Abstract
Emerging evidence suggests that gut microbiota-derived short-chain fatty acids (SCFAs; acetate, propionate, and butyrate) are important modulators of the inflammatory state in diseases such as asthma. However, the functional expression of the Gi protein-coupled free fatty acid receptors (FFAR2/GPR43 and FFAR3/GPR41) has not been identified on airway smooth muscle (ASM). Classically, acute activation of Gi-coupled receptors inhibits cyclic AMP (cAMP) synthesis, which impairs ASM relaxation and can also induce crosstalk between Gi- and Gq-signaling pathways, potentiating increases in intracellular Ca2+ concentration ([Ca2+]i), favoring ASM contraction. In contrast, chronic activation of Gi-coupled receptors can sensitize adenylyl cyclase resulting in increased cAMP synthesis favoring relaxation. We questioned whether the Gi-coupled FFAR2 or FFAR3 is expressed in human ASM, whether they modulate cAMP and [Ca2+]i, and whether SCFAs modulate human ASM tone. We detected the protein expression of FFAR3 but not FFAR2 in native human ASM and primary cultured human airway smooth muscle (HASM) cells. In HASM cells, acute activation of FFAR3 with SCFAs inhibited forskolin-stimulated cAMP accumulation, but chronic activation did not sensitize cAMP synthesis. SCFAs induced [Ca2+]i increases that were attenuated by pertussis toxin, gallein, U73122, or xestospongin C. Acute treatment with SCFAs potentiated acetylcholine-stimulated [Ca2+]i increases and stress fiber formation in cells and contraction of ex vivo human airway tissues. In contrast, chronic pretreatment of human ASM with propionate did not potentiate airway relaxation. Together, these findings demonstrate that FFAR3 is expressed in human ASM and contributes to ASM contraction via reduced cAMP and increased [Ca2+]i.
Keywords: actin, cyclic AMP, immunohistochemistry, intracellular calcium, siRNA
INTRODUCTION
Asthma affects more than 330 million people worldwide and an estimated 25 million people in the United States (47). Asthma susceptibility and severity are increased by a lack of early childhood exposure to symbiotic microorganisms (e.g., gut microbiota) or the overuse of antibiotics (34, 36). Epidemiological studies have suggested that the microbial diversity of the gut flora is important for preventing asthma (the so-called “hygiene hypothesis”) (8, 11). The gut microbiota of mammals and their hosts have evolved a symbiotic relationship, and a growing body of evidence indicates that gut microbiota are an important environmental factor that regulates several organ systems by signaling through metabolic byproducts including short-chain fatty acids (SCFAs; acetate, propionate, and butyrate), which are distributed systemically after colonic absorption (10). SCFAs are endogenous ligands for the G protein-coupled free fatty acid receptor 2 (FFAR2/GPR43) and the free fatty acid receptor 3 (FFAR3/GPR41), which are expressed on many peripheral cell types (3). These receptors differ in their intracellular signaling pathways, with FFAR2 coupling to either Gq or Gi proteins and FFAR3 exclusively activating the Gi pathway (27). In the airways, both FFAR2 protein and FFAR3 protein have been identified on human nasal and bronchial epithelial cell lines (21, 28) and human pulmonary fibroblasts (37), and mRNAs encoding both FFAR2 and FFAR3 were also identified in human airway smooth muscle (HASM) cells (2, 12). However, the functional expression of FFAR2 and FFAR3 has never been described on native airway smooth muscle itself. Classically, acute activation of Gi-coupled receptors inhibits the activity of adenylyl cyclase, thereby attenuating cyclic AMP (cAMP) production, resulting in impaired airway smooth muscle relaxation. In contrast, chronic activation of Gi-coupled receptors induces a paradoxical enhancement of adenylyl cyclase activity (heterologous sensitization), which potentiates airway smooth muscle relaxation (6, 30, 49). Moreover, it was well established that not only Gq-coupled receptors but also Gi-coupled receptors can contract airway smooth muscle through the release of Ca2+ from the sarcoplasmic reticulum (SR) (1, 14) and that the activation of Gi-coupled receptor potentiates Gq-coupled receptor-mediated airway smooth muscle contraction (Gi-Gq crosstalk) (29). These findings led us to hypothesize that SCFAs could modulate airway smooth muscle tone through FFAR2 and FFAR3 expressed on airway smooth muscle, which would have implications for obstructive lung diseases [e.g., asthma, chronic obstructive pulmonary disease (COPD)]. In the present study, we questioned whether SCFA receptors are expressed on human airway smooth muscle and whether they modulate airway smooth muscle tone through the regulation of cAMP and intracellular Ca2+ concentration ([Ca2+]i) signaling.
METHODS
Materials.
Lysates of human kidney protein were obtained from BD Biosciences. Pertussis toxin was obtained from Calbiochem. Protease inhibitor cocktail III was purchased from EMD Millipore. Antibiotic-antimycotic mix, DMEM/F-12 medium, M199 medium, fetal bovine serum (FBS), Fluo-4 AM, Opti-MEM, Pluronic F-127, predesigned small interfering (si)RNA targeting human FFAR2 (Silencer Select predesigned siRNA no. s223804), FFAR3 (Silencer Select predesigned siRNA no. s6078), OR51E2 (Silencer Select predesigned siRNA no. s37522), and a nontargeting control siRNA (AM4611) were obtained from Thermo Fisher Scientific. All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Cell culture.
Primary cultured HASM cells from three different donors (a 60-yr-old white male, a 56-yr-old white male, and a 27-yr-old white male), which were deidentified cell lines, were purchased from Lonza (CC-2576 and 00194850). Cells were grown in DMEM/F12 culture medium, supplemented with 10% FBS and an antibiotic-antimycotic mix (100 U/mL penicillin G sodium, 100 µg/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B) at 37°C in an atmosphere of 5% CO2-95% air.
Preparation of human trachea.
Studies were reviewed by Columbia University’s Institutional Review Board (IRB) and deemed not human subjects research under 45 CFR 46. Human tracheas were obtained from discarded regions of healthy donor lungs harvested for lung transplantation at Columbia University. Human tracheal tissue was transported to the laboratory in cold (4°C) M199 medium and oxygenated (95% O2-5% CO2) overnight at 4°C. The exterior of the human trachea was carefully dissected free of adherent connective tissue under a microscope. Airway epithelium was removed for immunoblotting and organ bath experiments.
Immunoblot analysis.
Freshly dissected native human airway smooth muscle tissue was homogenized (Tekmar Ultra Turrax T25 high-speed homogenizer set at top speed for 30 s) in cold (4°C) buffer (50 mM Tris, 10 mM HEPES, and 1 mM EDTA with a 1:200 dilution of protease inhibitor cocktail III, pH 7.4). The homogenate was filtered through 125-µm Nitex mesh and centrifuged twice at 500 g for 15 min. The supernatant was transferred into new tubes and centrifuged at 50,000 g for 30 min at 4°C. The final membrane pellet was resuspended in the same buffer for protein concentration determinations using Pierce BCA reagents (Thermo Fisher Scientific) and stored at −80°C.
Primary cultured HASM cells were grown to confluency in T75 culture flasks. Cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and mechanically scraped in cold PBS supplemented with protease inhibitor cocktail III. Cells were pelleted (500 g, 10 min, 4°C) and lysed in ice-cold lysis buffer [20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin, and 1:200 dilution of protease inhibitor cocktail III]. Lysed cells were centrifuged (15,000 g, 15 min, 4°C), and an aliquot of the supernatant was subjected to protein concentration determination and storage at −80°C.
Each sample was solubilized by heating at 95°C for 10 min in sample buffer (final concentrations: 50 mM Tris·HCl pH 6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, and bromophenol blue) before use. Lysates were electrophoresed on a 10% Mini-Protean TGX precast gel (Bio-Rad) at 200 V for 30 min. Semidry transfer to PVDF membranes was performed using a Trans-Blot Turbo transfer system (Bio-Rad) with “mini TGX program” (3 min at 25 V). The PVDF membrane was blocked for 1 h at room temperature with 5% membrane blocking agent (RPN418; GE Healthcare) in Tris-buffered saline with 0.1% Tween 20 (TBST). Membranes were then probed with antibodies directed against the FFAR2 receptor protein (rabbit polyclonal, 1:1,000; Santa Cruz, sc-32906) or the FFAR3 receptor protein (rabbit polyclonal, 1:1,000; Santa Cruz, sc-98332) supplemented with 1% membrane blocking agent overnight at 4°C. After being washed three times with TBST, membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-labeled secondary anti-rabbit antibodies (1:5,000; GE Healthcare, NA934V). The signals from the immunoreactive bands were detected by ECL Prime (GE Healthcare), and the signal was captured using a chemiluminescent image analyzer (LAS 4000 Mini; GE Healthcare). The same PVDF membranes were stripped and reprobed with the antibody against the GAPDH protein (rabbit monoclonal, 1:1,000, CST no. 5174) to demonstrate the variation in protein loading on the gels.
Small interfering RNA transfection.
HASM cells cultured in DMEM/F12 growth medium (supplemented with 10% FBS) without antibiotics were grown in 6-well plates (seeded in 2.5 ml of the growth medium) or white 96-well plates (seeded in 100 µL of the growth medium) until they reached 50% confluence. Cells were then transfected with the predesigned siRNA targeting human FFAR2, FFAR3, OR51E2, or the nontargeting siRNA (as negative control) using Lipofectamine RNAiMAX (Thermo Fisher Scientific) in serum-free Opti-MEM according to the manufacturer’s instructions. Briefly, Lipofectamine RNAiMAX was diluted with Opti-MEM in a 1:50 ratio. Opti-MEM was used to dilute siRNAs at a ratio of 1:100. Diluted siRNA and reagent were mixed in one tube and incubated for 20 min at room temperature to allow the siRNA-Lipofectamine RNAiMAX complexes to form. The siRNA-Lipofectamine RNAiMAX complexes (typically 500 µL/well in 6-well plates or 20 µL/well in 96-well plates) were added to the wells and incubated at 37°C in a 5% CO2 incubator for 24 h. The final siRNA concentration was 10 nM. At 24 h after transfection, the antibiotic-free medium was replaced with standard growth medium. Once the cells reached near confluence (2–3 days after transfection), the cells were used for subsequent cAMP assays.
Isolation of RNA and reverse transcriptase-polymerase chain reaction.
Total RNA was extracted from primary cultured HASM cells using the RNeasy mini kit (Qiagen) according to the manufacturer’s recommendation. RNA was transcribed into cDNA using the SuperScript VILO cDNA synthesis kit (Thermo Fisher Scientific) according to the manufacturer’s recommendation. PCR was performed using Advantage 2 PCR kits (Clontech) with sense and antisense primers [human FFAR2: forward primer, 5′-GCTACCTGGGAGTGGCTTTC-3′ and reverse primer, 5′-CATAACCCAGGCCACCAGAG-3′, amplicon size, 89 bp; human FFAR3: forward primer, 5′-GCACTAGGTCTGGAGAGACAGCAAGGT-3′ and reverse primer, 5′-GGAAAGTGAGAAGGTACACCGAGAAGACGAA-3′, amplicon size, 153 bp; human OR51E2: forward primer, 5′-CAGCCATTGACCTGGCCTTA-3′ and reverse primer, 5′-GAAGGCGAGTACCACACCAA-3′, amplicon size, 554 bp; and human GAPDH: forward primer, 5′-CCAGGGCTGCTTTTAACTCTGGTAAAGTGGATA-3′ and reverse primer, 5′-CATCGCCCCACTTGATTTTGGAGGGA-3′, amplicon size, 213 bp]. Two-step PCR was performed with a Mastercycler ep Gradient S thermal cycler (Eppendorf) for all PCR reactions and followed by 40 cycles of denaturation (94°C for 10 s) and annealing/extension at 72°C for 1 min. The human FFAR3 primer was designed to anneal in two separate exons spanning an intron to ensure that amplified PCR products resulted from amplified cDNA and not contaminating genomic DNA. Since the human FFAR2 and OR51E2 genes lack introns, the extracted RNA was treated enzymatically with DNase I (Thermo Fisher Scientific) to digest contaminating genomic DNA according to the manufacturer’s instruction. Complete removal of genomic DNA from the RNA samples was verified by performing negative control reactions in which reverse transcriptase was omitted from the cDNA synthesis reaction (−RT controls). PCR products were electrophoresed on 5% nondenaturing polyacrylamide gel in Tris-acetate-EDTA buffer. The gel was stained with ethidium bromide and visualized using ultraviolet illumination. The gel images were captured with a PowerShot A570 digital camera (Canon).
cAMP assays.
Cyclic AMP (cAMP) synthesis in HASM cells was measured using a HitHunter cAMP Assay for Small Molecules kit (DiscoverX) according to the manufacturer's instructions. Briefly, HASM cells grown in white 96-well plates were washed twice with warm PBS (37°C). In some experiments, the cells were pretreated for 15 min with the FFAR2 antagonist CATPB (10 µM) (5, 20), 4 h with the Gi protein inhibitor pertussis toxin (PTX) (100 ng/mL) (29), or 30 min with trichostatin A (TSA) (1 µM) (50) in cell culture medium before being washed with PBS. In acute experiments, the cells were incubated for 15 min at 37°C in the absence (basal activity) or presence of forskolin (10 µM) ± SCFAs (sodium acetate, sodium propionate, or sodium butyrate; 1 µM–10 mM) as previously described (30). In chronic experiments, HASM cells were initially treated for 60 min with 10 mM of SCFAs in DMEM/F12 cell culture medium at 37°C in a humidified incubator with 5% CO2. The cells were then washed twice with PBS and stimulated with 10 µM forskolin for 15 min at 37°C as previously described (30). After the incubation, the cAMP antibody reagent followed by the cAMP working solution (mixture of enzyme donor/lysis buffer/Emerald II/Galacton) was added to each well and incubated for 60 min at room temperature. Cells were further incubated with the enzyme acceptor reagent for 3 h at room temperature. Luminescence signals were detected using a multimode microplate reader (Appliskan, Thermo Fisher Scientific). The data from triplicate wells were averaged for each sample.
Measurement of [Ca2+]i.
Confluent HASM cells grown in 96-well plates were incubated in modified Hanks’ balanced salt solution (HBSS) (in mM: 138 NaCl, 5.3 KCl, 2.5 CaCl2, 0.4 MgSO4, 0.49 MgCl2, 0.34 Na2HPO4, 4.2 NaHCO3, 0.44 KH2PO4, 5.5 dextrose, and 20 HEPES, pH 7.4) in 100 µl/well containing 5 µM Fluo-4 AM, 0.05% Pluronic F-127, and 2.5 mM probenecid for 45 min at 37°C. Once the cells were loaded, the cells were washed twice with modified HBSS containing 2.5 mM probenecid and left for an additional 30 min at room temperature to allow complete deesterification of the intracellular Fluo-4 AM esters. This buffer was exchanged (100 µl/well) just before starting the measurement of fluorescence. The fluorescence was then continuously recorded every 5 s at wavelengths of 485-nm excitation and 528-nm emission using a fluorescence microplate reader (Appliskan; Thermo Fisher Scientific). Duplicate wells were simultaneously measured, and values were averaged for each data point. After a stable baseline was established for 2 min, SCFAs (sodium acetate, sodium propionate, or sodium butyrate; 10 mM), vehicle (modified HBSS), or acetylcholine (ACh; 1 µM) was added with the autoinjector to HASM cells, and the fluorescence intensity was recorded for 300 s. In separate experiments, HASM cells were pretreated with inhibitors [gallein (10 mM; 30 min), a Gβγ subunit signaling inhibitor; U73122 (5 µM; 30 min), an inhibitor of phospholipase (PLC)-β; and xestospongin C (20 µM; 30 min), a cell-permeable inhibitor of the inositol 1,4,5-triphosphate (IP3) receptor] or vehicle (modified HBSS) before addition of sodium propionate (10 mM). In an attempt to elucidate the possible mechanism of SCFA-induced potentiation of ACh-stimulated [Ca2+]i increase in HASM cells, the cells were treated with SCFAs (10 mM) for 1 min and then exposed to ACh (1 µM) with the autoinjector, and the fluorescence intensity was recorded for 400 s. For experiments using PTX (100 ng/mL; 4 h), cells were pretreated with PTX for 3 h before loading with Fluo-4 AM so that the total pretreatment duration of PTX would be 4 h. In all studies, the fluorescence intensity is presented as the change (ΔF) from baseline fluorescence (Fo).
Filamentous-to-globular actin ratio measurements.
Basal and agonist-induced ratios of filamentous (F) and globular (G) actin were determined as previously described (19, 43). Briefly, after serum starvation for 24 h, HASM cells at 70–90% confluence on eight-chamber microscope slides were exposed to vehicle (water) or SCFAs (sodium acetate, sodium propionate, or sodium butyrate; 10 mM) for 5 min. In an attempt to elucidate the possible mechanism of SCFA-induced potentiation of ACh-stimulated stress fiber formation in HASM cells, the cells were treated with SCFAs (10 mM) for 5 min and then exposed to ACh (1 µM) for 5 min. After the exposure, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and washed three times with PBS. After permeabilization (0.2% Triton X-100 in PBS for 5 min) and blocking (1% bovine serum albumin in 0.1% Triton X-100 in PBS for 15 min), cells were stained with rhodamine (540-nm excitation, 565-nm emission)-conjugated phalloidin (1 U/mL) and Alexa Fluor 488 (495-nm excitation, 519-nm emission)-conjugated DNase I (10 µg/mL) in 300 µl of 1% bovine serum albumin in PBS in the dark for 20 min. After being washed twice with PBS, a coverslip was mounted with Prolong gold antifade reagent (Thermo Fisher Scientific) and visualized with an inverted fluorescent microscope (DMI-4000; Leica). Digitized images were captured with MetaMorph software (Molecular Devices). To standardize the fluorescence intensity measurements among experiments, we optimally adjusted the duration of image capture (300 ms), the image intensity gain, the image enhancement, and the image black level and kept them constant. An increase in the F- to G-actin ratio (F/G actin) indicated an increase in stress fiber formation.
Organ bath study.
Force measurements were performed on human airway smooth muscle strips suspended in organ baths as previously described (9, 15). Briefly, the strips were attached inferiorly to a fixed hook and superiorly to a Grass FT03 force transducer (Grass-Telefactor), which was coupled to a computer via Biopac hardware and Acknowledge 7.3.3 software (Biopac Systems), such that muscle contraction would align in the vertical plane between the anchoring hook below and transducer above to give continuous digital recordings of muscle tension over time. The rings were suspended under isotonic force (1.5 g) in organ baths and equilibrated in Krebs-Henseleit (KH) buffer (composition in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.24 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, and 5.6 d-glucose; pH 7.4) with 10 µM indomethacin (DMSO vehicle final concentration of 0.01%) at 37°C. KH buffer was continuously bubbled with 95% O2-5% CO2, and buffer was exchanged every 15 min for 1 h during equilibration of airway smooth muscle strips at 1.5 g resting tension. Then, three cycles of cumulatively increasing concentrations of acetylcholine dose responses (100 nM to 100 µM) were performed with extensive buffer washes between cycles. The resting tension was reset to 1.5 g and tetrodotoxin (1 µM), pyrilamine (10 µM) and MK571 (10 µM) were added to the buffer in the baths to eliminate the confounding effects of airway nerves and histamine or leukotriene receptors, respectively. Thereafter, sodium propionate (10 mM) was added to the buffer in the baths to examine its effect on baseline human airway smooth muscle tone.
In separate experiments, human airway smooth muscle strips were contracted with ACh (an individual EC50 calculated for each ring). Following the achievement of a stable contraction (typically 15 min) with ACh (EC50), sodium propionate (10 mM) was added to the buffer in the baths. Control strips received vehicle (KH buffer) to serve as time controls. Values reported are the percentage of remaining contractile force of the ACh (EC50)-induced contraction at 15 min, with the force obtained after a plateaued ACh (EC50)-induced contraction representing 100% of muscle force and the baseline tension representing 0% (9). In chronic experiments, human airway smooth muscle strips were contracted with ACh (EC50). Following the achievement of a stable contraction (typically 15 min) with ACh (EC50), human airway smooth muscle strips were chronically pretreated with sodium propionate (10 mM) for 60 min. Thereafter, cumulative concentrations of forskolin (1–10 µM) were added to the buffer in the baths. Control strips received vehicle (ethanol) to serve as time controls. In separate experiments, human airway smooth muscle strips were chronically pretreated with sodium propionate (10 mM) for 60 min, and cumulative increasing concentrations of isoproterenol (0.1 nM – 10 µM) were added to the buffer in the baths in half-log increments at 7-min intervals.
Statistical analysis.
Measurements of cAMP and [Ca2+]i were performed in >100 cells in cell culture plates, and each of these experiments were performed on at least 4 different passages of HASM cells derived from three different subjects. In F/G actin ratio measurements, the fluorescence intensities were calculated from a view containing at least 15 cells. The n values for cell-based experiments represent the number of days on which the experiments were conducted on separate cell culture plates. In the organ bath studies, tracheal tissue samples from five different donors were used for experiments. The n values indicated in the results and figure legends represent the number of tissue samples. Statistical analysis was performed using the two-tailed paired t test when comparing means between two groups or repeated measures ANOVA followed by Bonferroni posttest comparison when comparing multiple groups using Prism 6 for Mac OS X software (GraphPad Software). Data are presented as means ± SE. P < 0.05 was considered significant.
RESULTS
Immunoblot analysis of FFAR2 and FFAR3 in human airway smooth muscle.
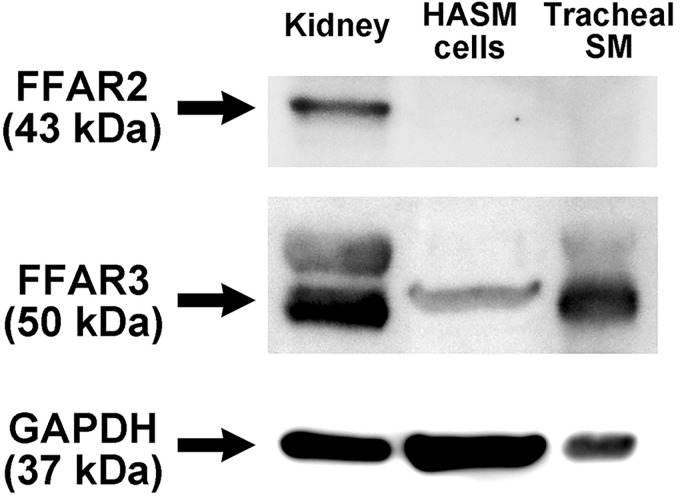
Initially, we assessed the protein expression of short-chain free fatty acid receptors (FFAR2 and FFAR3) in human airway smooth muscle. Lysates prepared from primary cultured HASM cells, and freshly dissected native human tracheal airway smooth muscle tissue were subjected to immunoblot analysis using specific antibodies against human FFAR2 and FFAR3. An immunoreactive band of FFAR3 (50 kDa) was detected in cultured HASM cells, freshly dissected native human airway smooth muscle tissue, and human whole kidney (positive control). In contrast, no immunoreactive band of appropriate molecular mass of FFAR2 (43 kDa) was identified in either cultured HASM cells or freshly dissected native human airway smooth muscle tissue but was detected in human whole kidney lysate (positive control) (Fig. 1).
Fig. 1.
Representative immunoblot image of free fatty acid receptor 2 (FFAR2) and FFAR3 protein expression in lysates prepared from primary cultured human airway smooth muscle (HASM) cells (50 µg), freshly dissected native human tracheal airway smooth muscle (SM; 50 µg), and human whole kidney (20 µg). Blots were reprobed for GAPDH expression to demonstrate relative lane loading. Image is representative of 4 experiments with cultured HASM cells from 3 individuals and native human ASM from 5 individual subjects.
SCFAs-induced cAMP activity in human airway smooth muscle cells.
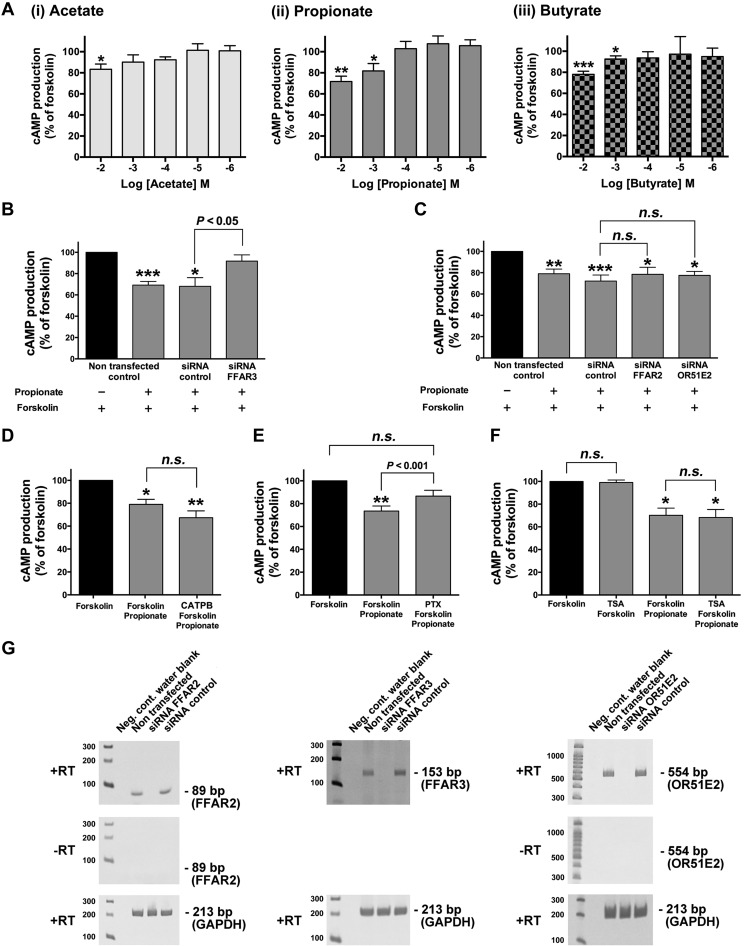
It is well established that acute activation of Gi-coupled receptors attenuates adenylyl cyclase activity, which leads to inhibition of cAMP synthesis (7). To examine whether acute treatment of the Gi-coupled FFAR3 receptor with SCFAs modulates cAMP levels in HASM cells, we measured cAMP synthesis in the presence or absence of SCFAs (acetate, propionate, or butyrate; 1 µM–10 mM). Acute treatment (15 min) of HASM cells with SCFAs (acetate, 10 mM; propionate and butyrate, 1–10 mM) significantly inhibited forskolin-stimulated cAMP production (acetate: P < 0.05, n = 6; propionate: P < 0.01, n = 14–19; butyrate: P < 0.01, n = 8) (Fig. 2A). To confirm propionate-induced inhibition of cAMP production is mediated through FFAR3, HASM cells were treated with human FFAR3-specific siRNA. Propionate-induced inhibition of cAMP activity was significantly attenuated in HASM cells transduced with FFAR3-specific siRNA compared with the cells transduced with nontargeting siRNA (P < 0.05, n = 7) (Fig. 2B). In contrast, propionate-induced inhibition of cAMP activity was not significantly attenuated in HASM cells transduced with FFAR2-specific siRNA compared with the cells transduced with nontargeting siRNA (n.s., n = 6) (Fig. 2C) nor pretreatment of the HASM cells with the FFAR2 antagonist (CATPB; 10 µM for 15 min; n.s., n = 6) (Fig. 2D). Recent findings have indicated that the olfactory receptor OR51E2, which is expressed on HASM cells also acts as the sensor of acetate and propionate (2). To examine whether OR51E2 contributes to the propionate-induced inhibition of cAMP production, HASM cells were transfected with human OR51E2-specific siRNA. Propionate-induced inhibition of cAMP activity was not significantly attenuated in HASM cells transduced with OR51E2-specific siRNA compared with the cells transduced with nontargeting siRNA (n.s., n = 6) (Fig. 2C). Furthermore, propionate-induced inhibition of cAMP synthesis was significantly blocked by pretreatment with the Gi protein inhibitor pertussis toxin (PTX) (100 ng/mL for 4 h; P < 0.001, n = 8) (Fig. 2E). Since SCFAs could exert several physiological effects through inhibition of histone deacetylase (HDAC) activity (42, 46), we examined if the HDAC inhibitor trichostatin A (TSA) could attenuate forskolin-induced cAMP production or propionate-induced inhibition of cAMP synthesis. TSA (1 µM for 30 min pretreatment) did not affect forskolin-stimulated cAMP production or propionate-induced inhibition of cAMP production (n.s., n = 8) (Fig. 2F). RT-PCR analyses confirmed that HASM cells treated with either FFAR2-, FFAR3-, or OR51E2-specific siRNA displayed a knockdown of the mRNA encoding these receptors, compared with nontransfected cells or cells treated with nontargeting siRNA (Fig. 2G).
Fig. 2.
A: dose-dependent effect of acute (15 min) pretreatment of human airway smooth muscle (HASM) cells with 1 µM to 10 mM short-chain fatty acids [SCFAs (i) acetate (n = 4–9). (ii) propionate (n = 4–19), or (iii) butyrate (n = 4–8)] before forskolin (10 µM)-stimulated (15 min) cAMP activity in HASM cells. B and C: involvement of FFAR3 (B) or either free fatty acid receptor 2 (FFAR2) or OR51E2 (C) in propionate-induced cAMP inhibition in HASM cells. HASM cells were transfected with either control nontargeting small-interfering RNA (siRNA) or FFAR3-specific siRNA (n = 7) (B) or either FFAR2- or OR51E2-specific siRNA (n = 6) (C) 2–3 days before analyses and then pretreated with propionate (10 mM) for 15 min before the addition of forskolin (10 µM) for 15 min. D–F: effect of the FFAR2 antagonist CATPB (10 µM, for 15 min pretreatment; n = 6) (D), pertussis toxin (PTX; 100 ng/mL, for 4 h pretreatment; n = 8) (E), or trichostatin A (TSA; 1 µM, for 30 min pretreatment; n = 8) (F) on propionate (10 mM)-induced attenuation of forskolin (10 µM)-stimulated cAMP activity in HASM cells. Data represent means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with forskolin alone. G: representative gel images of RT-PCR analyses of total RNA using primers specific for human FFAR2, FFAR3, or OR51E2. Total RNA extracted from nontransfected HASM cells, HASM cells that were transfected with either control nontargeting small-interfering RNA (siRNA control), FFAR2-, FFAR3-, or OR51E2-specific siRNA were analyzed. −RT, cDNA synthesis reactions performed in the absence of reverse transcriptase (RT) confirming that PCR products were not arising from contaminating gDNA. GAPDH was used as a control of relative RNA input among samples.
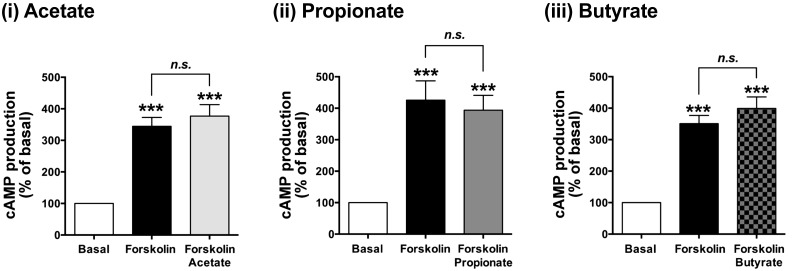
It is also well established that chronic stimulation of Gi-coupled receptors often sensitizes adenylyl cyclase enzyme activity, which potentiates subsequent cAMP synthesis (6, 23, 30, 48). Therefore, we examined whether chronic treatment of HASM cells with SCFAs potentiated cAMP synthesis. However, chronic pretreatment (60 min) of HASM cells with SCFAs did not significantly alter forskolin-stimulated cAMP activity (acetate: n.s., n = 8; propionate: n.s., n = 22; butyrate: n.s., n = 9) (Fig. 3).
Fig. 3.
Effect of chronic (60 min) pretreatment of human airway smooth muscle (HASM) cells with 10 mM short-chain fatty acids (SCFAs) [(i) acetate (n = 8), (ii) propionate (n = 22), or (iii) butyrate (n = 9)] on forskolin (10 µM)-stimulated cAMP activity in HASM cells. The cells were pretreated with SCFAs for 60 min followed by forskolin for 15 min before measurement of cAMP activity. Data represent means ± SE. ***P < 0.001 compared with basal.
SCFAs induced [Ca2+]i mobilization in human airway smooth muscle cells.
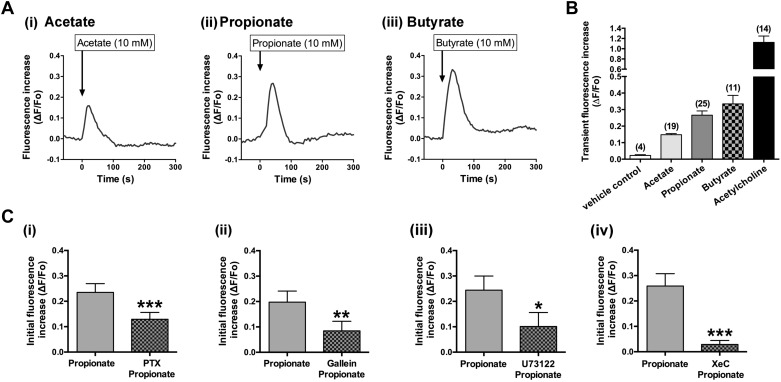
A variety of airway contractile agents, such as acetylcholine, histamine, and tachykinins, induce airway smooth muscle contraction through the activation of their specific Gq-coupled receptors. However, Gi-coupled receptors also contribute to airway smooth muscle contraction through the Gi protein subunits that crosstalk to activate PLC-β and release IP3, which mobilize Ca2+ from the sarcoplasmic reticulum (SR) (14, 29). Furthermore, SCFA increases [Ca2+]i through FFAR3 (27). Therefore, we measured changes of [Ca2+]i in cultured HASM cells following exposure to SCFAs. SCFAs (acetate, propionate, or butyrate; 10 mM) caused transient [Ca2+]i increases in HASM cells, although the magnitude of these increases was much smaller than the Gq-coupled receptor agonist acetylcholine (ACh; 1 µM) (Fig. 4, A and B). Pretreatment of HASM cells with PTX (100 ng/mL; 4 h) significantly blocked the transient increase in [Ca2+]i in response to 10 mM propionate (P < 0.001, n = 11) (Fig. 4C).
Fig. 4.
Effects of short-chain fatty acids (SCFAs; acetate, propionate, or butyrate) on intracellular Ca2+ concentration ([Ca2+]i) in human airway smooth muscle (HASM) cells. A: representative traces of fluorescence intensity [change in fluorescence (ΔF) from baseline fluorescence (Fo)] illustrating the characteristics of acetate (i; 10 mM), propionate (ii; 10 mM), or butyrate (iii; 10 mM)-induced [Ca2+]i increases in HASM cells. B: effects of acetate (10 mM), propionate (10 mM), butyrate (10 mM), acetylcholine (1 µM; positive control), or vehicle (H2O) on transient (initial) [Ca2+]i increases in HASM cells. Data are means ± SE presented as ΔF/Fo. Number of experiments is shown in parentheses. C: effect of pertussis toxin (PTX) (i; 100 ng/mL, for 4-h pretreatment, n = 11), gallein (ii; 10 µM, for 30 min pretreatment, n = 7), U73122 (iii; 5 µM, for 30 min pretreatment, n = 9), or xestospongin C (XeC) (iv; 20 µM, for 30 min pretreatment, n = 9) on propionate (10 mM)-induced transient [Ca2+]i increases in HASM cells. Data represent means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with propionate alone.
Stimulation of Gi-coupled receptors activates PLC-β by the liberated Giβγ subunits. PLC-β further promotes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG) and IP3, and IP3 in turn liberates Ca2+ from SR. Therefore, we examined whether Gβγ subunits, PLC-β, and the IP3 receptor contribute to the [Ca2+]i increases induced by propionate. In the presence of gallein (10 µM for 30 min pretreatment; a Gβγ subunit signaling inhibitor), U73122 (5 µM for 30 min pretreatment; an inhibitor of PLC-β), or xestospongin C (Xest C; 20 µM for 30 min pretreatment; an inhibitor of IP3 receptor), the propionate (10 mM)-induced transient increases in [Ca2+]i were significantly inhibited (gallein: P < 0.01, n = 7; U73122: P < 0.05, n = 9; Xest C: P < 0.001, n = 9) (Fig. 4C).
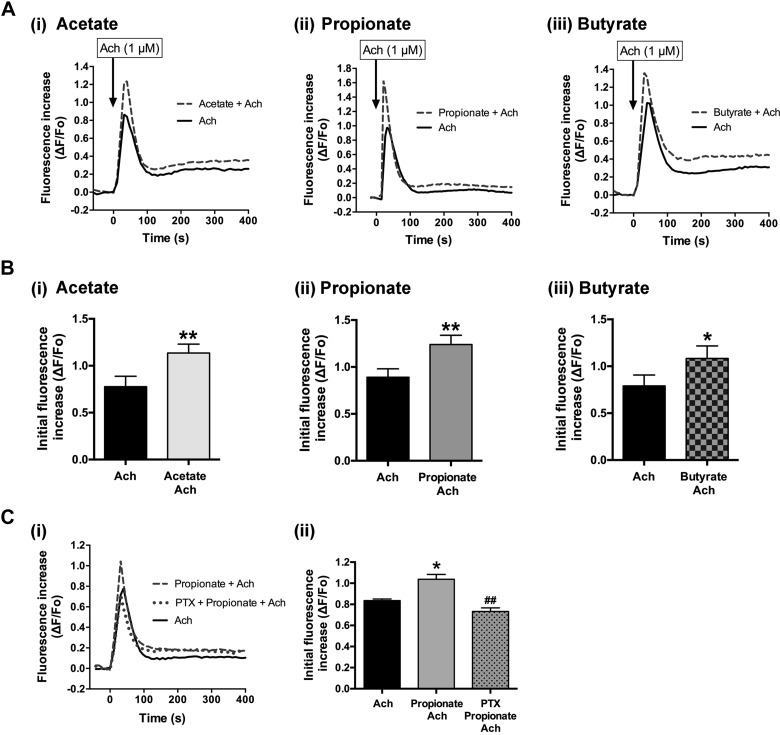
SCFAs potentiated acetylcholine-induced [Ca2+]i mobilization in human airway smooth muscle cells.
Activation of Gi-coupled receptors could augment airway smooth muscle contraction mediated via the activation of Gq-coupled receptors, as Gβγ subunits dissociated from Gi proteins are responsible for crosstalk between Gi- and Gq-coupled receptors (14, 29). Therefore, we examined whether SCFAs potentiate Gq-coupled receptor-mediated [Ca2+]i mobilization in HASM cells. SCFAs (acetate, propionate, or butyrate; 10 mM respectively) significantly potentiated ACh (1 µM)-induced increases in the initial transient phase of [Ca2+]i in HASM cells (acetate: P < 0.01, n = 9; propionate: P < 0.01, n = 10; butyrate: P < 0.05, n = 11) (Fig. 5, A and B). Pretreatment of HASM cells with pertussis toxin (100 ng/mL for 4 h) attenuated the propionate (10 mM)-induced potentiation of ACh-induced [Ca2+]i mobilization (P < 0.01, n = 5) (Fig. 5C), while pretreatment of HASM cells with pertussis toxin by itself did not affect ACh-induced [Ca2+]i mobilization (n.s, n = 5) (data not shown).
Fig. 5.
Effects of short-chain fatty acids (SCFAs; acetate, propionate, or butyrate) on acetylcholine (ACh; 1 µM)-stimulated increase of intracellular Ca2+ concentration ([Ca2+]i) in human airway smooth muscle (HASM) cells. A: representative traces of fluorescence intensities [change in fluorescence (ΔF) from baseline fluorescence (Fo)] illustrating the SCFA-induced potentiation of ACh (1 µM)-stimulated [Ca2+]i increases in HASM cells. B: effects of acetate (i; n = 9), propionate (ii; n = 10), or butyrate (iii; n = 11) on ACh (1 µM)-stimulated transient (initial) [Ca2+]i increases in HASM cells. Data represent means ± SE. *P < 0.05, and **P < 0.01 compared with ACh alone. Ci: representative traces of fluorescence intensities illustrating ACh (1 µM) alone and propionate (10 mM)-induced potentiation of ACh (1 µM)-stimulated [Ca2+]i increases in HASM cells in the presence or absence of pertussis toxin (PTX; 100 ng/mL, for 4 h pretreatment). Cii: effect of PTX on propionate-induced potentiation of ACh-stimulated [Ca2+]i increases in HASM cells. Data represent means ± SE. *P < 0.05 compared with ACh alone. ##P < 0.01 compared with propionate + ACh.
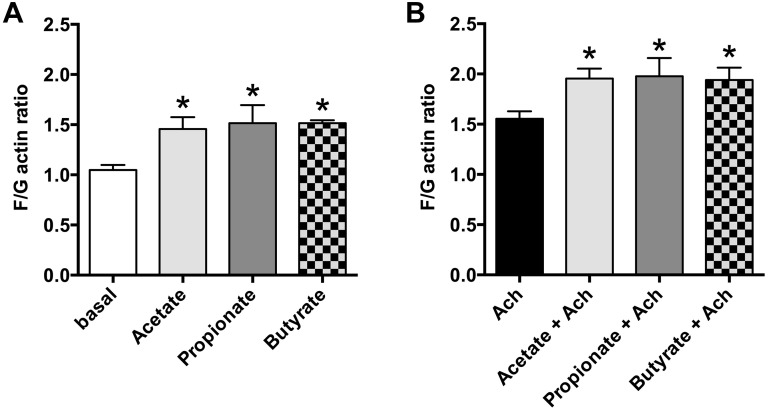
SCFAs induced actin reorganization in HASM cells.
The increase in intracellular Ca2+ in HASM cells favors airway smooth muscle contraction, and actin reorganization is a process required for smooth muscle contraction (17). Therefore, we examined whether SCFAs (acetate, propionate, or butyrate; 10 mM, respectively) induce actin reorganization in HASM cells. Acetate, propionate, and butyrate significantly induced actin reorganization reflected in an increase in the F/G actin ratio (acetate: P < 0.05; propionate: P < 0.05; butyrate: P < 0.05; n = 6) (Fig. 6A). We further examined whether SCFAs potentiate Gq-coupled receptor-mediated actin reorganization in HASM cells. SCFAs (acetate, propionate, or butyrate; 10 mM, respectively) significantly potentiated ACh (1 µM)-induced actin reorganization in HASM cells (acetate: P < 0.05; propionate: P < 0.05; butyrate: P < 0.05, n = 6) (Fig. 6B).
Fig. 6.
Effects of short-chain fatty acids (SCFAs; acetate, propionate, or butyrate) on fluorescent staining ratio of filamentous and globular actin (F/G actin) in human airway smooth muscle (HASM) cells. A: effects of acetate, propionate, or butyrate (10 mM; n = 6) on stress fiber formation in HASM cells. Data are means ± SE. *P < 0.05 compared with basal. B: effects of acetate, propionate, or butyrate (10 mM; n = 6) on acetylcholine (ACh; 1 µM)-induced stress fiber formation in HASM cells. An increase in F/G actin indicates an increase in filamentous actin, which is a component of the cytoskeletal contribution to smooth muscle cell contraction. Data are means ± SE. *P < 0.05 compared with ACh alone.
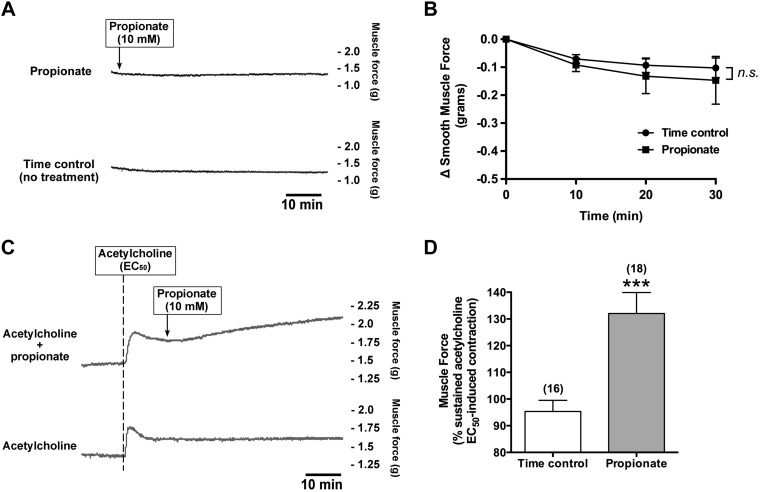
Propionate potentiated acetylcholine-induced human airway contraction.
We examined whether the activation of FFAR3 by propionate modulates basal human airway smooth muscle tone. Propionate (10 mM) did not alter human airway smooth muscle tone (n.s. compared with time control, n = 3) (Fig. 7, A and B). Next, we examined whether the activation of FFAR3 by propionate potentiates acetylcholine-induced human airway smooth muscle contraction. Human tracheal rings suspended in organ baths were contracted with acetylcholine (EC50) and at a plateau of the acetylcholine-induced contractile force, and rings were left untreated or treated with propionate (10 mM). Propionate significantly potentiated the ACh-induced contraction of human airway smooth muscle (132 ± 7.86% of sustained acetylcholine EC50-induced contraction; P < 0.001 compared with time control, n = 16–18) (Fig. 7, C and D).
Fig. 7.
Propionate by itself did not alter the basal human airway smooth muscle tone but potentiated acetylcholine (ACh)-induced contractions in human airway smooth muscle strips. A: representative tracings of muscle force recordings in human airway smooth muscle strip treated with propionate (10 mM). B: propionate did not alter basal human airway smooth muscle tone (n = 3). Data are means ± SE. C: representative tracings of muscle force recordings in human airway smooth muscle strip contracted with ACh (EC50 concentration), followed by propionate (10 mM). D: propionate significantly potentiated the ACh (EC50)-induced contraction. Data are means ± SE. ***P < 0.001 compared with time control. Numbers of experiments are shown in parentheses.
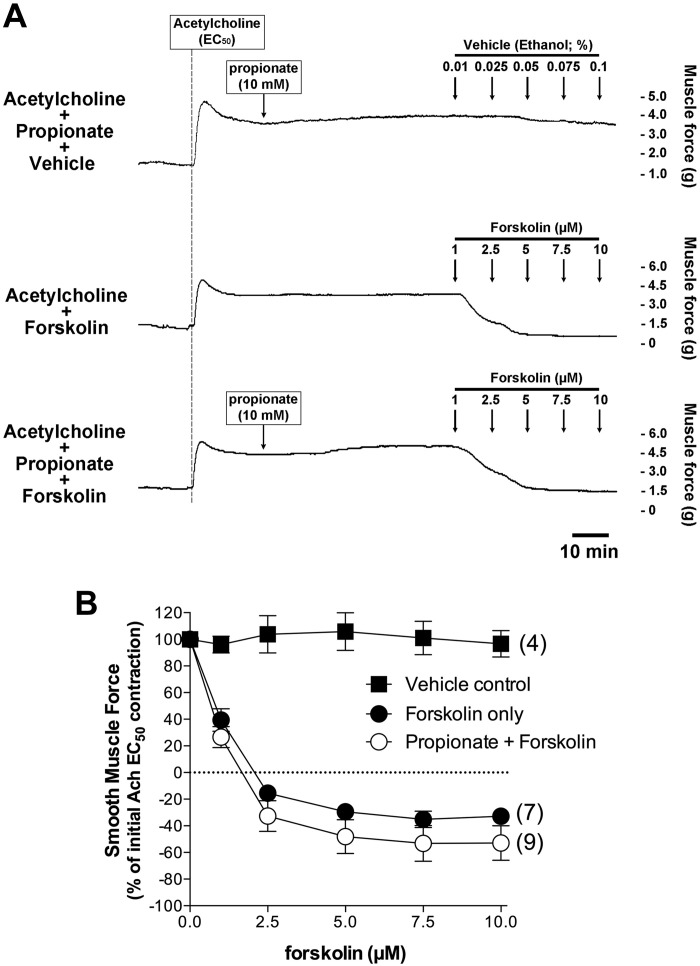
Propionate did not potentiate human airway smooth muscle relaxation induced by forskolin or isoproterenol.
We further examined whether chronic activation of FFAR3 by propionate would sensitize adenylyl cyclase to augment relaxation. We evaluated this by directly stimulating adenylyl cyclase with forskolin or by stimulating the β-adrenoceptor coupled through Gs to adenylyl cyclase after chronic pretreatment with propionate. Chronic treatment of the human tracheal rings with propionate (10 mM for 60 min) did not potentiate relaxation induced by incremental concentrations of forskolin (1–10 µM) (Fig. 8) or by incremental concentrations of isoproterenol (0.1 nM to 10 µM) (Fig. 9).
Fig. 8.
Chronic treatment of the human airway smooth muscle strips with propionate did not potentiate forskolin-induced relaxation. A: representative muscle force tracings in human airway smooth muscle strips illustrating that forskolin-induced relaxation following an acetylcholine (EC50)-induced contraction was not potentiated by chronic pretreatment with propionate (10 mM). Top tracing (vehicle control): propionate (10 mM) 60 min pretreatment followed by vehicle (ethanol) treatment; middle tracing: cumulative forskolin (1–10 µM) treatment; bottom tracing: propionate (10 mM) 60 min pretreatment followed by cumulative forskolin treatment. B: chronic pretreatment with propionate (10 mM) does not potentiate the relaxation induced by cumulative concentrations of forskolin (1–10 µM) indicating an absence of adenylyl cyclase sensitization by chronic propionate activation of Gi. Data represent means ± SE. Numbers of experiments are shown in parentheses.
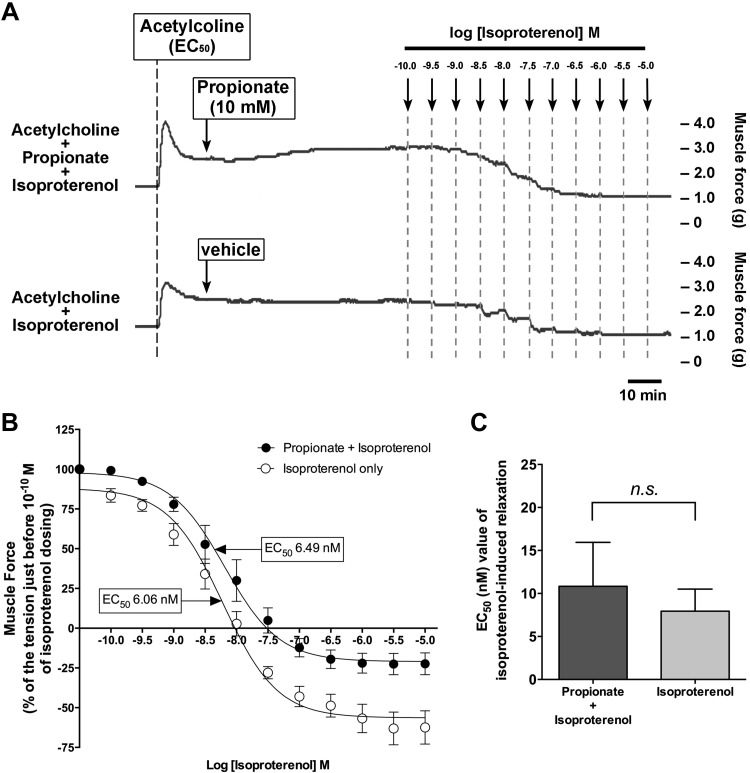
Fig. 9.
Chronic pretreatment of the human airway smooth muscle strips with propionate did not potentiate isoproterenol-induced relaxation. A: representative tension tracings in human airway smooth muscle strips after an acetylcholine (EC50) concentration followed by increasing concentrations (0.1 nM–10 µM) of the β-adrenoceptor agonist isoproterenol in the presence or absence of propionate (60 min pretreatment; 10 mM), illustrating that isoproterenol-induced relaxation of ACh (EC50) contraction was not potentiated by chronic pretreatment with propionate (10 mM) indicating an absence of adenylyl cyclase sensitization by chronic propionate activation of Gi. B: isoproterenol concentration response relaxation curves in the presence or absence of propionate (60 min pretreatment; 10 mM). Data represent means ± SE; n = 6. C: EC50 value of isoproterenol-induced relaxation with or without propionate pretreatment (60 min pretreatment; 10 mM). Data represent means ± SE; n = 6.
DISCUSSION
The main findings of the present study are that the functional short-chain free fatty acid receptor FFAR3 is expressed in human airway smooth muscle. SCFAs inhibit forskolin-stimulated cAMP accumulation via Gi protein-coupled FFAR3. SCFAs induced transient [Ca2+]i increases in HASM cells through the Giβγ-PLC-IP3 pathway and potentiated the acetylcholine-stimulated transient [Ca2+]i increases in HASM cells. Furthermore, SCFA induced stress fiber formation and potentiated acetylcholine-induced contraction of human airway smooth muscle strips without sensitizing adenylyl cyclase activation during chronic FFAR3 receptor activation.
Gut microbiota are the primary source of SCFAs in the plasma (45), and previous reports documented that plasma concentrations of SCFAs are between 0.1 and 10 mM (26, 32). SCFAs consist of one to six carbons of which acetate (C2), propionate (C3), and butyrate (C4) are the most abundant (10). Although these SCFAs are an important energy source for gut epithelium and peripheral tissues, SCFAs also affect immune functions, the colonic environment, insulin secretion, and lipid levels (4). Interestingly, recent findings have suggested that airway microbiota can produce SCFAs (28) and SCFAs are present in sputum samples from cystic fibrosis patients in millimolar concentrations (16). Collectively, both gut-derived and airway-derived SCFAs could contribute to the pathogenesis of airway diseases. SCFAs have been shown to exert their effect through their specific G protein-coupled receptors (GPCRs) FFAR2 and FFAR3 (3). FFAR2 is mainly expressed in immune cells, including neutrophils, eosinophils, dendritic cells, and monocytes, while FFAR3 is mainly expressed on pancreas, spleen, adipose tissue, and immune cells (25). In the airways, protein expression of both FFAR2 and FFAR3 has been identified in human nasal and bronchial epithelial cells (21, 28), and mRNA encoding both FFAR2 and FFAR3 was also identified in HASM cells (2). In accordance with the previous findings, we identified the protein expression of FFAR3 in human airway smooth muscle by immunoblot analysis. The predominant immunoreactive bands for the human FFAR3 in human tracheal smooth muscle tissue and HASM cells (50 kDa) migrated at a larger molecular mass than the theoretical predicted molecular mass (39 kDa), suggesting that the FFAR3 protein may undergo posttranslational modifications or may exist in larger splice variants. It has been reported that the FFAR3 protein sequence contains several potential phosphorylation and glycosylation sites (24). Therefore, it is possible that the molecular mass at which FFAR3 migrates on a gel is larger than its predicted molecular mass based on amino acid sequence alone (39). In contrast, although mRNA expression of FFAR2 has been reported in HASM cells (2), and we have also identified the mRNA expression of FFAR2 in primary cultured HASM cells, protein expression of FFAR2 on human airway smooth muscle was not identified in the present study. One possible explanation for this discrepancy of the expression of mRNA and protein of FFAR2 on human airway smooth muscle is that small amounts of FFAR2 mRNA detected by sensitive RNAseq or RT-PCR techniques may not translate into detectable levels of FFAR2 protein, as several studies have described the discrepancy between mRNA and protein expression levels in mammalian cells (41). In Fig. 1, the band intensity of GAPDH in human tracheal smooth muscle was slightly weaker than the band in HASM cells, although an equal amount of protein (50 µg) was loaded on the gels. It is likely that the fresh tracheal tissue has protein from other extracellular matrices that contribute to the protein assay but do not contain GAPDH.
Molecular identification of FFAR3 on human airway smooth muscle itself led us to hypothesize that FFAR3 could modulate human airway smooth muscle tone. In the airways, cAMP is one of the key molecules that modulate airway smooth muscle tone, and it is well established that the acute activation of Gi-coupled receptors inhibits adenylyl cyclase activity, which leads to reduce cAMP production (7). Consistent with previous findings, the present study showed that acute stimulation (15 min) of HASM cells with physiological concentration of SCFAs (1–10 mM) significantly inhibited forskolin-stimulated cAMP activity in HASM cells in a pertussis toxin-sensitive manner implicating coupling through a Gi/o pathway. Furthermore, in HASM cells in which FFAR3 expression was reduced by siRNA treatment, propionate-induced cAMP reduction was significantly inhibited compared with HASM cells transfected with nontargeting siRNA. In contrast, propionate-induced cAMP reduction was not affected by either knockdown of FFAR2 by siRNA or pretreatment the cells with the FFAR2 antagonist CATPB. These findings are consistent with the present study that FFAR2 protein was not detected in human airway smooth muscle tissue and cells by immunoblotting. Recent findings have suggested that mRNA of several olfactory receptors is expressed in HASM cells and OR51E2, which is the most abundantly expressed olfactory receptor in HASM cells, acts as the sensor of acetate and propionate (2). However, in the present study, propionate-induced cAMP reduction in HASM cells was not affected by knockdown of OR51E2 by siRNA. Collectively, these results suggest that, in HASM cells, acute activation of SCFAs induces inhibition of cAMP production solely through Gi-coupled FFAR3.
It is also well accepted that chronic activation of Gi-coupled receptors results in a paradoxical enhancement of cAMP production due to sensitization of adenylyl cyclase activity (49). However, chronic stimulation (1 h) of HASM cells with SCFAs did not increase cAMP production in the present study. This result is consistent with the previous finding that chronic exposure (4 h) of HASM cells with propionate or acetate did not increase cAMP production in HASM cells (2). Taken together, although acute activation of Gi-coupled FFAR3 with SCFAs inhibited cAMP production, chronic activation of FFAR3 did not enhance cAMP production in HASM cells. Furthermore, chronic pretreatment of the human tracheal smooth muscle strips with propionate also did not potentiate forskolin- or isoproterenol-induced airway smooth muscle relaxation. Similar findings were reported that chronic pretreatment (4 h) of HASM cells with physiological concentration of SCFAs in the serum and lung (0.1–10 mM) did not affect histamine-induced single HASM cell stiffness (2).
Intracellular Ca2+ mobilization is a key component of airway smooth muscle contraction. In the present study, SCFAs alone induced [Ca2+]i increases and stress fiber formation in HASM cells. Furthermore, the propionate-induced transient [Ca2+]i increase was attenuated by pertussis toxin (an inhibitor of Gi protein), gallein (a Gβγ signaling inhibitor), U73122 (a PLC-β inhibitor), and xestospongin C (an IP3 receptor inhibitor). Recent findings have suggested that G protein-coupled receptor-induced [Ca2+]i mobilization in HASM cells is mediated by both Gq- and Gi-coupled receptors and both receptors can merge onto the same signaling pathway, as Giβγ subunits dissociated from Gi-coupled receptors activate the classical Gq-PLC-β/IP3 pathway, which mobilizes calcium from the SR (14, 29). It has also reported that Gβγ subunits released following FFAR3 receptor activation directly activate PLC-β (22). These results, taken together, suggest that SCFAs induced transient [Ca2+]i increases via Gi-coupled FFAR3, which liberates Giβγ subunits to activate the PLC-β/IP3 pathway, releasing calcium from the SR. However, contrary to these cell-based findings, propionate alone did not alter the basal human airway smooth muscle tone ex vivo. However, under normal in vivo conditions, airway smooth muscle is never under zero contractile tone. Endogenous mediators always induce some degree of contractile tone. In human airway smooth muscle, endogenous histamine and leukotrienes induce intrinsic contractile tone (13). Therefore, it is most functionally relevant to evaluate potentiators of contraction under conditions in which some tone is present, such as was done with the potentiation of acetylcholine shown in Fig. 7, C and D.
In the present study, SCFAs potentiated the [Ca2+]i mobilization and stress fiber formation induced by ACh, a Gq-coupled muscarinic M3 receptor agonist, in a pertussis toxin-sensitive manner. Furthermore, propionate potentiated ACh-induced human tracheal smooth muscle contraction. These findings indicate that SCFAs could potentiate preexisting Gq-coupled receptor-mediated human airway smooth muscle contraction, which could augment airway narrowing. We have reported similar findings that activation of the Gi-coupled GABAB receptor potentiated the [Ca2+]i mobilization and the airway smooth muscle contraction mediated via the activation of Gq-coupled receptors (29). Such crosstalk between the Gi- and Gq-coupled receptor might not require their physical interaction but might relate to functional crosstalk between their signaling pathways (33, 35).
In asthmatic patients, the intake of a soluble fiber diet, which is fermented by commensal bacteria, attenuates airway inflammation (18). Increasing evidence has suggested that SCFAs produced by gut microbiota could be a potential therapeutic candidate for the treatment of asthma (31, 40) due to their effects on inflammation, but the present study suggests a potential detrimental effect on airway smooth muscle tone. Although FFAR2/3 is a promising target to attenuate asthma symptoms, it is still controversial whether FFAR2 and/or FFAR3 have therapeutic effects on airway diseases. Trompette et al. (44) reported that the short-chain fatty acid propionate elicits a protective effect against allergic airway inflammation through FFAR3, and Maslowski et al. (26) reported that FFAR2-knockout mice showed an exacerbation of allergic lung inflammation. In contrast, Mirković et al. (28) demonstrated that FFAR3 expressed on human bronchial epithelial cells could induce excessive IL-8 production, which promotes airway inflammation especially in patients with cystic fibrosis. Rutting et al. (38) also reported that SCFAs potentiated TNFα-induced release of the proinflammatory cytokines IL-6 and IL-8 in human lung fibroblast and HASM cells and the response in human lung fibroblast was mediated through FFAR3. In the present study, FFAR3 expressed on airway smooth muscle contributed to airway smooth muscle contraction, which could worsen asthma symptoms. However, a limitation of our study is that we have not examined whether gut microbiota-derived SCFAs could modulate airway smooth muscle tone through FFAR3 and we have not addressed whether the diversity and composition of gut microbiota could affect airway smooth muscle tone. It is also unknown whether the beneficial anti-inflammatory effects of SCFAs in allergic lung inflammation could outweigh any detrimental effects of SCFAs on airway tone. Further studies are required to characterize whether SCFAs produced by gut microbiota have either beneficial or detrimental effects in asthmatic patients.
In summary, we have demonstrated the functional expression of FFAR3 in human airway smooth muscle. Although SCFAs have been reported to be beneficial for asthmatics to attenuate airway inflammation, our findings demonstrated that SCFAs can directly regulate the cAMP production and mobilization of calcium in human airway smooth muscle through FFAR3, which favors airway smooth muscle-mediated bronchoconstriction.
GRANTS
This work was supported by the National Institutes of Health Grants GM-065281 and HL-122340 (to C. W. Emala), Grants-in-Aid from the Japan Society for the Promotion of Science 26560376 and 18K09783 (to K. Mizuta) and 17K11893 (to A. Matoba), and a research grant from The Mishima Kaiun Memorial Foundation (to K. Mizuta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M. and C.W.E.S. conceived and designed research; K.M., H.S., Y.Z., A.M., and C.W.E.S. performed experiments; K.M., H.S., Y.Z., and C.W.E.S. analyzed data; K.M., H.S., and C.W.E.S. interpreted results of experiments; K.M. and H.S. prepared figures; K.M. and C.W.E.S. drafted manuscript; K.M., H.S., Y.Z., and C.W.E.S. edited and revised manuscript; K.M., H.S., Y.Z., A.M., and C.W.E.S. approved final version of manuscript.
REFERENCES
- 1.Abebe W, Mustafa SJ. A1 adenosine receptor-mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 275: L990–L997, 1998. doi: 10.1152/ajplung.1998.275.5.L990. [DOI] [PubMed] [Google Scholar]
- 2.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, Natarajan N, Yong HM, De Santiago B, Oh JJ, Yoon AR, Panettieri RA, Homann O, Sullivan JK, Liggett SB, Pluznick JL, An SS. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep 6: 38231, 2016. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Curto E, Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol 114: 3–13, 2016. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation–protective or causative? Front Immunol 7: 28, 2016. doi: 10.3389/fimmu.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang Z, Xiong D, Wu M, Ding JL. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J 32: 289–303, 2018. doi: 10.1096/fj.201700252RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billington CK, Hall IP, Mundell SJ, Parent JL, Panettieri RA Jr, Benovic JL, Penn RB. Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am J Respir Cell Mol Biol 21: 597–606, 1999. doi: 10.1165/ajrcmb.21.5.3759. [DOI] [PubMed] [Google Scholar]
- 7.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4: 2, 2003. doi: 10.1186/rr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol 13: 70–77, 2013. doi: 10.1097/ACI.0b013e32835ad0d2. [DOI] [PubMed] [Google Scholar]
- 9.Brown A, Danielsson J, Townsend EA, Zhang Y, Perez-Zoghbi JF, Emala CW Sr, Gallos G. Attenuation of airway smooth muscle contractility via flavonol-mediated inhibition of phospholipase-Cβ. Am J Physiol Lung Cell Mol Physiol 310: L747–L758, 2016. doi: 10.1152/ajplung.00215.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E; GABRIELA Transregio 22 Study Group . Exposure to environmental microorganisms and childhood asthma. N Engl J Med 364: 701–709, 2011. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 12.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA 105: 5230–5235, 2008. doi: 10.1073/pnas.0801319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis JL, Undem BJ. Role of cysteinyl-leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. Am J Respir Crit Care Med 149: 118–122, 1994. doi: 10.1164/ajrccm.149.1.8111568. [DOI] [PubMed] [Google Scholar]
- 14.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 35: 496–502, 2006. doi: 10.1165/rcmb.2005-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol 304: L191–L197, 2013. doi: 10.1152/ajplung.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TM, Palaniyar N, Grasemann H. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 46: 1033–1045, 2015. doi: 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- 17.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients 9: 57, 2017. doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol 277: L653–L661, 1999. doi: 10.1152/ajplung.1999.277.3.L653. [DOI] [PubMed] [Google Scholar]
- 20.Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem 287: 41195–41209, 2012. doi: 10.1074/jbc.M112.396259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imoto Y, Kato A, Takabayashi T, Sakashita M, Norton JE, Suh LA, Carter RG, Weibman AR, Hulse KE, Stevens W, Harris KE, Peters AT, Grammer LC, Tan BK, Welch K, Conley DB, Kern RC, Fujieda S, Schleimer RP. Short-chain fatty acids induce tissue plasminogen activator in airway epithelial cells via GPR41&43. Clin Exp Allergy 48: 544–554, 2018. doi: 10.1111/cea.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue D, Kimura I, Wakabayashi M, Tsumoto H, Ozawa K, Hara T, Takei Y, Hirasawa A, Ishihama Y, Tsujimoto G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett 586: 1547–1554, 2012. doi: 10.1016/j.febslet.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Johnston CA, Watts VJ. Sensitization of adenylate cyclase: a general mechanism of neuroadaptation to persistent activation of Galpha(i/o)-coupled receptors? Life Sci 73: 2913–2925, 2003. doi: 10.1016/S0024-3205(03)00703-3. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Su H, Zhou Z, Yao W. Identification of the porcine G protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS One 9: e97342, 2014. doi: 10.1371/journal.pone.0097342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, van Esch BC, Wagenaar GT, Garssen J, Folkerts G, Henricks PA. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol 831: 52–59, 2018. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS One 9: e107388, 2014. doi: 10.1371/journal.pone.0107388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirković B, Murray MA, Lavelle GM, Molloy K, Azim AA, Gunaratnam C, Healy F, Slattery D, McNally P, Hatch J, Wolfgang M, Tunney MM, Muhlebach MS, Devery R, Greene CM, McElvaney NG. The role of short-chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway. Am J Respir Crit Care Med 192: 1314–1324, 2015. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA Jr, Emala CW. Gi-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232–1238, 2011. doi: 10.1165/rcmb.2011-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuta K, Zhang Y, Xu D, Masaki E, Panettieri RA Jr, Emala CW. The dopamine D(2) receptor is expressed and sensitizes adenylyl cyclase activity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L316–L324, 2012. doi: 10.1152/ajplung.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozturk AB, Turturice BA, Perkins DL, Finn PW. The potential for emerging microbiome-mediated therapeutics in asthma. Curr Allergy Asthma Rep 17: 62, 2017. doi: 10.1007/s11882-017-0730-1. [DOI] [PubMed] [Google Scholar]
- 32.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional cross talk between GPCRs: with or without oligomerization. Curr Opin Pharmacol 10: 6–13, 2010. doi: 10.1016/j.coph.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol 11: 35, 2015. doi: 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prézeau L. Cross talk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J 28: 2195–2208, 2009. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 13: 440–447, 2012. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutting S, Xenaki D, Lau E, Horvat J, Wood LG, Hansbro PM, Oliver BG. Dietary omega-6, but not omega-3, polyunsaturated or saturated fatty acids increase inflammation in primary lung mesenchymal cells. Am J Physiol Lung Cell Mol Physiol 314: L922–L935, 2018. doi: 10.1152/ajplung.00438.2017. [DOI] [PubMed] [Google Scholar]
- 38.Rutting S, Xenaki D, Malouf M, Horvat JC, Wood LG, Hansbro PM, Oliver BG. Short-chain fatty acids increase TNFα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am J Physiol Lung Cell Mol Physiol 316: L157–L174, 2019. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 39.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res 30: 149–156, 2009. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 40.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40: 833–842, 2014. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dai H, Thorsson V, Eng J, Goodlett D, Berger JP, Gunter B, Linseley PS, Stoughton RB, Aebersold R, Collins SJ, Hanlon WA, Hood LE. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics 3: 960–969, 2004. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Tian X, Hellman J, Horswill AR, Crosby HA, Francis KP, Prakash A. Elevated gut microbiome-derived propionate levels are associated with reduced sterile lung inflammation and bacterial immunity in mice. Front Microbiol 10: 159, 2019. [Erratum in Front Microbiol 10: 518, 2019.] doi: 10.3389/fmicb.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togashi H, Emala CW, Hall IP, Hirshman CA. Carbachol-induced actin reorganization involves Gi activation of Rho in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 274: L803–L809, 1998. doi: 10.1152/ajplung.1998.274.5.L803. [DOI] [PubMed] [Google Scholar]
- 44.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 45.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem 19: 587–593, 2008. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Walter H, Sadeque-Iqbal F, Ulysse R, Castillo D, Fitzpatrick A, Singleton J. The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol. JBI Database Syst Rev Implement Rep 13: 69–81, 2015. doi: 10.11124/jbisrir-2015-2335. [DOI] [PubMed] [Google Scholar]
- 48.Watts VJ. Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther 302: 1–7, 2002. doi: 10.1124/jpet.302.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol Ther 106: 405–421, 2005. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Zhao X, Liu H, Jin H, Ji Y. Trichostatin A inhibits proliferation of PC3 prostate cancer cells by disrupting the EGFR pathway. Oncol Lett 18: 687–693, 2019. doi: 10.3892/ol.2019.10384. [DOI] [PMC free article] [PubMed] [Google Scholar]