Abstract
Bronchopulmonary dysplasia (BPD), a long-term respiratory morbidity of prematurity, is characterized by attenuated alveolar and vascular development. Supplemental oxygen and immature antioxidant defenses contribute to BPD development. Our group identified thioredoxin reductase-1 (TXNRD1) as a therapeutic target to prevent BPD. The present studies evaluated the impact of the TXNRD1 inhibitor aurothioglucose (ATG) on pulmonary responses and gene expression in newborn C57BL/6 pups treated with saline or ATG (25 mg/kg ip) within 12 h of birth and exposed to room air (21% O2) or hyperoxia (>95% O2) for 72 h. Purified RNA from lung tissues was sequenced, and differential expression was evaluated. Hyperoxic exposure altered ~2,000 genes, including pathways involved in glutathione metabolism, intrinsic apoptosis signaling, and cell cycle regulation. The isolated effect of ATG treatment was limited primarily to genes that regulate angiogenesis and vascularization. In separate studies, pups were treated as described above and returned to room air until 14 days. Vascular density analyses were performed, and ANOVA indicated an independent effect of hyperoxia on vascular density and alveolar architecture at 14 days. Consistent with RNA-seq analyses, ATG significantly increased vascular density in room air, but not in hyperoxia-exposed pups. These findings provide insights into the mechanisms by which TXNRD1 inhibitors may enhance lung development.
Keywords: acute lung injury, acute respiratory distress syndrome, aurothioglucose, bronchopulmonary dysplasia, thioredoxin reductase
INTRODUCTION
In critically ill neonates, there exists a delicate balance between oxygen needs for tissue survival and damage caused by excessive oxygen exposure. Hypoxemia is detrimental, while hyperoxia leads to increased levels of reactive oxygen species. Insufficient and immature antioxidant defenses further the therapeutic impact of oxygen. Bronchopulmonary dysplasia (BPD) represents a multifactorial disorder characterized by enlarged alveoli and decreased lung function. Thioredoxin reductase-1 (TXNRD1), an NADPH-dependent selenocysteine-containing oxidoreductase, catalyzes the reduction of oxidized thioredoxin-1 (38). In newborn mice exposed to 85% O2 for 14 days, TXNRD1 inhibition improves lung development and enhances hyperoxia-induced nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant responses in C3H/HeN, but not C57BL/6, mice (6, 19, 30). Aurothioglucose (ATG) also increased alveolar number in room air-exposed newborn C3H/HeN mice. The protective effects of TXNRD1 inhibition in C3H/H3N mice are likely mediated by enhanced glutathione (GSH)-dependent antioxidant defenses (32). In C57BL/6 mice, the lack of a protective effect of TXNRD1 inhibition was most reasonably attributable to baseline decreases in Nrf2-dependent responses (9, 10)
Vascular development is impaired in patients with BPD. Vascular remodeling is present in 30–50% of patients with BPD, and decreased pulmonary vascular development contributes to pulmonary hypertension in animal models and in patients (2, 5, 26). Rodent models established a link between alveolar and vascular development (1, 14). Inhibition of angiogenesis in neonatal rats causes alveolar simplification similar to that seen in severe BPD (26). In hyperoxic BPD models, proangiogenic factors rescue abnormal lung development (17, 29).
Short-term acute hyperoxic exposure of neonatal mice causes durable effects on lung development (24). ATG improves pulmonary outcomes in adult mouse models of acute lung injury (6, 30). The present studies investigated the effects of ATG in a neonatal murine model of short-term acute high-level hyperoxia in C57BL/6 mice.
MATERIALS AND METHODS
Animal models.
Animal protocols were approved by the Institutional Animal Care and Use Committee at the Research Institute at University of Alabama at Birmingham. Mice were handled in accordance with the National Institutes of Health guidelines. C57BL/6 mice were bred, and at least two dams were required to deliver within 12 h of each other. Once pups were born, pups received either 25 mg/kg aurothioglucose (ATG) or saline intraperitoneally. Pups of both sexes were randomly and equally distributed between two dams and exposed to either room air (RA; 21% O2) or hyperoxia (>95% O2) for 72 h. In separate studies, pups were removed from hyperoxia at 72 h and allowed to recover in RA until 14 days. All hyperoxic exposures and euthanasia were performed as previously published (18). Morphometric analyses were performed (19, 20).
RNA sequencing analyses.
RNA was isolated from bronchoaleolar lavage fluid removed from snap-frozen lungs (n = 3) using RNeasy mini kit. RNA purity was confirmed (Spectramax, i3x multi-mode microplate reader; San Jose, CA), and sequencing was performed at the DNA Sequencing and Genotyping Core at Cincinnati Children’s Hospital Medical Center using an established next-generation sequencing pipeline. Total RNA was used to generate amplified cDNA (NuGen Ovation RNA-Seq System V2 kit, Redwood City, CA). A sequencing library was prepared (Illumina Nextera XT DNA sample preparation kit; San Diego, CA), and next-generation sequencing of equimolar pools of cDNA libraries was performed using a paired-end 75-bp rapid flow cell (Illumina HiSeq 2500 sequencing platform). Resulting fastq files were aligned to mm10 RefSeq using Bowtie2 (15). Raw gene counts were obtained using Bioconductor’s Genomic Alignment, and normalized RPKM values were generated using Cufflinks (16, 31). DeSeq was used to analyze the raw gene counts and calculate differentially expressed genes (3). Genes were deemed differentially expressed with a fold change >1.2×, nbinomTest P < 0.05 and RPKMs (reads per kilobase per million mapped reads) > 1 for 50% of the samples in at least one condition being compared. ToppFun was used to identify functional enrichment hits of significantly altered RNAs (7). Raw data can be accessed via Geo accession number GSE145912.
Immunohistochemistry and vascular density determinations.
Immunohistochemistry for von Willebrand Factor (vWF) was performed as published in Ref. 13.
Statistics.
All nonsequencing data were tested for homogeneity of variances and log-transformed when indicated. Parametric data were analyzed by ANOVA followed by Tukey’s multiple-comparison tests post hoc (GraphPad Prism 8.0, La Jolla, CA). Significance was accepted at P < 0.05.
RESULTS
Hyperoxia alters genes associated with apoptosis, glutathione metabolism, and DNA synthesis.
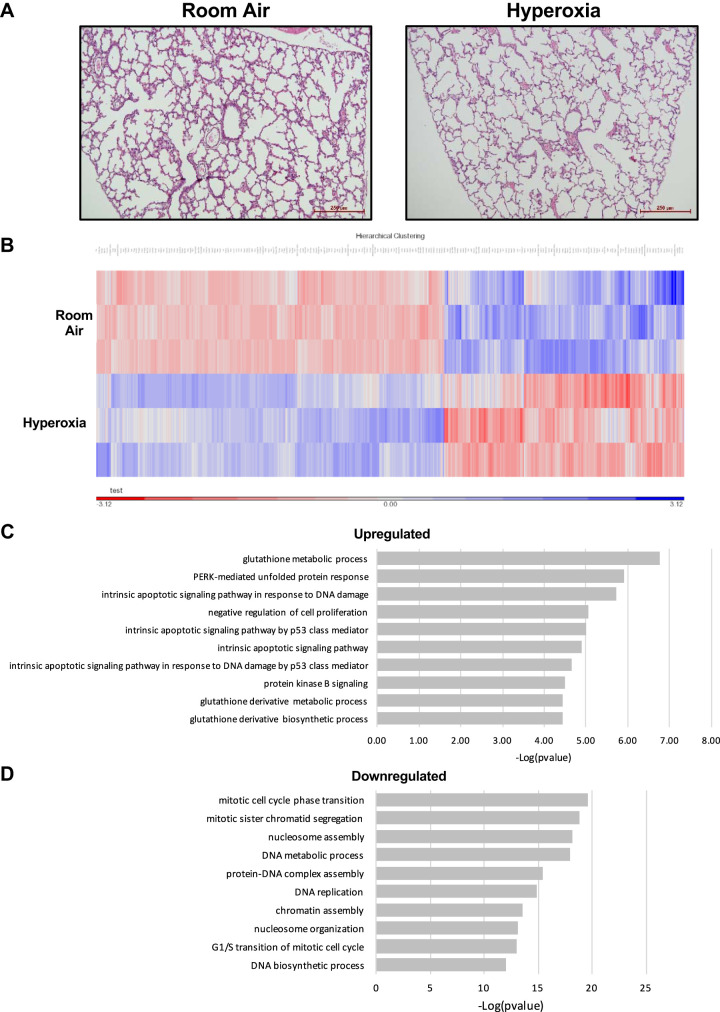
Qualitatively, 3-day hyperoxia minimally altered lung structure (Fig. 1A). RNA sequencing of peripheral lung sections from mice exposed to room air or >95% O2 for 72 h indicated that hyperoxic exposure altered the expression of ~2,000 genes (Fig. 1B). Four of the top 10 pathways upregulated by hyperoxia govern intrinsic apoptotic pathways (Fig. 1C). Specifically, cyclin-dependent kinase inhibitor 1A (Cdkn1A; 7.6-fold), zinc finger matrin-type 3 (Zmat3; 4.5-fold), and DNA damage-inducible transcript 4 (Ddit4, 1.7-fold) were represented in each pathway. Three of the top 10 upregulated pathways were associated with glutathione metabolism, including glutathione S-transferase alpha 3 (Gsta3, 2.5-fold), glutathione S-transferase mu 1 (Gstm1; 1.8-fold), glutathione S-transferase omega 1 (Gsto1; 1.6-fold), and glutathione S-transferase P (Gstp1; 1.6-fold). Among 10 pathways most downregulated by hyperoxia, the majority regulate DNA synthesis and cell cycle progression (Fig. 1D). These include H2B clustered histone 11 (Hist1h2bj; −2.6-fold), H2B clustered histone 14 (H2bc14; −2.9-fold), H2B histone cluster 15 (H2bc15; −2.2-fold), and minichromosome maintenance complex component 2 (Mcm2; −1.5-fold), and 5 (Mcm5; −1.9-fold).
Fig. 1.
Impact of 3 days of hyperoxia on lung development and transcription. Newborn C57BL/6 mice were exposed to room air (21% O2) or hyperoxia (>95% O2) for 72 h. RNA was isolated and purified from snap-frozen peripheral lungs, and RNA-sequencing analysis was performed. A: representative hematoxylin-and-eosin-stained images. B: heat map and hierarchical clustering of upregulated (blue) and downregulated genes (red). C: gene ontology analysis was performed, and the top 10 upregulated pathways associated with genes altered by hyperoxic exposure are shown. D: top 10 downregulated pathways associated with genes that were altered by hyperoxic exposure are shown.
Transcriptomic alterations are predominantly related to hyperoxic exposure.
RNA sequencing was performed on ATG-treated room air and hyperoxia-exposed mouse lungs and compared with non-ATG-treated samples of similar exposure. A heat map of differentially altered genes from pairwise comparisons is shown (Fig. 2A). In general, changes most strongly correlated with hyperoxia and not ATG treatment. Principal component analyses (Fig. 2B) demonstrated segregation of room air and hyperoxia exposure groups with overlap between saline and ATG treatment within each exposure group.
Fig. 2.

Transcriptomic alterations in lungs after 3 days of hyperoxia and/or aurothioglucose (ATG) treatment. Newborn C57BL/6 mice were treated with saline (SAL) or 25 mg/kg ATG within 12 h of birth and exposed to room air (RA; 21% O2) or hyperoxia (HYP; >95% O2) for 72 h. RNA was isolated and purified from snap-frozen peripheral lungs, and RNA-sequencing analysis was performed. A: heat map and hierarchical clustering of upregulated (blue) and downregulated genes (red). B: principal component analysis (PCA) demonstrating segregation of groups (ovals).
ATG specifically upregulates proangiogenic and vascular development signaling pathways.
Lung sections from ATG-treated mice exposed to room air or hyperoxia were qualitatively indistinguishable from each other (Fig. 3A) and from respective non-ATG controls (Fig. 1A). Within the room air exposure group, 308 genes were altered by ATG treatment, and 276 genes were altered in the lungs of ATG + hyperoxia pups (Fig. 3B). Fifty-two genes were altered in the same direction by ATG independent of exposure (Fig. 3C). Genes that regulate endothelial cell signaling and vascular function, including phosphodiesterase 4b (Pde4b; 2.9-fold) and apolipoprotein L domain containing 1 (Apold1; 1.7-fold), were increased. Biological processes upregulated by ATG (Fig. 3D) included angiogenesis (P = 0.00002), blood vessel development (P = 0.00004), vasculature development (P = 0.00005), blood vessel morphogenesis (P = 00007), endocardial cushion development (P = 0.0001), and cardiac muscle cell contraction (P = 0.0003).
Fig. 3.
Effect of aurothioglucose (ATG) at 3 days in newborn mouse lungs from room air or hyperoxia-exposed neonatal mice. Newborn C57BL/6 mice were treated with saline or 25 mg/kg ATG within 12 h of birth and exposed to room air (21% O2) or hyperoxia (>95% O2) for 72 h. RNA was isolated and purified from snap-frozen peripheral lungs, and RNA-sequencing analysis was performed. A: representative hematoxylin-and-eosin-stained images from ATG-treated mice exposed to room air or hyperoxia. B: Venn diagram of differentially altered genes in lungs from saline vs. ATG-treated mice exposed to room air (left) and hyperoxia (right). Of the differentially altered genes, 52 were specific to ATG (center). C: genes altered by ATG. D: pathways specifically upregulated by ATG treatment (gene ontology analysis).
Acute neonatal hyperoxia attenuates lung vascular and alveolar development while ATG increases vascular density in room air-exposed mice at 14 days.
To evaluate the functional impact of acute hyperoxia and ATG administration on lung vascular and alveolar development, mice treated with saline or ATG and exposed to >95% oxygen for the first 72 h of life were returned to room air until 14 days of age. Immunostaining for the endothelial cell marker von Willebrand factor (vWF) was performed in paraffin-embedded lung sections to determine vascular density (Fig. 4A) (8, 28, 37). Two-way ANOVA revealed an independent effect of hyperoxia (P < 0.0001), but not ATG (P = 0.07), and an interaction between hyperoxia and ATG (P = 0.0002) (Fig. 4B). In room air, ATG significantly increased vessel density at 14 days when compared with saline-treated controls (13.9 ± 0.7 vs. 11.36 ± 1.1, P = 0.0004).
Fig. 4.
Effects of aurothioglucose (ATG) treatment and hyperoxic exposure on lung vessel density. Newborn C57BL/6 mice were treated with saline (SAL) or 25 mg/kg aurothioglucose (ATG) within 12 h of birth, exposed to room air (RA; 21% O2) or hyperoxia (HYP; >95% O2) for 72 h, then returned to RA to 14 days. A: representative images of von Willebrand factor (vWF)-stained lung slices (scale bars = 100 μm). B: vessel density. *P = 0.0004 vs. SAL/RA. #P < 0.0001 vs. ATG/RA. C: radial alveolar count. Data are expressed as means ± SD; n = 6–9. Analysis was performed by two-way ANOVA, followed by Tukey’s post hoc test.
To quantify the durable impact of short-term perinatal hyperoxia and ATG treatment on lung architecture, radial alveolar count (RAC) and mean linear intercept (MLI) were calculated at 14 days (Fig. 4C). Two-way ANOVA indicated an independent effect of hyperoxia but not ATG on RAC (P = 0.0095). Neither hyperoxia nor ATG independently altered MLI (not shown).
DISCUSSION
The present studies investigated the effects of ATG in a neonatal murine model of short-term acute high-level hyperoxia using C57BL/6 mice. We performed RNA sequencing and functional pathway analyses of lungs taken from mice exposed to >95% hyperoxia in the first 72 h of life in the presence and absence of systemic ATG pretreatment. Our primary findings indicate 1) that hyperoxia predominantly increases the activation of pathways that regulate intrinsic apoptosis and glutathione metabolism, while downregulating pathways that regulate DNA synthesis and cell cycle progression (Fig. 1); 2) that alterations in transcription are disproportionally affected by hyperoxia and not ATG (Fig. 2); 3) that the 53 genes specifically altered by ATG predominantly regulate angiogenesis and vascular development (Fig. 3); 4) that early hyperoxia causes persistent defects in lung vascular and alveolar development (Fig. 4); and 5) that ATG increases blood vessel density in room air exposed mice at 14 days.
Our previous data in C3H/HeN mice demonstrated that ATG + 85% oxygen exposure enhanced Nrf2-dependent responses, including higher GSH levels in treated and exposed lungs (32). This therapeutic effect was not observed in C57BL/6 mice (18). Strain-dependent differences in Nrf2-dependent responses to hyperoxia likely explain the lack of efficacy in the 14-day developmental BPD model (11). The present studies did not identify Nrf2-dependent pathways among those upregulated by a 3-day exposure to >95% in C57BL/6 mice. Unexpectedly, upregulation of GSH-dependent responses occurred in the absence of evidence of Nrf2 activation by hyperoxia. The rate-limiting step in de novo GSH synthesis is Nrf2-regulated, meaning that our data have identified Nrf2-independent control of GSH-dependent responses upon acute neonatal hyperoxic exposure. We speculate that Nrf2-independent responses are insufficient to prevent durable impacts on alveolarization given the independent effect of oxygen exposure on lung development at 14 days (Fig. 4B). In addition, the hyperoxia-induced upregulation of glutathione S-transferase (Gst) is associated with findings of enhanced intrinsic apoptotic signaling, DNA damage, and protection of cell cycle pathways, suggesting compensatory increases in GSH-dependent responses to limit DNA damage and to enhance repair mechanisms (4, 33, 34). Gstp1, Gstm1, and Gstt1 were upregulated by hyperoxia in our studies. Susceptibility to BPD has been linked to human polymorphisms in GSTP1, GSTM1 and GSTT1 (21, 34).
Of the >2,000 genes altered in our experimental conditions, the majority was altered by hyperoxia alone (Fig. 2). Although the impact of hyperoxia on transcriptional response in the lung is not a surprising finding, the trivial impact of ATG treatment was unexpected (12, 27). In our current working model, the protective effect of ATG on lung development in C3H/HeN, but not C57BL/6 mice, is likely attributable the relative differences in Nrf2 haplotypes between strain. The present studies used a higher concentration of oxygen, based upon the underlying hypothesis that 85% oxygen was not potent enough to elicit Nrf2-dependent responses in C57BL/6 mice. The present studies indicate that >95% oxygen is also not potent enough to activate Nrf2-dependent pathways, as our analyses failed to identify Nrf2 responses. Although the database included Nrf2-regulated genes, glutathione peroxidase (Gpx), heme oxygensase-1 (Hmox1), gamma-glutamate cysteine ligase modifier (Gclm) and catalytic (Gclc) subunits, and NADPH quinone oxidoreductse-1 (Nqo1), only Gclc (2.4-fold; P < 0.0001) and Nqo1 (1.9-fold; P = 0.0005) were significantly upregulated by hyperoxia. Thus, it is not surprising that ATG did not significantly alter pulmonary responses to hyperoxia in the present studies (6, 19, 20, 30).
ATG specifically altered 53 genes across all treatment/exposure groups (Fig. 3). Among these genes, many positively regulate endothelial cell signaling and vascular development. In our C3H/HeN neonatal hyperoxia studies, ATG independently increased alveolar development in room air-exposed mice (19). Vascular and alveolar development are inextricably linked (1). Given this link and the upregulation of vascular development-related pathways by ATG, our finding of increased vascular density in room air-exposed mice is most likely attributable to the proangiogenic effects of ATG.
Acute perinatal hyperoxic exposure caused persistent defects in lung vascular and alveolar architecture despite room air recovery. The independent effect of hyperoxic exposure, as evidenced by decreased vessel density (Fig. 4B) and RAC (Fig. 4C), supports the durable impact of short-term hyperoxia limited to the saccular stage, as previously demonstrated (25, 36). Pde4b was most upregulated by ATG. The PDE family of enzymes regulates cAMP levels (23). Pde4 is upregulated by hyperoxia, while the biological impact of PDE4 inhibition on hyperoxia-induced deficits in alveolar development is uncertain (22, 23, 35).
The present findings provide what appears to be conflicting data regarding TXNRD1 as a therapeutic target to prevent BPD. In reality, our findings reflect the complexities of therapeutic development using rodent hyperoxia models. We have established that TXNRD1 inhibition elicits pulmonary protection by enhancing endogenous antioxidant responses via Nrf2 (6, 19, 20, 30). Deficient hyperoxia-induced Nrf2 activation in C57BL/6 mice is confirmed by our transcriptomic analyses despite using the most acute hyperoxic exposure possible. We will leave the implications of these findings in relation to other preclinical studies in C57BL/6 mice to the reader. At the least, our data provide scientific rationale for the consideration of murine strain as a biological variable when using mouse models to study BPD. Our group is currently completing large-scale hyperoxic studies using unbiased methodological approaches to define the impacts of strain, exposure, treatment, and sex on pulmonary responses to hyperoxia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-119280 (to T.E.T.) and R01-HL-119280-S1 (to T.E.T.) and K08-HL-132014-01 (to C.D.) and American Heart Association Grant AHA19PRE343805000 (to K.D.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.D.-R. and T.E.T. conceived and designed research; K.D.-R., V.L., M.S., S.B.W., R.L., J.G., A.S., J.S., and Q.L. performed experiments; K.D.-R., V.L., M.S., S.B.W., T.N., J.S., and C.D. analyzed data; K.D.-R., J.S., and T.E.T. interpreted results of experiments; K.D.-R., J.S., C.D., and T.E.T. prepared figures; K.D.-R. and T.E.T. drafted manuscript; K.D.-R., V.L., M.S., S.B.W., J.S., C.D., and T.E.T. edited and revised manuscript; K.D.-R., V.L., M.S., S.B.W., R.L., J.G., T.N., A.S., J.S., C.D., Q.L., and T.E.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jeffrey Whitsett for advice on experimental design.
REFERENCES
- 1.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 164: 1755–1756, 2001. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 2.Alvira CM. Aberrant Pulmonary Vascular Growth and Remodeling in Bronchopulmonary Dysplasia. Front Med (Lausanne) 3: 21, 2016. doi: 10.3389/fmed.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders S, Huber W. Differential expression of RNA-Seq data at the gene level–the DESeq package. Heidelberg, Germany: European Molecular Biology Laboratory (EMBL), 2012. [Google Scholar]
- 4.Baden J, Adams S, Astacio T, Jones J, Markiewicz J, Painter J, Trust C, Wang Y, Green G. Predicting prostate biopsy result in men with prostate specific antigen 2.0 to 10.0 ng/ml using an investigational prostate cancer methylation assay. J Urol 186: 2101–2106, 2011. doi: 10.1016/j.juro.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology 107: 344–351, 2015. doi: 10.1159/000381129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt RD Jr, Velten M, Locy ML, Rogers LK, Tipple TE. The thioredoxin reductase-1 inhibitor aurothioglucose attenuates lung injury and improves survival in a murine model of acute respiratory distress syndrome. Antioxid Redox Signal 20: 2681–2691, 2014. doi: 10.1089/ars.2013.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305-11, 2009. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi X, Guo N, Yao W, Jin Y, Gao W, Cai J, Hei Z. Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. J Transl Med 14: 35, 2016. doi: 10.1186/s12967-016-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HY, Jedlicka AE, Gladwell W, Marzec J, McCaw ZR, Bienstock RJ, Kleeberger SR. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxid Redox Signal 22: 325–338, 2015. doi: 10.1089/ars.2014.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HY, Kleeberger SR. Association of Nrf2 with airway pathogenesis: lessons learned from genetic mouse models. Arch Toxicol 89: 1931–1957, 2015. doi: 10.1007/s00204-015-1557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HY, van Houten B, Wang X, Miller-DeGraff L, Fostel J, Gladwell W, Perrow L, Panduri V, Kobzik L, Yamamoto M, Bell DA, Kleeberger SR. Targeted deletion of nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxid Redox Signal 17: 1066–1082, 2012. doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K. Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol 313: L991–L1005, 2017. doi: 10.1152/ajplung.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaney C, Sherlock L, Fisher S, Maltzahn J, Wright C, Nozik-Grayck E. Serotonin 2A receptor inhibition protects against the development of pulmonary hypertension and pulmonary vascular remodeling in neonatal mice. Am J Physiol Lung Cell Mol Physiol 314: L871–L881, 2018. doi: 10.1152/ajplung.00215.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 15.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence M, Huber W, Pagès H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, Carey VJ. Software for computing and annotating genomic ranges. PLOS Comput Biol 9: e1003118, 2013. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562, 2002. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Li R, Wall SB, Dunigan K, Ren C, Jilling T, Rogers LK, Tipple TE. Aurothioglucose does not improve alveolarization or elicit sustained Nrf2 activation in C57BL/6 models of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 314: L736–L742, 2018. doi: 10.1152/ajplung.00539.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Wall SB, Ren C, Velten M, Hill CL, Locy ML, Rogers LK, Tipple TE. Thioredoxin Reductase Inhibition Attenuates Neonatal Hyperoxic Lung Injury and Enhances Nuclear Factor E2-Related Factor 2 Activation. Am J Respir Cell Mol Biol 55: 419–428, 2016. doi: 10.1165/rcmb.2015-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locy ML, Rogers LK, Prigge JR, Schmidt EE, Arnér ES, Tipple TE. Thioredoxin reductase inhibition elicits Nrf2-mediated responses in Clara cells: implications for oxidant-induced lung injury. Antioxid Redox Signal 17: 1407–1416, 2012. doi: 10.1089/ars.2011.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manar MH, Brown MR, Gauthier TW, Brown LA. Association of glutathione-S-transferase-P1 (GST-P1) polymorphisms with bronchopulmonary dysplasia. J Perinatol 24: 30–35, 2004. doi: 10.1038/sj.jp.7211020. [DOI] [PubMed] [Google Scholar]
- 22.Méhats C, Bourbon J, Jarreau PH. Does PDE4 inhibition improve alveolarisation in hyperoxia-exposed immature rodents? Eur Respir J 33: 1236, 2009. doi: 10.1183/09031936.00002809. [DOI] [PubMed] [Google Scholar]
- 23.Méhats C, Franco-Montoya ML, Boucherat O, Lopez E, Schmitz T, Zana E, Evain-Brion D, Bourbon J, Delacourt C, Jarreau PH. Effects of phosphodiesterase 4 inhibition on alveolarization and hyperoxia toxicity in newborn rats. PLoS One 3: e3445, 2008. doi: 10.1371/journal.pone.0003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols JL, Gladwell W, Verhein KC, Cho HY, Wess J, Suzuki O, Wiltshire T, Kleeberger SR. Genome-wide association mapping of acute lung injury in neonatal inbred mice. FASEB J 28: 2538–2550, 2014. doi: 10.1096/fj.13-247221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Reilly MA, Yee M, Buczynski BW, Vitiello PF, Keng PC, Welle SL, Finkelstein JN, Dean DA, Lawrence BP. Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol 181: 441–451, 2012. doi: 10.1016/j.ajpath.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623–661, 2005. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 27.Sucre JMS, Vickers KC, Benjamin JT, Plosa EJ, Jetter CS, Cutrone A, Ransom M, Anderson Z, Sheng Q, Fensterheim BA, Ambalavanan N, Millis B, Lee E, Zijlstra A, Königshoff M, Blackwell TS, Guttentag SH. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am J Respir Crit Care Med In press. doi: 10.1164/rccm.201908-1513OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahara T, Sun J, Igarashi K, Taketani S. Heme-dependent up-regulation of the α-globin gene expression by transcriptional repressor Bach1 in erythroid cells. Biochem Biophys Res Commun 324: 77–85, 2004. doi: 10.1016/j.bbrc.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 30.Tipple TE, Welty SE, Rogers LK, Hansen TN, Choi YE, Kehrer JP, Smith CV. Thioredoxin-related mechanisms in hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 37: 405–413, 2007. doi: 10.1165/rcmb.2006-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall SB, Wood R, Dunigan K, Li Q, Li R, Rogers LK, Tipple TE. Thioredoxin reductase-1 inhibition augments endogenous glutathione-dependent antioxidant responses in experimental bronchopulmonary dysplasia. Oxid Med Cell Longev 2019: 7945983, 2019. doi: 10.1155/2019/7945983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem 276: 20999–21003, 2001. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Li W, Liu W, Cai B, Cheng T, Gao C, Mo L, Yang H, Chang L. GSTM1 and GSTT1 gene polymorphisms as major risk factors for bronchopulmonary dysplasia in a Chinese Han population. Gene 533: 48–51, 2014. doi: 10.1016/j.gene.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Rühlmann A, Weissmann N, Ghofrani HA, Günther A, Seeger W, Grimminger F, Morty RE, Schermuly RT. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J 33: 861–870, 2009. doi: 10.1183/09031936.00109008. [DOI] [PubMed] [Google Scholar]
- 36.Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, Dean DA, Notter RH, O’Reilly MA. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol 297: L641–L649, 2009. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol 27: 6962–6971, 2007. doi: 10.1128/MCB.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Zhang J, Peng S, Liu R, Li X, Hou Y, Han X, Fang J. Thioredoxin reductase inhibitors: a patent review. Expert Opin Ther Pat 27: 547–556, 2017. doi: 10.1080/13543776.2017.1272576. [DOI] [PubMed] [Google Scholar]