Dear Editor,
Coronavirus disease 2019 (COVID-19) is associated with a high fatality rate in patients requiring invasive mechanical ventilation (IMV) [1]. COVID-19-related acute respiratory distress syndrome (COVID-ARDS) might exhibit vascular insults, resulting in loss of hypoxic pulmonary vasoconstriction, and subsequent dissociation between profound hypoxemia and preserved static compliance of the respiratory system (Cst-rs) [2]. Experts recently distinguished two phenotypes of COVID-ARDS according to their Cst-rs [2]: patients were classified as groups L (low elastance (or high Cst-rs) and low recruitability) and H (high elastance and high recruitability). They recommended different ventilatory approaches [3], contrary to Sepsis Surviving Campaign guidelines [4]. We describe characteristics and outcomes in patients with different initial Cst-rs, but all receiving IMV following ARDS guidelines.
We report the courses of respiratory parameters and outcomes in an observational cohort of 36 patients who developed COVID-ARDS requiring IMV from March 17 to April 18, 2020. Patients were divided into two groups (low and high Cst-rs) according to their initial Cst-rs was above or below the median value. We applied institutional ARDS procedures to all patients. Our management was based on the systematic use of neuromuscular blockers for at least 48 h, positive end-expiratory pressures (PEEP) titrated on oxygenation, and prone positioning sessions if PaO2/FiO2 ratio dropped below 150. Patients’ data were analyzed until patients were discharged from the intensive care unit or died. Courses of Cst-rs, PEEP, and tidal volumes were analyzed using a linear mixed model.
The median baseline Cst-rs was 36 mL/cmH2O [interquartile range (IQR) 29–44]. Characteristics of the patients at baseline, therapeutic interventions, and outcome measures are provided in Table 1. Twenty-nine patients (80.6%) in whom PaO2/FiO2 ratio dropped below 150 were placed in prone position. Courses of Cst-rs, PEEP levels, and tidal volumes are provided in Fig. 1. Cst-rs did not vary over time in both groups and remained higher in the high Cst-rs group (mean difference 17.7 mL/cmH2O [95% CI 11.3–24.0] compared to the low Cst-rs group, P < 0.001). Tidal volumes were higher in the high Cst-rs group (mean difference 0.90 mL/kg [95% CI 0.31–1.50] compared to the low Cst-rs group, P = 0.005). PEEP levels were not different between groups and decreased over time.
Table 1.
Baseline characteristics, therapeutic interventions, and outcomes of patients, according to respiratory compliance
| Overall (N = 36), no. (%) of patientsa | High respiratory compliance (N = 17), no. (%) of patientsa | Low respiratory compliance (N = 19), no. (%) of patientsa | P value between groups | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, mean ± SD, years | 53.4 ± 10.2 | 56.1 ± 7.1 | 50.9 ± 12.1 | 0.12 |
| Male sex | 30 (83.3) | 16 (94.1) | 14 (73.7) | 0.18 |
| Obesityb | 14 (38.9) | 3 (17.7) | 11 (57.9) | 0.02 |
| Diabetes mellitus | 11 (30.6) | 5 (29.4) | 6 (31.6) | 1.0 |
| Arterial hypertension | 16 (44.4) | 11 (64.7) | 5 (26.3) | 0.19 |
| SAPS 2 score, median [IQR] | 31 [27–36] | 31 [29–36] | 29 [22–39] | 0.51 |
| SOFA score, median [IQR] | 5 [4–7] | 6 [4–7] | 4 [3–6] | 0.04 |
| Tidal volume, mean ± SD, mL/kg | 6.1 ± 0.6 | 6.2 ± 0.3 | 6.0 ± 0.7 | 0.02 |
| Respiratory frequency, median [IQR], breaths/min | 25 [24–27] | 25 [24–26] | 26 [24–28] | 0.64 |
| FiO2, median [IQR], % | 65 [50–100] | 60 [40–80] | 70 [60–100] | 0.07 |
| PaO2/FiO2 ratio, median [IQR] | 152 [112–240] | 209 [150–256] | 117 [83–201] | 0.02 |
| PEEP, mean ± SD, cmH2O | 13.4 ± 3.2 | 13.4 ± 3.6 | 13.4 ± 3.1 | 0.92 |
| Respiratory compliance, mean ± SD, mL/cmH2Oc | 39.4 ± 16.9 | 51.8 ± 16.4 | 28.3 ± 6.1 | |
| Therapeutic interventions | ||||
| Prone positioning | 29 (80.6) | 12 (70.6) | 17 (89.5) | 0.22 |
| Number of sessions, median [IQR] | 4.0 [2.0–6.0] | 4.0 [2.5–5.0] | 5.0 [1.7–6.0] | 0.91 |
| Inhaled nitric oxide | 9 (25.0) | 2 (11.8) | 7 (36.8) | 0.13 |
| Venovenous ECMO | 7 (19.4) | 0 (0.0) | 7 (36.8) | 0.008 |
| Vasopressors | 31 (86.1) | 17 (100.0) | 14 (73.7) | 0.048 |
| Renal replacement therapy | 7 (19.4) | 3 (17.7) | 4 (21.1) | 1.0 |
| Hydroxychloroquine | 32 (88.9) | 16 (94.1) | 16 (84.2) | 0.6 |
| Steroïds | 11 (30.6) | 5 (29.4) | 6 (31.6) | 1.0 |
| Outcomes | ||||
| Ventilator-free days, median [IQR] | 3.0 [0.0–14.5] | 10.0 [0.0–17.2] | 0.0 [0.0–8.0] | 0.04 |
| Mortality at day 28 | 4 (11.1) | 1 (5.9) | 3 (15.8) | 0.61 |
Abbreviations: ECMO extracorporeal membrane oxygenation, FiO2 fraction of inspired oxygen, PEEP positive end-expiratory pressure
aUnless otherwise indicated
bObesity is defined by a body mass index above 30 kg/m2. The formula for body mass index is weight in kilograms divided by height in meters squared
cNo statistical comparison performed
Fig. 1.
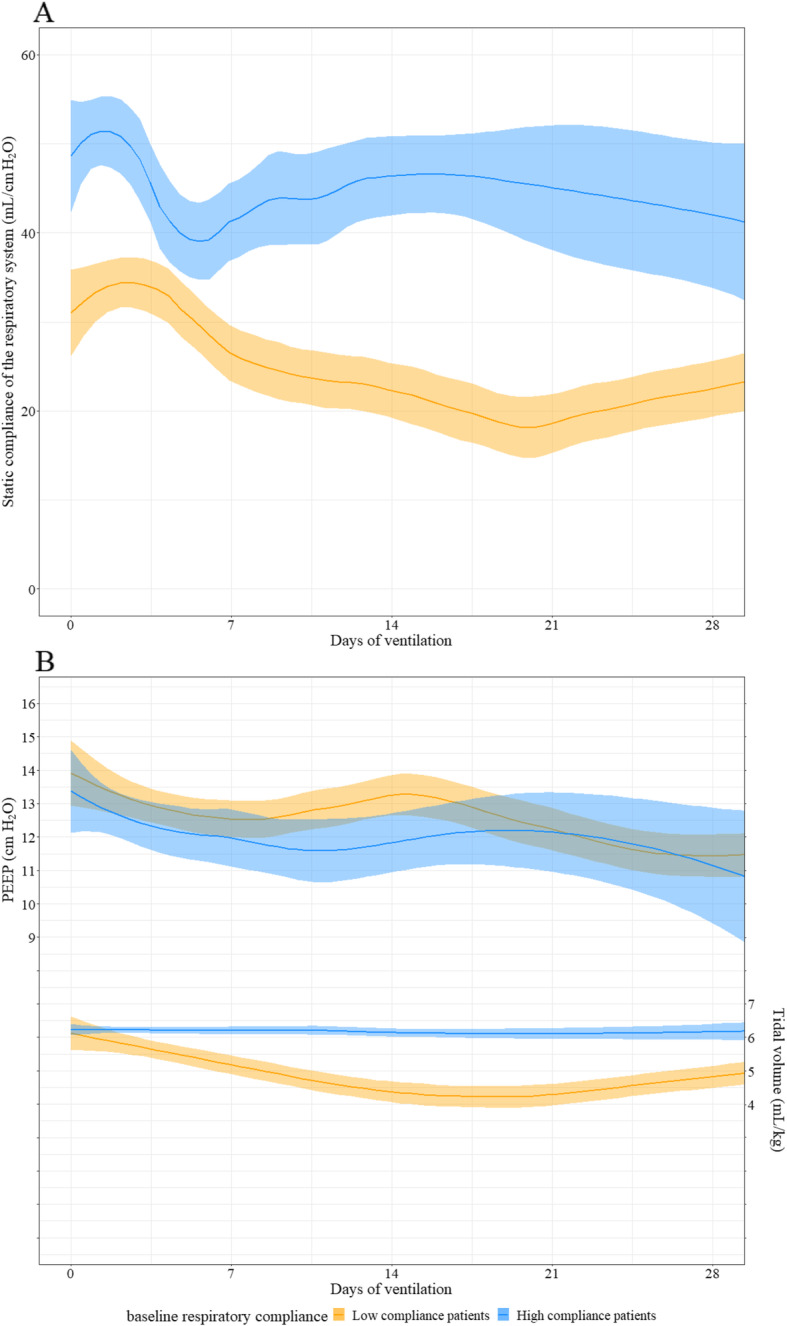
Course of the respiratory system static compliances (Cst-rs), positive end-expiratory pressures (PEEP), and tidal volumes (Vt). The means and 95% confidence intervals are represented respectively by solid lines and colored areas. Results are expressed in mean differences [95% CI]. a Cst-rs remained higher in the high initial Cst-rs group. There was no significant effect of time on Cst-rs (slope = − 0.03 mL/cmH2O/day of ventilation [95% CI − 0.17 to 0.12], P = 0.70). b PEEP levels did not differ between groups (high vs. low Cst-rs group − 0.69 cmH2O [95% CI − 2.05 to 0.66], P = 0.33). There was a statistically significant effect of time on PEEP (slope = − 0.10 cmH2O/day of ventilation [95% CI − 0.13 to − 0.06], P < 0.001). Vt were higher in the high Cst-rs group. There was no significant effect of time on Vt (slope = − 0.006 [− 0.02 to 0.007], P = 0.375)
On day 28, 32 patients (88.9%) survived and 25 (69.4%) were discharged from the intensive care unit. As of May 30, 2020, weaning from mechanical ventilation was effective in 16 high Cst-rs patients (94.1%) and 13 low Cst-rs patients (68.4%) (P = 0.09).
As previously suggested [3], some COVID-ARDS patients exhibit high initial Cst-rs. However, the median baseline Cst-rs was not different from Cst-rs observed in “typical” non-COVID-ARDS, as demonstrated in another study [5]. The high Cst-rs did not drop and remained different from the initial low Cst-rs during the first 28 days, suggesting a lack of transition from a high to a low Cst-rs phenotype in patients receiving neuromuscular blockers. We therefore hypothesize that if this transition exists, self-inflicted lung injury during spontaneous ventilation or asynchronies is one of its main determinants.
Although therapeutic management of low Cst-rs patients is not disputed [2, 6], a low-PEEP, high-FiO2, liberal tidal volume approach has been suggested for high Cst-rs patients. Using established ARDS therapies [3] with either low or high Cst-rs, the survival rate is better than initially reported [1], following a recent publication using the same strategy [5]. A low initial Cst-rs could be a marker of severity, as suggested by more extracorporeal membrane oxygenation requirement and less ventilator-free days at day 28.
Limitations include the small number of patients and the retrospective design. While further study is needed, our findings provide arguments to treat all COVID-ARDS with established ARDS therapies, whatever the initial value of Cst-rs.
Acknowledgements
We thank all clinical and nursing staff of Marie Lannelongue Hospital for their flawless commitment during this outbreak. We also thank Sebastien Morisset, MSc, for performing the statistical analysis, and Vincent Roth, MD (Easy CRF SAS®), for providing us the database.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- Cst-rs
Static compliance of the respiratory system
- IMV
Invasive mechanical ventilation
- PEEP
Positive end-expiratory pressure
Authors’ contributions
FL and FS have designed the work. All authors have drafted the work. All authors have made the acquisition, analysis, and interpretation of the data. All contributors read and approved the manuscript.
Funding
The present study was supported solely by institutional sources.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The entire project was approved by the Groupe Hospitalier Paris Saint Joseph Ethics Board (IRB 00012157. Project number: 20-37816004), which waived informed consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:20. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020; 10.1001/jama.2020.6825. [DOI] [PubMed]
- 4.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020. 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed]
- 5.Haudebourg A-F, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, et al. Respiratory mechanics of COVID-19 vs. non-COVID-19 associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed]
- 6.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020; 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


