b-BG. Virus was packaged by Virovek (Hayward, CA) with serotype AAV5.
To produce AAV1-CAG-DIO-Ace2N-4AA-mNeon (2.23E+13 vg/mL), we received pAAV-CAG-DIO-Ace2N-4AA-mNeon from Mark J. Schnitzer (Stanford University). Virus was packaged by Virovek with serotype AAV1.
Surgery
Male and female mice were anaesthetized using isoflurane (2.5% induction, 1.2–1.5% maintenance, in 95% oxygen) and placed in a stereotaxic frame (David Kopf Instruments). Body temperature was maintained using a heating pad. An incision was made to expose the skull for stereotaxic alignment using bregma and lambda as vertical references. The scalp and periosteum were removed from the dorsal surface of the skull and scored with a scalpel to improve implant adhesion. Viruses were infused at 100–150 nL/min through a 35-gauge, beveled injection needle (World Precision Instruments) using a microsyringe pump (World Precision Instruments, UMP3 UltraMicroPump). After infusion, the needle was kept at the injection site for 5–10 min and then slowly withdrawn. After surgery, mice were allowed to recover until ambulatory on a heated pad, then returned to their homecage.
For behavioral experiments using Cre-dependent optogenetic opsins, mice were injected bilaterally in the mPFC, near the border between the prelimbic and infralimbic cortices (1.7 anterior-posterior (AP), ±0.3 mediolateral (ML), and −2.75 dorsoventral (DV) millimeters relative to bregma) with 1 μL of AAV5-EF1α-DIO-ChR2-eYFP (7.4E+ 12 vg/mL; UNC Virus Core) or 1 μL of AAV5-I12b-BG-DIO-ChR2-eYFP or 1 μL of AAV5-EF1α-DIO-eYFP (6E+ 12 vg/mL; UNC VIrus Core) per hemisphere, to selectively target neurons expressing Cre. Dlx5/6, Sst-Cre mice were injected bilaterally in the mPFC (1.7 (AP), ±0.3 (ML), and −2.75 (DV)) with 1 μL of AAV5- EF1α-DIO-ChR2-eYFP or 1 μL of AAV5-EF1α-DIO-eYFP per hemisphere. After injection of virus, a 200/240 μm (core/outer) diameter, NA=0.22, dual fiber-optic cannula (Doric Lenses, DFC_200/240–0.22_2.3mm_GS0.7_FLT) was slowly inserted into mPFC until the tip of the fiber reached a DV depth of −2.25. Implants were affixed onto the skull using Metabond Quick Adhesive Cement (Parkell). We waited at least 5 weeks after injection before behavioral experiments to allow for virus expression. For experiments using LFP recordings, standard-tip 0.4 MΩ-impedance tungsten microelectrodes (Microprobes) were used. The coordinates were adjusted to accommodate experiments whereby LFP electrodes were affixed to the fiber implant and protruded 200–300 μm beyond the fiber tip. A common reference screw was implanted into the cerebellum: −5 (AP), 0 (ML) and a ground screw was implanted at −5 (AP), −3 (ML). After affixing the electrodes in place using Metabond (Parkell), connections were made to the headstage of a multi-channel recording system (Pinnacle Technology, Inc.).
For behavioral experiments used in photometry experiments, mice were injected unilaterally at 4 depths (DV: −2.75, −2.5, −2.25, −2.0) at the following AP/ML for mPFC: 1.7 AP, 0.3 ML with 4 × 0.2 μL of AAV2/1-Syn-FLEX-GCaMP6f-WPRE-SV40 (2.28E+ 13 vg/mL; UPenn Virus Core). After injection of virus, a 400/430 μm (core/outer) diameter, NA=0.48, multimode fiber implant (Doric Lenses, MFC_400/430–0.48_2.8mm_ZF2.5_FLT) was slowly inserted into the mPFC until the tip of the fiber reached a DV depth of −2.25. We waited at least 4 weeks after injection before behavioral experiments to allow for virus expression.
For behavioral experiments used in dual-site TEMPO experiments, mice were injected bilaterally at 3 depths (DV: −2.5, −2.25, −2.0) at the following AP/ML for mPFC: 1.7 AP, ±0.3 (ML) with 3 × 0.2 μL of AAV1-CAG-DIO-Ace2N-4AA-mNeon (Virovek) or with the addition of 0.1 μL per depth of AAV2-Syn-tdTomato (1.23E+ 12 vg/mL; SignaGen Laboratories). After injection of virus, a 400/430 μm (core/outer) diameter, NA=0.48, multimode fiber implant (Doric Lenses, MFC_400/430–0.48_2.8mm_ZF1.25_FLT) was slowly inserted into the mPFC at a 12° angle using the following coordinates: 1.7 (AP), ±0.76 (ML), −2.13 (DV). We waited at least 5 weeks after injection before behavioral experiments to allow for virus expression.
For in vivo awake head-fixed recordings, PV-Cre mice were injected unilaterally in the mPFC, near the border between the prelimbic and infralimbic cortices (1.7 anterior-posterior (AP), +0.3 mediolateral (ML), and −2.75 dorsoventral (DV) millimeters relative to bregma) with 1 μL of AAV5-EF1α-DIO-ChR2-eYFP (7.4E+ 12 vg/mL; UNC Virus Core), to selectively target neurons expressing Cre. At least 3 weeks later, mice were implanted with a circular head bar at least 2 weeks before the day of the recording. The animals were anesthetized with 2% isoflurane, the scalp was removed and the skull was disinfected with alcohol and povidone iodine and scored with bone scraper. The edge of the skin was glued to the skull and the metal head bar was sterilized and mounted using dental cement Relyx Unicem 2 automix (3M ESPE). The head bar was stereotactically mounted with the help of an inclinometer (Digi-Key electronics 551–1002-1-ND). The inclinometer was instrumental in calibrating the angle of the two axes of the head bar in relation to the sagittal and medio-lateral axes of the head. Following the bar implantation, black dental cement was used to build a recording well surrounding the recording site. The surface of the skull above the right PFC was not covered with dental cement but coated with a thin layer of transparent cyanoacrylate glue. Animals were injected subcutaneously with 0.1mg/kg buprenorphine and checked daily after the head bar surgery. For at least 4 days before recording, mice were habituated to head fixation within the recording setup.
On the day before recording, mice were anesthetized with 2% isoflurane and the layer of cyanoacrylate glue covering the recording sites was drilled off. The dura was not removed, and the exposed brain was kept moist with artificial cerebrospinal fluid (ACSF; 140mM NaCl, 5mM KCl, 10mM d-glucose, 10mM HEPES, 2mM CaCl2, 2mM MgSO4, pH 7.4). 3 out of 4 animals were recorded a second time at least 8 hours after the first recording. The electrode was moved from 0.3 ML, 1.5 AP to 0.3 ML, 1.7 AP, so more anterior. The depth of the recorded units varied between 1.78 to 2.42 mm from pia.
Rule shift task
This cognitive flexibility task was described in Cho et al., 2015. Briefly, mice are singly-housed and habituated to a reverse light/dark cycle and food intake is restricted until the mouse is 80–85% of the ad libitum feeding weight. At the start of each trial, the mouse was placed in its home cage to explore two bowls, each containing one odor and one digging medium, until it dug in one bowl, signifying a choice. As soon as a mouse began to dig in the incorrect bowl, the other bowl was removed, so there was no opportunity for “bowl switching.” (Digging is defined as the sustained displacement of the media within a bowl). The bait was a piece of a peanut butter chip (approximately 5–10 mg in weight) and the cues, either olfactory (odor) or somatosensory and visual (texture of the digging medium which hides the bait), were altered and counterbalanced. All cues were presented in small animal food bowls (All Living Things Nibble bowls, PetSmart) that were identical in color and size. Digging media were mixed with the odor (0.01% by volume) and peanut butter chip powder (0.1% by volume). All odors were ground dried spices (McCormick garlic and coriander), and unscented digging media (Mosser Lee White Sand Soil Cover, Natural Integrity Clumping Clay cat litter).
After mice reached their target weight, they underwent one day of habituation. On this day, mice were given ten consecutive trials with the baited food bowl to ascertain that they could reliably dig and that only one bowl contained food reward. All mice were able to dig for the reward. Mice do not undergo any other specific training before being tested on the task. Then, on Days 1 and 2 (and in some cases, on additional days as well), mice performed the task (this was the testing done for experiments). After the task was done for the day, the bowls were filled with different odor-medium combinations and food was evenly distributed among these bowls and given to the mouse so that the mouse would disregard any associations made earlier in the day.
Mice were tested through a series of trials. The determination of which odor and medium to pair and which side (left or right) contained the baited bowl was randomized (subject to the requirement that the same combination of pairing and side did not repeat on more than 3 consecutive trials) using http://random.org. On each trial, while the particular odor-medium combination present in each of the two bowls may have changed, the particular stimulus (e.g., a particular odor or medium) that signaled the presence of food reward remained constant over each portion of the task (initial association and rule shift). If the initial association paired a specific odor with food reward, then the digging medium would be considered the irrelevant dimension. The mouse is considered to have learned the initial association between stimulus and reward if it makes 8 correct choices during 10 consecutive trials. Each portion of the task ended when the mouse met this criterion. Following the initial association, the rule shift portion of the task began, and the particular stimulus associated with reward underwent an extra-dimensional shift. For example, if an odor had been associated with reward during the initial association, then a digging medium was associated with reward during the rule shift portion of the task. The mouse is considered to have learned this extra-dimensional rule shift if it makes 8 correct choices during 10 consecutive trials. When a mouse makes a correct choice on a trial, it is allowed to consume the food reward before the next trial. Following correct trials, the mouse is transferred from the home cage to a holding cage for about 10 seconds while the new bowls were set up (intertrial interval). After making an error on a trial, a mouse was transferred to the holding cage for about 2 minutes (intertrial interval). All animals performed the initial association in a similar number of trials (average: 10–15 trials). We were blind to genotype and/or virus injected. Videos were manually scored with a temporal resolution of 1 second.
For analyses (described below), we chose the onset of digging as the time of a decision for two reasons. First, as noted above, once a mouse began to dig in the incorrect bowl, the other (correct) bowl was removed. Second, only upon the commencement of digging could a mouse determine whether reward was present in the chosen bowl and obtain feedback about whether or not it had made a correct choice. We regarded the end of digging (i.e., the beginning of a sustained period of not digging) as the end of the trial because immediately following this timepoint, the mouse was removed from the test cage and placed in a holding cage for the intertrial interval.
Rule reversal task
This cognitive flexibility task was described in Cho et al., 2015. Similar to the mechanics of the rule shift task described above, following the initial association, the rule reversal portion of the task began, and the particular stimulus associated with reward underwent an intra-dimensional shift. For example, if an odor had been associated with reward during the initial association, then the previously uncued odor was associated with reward during the rule reversal portion of the task. The mouse is considered to have learned this intra-dimensional rule reversal if it makes 8 correct choices during 10 consecutive trials.
Mice that were involved in both the rule shift and rule reversal tasks were randomly assigned the order of tasks over the course of two days.
In vivo optogenetic stimulation
In-phase ChR2 stimulation: A 473 nm blue laser (OEM Laser Systems, Inc.) was coupled to the dual fiber-optic cannula (Doric Lenses) through a 200 μm diameter dual fiber-optic patchcord with guiding socket (Doric Lenses, Inc.) and 1×2 intensity division fiber-optic rotary joint (Doric Lenses, Inc.), and adjusted such that the final light power was ~0.5 mW total, summed across both fibers and averaged over light pulses and the intervening periods. A function generator (Agilent 33500B Series Waveform Generator) connected to the laser generated a 40 Hz train of 5 ms pulses.
Out-of-phase ChR2 stimulation: The stereotaxically implanted dual fiber-optic cannula was coupled to two separate 473 nm blue lasers via a dual fiber-optic patch cord with fully-separated optical paths that were each connected to separate fiber-optic rotary joints. Again, light power was adjusted such that the final output was ~0.5 mW across both fibers. Different function generators connected to each laser, in order to generate out-of-phase stimulation. For the experiments shown in Figures 4d–e and Figures 6d–f, these two function generators were not connected in any way, except that we verified (by eye) that the light pulses were delivered at non-overlapping times, producing phase differences between 72 and 288 degrees. For the experiments shown in Figures 4a–c and Figures 4f–h, one function generator was triggered at the time when the other function generator switched off, so the phase difference was exactly 72 degrees. Stimulation was generated using either a 20 Hz train of 10 ms pulses or a 40 Hz train of 5 ms pulses.
For all experiments in which we delivered optogenetic stimulation to behaving mice, light stimulation began once mice reached the 80% criterion during the initial association portion of the task. Mice then performed three additional initial association trials with the light stimulation before the rule shift portion of the task began. The light stimulation did not alter the performance or behavior of the mice during these three extra trials of the initial association. Experiments were performed blind to genotype and/or virus injected.
Drug administration
Clonazepam at indicated concentrations (0.0625 mg/kg, Sigma) was diluted in the vehicle solution (PBS with 0.5% methylcellulose) then injected (I.P.) in a volume of 0.01 ml/kg 30 min prior to behavioral testing.
Fiber photometry design and recording
The photometry apparatus and analysis was based on Lerner et al., 2015, with some modifications described below.
A fiber-optic stub (400 μm core, NA=0.48, low-autofluorescence fiber; Doric Lenses, Quebec, Canada, MFC_400/430–0.48_2.3mm_ZF2.5_FLT) was stereotaxically implanted in mPFC. A single fiber was used to both deliver excitation light and collect emitted fluorescence from the recording site. A matching fiber-optic patch cord (Doric Lenses, MFP_400/430/1100–0.48_2m_FC-ZF2.5) provided a light path between the animal and a miniature, permanently-aligned optical bench, or ‘mini cube’ (Doric Lenses, FMC2_AF405-GCaMP_FC). Two excitation LEDs (470 nm ‘blue’ and 405 nm ‘violet’, Thorlabs M470F1 and M405FP1) were connected to the ‘mini cube’ by a patch cord (200 μm core, NA = 0.39, Doric Lenses) and controlled by an LED driver (Thorlabs DC4104), and connected to an RX-8 real-time processor (Tucker Davis Technologies). Excitation light is delivered at 470 nm to stimulate GCaMP6f fluorescence in a Ca2+-dependent manner and at 405 nm, an excitation isosbestic wavelength for GCaMP6f, to perform ratiometric measurements of GCaMP6f activity, correcting for bleaching and artifactual signal fluctuations. Blue excitation was sinusoidally-modulated at 210 Hz and violet excitation was modulated at 330 Hz. The GCaMP6f emission signal was collected through a patchcord (Doric Lenses, MFP_600/630/LWMJ-0.48_0.5m_FC-FC) and focused onto a femtowatt photoreceiver (Newport, Model 2151) with a lensed, permanently-aligned FC coupler (Doric Lenses). Each of the two modulated signals generated by the two LEDs was independently recovered using standard synchronous demodulation techniques implemented on the RX-8 real-time processor. The commercial software Synapse (Tucker-Davis Technologies) running on a PC was used to control the signal processor, write data streams to disk, and to record synchronized video from a generic infrared USB webcam (Ailipu Technology, Shenzhen, China, ELP-USB100W05MT-DL36). Files were then exported for analysis to MATLAB (Mathworks).
For every experiment, the far end of the patch cord and the 2.5 mm diameter zirconia optical implant ferrule were cleaned with isopropanol before each recording, then securely attached via a zirconia sleeve.
LFP recording
Data was recorded at 1 kHz and analog signals were digitized by a multichannel real-time signal processor (Tucker-Davis Technologies, Alachua, Florida; RX-8). The commercial software Synapse (Tucker-Davis Technologies) running on a PC was used to control the signal processor, write data streams to disk, and to record synchronized video from a generic infrared USB webcam (Ailipu Technology, Shenzhen, China, ELP-USB100W05MT-DL36). Channels shared a common reference (cerebellum). In one experiment, LFPs were recorded in freely moving mice in their home cage, either when optogenetic stimulation was absent (baseline period) or during delivery of optogenetic stimulation to prefrontal PV interneurons. Patterns of optogenetic stimulation and methods for expressing ChR2 in PV interneurons were the same as in behavioral experiments (see above). In another experiment, we recorded LFPs from the left and right mPFC while mice performed the rule shift task.
In vivo awake head-fixed recordings
Extracellular recordings from PFC were performed using opto silicon probes: ASSY-37 H4 (acute 32 channel H4 opto-electrode, 1 shank, 9 mm length). The recording electrodes were controlled with Luigs & Neumann micromanipulators and stained with DiI lipophilic dyes (Life Technologies) for post hoc identification of the electrode track. We recorded the signals at 30 kHz using an Intan system (RHD2000 USB Interface Board, Intan Technologies). Automated spike sorting was then carried out using KiloSort (https://github.com/cortex-lab/Kilosort) by manual curation of the units using Phy (http://phy-contrib.readthedocs.io/en/latest/template-gui/). Light power was adjusted such that the final output was ~0.25 mW when delivering 40 Hz, 5ms pulses for DIO-ChR2 activation. Units were identified and all following analysis was carried out using routines written in Matlab. We excluded units with refractory period violations greater than 1%. Neurons were considered as putative PV neurons when their firing increased and was significantly different from the baseline activity (P < 0.01) during 5 ms blue light pulse activation at 1Hz. Regular spiking (RS) and fast spiking (FS) neurons were identified based on spike shape. The average peristimulus time histogram of PV cell responses was calculated from minute 1 through 4 of the 40 Hz, 5 ms ChR2 activation.
Dual-site voltage-sensor photometry (TEMPO)
High-bandwidth bandwidth time-varying bulk fluorescence signals were measured at each recording site using the TEMPO technique described in Marshall et al., 2016, with some modifications as described below.
Optical apparatus
A fiber-optic stub (400 μm core, NA=0.48, low-autofluorescence fiber; Doric Lenses, MFC_400/430–0.48_2.8mm_ZF1.25_FLT) was stereotaxically implanted in each targeted brain region. A matching fiber-optic patch cord (Doric Lenses, MFP_400/430/1100–0.48_2m_FC-ZF1.25) provided a light path between the animal and a miniature, permanently-aligned optical bench, or ‘mini-cube’ (Doric Lenses, FMC5_E1(460–490)_F1(500–540)_E2(555–570)_F2(580–680)_S). A single fiber was used to both deliver excitation light to and collect emitted fluorescence from each recording site. The far end of the patch cord and each 1.25mm diameter zirconia optical implant ferrule were cleaned with isopropanol before each recording, then securely attached via a zirconia sleeve.
The mini-cube optics allow for the simultaneous monitoring of two spectrally-separated fluorophores, with dichroic mirrors and cleanup filters chosen to match the excitation and emission spectra of the voltage sensor and reference fluorophores in use (‘mNeon’ voltage sensor channel: Ex. 460–490 nm, Em. 500–540 nm; ‘Red’ control fluorophore: Ex. 555–570 nm, Em. 580–680 nm). The mini-cube optics are sealed and permanently aligned and all 5 ports (sample to animal, 2 excitation lines, 2 emission lines) are provided with matched coupling optics and FC connectors to allow for a modular system design.
Excitation light for each of the two color channels was provided by a fiber-coupled LED (Center wavelengths 490 nm and 565 nm, Thorlabs M490F3 and M565F3) connected to the mini-cube by a patch cord (200 μm, NA=0.39; Thorlabs M75L01). Using a smaller diameter for this patch cord than for the patch cord from the cube to the animal is critical to reduce the excitation spot size on the output fiber face and thus avoid cladding autofluorescence. LEDs were controlled by a 4-channel, 10kHz-bandwidth current source (Thorlabs DC4104). LED current was adjusted to give a final light power at the animal (averaged during modulation, see below) of approximately 200 μW for the mNeon channel (460–490 nm excitation), and 100 μW for the Red channel (555–570 nm excitation).
Each of the two emission ports on the mini-cube was connected to an adjustable-gain photoreceiver (Femto, Berlin, Germany, OE-200-Si-FC; Bandwidth set to 7kHz, AC-coupled, ‘Low’gain of ~5×10^7 V/W) using a large-core high-NA fiber to maximize throughput (600 μm core, NA=0.48 (Doric lenses, MFP_600/630/LWMJ-0.48_0.5m_FC-FC).
Note that, for dual-site recordings, two completely independent optical setups were employed, with separate implants, patch cords, mini-cubes, LEDs, photoreceivers, and lock-in amplifiers.
Modulation and lock-in detection
At each recording site, each of the two LEDs was sinusoidally modulated at a distinct carrier frequency to reduce crosstalk due to overlap in fluorophore spectra. The corresponding photoreceiver outputs were then demodulated using lock-in amplification techniques. A single instrument (Stanford Research Systems, SR860) was used to generate the modulation waveform for each LED and to demodulate the photoreceiver output at the carrier frequency. To further reduce cross-talk between recording sites, distinct carrier frequencies were used across at each site (mNeon site 1: 2 kHz; mNeon site 2: 2.5 kHz; Red site 1: 3.5 kHz; Red site 2: 4 kHz). Low-pass filters on the lock-in amplifiers were selected to reject noise above the frequencies under study (cascade of 4 Gaussian FIR filters with 84 Hz equivalent noise bandwidth; final attenuation of signals are approximately −1dB (89% of original magnitude) at 20 Hz, −3dB (71% of original magnitude) at 40 Hz, and −6dB (50% of original magnitude) at 60 Hz).
TEMPO recording
Analog signals were digitized by a multichannel real-time signal processor (Tucker-Davis Technologies, Alachua, Florida; RX-8). The commercial software Synapse (Tucker-Davis Technologies) running on a PC was used to control the signal processor, write data streams to disk, and to record synchronized video from a generic infrared USB webcam (Ailipu Technology, Shenzhen, China, ELP-USB100W05MT-DL36). Lock-in amplifier outputs were digitized at 3 kHz.
Histology and imaging
All mice used for behavioral and imaging experiments were anesthetized with Euthasol and transcardially perfused with 30 mL of ice-cold 0.01 M PBS followed by 30 ml of ice-cold 4% paraformaldehyde (PFA) in PBS. Brains were extracted and stored in 4% PFA for 24 h at 4 °C before being stored in PBS. 50 μm and 70 μm slices were obtained on a Leica VT100S and mounted on slides. All imaging was performed on an Olympus MVX10, Nikon Eclipse 90i, Zeiss LSM510, and Zeiss Axioskop2. Representative images for all figures are enlarged in Supplementary Figure 1. We verified that all mice had virus-driven expression and optical fibers located in the mPFC.
Fiber photometry analysis
For calculating peak GCaMP6f signals during activity time-locked to task-related events: the beginning of each trial, the time of each decision (indicated by the mouse beginning to dig in one bowl), and the beginning and end of each intertrial interval, a least-squares linear fit was applied to the 405 nm control signal to align it to the 470 nm signal. The dF/F time series was then calculated for each behavioral session as: ((470 nm signal - fitted 405 nm signal) / fitted 405 nm signal). For points of interest (e.g., time of decision), peak dF/F was calculated as the most extreme dF/F value at time 0–4 seconds (positive or negative) for PV-Cre experiments and at time 4–8 seconds (positive or negative) for Sst-Cre experiments. Experiments were performed, scored, and analyzed blind to genotype.
LFP analysis
To analyze changes in power in our LFP data, we computed spectrograms from completely non-overlapping time windows and compared unnormalized power (measured in log units) during 40 Hz optical stimulation to the power during the baseline period.
To quantify zero-phase lag synchrony between LFPs recorded from the left and right mPFC, we calculated the square of the real part of the wavelet cross spectrum, obtained from the wcoherence function in Matlab.
TEMPO analysis
Analysis of TEMPO data was facilitated using the signal processing toolbox and MATLAB (Mathworks), using the following functions: fir1, filtfilt, and regress. All four signals during the entire time series of the experiment (left mNeon, left tdTomato, right mNeon, right tdTomato) were first filtered around a frequency of interest. To quantify zero-phase lag cross-hemispheric synchronization between left and right mNeon signals, we performed a linear regression analysis to predict the right mNeon signal using the following inputs: left mNeon signal, left tdTomato signal, and right tdTomato signal. The goodness of fit is compared to how well the regression works if the left mNeon signal is shuffled, i.e., if we use a randomly chosen segment of the original left mNeon signal, instead of the segment recorded at the same time as the right mNeon signal. R2 values are calculated as a function of time using one second segments and compared to the 99th percentile of the distribution of R2 values obtained from 100 fits to randomly-shuffled data. The fraction of timepoints at which the R2 obtained from actual data exceeds the 99th percentile of the R2 values obtained from shuffled data was used to measure zero-phase lag synchronization between the left and right mNeon signals.
We performed this analysis at the time of the decision (e.g., immediately following the beginning of digging in one bowl, until the end of digging), and smoothed measurements over a 5 minute time window following the time point of interest. We also confirmed that our TEMPO findings did not change when we did not smooth over any window but instead simply averaged R2 values from the start of the trial until the end of the subsequent intertrial interval. To measure 90 degree out-of-phase synchronization, the filtered left mNeon signal was simply advanced 90 degrees relative to the right mNeon signal, before performing the analysis described above. Experiments were performed, scored, and analyzed blind to genotype. Note: mice that did not make both correct and incorrect decisions in the first 5 trials of a task could not be used for analyses which compared activity on correct vs. incorrect decisions.
Data analyses and statistics
Statistical analyses were performed using Prism 7 (GraphPad) and detailed in the corresponding figure legends. Quantitative data are expressed as the mean and error bars or shaded areas represent the standard error of the mean (s.e.m.). Group comparisons were made using one-way repeated measures or two-way ANOVA followed by Tukey’s or Bonferroni post-hoc tests to control for multiple comparisons, respectively. Paired and unpaired two-tailed Student’s t-tests were used to make single-variable comparisons or with Welch’s correction for unequal variance. Similarity of variance between groups was confirmed by the F test. Measurements were taken from distinct samples. P = *, < 0.05; **, < 0.01; ***, < 0.001; ****, < 0.0001. Comparisons with no asterisk had P > 0.05 and were considered not significant. No statistical methods were used to pre-determine sample sizes but our sample size choice was based on previous studies (Cho et al., 2015) and are consistent with those generally employed in the field. Data distribution was assumed to be normal but this was not formally tested.
Extended Data
Extended Data Fig. 1:
Photometry signals from PV interneurons and behavior during rule shifts in mice used for photometry experiments. a, PV-Cre mice had a unilateral FLEX-GCaMP6f injection and fiber-optic implant in mPFC for photometry (scale bar, 100 μm). b, Rule-shift (RS) performance of PV-Cre mice (n = 8) used for photometry experiments. c, Numbers of perseverative (P) or random (R) errors during the rule shift of PV-Cre mice (n = 8). d, Averaged PV interneuron photometry signal (dF/F), aligned to the start of each trial, for correct (white line) vs. incorrect trials (black line; n = 8). e, Averaged PV interneuron photometry signal (dF/F), aligned to the start of the intertrial interval (ITI), for correct (white line) vs. incorrect trials (black line; n = 8) for the first ten seconds of ITIs. f, Peak dF/F during the entire intertrial interval (ITI), usually lasting around two minutes. Signals are significantly higher on incorrect rule shift trials than correct rule shift trials (n = 8 mice; two-way ANOVA; main effect of outcome: F1,14 = 26.53, ***P = 0.0001; task X outcome interaction: F1,14 = 7.47, *P = 0.016; RS correct versus incorrect, post hoc t(14) = 5.58, ***P = 0.0001; IA correct versus incorrect, post hoc t(14) = 1.71, P = 0.22). g, Mouse behavior scored at the time of the peak dF/F signal during rule shift error trials (n = 31 error trials of 8 mice). h, Mouse behavior scored at the time of the peak dF/F signal during initial association error ITI (n = 21 error ITIs of 8 mice). i, Mouse behavior scored at the time of the peak dF/F signal during rule shift error ITI (n = 31 error ITIs of 8 mice). Data are shown as means (b, c); error bars (b, c) and shading (d, e) denote s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons were used. Comparisons were not significant unless otherwise noted.
Extended Data Fig. 2:
Cross-hemispheric synchrony of PV interneurons at non-zero phase lag. a, Schematic: analysis to measure out-of-phase synchrony. In this case, one Ace-mNeon is signal is shifted 90 degrees out-of-phase relative to the other signals, before following the procedure outlined in Figure 2e. b–d, Out-of-phase 30–50 Hz synchrony (n = 12 mice) did not differ between the baseline period and RS correct trials (two-way ANOVA; frequency X condition interaction: F2,33 = 0.16, P = 0.86; post hoc t(33) = 1.35, P = 0.56), baseline period and RS incorrect trials (two-way ANOVA; frequency X condition interaction: F2,33 = 0.06, P = 0.94; post hoc t(33) = 0.05, P > 0.99), or correct and incorrect trials (two-way ANOVA; frequency X condition interaction: F2,33 = 0.05, P = 0.95; post hoc t(33) = 1.53, P = 0.41). Two-way ANOVA followed by Bonferroni post hoc comparisons were used. Comparisons were not significant.
Extended Data Fig. 3:
Learning during rule shifts in mice used for TEMPO measurements from Sst interneurons, and photometry signals from Sst interneurons during rule shifts. a, Sst-Cre, Ai14 mice had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. b, Representative images of tdTomato (red) and Ace-mNeon (green) fluorescence in a coronal section of mPFC (left), alongside a high power image (right). Scale bars: 100 μm and 25 μm, respectively. c, Rule-shift (RS) performance of Sst-Cre, Ai14 mice (n = 5) used for dual-site TEMPO imaging. d, Number of perseverative (P) and random (R) errors during the rule shift of Sst-Cre, Ai14 mice (n = 5). e, Sst-Cre mice had a unilateral FLEX-GCaMP6f injection and fiber-optic implant in mPFC for photometry (scale bar, 100 μm). f, Rule-shift (RS) performance of Sst-Cre mice (n = 4) used for photometry experiments. g, Numbers of perseverative (P) or random (R) errors during the rule shift Sst-Cre mice (n = 4). h, Averaged Sst interneuron photometry signal (dF/F), aligned to the time of dig, which indicates a decision, for correct (white line) vs. incorrect trials (purple line; n = 4). i, Peak dF/F during the 4–8 sec following the decision (this was the time at which peak Sst activity occurred). Sst interneuron photometry signals are significantly higher on incorrect than correct trials (n = 4 mice; two-tailed, paired t-test; t(3) = 4.65, *P = 0.02). Data are shown as means (c, d, f, g, h); error bars (c, d, f, g) and shading (h) denote s.e.m.
Extended Data Fig. 4: Cross-hemispheric synchrony between prefrontal PV interneurons for various frequency bands and types of trials.
a, PV-Cre, Ai14 mice had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. Experimental design: Day 1: Initial association (IA) followed by rule shift (RS) or rule reversal (RR); Day 2: IA followed by the rule change (RS or RR) that was not performed on Day 1. b, During learning of the IA that preceded the RS, synchrony was not different after correct decisions vs. during the baseline period (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.51, P = 0.48; frequency X condition interaction: F2,18 = 0.20, P = 0.82). c, During learning of this IA, synchrony was not different after incorrect decisions vs. during the baseline period (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.39, P = 0.54; frequency X condition interaction: F2,18 = 0.07, P = 0.94). d, IA performance was not different across days (n = 7 mice; two-tailed, paired t-test; t(6) = 1.29, P = 0.25). e, There was no difference in synchrony after correct trials during learning of the IA on Day 1 vs. 2 (n = 7 mice; two-way ANOVA; main effect of day: F1,18 = 0.02, P = 0.89; frequency X condition interaction: F2,18 = 1.48, P = 0.26). f, There was no difference in synchrony after incorrect trials during learning of the IA on Day 1 vs. 2 (n = 7 mice; two-way ANOVA; main effect of day: F1,18 = 3.05, P = 0.10; frequency X condition interaction: F2,18 = 0.03, P = 0.97). g, During the RR, synchrony was not different after correct decisions vs. during the baseline period (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.28, P = 0.60; frequency X condition interaction: F2,18 = 1.24, P = 0.31). h, During the RR, synchrony was not different after incorrect decisions vs. the baseline period (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.09, P = 0.77; frequency X condition interaction: F2,18 = 0.30, P = 0.74). i, Synchrony after correct decisions did not differ between the IA vs. RS (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.13, P = 0.73; frequency X condition interaction: F2,18 = 1, P = 0.39). j, Synchrony after correct decisions did not differ between the RR vs. RS (n = 7 mice; two-way ANOVA; main effect of condition: F1,18 = 0.16, P = 0.70; frequency X condition interaction: F2,18 = 2.55, P = 0.11). k, The plot shows the average gamma synchrony on correct vs. incorrect trials, during the first 2 (‘early’) or next 3 (‘late’) trials of a rule shift (RS) or rule reversal (RR). In order to average together values from different mice (n = 7), each synchrony value was computed relative to the average gamma synchrony measured during the first 5 RS and RR trials from the same mouse. We performed ANOVA on the gamma synchrony from each of the first 5 trials during a rule shift (RS) or rule reversal (RR), including the following factors and interaction terms: mouse (F6,59 = 1.72, P = 0.13), type of rule change (RS vs. RR) (F1,59 = 3.74, P = 0.06), correct vs. incorrect trial outcome (F1,59 = 2.64, P = 0.11), an interaction of correct-incorrect X RS-RR (F1,59 = 11.12, **P = 0.0015), and an interaction of correct-incorrect X RS-RR X early vs. late trials (i.e. first 2 vs. next 3 trials) (F1,59 = 4.28, *P = 0.043). l, Following errors, synchrony during the intertrial interval (ITI) was specifically higher in the 30–50 Hz band during the RS than during the IA (n = 7 mice; two-way ANOVA; condition X frequency interaction: F4,36 = 3.217, *P = 0.023; 30–50 Hz post hoc t(6) = 3.55, *P = 0.036) or RR (two-way ANOVA; 30–50 Hz post hoc t(6) = 3.97, *P = 0.022). m, Mouse behavior scored during the initial association error ITI for the first 5 IA dual-site TEMPO trials (n = 10 error ITIs of 8 mice). n, Mouse behavior scored during the rule shift error ITI for the first 5 RS dual-site TEMPO trials (n = 17 error ITIs of 8 mice). There is no difference in seconds of movement between IA and RS (two-tailed, paired t-test; t(6) = 0.52, P = 0.62) nor in seconds of not moving between IA and RS (two-tailed, paired t-test; t(6) = 0.56, P = 0.59). Two-way ANOVA followed by Bonferroni post hoc comparisons were used in panels b–c and e–j, l. Comparisons were not significant unless otherwise noted.
Extended Data Fig. 5: Types of errors during rule shifts (RS) in the presence of various forms of optogenetic stimulation in PV-Cre mice.
a, d, PV-Cre, Ai14 mice had bilateral AAV-DIO-eYFP or AAV-DIO-ChR2 injections and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 40 Hz stimulation during the rule shift (RS); Day 2: no stimulation. b, e, Representative images showing mPFC expression of eYFP (b) or ChR2-eYFP (e) (scale bar, 100 μm). c, f, Optogenetic stimulation increases perseverative errors in ChR2-expressing mice (n = 5) compared to eYFP-expressing (n = 5 mice) controls (two-way ANOVA; main effect of day: F1,8 = 20.8, **P = 0.0018; main effect of virus: F1,8 = 8.96, *P = 0.017; day X virus interaction: F1,8 = 14.89, **P = 0.0048). There is no change in random errors (two-way ANOVA; main effect of day: F1,8 = 0, P > 0.99; main effect of virus: F1,8 = 0, P > 0.99; day X virus interaction: F1,8 = 0.89, P = 0.37). c, Light delivery does not affect the number of perseverative or random errors in eYFP-expressing controls (perseverative: post hoc t(8) = 0.50, P > 0.99; random: post hoc t(8) = 0.67, P > 0.99). f, Optogenetic stimulation of PV interneurons on Day 1 increased the number of perseverative errors compared to no stimulation on Day 2 (post hoc t(8) = 5.95, ***P = 0.0007), but does not affect random errors (post hoc t(8) = 0.67, P > 0.99). g, PV-Cre mice had bilateral AAV-DIO-ChR2-eYFP injections and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 20 Hz stimulation; Day 2: out-of-phase 40 Hz stimulation; Day 3: in-phase 40 Hz stimulation. h, Representative image showing mPFC ChR2 expression (scale bar, 100 μm). i, Out-of-phase 40 Hz stimulation (Day 2) increases perseverative errors but does not affect random errors, relative to out-of-phase 20 Hz stimulation (Day 1) or in-phase 40 Hz stimulation (Day 3) (n = 5 mice; two-way ANOVA; main effect of day: F2,16 = 13.5, ***P = 0.0004; Day 1 vs. Day 2 perseverative: ***P = 0.0002, Day 2 vs. Day 3 perseverative: ****P = 0.00003, Day 1 vs. Day 3 perseverative: P > 0.99; Day 1 vs. Day 2 random: P > 0.99, Day 2 vs. Day 3 random: P > 0.99, Day 1 vs. Day 3 random: P > 0.99). Two-way ANOVA followed by Bonferroni post hoc comparisons were used.
Extended Data Fig. 6: Behavior and types of errors during rule shifts (RS) in the presence of various forms of optogenetic stimulation in Dlx5/6+/−, Sst-Cre and Sst-Cre mice.
a, e, Sst-Cre mice had bilateral injections of AAV-DIO-eYFP (a, ‘Sst-eYFP’) or AAV-DIO-ChR2-eYFP (e, ‘Sst-ChR2’) along with fiber-optic implants in mPFC. Experimental design: Day 1: no light stimulation; Day 2: out-of-phase 20 Hz stimulation; Day 3: out-of-phase 40 Hz stimulation. b, Representative image showing mPFC ChR2 expression (scale bar, 100 μm). c, g, Light delivery did not affect performance in either Sst-eYFP (c; n = 4 mice) or Sst-ChR2 (g; n = 5) mice (two-way ANOVA; main effect of day: F2,14 = 2.53, P = 0.12; main effect of virus: F1,7 = 0.01, P = 0.92; day X virus interaction: F2,14 = 0.59, P = 0.57). d, Sst-eYFP mice showed no change in perseverative or random errors from Day 1 to Day 2 to Day 3 (n = 4 mice; two-way ANOVA; day X type of error interaction: F2,9 = 1.18, P = 0.35). h, Sst-ChR2 mice showed no change in perseverative or random errors from Day 1 to Day 2 to Day 3 (n = 5 mice; two-way ANOVA; day X type of error interaction: F2,16 = 0.81, P = 0.46). i,m, Dlx5/6+/−, Sst-Cre mice had bilateral control virus (AAV-DIO-eYFP or AAV-i12b-DIO-eYFP) or AAV-DIO-ChR2-eYFP (m) injections and fiber-optic implants in mPFC. Experimental design: Day 1: no stimulation; Day 2: 40 Hz stimulation. j,n, Representative eYFP (j) and ChR2-eYFP (n) expression in the mPFC of Dlx5/6+/−, Sst-Cre mice (scale bar, 100 μm). k,o, Light delivery did not affect performance in Sst-eYFP-expressing (k; n = 4 mice) or Sst-ChR2-expressing (o; n = 8) mutant mice (two-way ANOVA; main effect of day: F1,10 = 0.08, P = 0.79; main effect of virus: F1,10 = 0.002, P = 0.96; day X virus interaction: F1,10 = 2.69, P = 0.13). l, Sst-eYFP expressing mutants showed no change in perseverative or random errors from Day 1 to Day 2 (n = 4 mice; two-way ANOVA; day X type of error interaction: F1,6 = 0.16, P = 0.70). p, Sst-ChR2 expressing mutants showed no change in perseverative or random errors from Day 1 to Day 2 (n = 8 mice; two-way ANOVA; day X type of error interaction: F1,14 = 0.64, P = 0.44). Two-way ANOVA followed by Bonferroni post hoc comparisons were used. Comparisons were not significant unless otherwise noted.
Extended Data Fig. 7: Single unit recordings from PV interneurons and regular spiking neurons in the mPFC of awake, head-fixed mice.
a, Schematic of opto-silicon probe recording PFC in awake head-fixed mice (top panel). Histology of the recording electrode (bottom panel, scale = 1mm). b, Example raster plot of a putative PV cell responding to ChR2 activation. Stimulation at 1 Hz (5 ms illumination, 0.25 mW). The blue line represents the duration of ChR2 stimulation (5 ms). c, Average peristimulus time histogram (PSTH) of PV cell responses during 40 Hz ChR2 stimulation (n = 8 PV cells, time bin = 1 ms). The blue line indicates the period of ChR2 stimulation (5 ms flash) at 40 Hz. PV cells fired 0.49 ± 10 spikes per 40 Hz cycle at a latency of 1.82 ± 0.34 ms following the onset of each light flash. d, Baseline subtracted and peak normalized activity of all putative PV cells (green line; n = 8 cells, 3 mice) and all the regular-spiking (RS) cells (black line; n = 237 cells, 4 mice). Mean firing rate of RS cells was 4.9 vs. 2.1 spikes/sec at the peak vs. trough of each cycle, respectively. The blue line indicates the period of ChR2 stimulation (5 ms flash) at 40 Hz. Data are shown as means (c, d) and shading (c, d) denote s.e.m.
Extended Data Fig. 8: Changes in the power spectra of prefrontal LFPs elicited by in- vs. out-of-phase stimulation of PV interneurons.
a–d, Difference between the power spectra for LFPs recorded during light stimulation vs. at baseline for control mice (PV-Cre mice injected with AAV-DIO-eYFP, n = 6 recordings from 3 mice) receiving (a) in- or (c) out-of-phase stimulation, or for PV-Cre mice (n = 12 recordings from 6 mice) injected with AAV-DIO-ChR2-eYFP receiving (b) in- or (d) out-of-phase stimulation. Positive (negative) values correspond to higher power during periods of stimulation (at baseline). e, Quantification of average change in 40 Hz power in various conditions (relative to baseline). The change was significantly different from 0 for PV-ChR2 in-phase condition (n = 12 recordings from 6 mice; two-tailed, paired t-test; t(11) = 2.24, *P = 0.047) and for PV-ChR2 out-of-phase condition (n = 12 recordings from 6 mice; two-tailed, paired t-test; t(11) = 2.34, *P = 0.039). f, Quantification of average change in 150–200 Hz power in various conditions (relative to baseline). The change was significantly different from 0 for PV-ChR2 in-phase condition (n = 12 recordings from 6 mice; two-tailed, paired t-test; t(11) = 2.40, *P = 0.036) and for PV-ChR2 out-of-phase condition (n = 12 recordings from 6 mice; two-tailed, paired t-test; t(11) = 2.65, *P = 0.023). Two-tailed, paired t-tests were used. Data are shown as means; shading (a–d) and error bars (e,f) denote s.e.m.
Extended Data Fig. 9: Out-of-phase 40 Hz stimulation does not disrupt the ability of PV-Cre mice to revert to an initial association.
a, Schematic for task: The mouse learns an initial association (IA), then a rule shift (RS). After the mouse learns the RS, the task reverts to the original rule, i.e., the rule learned during the IA with out-of-phase 40 Hz stimulation. b, PV-Cre mice had bilateral AAV-DIO-eYFP (‘PV-eYFP’) or AAV-DIO-ChR2-eYFP (‘PV-ChR2’) injections and fiber-optic implants in mPFC. Experimental design: no stimulation during learning of the IA or RS. Then, when the rule reverts to the IA, out-of-phase 40 Hz stimulation is delivered. c, In PV-eYFP mice (n = 5), there was no difference in the number of trials needed to reach the criterion during initial learning of the IA (when no light was delivered) vs. when reverting to the IA after learning the RS (when light stimulation was delivered; two-tailed, paired t-test; t(4) = 1.31, P = 0.261). d, In PV-ChR2 mice (n = 5), there was no difference in the number of trials needed to reach the criterion during initial learning of the IA (when no light was delivered) vs. when reverting to the IA after learning the RS (when light stimulation was delivered; two-tailed, paired t-test; t(4) = 0.466, P = 0.666). Two-tailed, paired t-tests were used. Comparisons were not significant unless otherwise noted.
Extended Data Fig. 10: Behavior and cross-hemispheric synchrony between prefrontal PV or Sst interneurons during rule shifts, baseline periods, or learning of initial associations in Dlx5/6+/− mice and wild-types.
a, Rule-shift (RS) performance for photometry experiments in Dlx5/6+/−, PV-Cre and Dlx5/6+/+, PV-Cre mice. Compared to wild-type littermates (n = 8), mutant mice (n = 7) make more perseverative errors (two-way ANOVA; main effect of genotype: F1,13 = 43.6, ****P = 0.00002; main effect of error type: F1,13 = 24.7, ***P = 0.0003; error type X genotype interaction: F1,13 = 10.5, **P = 0.006; post hoc t(26) = 6.55, ****P = 0.000001), but similar numbers of random errors (post hoc t(26) = 1.36, P = 0.37). b, Rule-shift performance for mice used in dual-site TEMPO experiments. Compared to wild-type mice (n = 12), mutant mice (n = 8) make more perseverative errors (two-way ANOVA; main effect of genotype: F1,18 = 89.4, ****P =0.00000002; main effect of error type: F1,13 = 137.3, ****P = 0.0000000007; type of error X genotype interaction: F1,18 = 46.5, ****P = 0.000002; post hoc t(36) = 11.6, ****P = 0.0000000000002), and random errors (post hoc t(36) = 2.43, *P = 0.04). c, Compared to wild-type mice, Dlx5/6+/−, Sst-Cre, Ai14 mice make more perseverative errors (n = 5 mice in each cohort; two-way ANOVA; main effect of genotype: F1,8 = 42.3, ***P = 0.0002; main effect of error type: F1,8 = 30.7, ***P = 0.0005; error type X genotype interaction: F1,8 = 17.0, **P = 0.003; post hoc t(16) = 7.12, ****P = 0.000005), but numbers of random errors are comparable (post hoc t(16) = 0.38, P > 0.99). d, Synchrony was not different between Dlx5/6++-, Sst-Cre, Ai14 and Dlx5/6+/−, Sst-Cre, Ai14 mice during the baseline period (n = 5 mice in each cohort; two-way ANOVA; main effect of genotype: F1,8 = 0.77, P = 0.41; frequency X genotype interaction: F2,16 = 0.05, P = 0.95). e, Dlx5/6+/−, PV-Cre mice had bilateral AAV-DIO-ChR2-eYFP injections and fiber-optic implants in mPFC. Experimental design: Day 1: no stimulation; Day 2: in-phase 40 Hz stimulation during the first 5 RS trials. f, In-phase 40 Hz stimulation on Day 2 reduces perseverative errors relative to no stimulation on Day 1 (n = 6 mice; two-way ANOVA; main effect of day: F1,10 = 18.32, **P = 0.0016; main effect of error type: F1,10 = 49.9, ****P =0.000034; post hoc t(10) = 3.98, **P = 0.005); there was no change in random errors (post hoc t(10) = 2.07, P = 0.13). g, Dlx5/6+/−, PV-Cre mice had bilateral AAV-DIO-ChR2-eYFP injections and fiber-optic implants in mPFC. Experimental design: Day 1: out-of-phase 20 Hz stimulation; Day 2: out-of-phase 40 Hz stimulation; Day 3: in-phase 40 Hz stimulation. h, Perseverative errors are reduced by in phase 40 Hz stimulation on Day 3, compared to either out-of-phase 20 Hz stimulation on Day 1 or out-of-phase 40 Hz stimulation on Day 2 (n = 5 mice; two-way ANOVA; main effect of day: F2,16 = 11.6, ***P = 0.0008; main effect of error type: F1,8 = 31.5, ***P = 0.0005; Day 1 vs. Day 2 perseverative: P > 0.99, Day 2 vs. Day 3 perseverative: ***P = 0.0005, Day 1 vs. Day 3 perseverative: **P = 0.0016). There are no changes in random errors across days (post hoc Day 1 vs. Day 2: P > 0.99, Day 2 vs. Day 3: P > 0.99, Day 1 vs. Day 3: P = 0.33). i, Dlx5/6+/−, PV-Cre, Ai14 mice had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. j, Representative images of tdTomato (red) and Ace-mNeon (green) fluorescence within PV interneurons in a coronal section of mPFC (left), alongside a high power image (right). Scale bars: 100 μm and 25 μm, respectively. k, In mutants, PV interneuron synchrony was not different after correct decisions vs. during the baseline period (n = 8 mice; two-way ANOVA; main effect of condition: F1,21 = 1.89, P = 0.18; frequency X condition interaction: F2,21 = 0.35, P = 0.71). l, PV interneuron synchrony was not different after incorrect decisions vs. during the baseline period (n = 8 mice; two-way ANOVA; main effect of condition: F1,21 = 3.31, P = 0.083; frequency X condition interaction: F2,21 = 0.04, P = 0.96). m, Dlx5/6+/−, Sst-Cre, Ai14 mice had bilateral AAV-DIO-Ace2N-4AA-mNeon ± AAV-Syn-tdTomato injections and fiber-optic implants in mPFC. n, Representative images of tdTomato (red) and Ace-mNeon (green) fluorescence in Sst interneurons within a coronal section of mPFC (left) from a Dlx5/6+/−, Sst-Cre, Ai14 mouse, alongside a high power image (right). Scale bars: 100 μm and 25 μm, respectively. o, In mutants, Sst interneuron synchrony was not different after correct decisions vs. during the baseline period (n = 5 mice; two-way ANOVA; main effect of frequency: F2,12 = 9.88, **P = 0.003; frequency X condition interaction: F2,12 = 0.58, P = 0.58). p, Sst interneuron synchrony was not different after incorrect decisions vs. during the baseline period (n = 5 mice; two-way ANOVA; main effect of frequency: F2,12 = 4.95, *P = 0.027; frequency X condition interaction: F2,12 = 0.44, P = 0.66). q, Learning of an initial association (IA) was similar in mutants (n = 6) and their wild-type (n = 11) littermates (two-tailed, unpaired t-test; n = 11; t(15) = 0.202, P = 0.842). r, There was no difference in cross-hemispheric PV interneuron synchronization between mutant (n = 6 mice) and wild-type (n = 11 mice) littermates at baseline (two-way ANOVA; main effect of genotype: F1,15 = 0.45, P = 0.51; genotype X frequency interaction F2,30 = 1.11, P = 0.34). s, During learning of an initial association, changes in PV interneuron synchrony following errors (relative to synchrony after correct decisions) is similar in mutants (n = 6 mice) and their wild-type (n = 11 mice) littermates (two-way ANOVA; main effect of genotype: F1,15 = 0.07, P = 0.80; genotype X frequency interaction: F2,30 = 0.16, P = 0.86). Data are shown as means (a–c); error bars (a–c) denote s.e.m. Two-way ANOVA followed by Bonferroni post hoc comparisons were used. Comparisons were not significant unless otherwise noted.
Supplementary Material
Acknowledgements
This work was supported by NIMH (R01MH106507 to V.S.S. and K99MH108720 to K.K.A.C.), the Brain and Behavior Foundation (NARSAD Young Investigator Award to K.K.A.C.), the McKnight Foundation (Memory and Cognitive Disorders award to V.S.S.), and the Brain Research Foundation (Scientific Innovations Award to V.S.S.). We appreciate helpful feedback on earlier versions of this manuscript from Loren Frank and Massimo Scanziani.
Footnotes
Competing interests Statement
The authors declare no competing interests.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Code availability
Scripts used to analyze dual-site TEMPO data are deposited at https://github.com/sohallab/dual-site-TEMPO
References
- 1.Young DA, Davila R, & Scher H. Unawareness of illness and neuropsychological performance in chronic schizophrenia. Schizophr. Res. 10, 117–124 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Shad MU, Tamminga CA, Cullum M, Haas GL, & Keshavan MS Insight and frontal cortical function in schizophrenia: a review. Schizophr. Res. 86, 54–70 (2006). [DOI] [PubMed] [Google Scholar]
- 3.von der Malsburg C. The correlation theory of brain function. Internal Report 81–82, Dept. Neurobiology, Max-Planck-Institute for Biophysical Chemistry (1981). [Google Scholar]
- 4.Abeles M, Prut Y, Bergman H, & Vaadia E. Synchronization in neuronal transmission and its importance for information processing. Prog. Brain Res. 102, 395–404 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 49, 111–125 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Salinas E & Sejnowski TJ Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 8, 539–550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries P. A mechanism for cognitive dynamics: neuronal communication through coherence. Trends Cogn. Sci. 9, 474–480 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Ann. Rev. of Neurosci. 32, 209–224 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Sohal VS, Zhang F, Yizhar O, & Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohal VS How close are we to understanding what (if anything) gamma oscillations do in cortical circuits? J. Neurosci. 36, 10489–10495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastos AM, Loonis R, Kornblith S, Lundqvist M, & Miller EK Laminar recordings in frontal cortex suggest distinct layers for maintenance and control of working memory. Proc. Natl. Acad. Sci. 115, 1117–1122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller EK, Lundqvist M, & Bastos AM Working memory 2.0. Neuron 100, 463–475 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho KKA et al. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6+/− mice. Neuron 85, 1332–1343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marton TF, Seifikar H, Luongo FJ, Lee AT, & Sohal VS Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J. Neurosci. 38, 2569–2578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson MP, Tervo DG, & Karpova AY Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science 338, 135–139 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Cardin JA Snapshots of the brain in action: Local circuit interactions through the lens of gamma oscillations. J. Neurosci. 36, 10496–10504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray S & Maunsell JH Do gamma oscillations play a role in cerebral cortex? Trends Cogn Sci. 19, 78–85 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissonette GB et al. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 28, 11124–11130 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellwood IT et al. Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. J. Neurosci. 37, 8315–8329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canetta S et al. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatry 21, 956–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao W et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron 97, 1253–1260 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Siegle JH, Pritchett DL, & Moore CI Gamma-range synchronization of fast-spiking interneurons can enhance detection of tactile stimuli. Nat. Neurosci. 17, 1371–1379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, & Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunaydin LA et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner TN et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TW et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall JD et al. Cell-type-specific optical recording of membrane voltage dynamics in freely moving mice. Cell 167, 1650–1662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carandini M et al. Imaging the awake visual cortex with a genetically encoded voltage indicator. J. Neurosci. 35, 53–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pi HJ et al. Cortical interneurons that specialize in disinhibitory control. Nature 7477, 521–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray S, Ni AM, & Maunsell JH Strength of gamma rhythm depends on normalization. PLoS. Biol. 11, e1001477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shadlen MN & Movshon JA Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24, 67–77 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Palmigiano A, Geisel T, Wolf F, & Battaglia D. Flexible information routing by transient synchrony. Nat. Neurosci. 7, 1014–1022 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Tukker JJ et al. Distinct dendritic arborization and in vivo firing patterns of parvalbumin-expressing basket cells in the hippocampal area CA3. J Neurosci, 33, 6809–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, Gan J, & Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 345, 1255263 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Otis JM et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature, 543, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bissonette GB & Roesch MR Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience, 345 64–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips EAK & Hasenstaub AR Asymmetric effects of activating and inactivating cortical interneurons. eLife 5, pii: e18383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Burgos G, Cho RY, & Lewis DA Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psy. 77, 1031–1040 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlhaas PJ & Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol. Psy. 77, 1001–1009 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Senkowski D & Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol. Psy. 77, 1010–1019 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Green MJ Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. Psych. 67, 36–42 (2006). [PubMed] [Google Scholar]
- 43.Minzenberg MJ & Carter CS Developing treatments for impaired cognition in schizophrenia. Trends Cogn. Sci. 16, 35–42 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Verret L et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iaccarino HF et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martorell AJ et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohmuro N et al. The relationship between cognitive insight and cognitive performance among individuals with at-risk mental state for developing psychosis. Schizophr. Res. 192, 281–286 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Lappin JM et al. Insight in individuals with an at risk mental state. Schizophr. Res. 90, 238–244 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.