Abstract
We have demonstrated previously that intracellular transport is impaired in cystic fibrosis (CF) epithelial cells. This impairment is related to both growth and inflammatory regulation in CF cell and animal models. Understanding how transport in CF cells is regulated and identifying means to manipulate that regulation are key to identifying new therapies that can address key CF phenotypes. It was hypothesized that resveratrol could replicate these benefits since it interfaces with multiple pathways identified to affect microtubule regulation in CF. It was found that resveratrol treatment significantly restored intracellular transport as determined by monitoring both cholesterol distribution and the distribution of rab7-positive organelles in CF cells. This restoration of intracellular transport is due to correction of both microtubule formation rates and microtubule acetylation in cultured CF cell models and primary nasal epithelial cells. Mechanistically, the effect of resveratrol on microtubule regulation and intracellular transport was dependent on peroxisome proliferator-activated receptor-γ signaling and its ability to act as a pan-histone deacetylase (HDAC) inhibitor. Resveratrol represents a candidate compound with known anti-inflammatory properties that can restore both microtubule formation and acetylation in CF epithelial cells.
Keywords: cystic fibrosis, ibuprofen, microtubule, resveratrol
INTRODUCTION
Previous work has identified perinuclear cholesterol accumulation due to impaired endosomal transport as a distinct phenotype in cystic fibrosis (CF) epithelial cells, a process that can be manipulated through a variety of pathways (6, 25, 27, 38, 53). Alterations to microtubule regulation, including reduced acetylation and slowed rates of formation, are directly responsible for the intracellular transport changes observed in CF cells (37). These changes to microtubule regulation and intracellular transport result in signaling cascades associated with inflammatory signaling and growth regulation in CF (20, 22, 36), so identifying easily accessible compounds that can reverse these alterations can have important consequences for therapeutic development.
Mechanisms controlling CF microtubule regulation have been identified. An early cellular event leading to reduced microtubule growth rates is the lower activity levels of the exchange protein activated by cAMP-1 (EPAC1) in CF cells (38). EPAC1 has multiple functions, but one is acting as a microtubule elongation factor (45). We have demonstrated that activation of EPAC1 leads to improved microtubule formation rates in primary CF epithelial cells, resulting in improved intracellular cholesterol distribution due to improved endosomal transport (38). Recent findings also demonstrate that ibuprofen treatment reverses the cholesterol accumulation phenotype by correction of CF microtubule dynamics through an adenosine monophosphate-activated protein kinase (AMPK)-dependent pathway (39). The efficacy of ibuprofen in regulating this pathway is significant, since multiple studies have shown that high-dose ibuprofen therapy is effective at controlling inflammation and reducing the rate of pulmonary function decline, particularly in younger CF patients (16–18, 21). However, the use of ibuprofen in the CF population is limited due to potential adverse effects, so new interventions are needed (2, 19, 24, 44). Finally, inhibition of histone deacetylase 6 (HDAC6), a deacetylase that regulates tubulin acetylation, restores CF microtubule acetylation, reestablishes intracellular transport, and reduces inflammatory signaling in CF models (37). A CF mouse model with Hdac6 expression depleted has restored growth and fertility, further demonstrating the potential therapeutic implications of targeting microtubule defects in CF (36). The goal of this study is to define other mechanisms of microtubule regulation that can be manipulated with a common compound for potential therapeutic use.
It is hypothesized in this study that resveratrol represents an appealing intervention for CF microtubule regulation based on the activation of sirtuins 1 (SIRT1) and 3 (SIRT3). SIRT1 and SIRT3 are deacetylases that regulate a number of cellular functions related to metabolism and inflammation (28, 48). Changes in CF metabolomics and mitochondrial function also point potentially for a need to activate sirtuins (12, 14, 49). Directly relevant to this study, activation of both SIRT1 and SIRT3 has been shown to promote microtubule polymerization and stability (8, 15, 42). Resveratrol also stimulates other pathways that may be relevant to microtubule stability in CF cells such as the stimulation of adenosine monophosphate-activated protein kinase (AMPK) activity (3). As previously noted, ibuprofen corrects CF microtubule dynamics through AMPK activation (39).
In this paper, the ability of resveratrol to improve intracellular transport as marked by improved endosomal trafficking through the regulation of microtubule dynamics and stability is shown. The mechanisms of action are explored, and novel regulatory interactions are defined. The use of resveratrol represents an easily accessible means of modulating microtubule regulation in CF cells and correcting aspects of CF cell biology.
METHODS
Cells.
9/HTEo− cells originally developed by Dr. Dieter Gruenert were human tracheal epithelial cells that transformed by simian virus 40. They were grown at 37°C in a 95% O2-5% CO2 incubator on 10-cm-diameter tissue culture dishes (Corning, Corning, NY) in 10 ml Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% FBS containing 2 mM l-glutamine, 0.04 mM HEPES, 1 U/ml penicillin, and 1 µg/ml streptomycin. IB3 cells (CF), human bronchial epithelial cells isolated and immortalized from a CF patient with ΔF508 mutation, and S9 cells [wild type (WT)], IB3 cell mutation corrected with full-length WT cystic fibrosis transmembrane conductance regulator (CFTR), were a gift from Pamela L. Zeitlin (Johns Hopkins University, Baltimore, MD). They were grown at 37°C in a 95% O2-5% CO2 incubator on 10-cm-diameter tissue culture dishes in LHC-8 basal medium (Gibco) supplemented with 5% FBS containing 1 U/ml penicillin and 1 µg/ml streptomycin. Primary tissue CF mouse nasal epithelium (MNE) was excised from WT and Cftr−/− mice. They were grown at 37°C in a 95% O2-5% CO2 incubator on T-75 flasks (Corning) in DMEM-Ham’s F-12 medium containing Y-27632, penicillin, and streptomycin. Human nasal epithelial cells were obtained from the CWRU CF Center cell culture core facility.
Western immunoblotting.
Acetylated-α-tubulin (mouse) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). α-Tubulin (rabbit) antibodies were obtained from Abcam (Cambridge, MA). Cells were grown to 95% confluency in 35-mm dishes and lysed with lysis buffer (50 mM Tris, pH 7.5, 1% Triton X-100, 50 mM NaF, 200 μM Na3VO4, and 10 μg/ml pepstatin and leupeptin) for 20 min at 4°C. Lysates were centrifuged at 10,000 revolutions/min for 5 min. Proteins were separated using SDS-PAGE containing 15–30 μg protein on 7.5% polyacrylamide gels. Samples were transferred to an Immobilon-P membrane (Millipore, Bedford, MA) at 15 volts for 30 min. Membranes were blocked in 10% nonfat dehydrated milk in PBS with 0.1% Tween 20 (PBS-T) for 1 h at room temperature and then incubated with primary antibodies diluted in 10% nonfat dehydrated milk in PBS-T overnight at 4°C. Membranes were washed three times for 10 min each with PBS-T, incubated with the respective secondary antibodies conjugated to horseradish peroxidase (1:3,000 dilution) in PBS-T, washed again with PBS-T, and visualized using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL) and the Chemidoc Imaging System (Bio-Rad, Hercules, CA). Quantification of protein expression was performed with Quality One software (Bio-Rad).
Filipin staining.
Cells were grown to 60–70% confluency on collagen-coated cover slips. Cells were rinsed three times with PBS, fixed with 4% paraformaldehyde for 30 min, and incubated with 0.05 mg/mL filipin (Sigma-Aldrich, St. Louis, MO) in PBS for 1 h on a shaker in the dark. Filipin was dissolved freshly in dimethyl sulfoxide before each experiment. Cells were rinsed and then mounted using SlowFade (Invitrogen) on slides. Cells were visualized in the ultraviolet range using a wide-field microscope with a ×40 oil objective on a Leica DM6000 upright microscope (×40 oil objective) with Improvision's Volocity software. The number of cells having or not having perinuclear cholesterol accumulation was counted, and quantification was determined by the ratio of cells with accumulation to total cells. Over 35 images were quantified for each condition.
Microtubule formation assay.
Cells were grown on collagen-coated cover slips to 75–80% confluency. Cells were placed on ice for 45–60 min for microtubule depolymerization. After the depolymerization period, prewarmed 37°C media (with carrier or drug) was added at designated time points. At the end of the time course, cells were rinsed with PBS and fixed with cold methanol immediately. Cells were rinsed with PBS and blocked with 5% goat serum for 30 min, incubated with α-tubulin antibodies (1:200 dilution) in 5% goat serum for 1 h, and incubated with Texas Red goat anti-rabbit IgG antibodies (1:4,000 dilution) in 5% goat serum in the dark. Cells were rinsed and mounted with SlowFade (Invitrogen) on slides. Cells were visualized in the appropriate range using a Leica DM6000 upright microscope (×40 oil objective) with Improvision's Volocity software. The number of cells having or not having an aster was counted, and quantification was determined by the ratio of cells with aster formation to total cells at various time points.
Immunostaining.
Rab7 (rabbit) antibodies were obtained from Abcam (Cambridge, MA). Cells were grown on collagen-coated cover slips to 75–80% confluency. Cells were rinsed and fixed with cold methanol for 10 min at −20°C. Cells were blocked in 5% goat serum for 30 min, incubated in 5% goat serum with Rab7 antibodies (1:150) for 1, and incubated with Texas Red goat anti-mouse IgG antibodies in 5% goat serum in the dark. Cells were rinsed and stained with1 µg/mL of 4',6-diamidino-2-phenylindole (DAPI) in PBS for 5 min in the dark. Cells were mounted with SlowFade (Invitrogen) on slides, and they were visualized in the appropriate range using a Leica DM6000 upright microscope (×40 oil objective) with Improvision's Volocity software.
Data analysis.
Using ImageJ software, perimeters were drawn around each nucleus, and partial perimeters highlighting the portion of the nucleus with endosomal accumulation were drawn on top of these. Measurements of the length of these segments were generated on the software and used to calculate the ratio of the covered nuclear circumference. A t test was performed to compare the ratios of perinuclear accumulation between resveratrol (RSV)-treated and mock-treated IB3 cells. Every cell with a nucleus fully visible in the frame of the image and that did not appear to be dividing was measured by this method and included in the analysis.
RESULTS
Resveratrol restores cholesterol processing in CF-model cells.
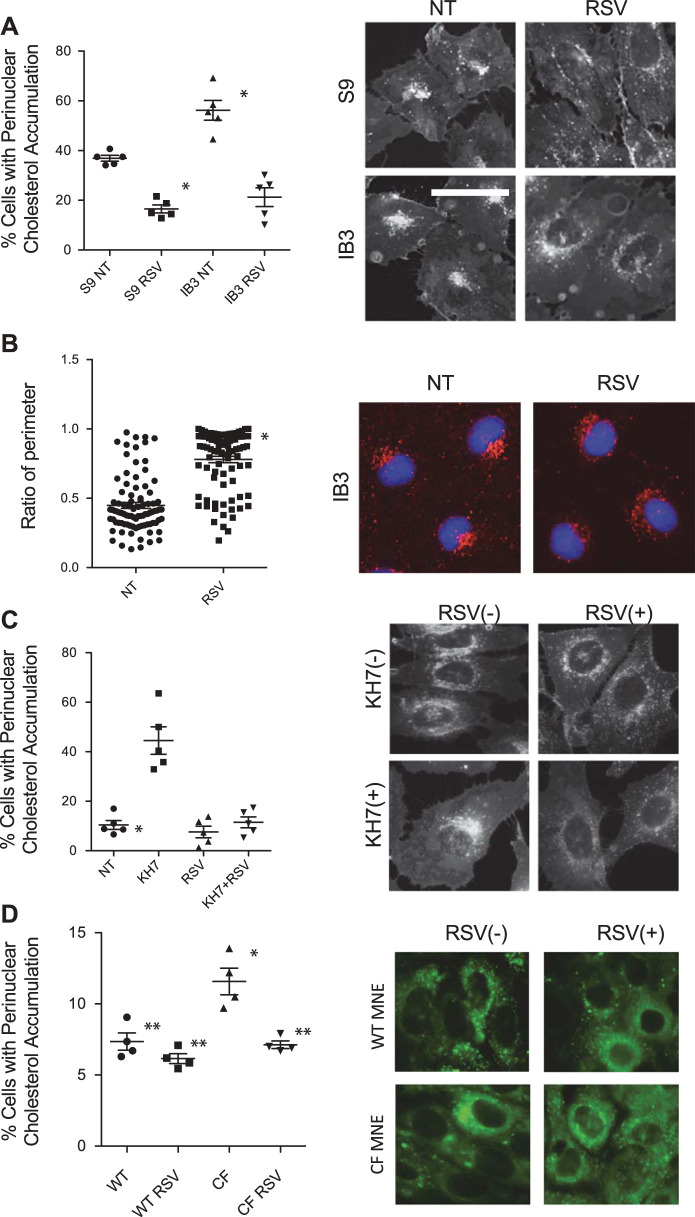
Two cultured cell models were used to initially test whether resveratrol treatment could restore cholesterol trafficking. We first used the immortalized CF cell line IB3 cells and corrected control S9 cells, since the cholesterol accumulation phenotype occurs in these cells (53). Resveratrol treatment (50 µM, 24 h) effectively reverses the perinuclear cholesterol accumulation, suggesting intracellular transport is restored (Fig. 1A). Cells with cholesterol accumulation decreased from 56.2 ± 8.9% to 21.3 ± 8.4% in IB3 cells when treated with RSV. In S9 cells, this number decreased from 36.9 ± 2.6% to 16.5 ± 3.6%. To test endosomal distribution directly, endosomal localization was determined by Rab7 immunostaining. In CF-model cells, it was shown that Rab7-positive organelles accumulate near the nucleus with a limited distribution, as shown previously (40). Resveratrol treatment leads to redistribution of the endosomes, consistent with improving intracellular transport (Fig. 1B). The mean percentage of Rab7-covered nuclear circumferences was 44.8% for mock-treated and 78.0% for RSV-treated IB3 cells. It was previously reported that inhibition of soluble adenylate cyclase with KH7 in WT epithelial cells mimics the CF phenotype of cholesterol accumulation and microtubule disruption (38). The ability of resveratrol to reverse the actions of KH7 on cholesterol transport was tested. Consistent with CF cell data, resveratrol (50 µM, 24 h) treatment completely restores cholesterol trafficking in KH7-treated cells (Fig. 1C). The percentage of cells with cholesterol accumulation was 10.4 ± 4.0% for cells treated with vehicle, 44.6 ± 12.5% for cells treated with KH7 only, 7.6 ± 5.2% for cells treated with RSV only, and 11.4 ± 5.0% for cells treated with both KH7 and RSV. These data demonstrate that resveratrol is an effective agent in reversing intracellular transport issues associated with CF-model cells.
Fig. 1.
Resveratrol restores cholesterol processing in cystic fibrosis (CF)-model cells. A: S9 and IB3 cells are treated with vehicle or with 50 µM of resveratrol (RSV) for 24 h and assessed for cholesterol distribution by filipin staining. Significance is determined by t test comparing vehicle vs. resveratrol treated for each cell line; *P < 0.001, n = 5 experiments. Representative images are shown for each condition. B: IB3 cells were treated with vehicle or 50 µM of RSV for 24 h, immunostained with Rab7 antibodies and DAPI. Images of cells were quantified as mentioned in Immunostaining. Over 85 cells of each condition from 5 separate experiments were measured. Significance was determined by t test to determine significance between groups; P < 0.001. Representative images are shown for each condition. C: 9/HTEo− cells were treated with RSV (50 µM) in the absence and presence of KH7 (50 µM) for 24 h and assessed for cholesterol distribution by filipin staining as mentioned in methods. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), P < 0.05 (n = 5 experiments). Representative images are shown for each condition. Scale bars represent 50 µm. D: wild-type (WT) and cystic fibrosis transmembrane conductance regulator (Cftr)−/− (CF) mouse nasal epithelium (MNE) cells were treated with RSV (50 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups, n = 4 separate experiments. *P < 0.05 compared with WT untreated. **P < 0.05 compared with CF untreated.
It has been reported that RSV can lead to CFTR activation. To test whether CFTR function was necessary for the intracellular transport correction in CF cells, we tested the ability of RSV to improve cholesterol mobilization in primary mouse nasal epithelial (MNE) cells isolated from Cftr−/− mice. RSV (50 µM) reduced cholesterol accumulation significantly in Cftr−/− MNE, showing CFTR is not necessary for RSV efficacy (Fig. 1D). We also examined the impact of RSV treatment on F508del CFTR function using human nasal epithelial (HNE) cells from F508del patients. No enhancement of F508del CFTR activity is observed with RSV (Table 1). Together these data indicate that modulation of CFTR function is not a mechanism of RSV efficacy.
Table 1.
Effect of RSV on CFTR function in F508del/F508del HNE cells
|
Isc |
P Value | ||
|---|---|---|---|
| Treatment | DMSO | RSV (50 µM) | |
| Baseline | 81.9 ± 33.4 | 114.1 ± 2.6 | NS |
| Amiloride (ΔIsc) | −1.6 ± 0.8 | −2.5 ± 0.4 | NS |
| Forskolin/vx-770 (ΔIsc) | −1.3 ± 2.3 | −3.3 ± 1.1 | NS |
| Inh172 (ΔIsc) | −3.5 ± 1.0 | −4.0 ± 0.3 | NS |
Values are means ± SE; n = 5 experiments for each group. Isc, short-circuit current. Units are µA/cm2. Resveratrol (RSV) has no effect on cystic fibrosis transmembrane conductance regulator (CFTR) function in primary cystic fibrosis human nasal epithelial (HNE) cells homozygous for the F508del mutation. Cells were treated with 50 µM RSV for 24 h. There were no significant (NS) differences in resistance values after any treatment. Significance determined by t test.
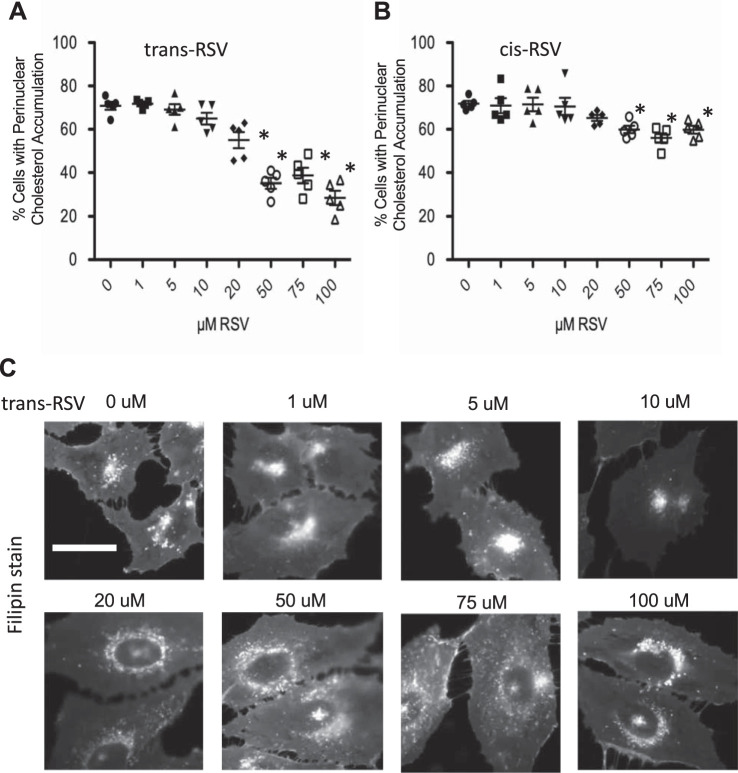
To determine if the efficacy of RSV was dose dependent, a dose-response curve examining cholesterol mobilization (Fig. 2, A and C) was performed. Results show a clear dose response, with the first near-maximal effect dose being 50 µM. These studies are done with trans-RSV, so we also examined the efficacy of cis-RSV in mediating increased endosomal transport in IB3 cells. cis-RSV is statistically effective in correcting the phenotype in CF-model cells at higher doses, although less efficiently than trans-RSV (Fig. 2B).
Fig. 2.
Differential effect of trans-resveratrol (RSV) and cis-RSV on cholesterol distribution in cystic fibrosis (CF) IB3 cells. A: dose response of trans-RSV on cholesterol distribution. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups, n = 5 separate experiments. *P < 0.05 compared with 0 µM RSV group. B: dose response of cis-resveratrol (RSV) on cholesterol distribution. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups, n = 5 separate experiments. *P < 0.05 compared with 0 µM RSV group. C: representative images of cholesterol distribution in IB3 cells with varying doses of trans-RSV. Scale bars represent 50 µm.
Determination of resveratrol-mediated signaling mechanisms controlling intracellular transport.
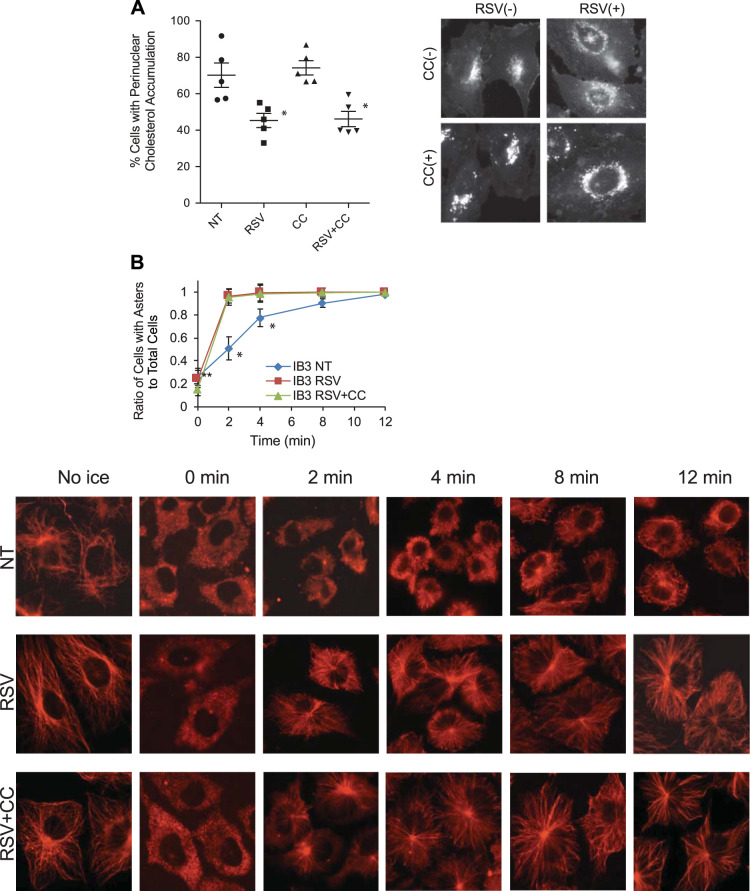
The above data demonstrate that resveratrol treatment significantly improves both microtubule structure and intracellular transport in CF-model cells. We have previously demonstrated that AMPK activation corrects microtubule formation rates in primary CF epithelial cells whether stimulated by ibuprofen or 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; see Ref. 39). The effect of these compounds on microtubule regulation was completely blocked by the AMPK inhibitor compound C (40). Because resveratrol is capable of stimulating AMPK function, the role of this pathway in mediating the effects of resveratrol was tested. IB3 cells were treated with resveratrol (50 µM, 24 h) in the absence and presence of compound C (10 µM). Compound C had no effect on the ability of resveratrol to correct cholesterol distribution in IB3 cells (Fig. 3A). The percentage of cells with cholesterol accumulation was 70.2 ± 14.9% for cells treated with vehicle, 45.4 ± 8.6% for cells treated with RSV only, 74.2 ± 8.8% for cells treated with compound C only, and 46.1 ± 9.4% for cells treated with both compound C and RSV. Previous work has demonstrated that the direct cause of impaired intracellular transport in CF cells is changes to microtubule acetylation and reformation rates (37–39). We tested directly whether resveratrol could influence microtubule reformation rates in CF-model IB3 cells. Results demonstrate that resveratrol treatment does indeed increase the rate of reformation in IB3 cells. However, compound C also had no influence on resveratrol stimulation of microtubule formation rates in IB3 cells, further demonstrating a lack of AMPK signaling in mediating the effects of resveratrol (Fig. 3B).
Fig. 3.
Compound C (CC) could not reverse the effect of resveratrol on correction of cholesterol distribution or stimulation of microtubule formation rates in IB3 cells. A: IB3 cells were treated with resveratrol (RSV, 50 µM) in the absence and presence of compound C (10 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5 experiments). Representative images are shown for each condition. B: IB3 cells were treated with RSV (50 µM) in the absence and presence of compound C (10 µM) for 24 h and analyzed by a microtubule formation assay. Images of cells were quantified as mentioned in Microtubule formation assay. The percentages of cells with asters in IB3 cells treated with vehicle, RSV, and RSV + compound C at various time points are shown. Microtubule formation rates were significantly increased in cells treated with RSV regardless of the presence of compound C at 2, 4, and 8 min (n = 5 experiments). Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups at each time point. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5). Representative images are shown for each condition. Scale bars represent 50 µm.
Resveratrol stimulates microtubule formation in primary human nasal epithelial cells.
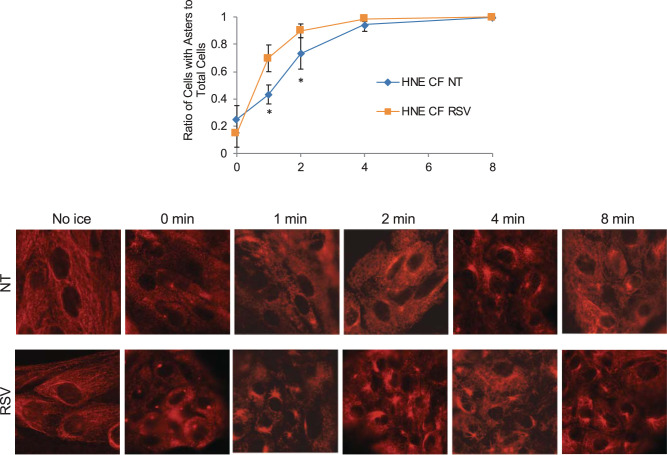
To determine if resveratrol had similar function in primary cells, F508del/F508del primary human nasal epithelial (HNE) cells were treated with resveratrol and assayed for microtubule reformation. Resveratrol significantly increases the rate of microtubule reformation, consistent with IB3 cell results (Fig. 4). These data demonstrate that the effect of resveratrol is not model specific and has direct impact on microtubule processes that we have previously shown to be related to intracellular transport (37–39).
Fig. 4.
Resveratrol stimulates microtubule formation in primary human nasal epithelial cells. Human nasal epithelial (HNE) cells were treated with resveratrol (RSV, 50 µM) for 24 h and analyzed by a microtubule formation assay. Images of cells were quantified as mentioned in Microtubule formation assay. The percentages of cells with asters in cells treated with vehicle or RSV at various time points are shown. Microtubule formation rates are significantly increased in cells treated with RSV at 1 and 2 min (n = 7 experiments). CF, cystic fibrosis; NT, no treatment (vehicle control). Significance is determined by t test for each time point between the two groups, *P < 0.01. Representative images of aster formation are shown for various time points. Scale bars represent 50 µm.
Phosphodiesterase inhibition likely not involved in resveratrol efficacy.
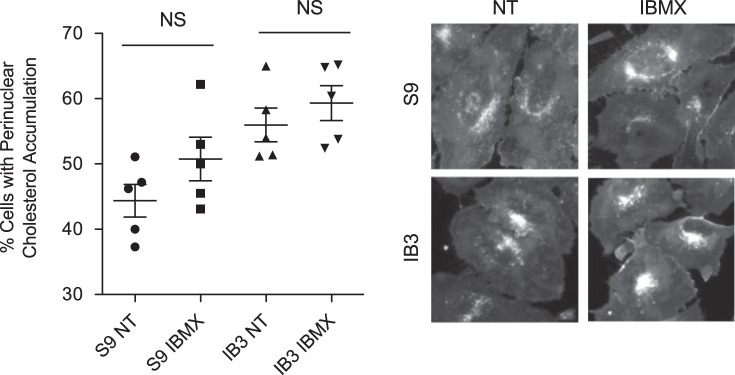
Resveratrol also acts as a cAMP phosphodiesterase (PDE) inhibitor (23, 35, 56), and we have demonstrated the ability of cAMP to modulate intracellular transport and microtubule dynamics in CF cells through EPAC1 (27, 38). Therefore, the ability of the PDE inhibitor isobutyl methylxanthine (IBMX, 200 µM, 24 h) to modulate cholesterol processing was examined. The percentages of cells with cholesterol accumulation were 44.4 ± 5.6% and 50.7 ± 7.5% for mock-treated and IBMX-treated S9 cells and 56.0 ± 5.8% and 59.3 ± 6.0% for mock-treated and IBMX-treated IB3 cells. There was no significant difference between mock-treated and IBMX-treated cells in either cell line. IBMX treatment had no impact on cholesterol accumulation, suggesting PDE inhibition is not a likely mechanism of resveratrol action (Fig. 5).
Fig. 5.
Isobutyl methylxanthine (IBMX) has on effect on cholesterol distribution in S9 and IB3 cells. S9 and IB3 cells were treated with vehicle or with 200 µM of IBMX for 24 h and assessed for cholesterol distribution by filipin staining. NT, no treatment (vehicle control). Significance was determined by t test to determine significance between groups. There was no significant difference (NS) between mock-treated and IBMX-treated cells in either cell line (n = 5 experiments). Representative images are shown for each condition. Scale bars represent 50 µm.
Sirtuin signaling does not mediate resveratrol effects.
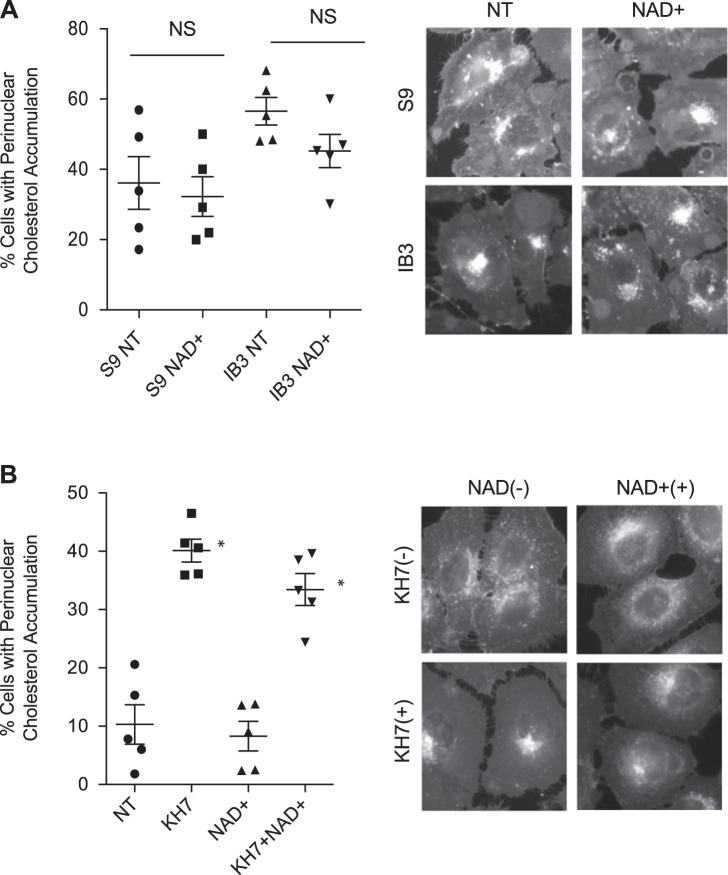
The best-characterized role of resveratrol is as a direct activator of SIRT1 (13). Given this primary function, the role of sirtuins in mediating the effects of resveratrol on intracellular transport were examined. Initially, the ability of the sirtuin cofactor NAD+ was tested for its ability to restore cholesterol transport in the CF-model IB3 cells or control S9 cells. IB3 cells exhibit increased cholesterol accumulation compared with control S9 cells, consistent with previous studies, but NAD+ (5 mM, 24 h) had no significant impact in either cell line (Fig. 6A). The percentages of cells with cholesterol accumulation were 36.1 ± 16.8% and 32.2 ± 12.6% for mock-treated and NAD+-treated S9 cells and 56.5 ± 8.8% and 45.2 ± 10.6% for mock-treated and NAD+-treated IB3 cells. The ability of NAD+ to reverse cholesterol accumulation induced by the soluble adenylate cyclase inhibitor KH7 was also examined. Consistent with CF IB3 cell results, no impact of NAD+ was seen (Fig. 6B). The percentage of cells with cholesterol accumulation was 10.3 ± 7.0% for cells treated with vehicle, 40.1 ± 4.4% for cells treated with KH7 only, 8.3 ± 5.7% for cells treated with NAD+ only, and 33.4 ± 6.1% for cells treated with both KH7 and NAD+.
Fig. 6.
Sirtuin signaling does not mediate resveratrol effects. A: mock-treated or NAD+ (5 mM, 24 h)-treated S9 and IB3 cells were assessed for cholesterol distribution by filipin staining. Significance was determined by t test to determine significance between groups. There was no significant difference (NS) between mock-treated and NAD+-treated cells in either cell line (n = 5 experiments). Representative images are shown for each condition. B: 9/HTEo− cells were treated with NAD+ (5 mM) in the absence and presence of KH7 (50 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5 experiments). No significant effect of NAD+ treatment was found. Representative images are shown for each condition. Scale bars represent 50 µm.
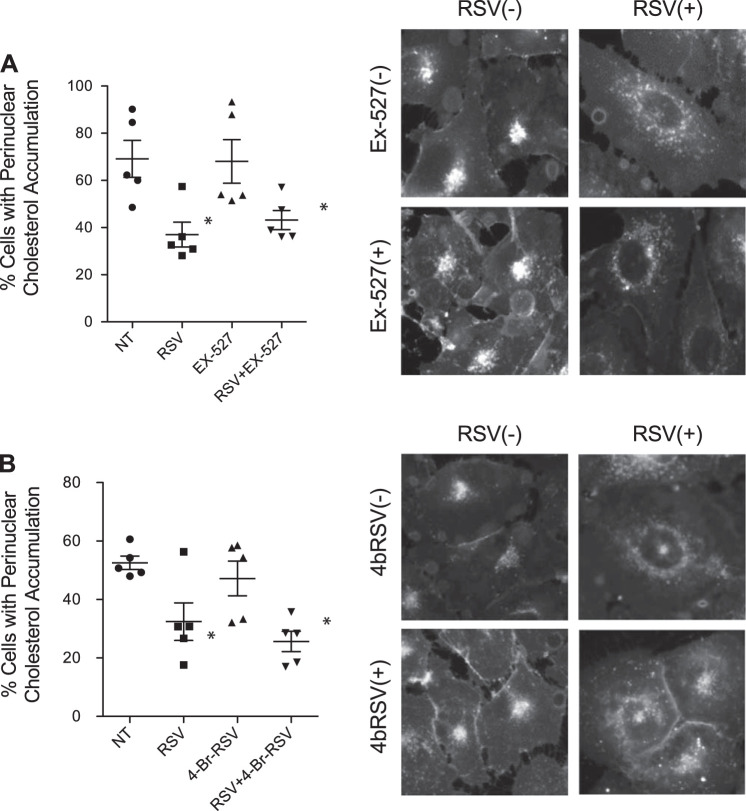
To more directly test the role of sirtuins in mediating the effects of resveratrol, the ability of the SIRT1 inhibitor EX-527 and the SIRT1/3 inhibitor 4-bromo-resveratrol (4bRSV) to block resveratrol-mediated cholesterol trafficking in IB3 cells was tested. The percentage of cells with cholesterol accumulation was 69.1 ± 17.6%, 37.0 ± 11.8%, 68.1 ± 20.7%, and 43.2 ± 9.1% for cells treated with vehicle, RSV, EX-527, and RSV in addition to EX-527, respectively. The difference between cells treated with RSV and cells treated with both RSV and EX-527 was not significant. Similarly, the percentage of cells with cholesterol accumulation was 52.6 ± 5.1%, 26.4 ± 5.4%, 47.2 ± 13.3%, and 25.7 ± 7.7% for cells treated with vehicle, RSV, 4bRSV, and RSV in addition to 4bRSV, respectively. The difference between cells treated with RSV and cells treated with both RSV and 4bRSV was not significant. Neither compound had a significant impact on resveratrol efficacy, suggesting sirtuin activation is not a key factor in the mechanisms (Fig. 7, A and B).
Fig. 7.
EX-527 or 4-bromo-resveratrol (4bRSV) could not reverse the effect of resveratrol (RSV) on correcting cholesterol distribution. A: IB3 cells were treated with RSV (50 µM) in the absence and presence of EX-527 (1 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5 experiments). Representative images are shown for each condition. B: IB3 cells were treated with RSV (50 µM) in the absence and presence of 4bRSV (15 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5 experiments). Representative images are shown for each condition. Scale bars represent 50 µm.
Inhibition of peroxisome proliferator-activated receptor-γ signaling blocks effect of resveratrol on CF cells.
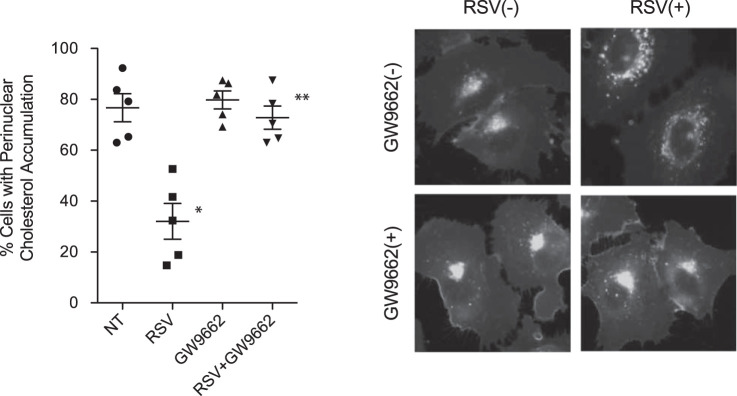
Resveratrol is also reportedly a selective activator of peroxisome proliferator-activated receptor-γ (PPARγ; see Ref. 33); thus, the role of the PPARγ pathway in mediating the effects of resveratrol on intracellular transport was examined. IB3 cells were treated with resveratrol (50 µM, 24 h) in the absence or presence of the PPARγ inhibitor GW-9662 (20 µM, 24 h). Unlike other interventions, GW-9662 successfully blocked the effect of resveratrol in reversing cholesterol accumulation in IB3 cells (Fig. 8). The percentage of cells with cholesterol accumulation was 76.7 ± 12.0%, 32.1 ± 15.7%, 79.8 ± 7.9%, and 72.8 ± 10.2% for cells treated with vehicle, RSV, GW-9662, and RSV in addition to GW-9662, respectively. These data demonstrate that resveratrol is acting at least in part through the PPARγ pathway to promote intracellular transport.
Fig. 8.
Peroxisome proliferator-activated receptor-γ (PPARγ) inhibition blocks the efficacy of resveratrol in restoring endosomal transport in cystic fibrosis (CF)-model cells. A: IB3 cells were treated with resveratrol (RSV, 50 µM) in the absence and presence of GW-9662 (20 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5 experiments). RSV + GW-9662 is significantly different from RSV treatment alone, **P < 0.05. Representative images are shown for each condition. Scale bars represent 50 µm.
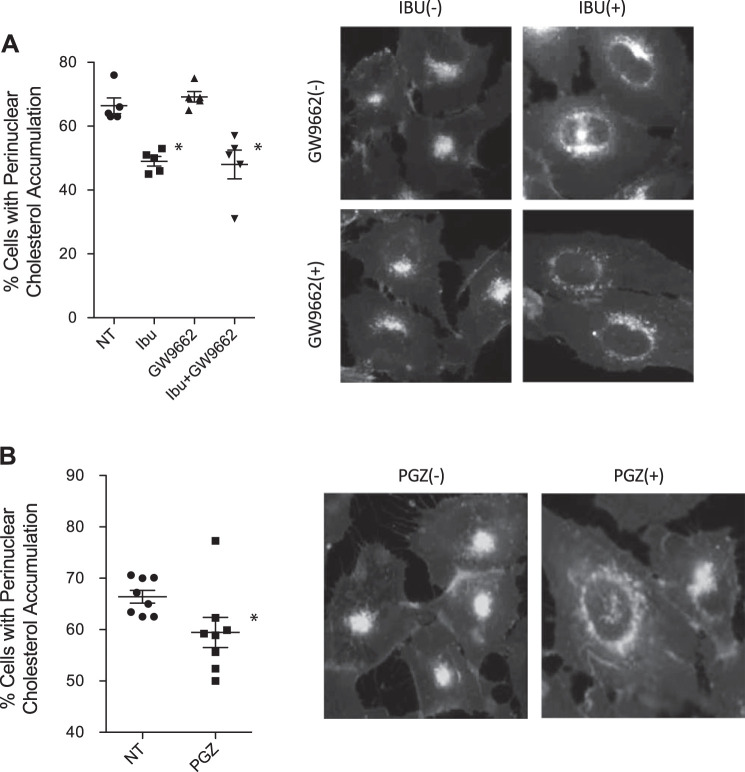
As a control, the ability of GW-9662 to inhibit ibuprofen-mediated correction of intracellular transport in IB3 cells was examined. GW-9662 treatment had no impact on ibuprofen-mediated correction of cholesterol transport in IB3 cells, demonstrating that resveratrol is acting through a mechanism independent of ibuprofen/AMPK and that GW-9662 is not initiating an effect of its own on intracellular transport (Fig. 9A). The percentage of cells with cholesterol accumulation was 66.4 ± 5.8%, 48.8 ± 3.1%, 69.4 ± 3.5%, and 48.1 ± 10.0% for cells treated with vehicle, ibuprofen, GW-9662, and both ibuprofen and GW-9662, respectively. Significant differences were found in cells treated with or without ibuprofen regardless of the presence of GW-9662, but GW-9662 had no impact on ibuprofen efficacy. Because the PPARγ inhibitor blocked the effects of resveratrol on intracellular transport, we tested whether PPARγ activation directly with the agonist pioglitazone could replicate the efficacy of resveratrol. CF-model IB3 cells were treated with pioglitazone (10 µM, 24 h) and assessed for cholesterol distribution by filipin staining. Pioglitazone treatment successfully reduces cholesterol distribution in IB3 cells (Fig. 9B). These data suggest that PPARγ signaling is necessary to mediate the effects of resveratrol treatment.
Fig. 9.
Peroxisome proliferator-activated receptor-γ (PPARγ) signaling is necessary but not sufficient to mediate the effects of resveratrol treatment. A: IB3 cells were treated with ibuprofen (IBU, 500 µM) in the absence and presence of GW-9662 (20 µM) for 24 h and assessed for cholesterol distribution by filipin staining. Significance was determined by ANOVA and Newman-Keuls multiple-comparison test to determine significance between groups. Significance is shown for groups compared with vehicle (NT), *P < 0.05 (n = 5). Representative images are shown for each condition. B: IB3 cells were treated with vehicle or with 10 µM of pioglitazone (PGZ) for 24 h and assessed for cholesterol by filipin staining. Significance was determined by t test to determine significance between groups; *P = 0.05 (n = 8 experiments). Representative images are shown for each condition. Scale bars represent 50 µm.
Resveratrol increases acetylated tubulin levels in CF cells.
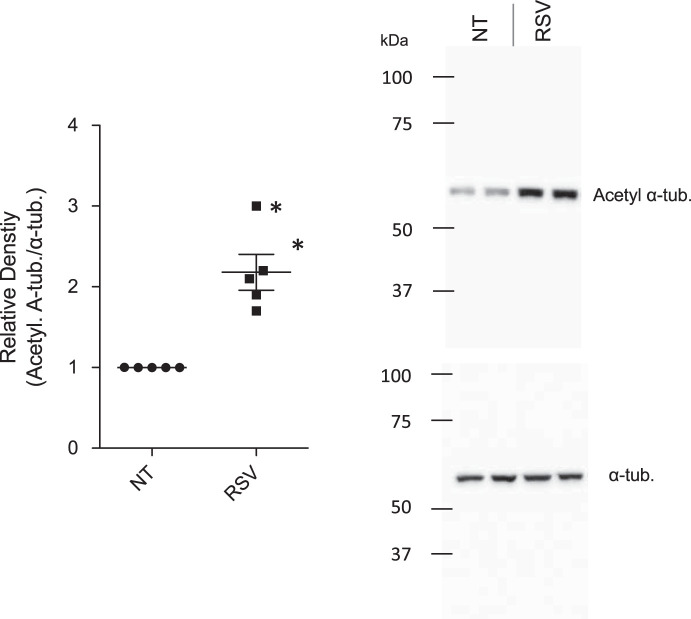
Because PPARγ activation alone is not sufficient to explain the efficacy of resveratrol in CF cells, we examined another pathway relevant to intracellular transport control in CF cells. We have previously shown that microtubule acetylation is reduced in CF cells and tissues (37). Inhibiting the primarily cytosolic histone deacetylase family member HDAC6 restores tubulin acetylation and intracellular transport in CF cells (37). We tested whether resveratrol could impact tubulin acetylation. Resveratrol treatment significantly increases tubulin acetylation in CF-model IB3 cells (Fig. 10). These data are consistent with a previous report suggesting resveratrol could act as a pan-HDAC inhibitor and suggest that resveratrol’s effects on both PPARγ activation and HDAC6 inhibition likely mediate its effects on microtubule regulation and subsequently intracellular transport.
Fig. 10.
Resveratrol increases acetylated tubulin content in cystic fibrosis-model IB3 cells. Expressions of acetylated α-tubulin (Acetyl α-tub) in mock-treated and resveratrol (RSV, 50 µM, 24 h)-treated IB3 cells were analyzed via Western blot (n = 5 experiments). α-Tubulin was used for normalization of protein loading. NT, no treatment (vehicle control). Representative Western blot images were shown. Significance was determined by t test, *P < 0.01.
DISCUSSION
We have demonstrated previously that intracellular transport in CF epithelial cells is impaired with regard to endosomal movement (26). This impairment is marked by free cholesterol accumulation in late endosomes and is linked to growth and inflammatory signaling. We have identified a number of mechanisms that contribute to microtubule regulation in CF, including EPAC1 and HDAC6 activity (38). Correction of microtubule dynamics and associated intracellular transport functions is also achieved with AMPK activation with either direct AMPK agonists or the anti-inflammatory ibuprofen (39). The finding that ibuprofen so effectively impacted the microtubule pathway strongly suggested that targeting this pathway could be an important anti-inflammatory therapeutic target for CF to provide the benefits of ibuprofen therapy without the potential adverse effects.
The overall goal of this research is to identify accessible compounds that can stimulate intracellular transport in CF cells through correction of microtubule regulation to be evaluated for anti-inflammatory properties. In this study, it is hypothesized that resveratrol would be an effective agonist to stimulate microtubule elongation in CF cells and restore intracellular transport. Resveratrol is an attractive candidate, since it reportedly interacts with a number of pathways already identified to influence microtubule dynamics in CF. Resveratrol is a known agonist of AMPK signaling, and we have demonstrated that AMPK agonists potently stimulate microtubule formation in both cultured and primary CF cells, correcting endosomal movement (3, 7, 39). Resveratrol also acts as a phosphodiesterase inhibitor, increasing cAMP levels in various studies (52). We have demonstrated that cAMP activation of EPAC1 can regulate CF microtubule dynamics (38). The most direct impact of resveratrol is the stimulation of sirtuins, particularly SIRT1 (13). Both SIRT1 and SIRT3 have been shown to influence microtubules and offer a potentially novel mechanism for control of microtubules in CF (15, 30).
Resveratrol has been studied as a potential therapy in CF before, mostly in the context of CFTR activation. One study found resveratrol to be effective in stimulating CFTR function in acquired CFTR deficiency due to hypoxia (54). Other studies extend this property to CF disease-causing mutations. Cho et al. recently demonstrate that resveratrol augments the activation of G551D CFTR in the presence of modulators (4). One study even found evidence that resveratrol could help stimulate F508del CFTR function (5). The issue with these studies, as well as the data presented in this manuscript, is that higher doses of resveratrol in the 50- to 100-µM range are needed to achieve CFTR activation and other biological events. Resveratrol is not very bioavailable, with plasma concentrations only reaching ~2 µM (51). Indeed, Jai et al. have shown that physiologically achievable concentrations of resveratrol are insufficient to activate F508del CFTR (11).
Testing of resveratrol revealed that resveratrol is indeed a potent stimulator of microtubule formation in cultured models and primary CF epithelial cells. These microtubule effects result in more efficient intracellular transport as measured by cholesterol and Rab7-positive organelle distribution in CF-model IB3 cells. In this sense, the hypothesis tested positively. However, mechanistic analysis revealed that resveratrol exerts its effects through unexpected avenues. Data demonstrate that neither AMPK signaling, cAMP levels, nor sirtuin activation play a role in the actions of resveratrol on microtubule and intracellular transport. The lack of involvement of these predicted pathways required the identification of a more novel mechanism of action.
It was identified that resveratrol was exerting its influence on microtubules through two other mechanisms, PPARγ activation and likely HDAC6 inhibition. There is precedent for both of these mechanisms. Resveratrol activation of PPARγ has been reported previously, particularly as a mechanism for antiproliferation effects in tumor cells (1). Our data demonstrate that inhibition of PPARγ signaling effectively blocks the ability of resveratrol to restore intracellular transport in CF cells, effectively mimicked by direct PPARγ activation with pioglitazone. These findings are consistent with previous reports that PPARγ activity is reduced in CF epithelium and that its activation can have a number of beneficial effects, including improved immune function (9, 32, 33, 43, 46).
Another aspect of microtubule dysregulation in CF is reduced tubulin acetylation (37), and it has been reported that resveratrol can act as a pan-HDAC inhibitor (50). An examination of tubulin acetylation influence by resveratrol in CF cells does show a significant increase. These data are consistent with our previous findings that HDAC6 inhibition restores microtubule acetylation and intracellular transport in CF cells (37). Depletion of HDAC6 expression from CF mouse models also restores growth and inflammatory responses, demonstrating the importance of intervening in this pathway (34, 36).
The conclusion of the study is that resveratrol is a very effective modulator of microtubule regulation in cultured and primary CF epithelial cells, resulting in improved intracellular transport. The positive impact of resveratrol on microtubule formation rates and acetylation in CF cells is somewhat surprising. Several analogs of resveratrol have been developed as antitumor therapeutic candidates based on their ability to block tubulin polymerization (47). Although these analogs may be acting through different mechanisms than resveratrol itself, it is possible that resveratrol is acting on pathways that are specifically altered in CF, allowing for such dramatic improvement in microtubule formation. No adverse effects of resveratrol were observed on WT cell microtubules, but no further benefit was seen either. We also find that resveratrol treatment increases acetylated tubulin levels, which is consistent with resveratrol acting as a pan-HDAC inhibitor, including HDAC6, as mentioned. However, another report by Misawa et al. demonstrates that resveratrol treatment prevents acetylated tubulin accumulation in response to mitochondrial damage, preventing inflammasome activation (29).
Although further studies are necessary, the ability of resveratrol to reverse CF cellular phenotypes in a manner not only consistent, but more efficiently and with broader impact than ibuprofen, suggests that resveratrol could be an effective anti-inflammatory therapy. The anti-inflammatory properties of resveratrol are well established in multiple studies under a variety of conditions (10, 31, 41, 55). As noted above, a limitation of this study is that plasma levels of resveratrol cannot reach the 50-µM dose found to be necessary in these cellular data. However, understanding how resveratrol is acting in CF could lead to a new therapeutic avenue and to a better understanding of CF cell signaling.
GRANTS
This work is supported by Cystic Fibrosis Foundation (CFF) Grants KELLEY17G0 and CFF RDP R447-CR11.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.K. conceived and designed research; B.L. and D.A.C. performed experiments; B.L., D.A.C., and T.J.K. analyzed data; B.L. and T.J.K. interpreted results of experiments; B.L. and T.J.K. prepared figures; B.L. and T.J.K. drafted manuscript; B.L., D.A.C., and T.J.K. edited and revised manuscript; B.L., D.A.C., and T.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the use of the Leica microscopes in the Genetics Department Imaging Facility at Case Western Reserve University, made available through a National Center for Research Resources Shared Instrumentation Grant (S10-RR-021228). We thank P. Bead for technical assistance.
REFERENCES
- 1.Aires V, Brassart B, Carlier A, Scagliarini A, Mandard S, Limagne E, Solary E, Martiny L, Tarpin M, Delmas D. A role for peroxisome proliferator-activated receptor gamma in resveratrol-induced colon cancer cell apoptosis. Mol Nutr Food Res 58: 1785–1794, 2014. doi: 10.1002/mnfr.201300962. [DOI] [PubMed] [Google Scholar]
- 2.Bell EA, Grothe R, Zivkovich V, Foote JM, Wellendorf J. Pyloric channel stricture secondary to high-dose ibuprofen therapy in a patient with cystic fibrosis. Ann Pharmacother 33: 693–696, 1999. doi: 10.1345/aph.18187. [DOI] [PubMed] [Google Scholar]
- 3.Chiang MC, Nicol CJ, Cheng YC. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int 115: 1–10, 2018. doi: 10.1016/j.neuint.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Cho DY, Zhang S, Lazrak A, Grayson JW, Peña Garcia JA, Skinner DF, Lim DJ, Mackey C, Banks C, Matalon S, Woodworth BA. Resveratrol and ivacaftor are additive G551D CFTR-channel potentiators: therapeutic implications for cystic fibrosis sinus disease. Int Forum Allergy Rhinol 9: 100–105, 2019. doi: 10.1002/alr.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhooghe B, Bouckaert C, Capron A, Wallemacq P, Leal T, Noel S. Resveratrol increases F508del-CFTR dependent salivary secretion in cystic fibrosis mice. Biol Open 4: 929–936, 2015. doi: 10.1242/bio.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentzsch M, Choudhury A, Chang XB, Pagano RE, Riordan JR. Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J Cell Sci 120: 447–455, 2007. doi: 10.1242/jcs.03350. [DOI] [PubMed] [Google Scholar]
- 7.Guo H, Zhang L. Resveratrol provides benefits in mice with type II diabetes-induced chronic renal failure through AMPK signaling pathway. Exp Ther Med 16: 333–341, 2018. doi: 10.3892/etm.2018.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkcom WT, Ghosh AK, Sung MS, Matov A, Brown KD, Giannakakou P, Jaffrey SR. NAD+ and SIRT3 control microtubule dynamics and reduce susceptibility to antimicrotubule agents. Proc Natl Acad Sci USA 111: E2443–E2452, 2014. doi: 10.1073/pnas.1404269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nat Med 16: 313–318, 2010. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H, Nakata R. Resveratrol targets in inflammation. Endocr Metab Immune Disord Drug Targets 15: 186–195, 2015. doi: 10.2174/1871530315666150316120316. [DOI] [PubMed] [Google Scholar]
- 11.Jai Y, Shah K, Bridges RJ, Bradbury NA. Evidence against resveratrol as a viable therapy for the rescue of defective ΔF508 CFTR. Biochim Biophys Acta 1850: 2377–2384, 2015. doi: 10.1016/j.bbagen.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseloff E, Sha W, Bell SC, Wetmore DR, Lawton KA, Milburn MV, Ryals JA, Guo L, Muhlebach MS. Serum metabolomics indicate altered cellular energy metabolism in children with cystic fibrosis. Pediatr Pulmonol 49: 463–472, 2014. doi: 10.1002/ppul.22859. [DOI] [PubMed] [Google Scholar]
- 13.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem 280: 17038–17045, 2005. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 14.Kelly-Aubert M, Trudel S, Fritsch J, Nguyen-Khoa T, Baudouin-Legros M, Moriceau S, Jeanson L, Djouadi F, Matar C, Conti M, Ollero M, Brouillard F, Edelman A. GSH monoethyl ester rescues mitochondrial defects in cystic fibrosis models. Hum Mol Genet 20: 2745–2759, 2011. doi: 10.1093/hmg/ddr173. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Gil NY, Zhang XH, Chun KH, Fang G, Kim J, Cho H, Jang CY, Cha HJ. Sirt1 regulates microtubule dynamics through negative regulation of Plk1 in mitosis. J Cell Biochem 116: 1888–1897, 2015. doi: 10.1002/jcb.25144. [DOI] [PubMed] [Google Scholar]
- 16.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 332: 848–854, 1995. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 17.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med 176: 1084–1089, 2007. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstan MW, VanDevanter DR, Sawicki GS, Pasta DJ, Foreman AJ, Neiman EA, Morgan WJ. Association of high-dose ibuprofen use, lung function decline, and long-term survival in children with cystic fibrosis. Ann Am Thorac Soc 15: 485–493, 2018. doi: 10.1513/AnnalsATS.201706-486OC. [DOI] [PubMed] [Google Scholar]
- 19.Kovesi TA, Swartz R, MacDonald N. Transient renal failure due to simultaneous ibuprofen and aminoglycoside therapy in children with cystic fibrosis. N Engl J Med 338: 65–66, 1998. doi: 10.1056/NEJM199801013380115. [DOI] [PubMed] [Google Scholar]
- 20.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1286–L1295, 2003. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr 151: 249–254, 2007. doi: 10.1016/j.jpeds.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Elmer HL, Ross KR, Kelley TJ. Isoprenoid-mediated control of SMAD3 expression in a cultured model of cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 31: 234–240, 2004. doi: 10.1165/rcmb.2003-0447OC. [DOI] [PubMed] [Google Scholar]
- 23.Liu LQ, Fan ZQ, Tang YF, Ke ZJ. The resveratrol attenuates ethanol-induced hepatocyte apoptosis via inhibiting ER-related caspase-12 activation and PDE activity in vitro. Alcohol Clin Exp Res 38: 683–693, 2014. doi: 10.1111/acer.12311. [DOI] [PubMed] [Google Scholar]
- 24.Mackey JE, Anbar RD. High-dose ibuprofen therapy associated with esophageal ulceration after pneumonectomy in a patient with cystic fibrosis: a case report. BMC Pediatr 4: 19, 2004. doi: 10.1186/1471-2431-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ. Regulatory role of β-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res 53: 1268–1276, 2012. doi: 10.1194/jlr.M021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson ME, Corey DA, Rymut SM, Kelley TJ. β-Arrestin-2 regulation of the cAMP response element binding protein. Biochemistry 50: 6022–6029, 2011. doi: 10.1021/bi200015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am J Physiol Lung Cell Mol Physiol 295: L809–L819, 2008. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes KL, Lelis DF, Santos SHS. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev 38: 98–105, 2017. doi: 10.1016/j.cytogfr.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Misawa T, Saitoh T, Kozaki T, Park S, Takahama M, Akira S. Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int Immunol 27: 425–434, 2015. doi: 10.1093/intimm/dxv018. [DOI] [PubMed] [Google Scholar]
- 30.Neo SH, Tang BL. Sirtuins as modifiers of Huntington’s Disease (HD) pathology. Prog Mol Biol Transl Sci 154: 105–145, 2018. doi: 10.1016/bs.pmbts.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev 31: 85–97, 2018. doi: 10.1017/S095442241700021X. [DOI] [PubMed] [Google Scholar]
- 32.Ollero M, Junaidi O, Zaman MM, Tzameli I, Ferrando AA, Andersson C, Blanco PG, Bialecki E, Freedman SD. Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol 200: 235–244, 2004. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 33.Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB. Peroxisome proliferator-activated receptor-γ in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol 295: L303–L313, 2008. doi: 10.1152/ajplung.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenjack J, Hodges CA, Darrah RJ, Kelley TJ. HDAC6 depletion improves cystic fibrosis mouse airway responses to bacterial challenge. Sci Rep 9: 10282, 2019. doi: 10.1038/s41598-019-46555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouse M, Younès A, Egan JM. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J Endocrinol 223: 107–117, 2014. doi: 10.1530/JOE-14-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rymut SM, Corey DA, Valerio DM, Erokwu BO, Flask CA, Kelley TJ, Hodges CA. Improved growth patterns in cystic fibrosis mice after loss of histone deacetylase 6. Sci Rep 7: 3676, 2017. doi: 10.1038/s41598-017-03931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rymut SM, Harker A, Corey DA, Burgess JD, Sun H, Clancy JP, Kelley TJ. Reduced microtubule acetylation in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 305: L419–L431, 2013. doi: 10.1152/ajplung.00411.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rymut SM, Ivy T, Corey DA, Cotton CU, Burgess JD, Kelley TJ. Role of exchange protein activated by cAMP 1 in regulating rates of microtubule formation in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 53: 853–862, 2015. doi: 10.1165/rcmb.2014-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rymut SM, Kampman CM, Corey DA, Endres T, Cotton CU, Kelley TJ. Ibuprofen regulation of microtubule dynamics in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 311: L317–L327, 2016. doi: 10.1152/ajplung.00126.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rymut SM, Lu B, Perez A, Corey DA, Lamb K, Cotton CU, Kelley TJ. Acetyl-CoA carboxylase inhibition regulates microtubule dynamics and intracellular transport in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 316: L1081–L1093, 2019. doi: 10.1152/ajplung.00369.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadeghi A, Seyyed Ebrahimi SS, Golestani A, Meshkani R. Resveratrol ameliorates palmitate-induced inflammation in skeletal muscle cells by attenuating oxidative stress and JNK/NF-κB pathway in a SIRT1-independent mechanism. J Cell Biochem 118: 2654–2663, 2017. doi: 10.1002/jcb.25868. [DOI] [PubMed] [Google Scholar]
- 42.Scherzberg MC, Kiehl A, Zivkovic A, Stark H, Stein J, Fürst R, Steinhilber D, Ulrich-Rückert S. Structural modification of resveratrol leads to increased anti-tumor activity, but causes profound changes in the mode of action. Toxicol Appl Pharmacol 287: 67–76, 2015. doi: 10.1016/j.taap.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62: 1551–1562, 2015. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott CS, Retsch-Bogart GZ, Henry MM. Renal failure and vestibular toxicity in an adolescent with cystic fibrosis receiving gentamicin and standard-dose ibuprofen. Pediatr Pulmonol 31: 314–316, 2001. doi: 10.1002/ppul.1047. [DOI] [PubMed] [Google Scholar]
- 45.Sehrawat S, Cullere X, Patel S, Italiano J Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell 19: 1261–1270, 2008. doi: 10.1091/mbc.e06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Chen L, Wang T, Wen F. PPARγ as a potential target to treat airway mucus hypersecretion in chronic airway inflammatory diseases. PPAR Res 2012: 256874, 2012. doi: 10.1155/2012/256874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas E, Gopalakrishnan V, Hegde M, Kumar S, Karki SS, Raghavan SC, Choudhary B. A novel resveratrol based tubulin inhibitor induces mitotic arrest and activates apoptosis in cancer cells. Sci Rep 6: 34653, 2016. doi: 10.1038/srep34653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vachharajani VT, Liu T, Wang X, Hoth JJ, Yoza BK, McCall CE. Sirtuins link inflammation and metabolism. J Immunol Res 2016: 8167273, 2016. doi: 10.1155/2016/8167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valdivieso AG, Clauzure M, Marín MC, Taminelli GL, Massip Copiz MM, Sánchez F, Schulman G, Teiber ML, Santa-Coloma TA. The mitochondrial complex I activity is reduced in cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS One 7: e48059, 2012. doi: 10.1371/journal.pone.0048059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venturelli S, Berger A, Böcker A, Busch C, Weiland T, Noor S, Leischner C, Schleicher S, Mayer M, Weiss TS, Bischoff SC, Lauer UM, Bitzer M. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS One 8: e73097, 2013. doi: 10.1371/journal.pone.0073097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci 1215: 9–15, 2011. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Chen L, Pan X, Chen J, Wang L, Wang W, Cheng R, Wu F, Feng X, Yu Y, Zhang HT, O’Donnell JM, Xu Y. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 7: 17380–17392, 2016. doi: 10.18632/oncotarget.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 292: L476–L486, 2007. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 54.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. Laryngoscope 125, Suppl 7: S1–S13, 2015. doi: 10.1002/lary.25335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu D, Li Y, Zhang B, Wang Y, Liu Y, Luo Y, Niu W, Dong M, Liu M, Dong H, Zhao P, Li Z. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int J Med Sci 13: 942–954, 2016. doi: 10.7150/ijms.16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao P, Chen SK, Cai YH, Lu X, Li Z, Cheng YK, Zhang C, Hu X, He X, Luo HB. The molecular basis for the inhibition of phosphodiesterase-4D by three natural resveratrol analogs. Isolation, molecular docking, molecular dynamics simulations, binding free energy, and bioassay. Biochim Biophys Acta 1834: 2089–2096, 2013. doi: 10.1016/j.bbapap.2013.07.004. [DOI] [PubMed] [Google Scholar]