Abstract
Autism spectrum disorders (ASD) are strongly associated with auditory hypersensitivity or hyperacusis (difficulty tolerating sounds). Fragile X syndrome (FXS), the most common monogenetic cause of ASD, has emerged as a powerful gateway for exploring underlying mechanisms of hyperacusis and auditory dysfunction in ASD. This review discusses examples of disruption of the auditory pathways in FXS at molecular, synaptic, and circuit levels in animal models as well as in FXS individuals. These examples highlight the involvement of multiple mechanisms, from aberrant synaptic development and ion channel deregulation of auditory brainstem circuits, to impaired neuronal plasticity and network hyperexcitability in the auditory cortex. Though a relatively new area of research, recent discoveries have increased interest in auditory dysfunction and mechanisms underlying hyperacusis in this disorder. This rapidly growing body of data has yielded novel research directions addressing critical questions regarding the timing and possible outcomes of human therapies for auditory dysfunction in ASD.
Keywords: auditory system, autism spectrum disorders, circuit development, Fragile X syndrome, hyperacusis, synaptic transmission
1 |. INTRODUCTION
Communication disorders and sensory hypersensitivity are prominent features of autism spectrum disorder (ASD). ASD is frequently diagnosed in individuals with Fragile X syndrome (FXS), a leading cause of intellectual disability. FXS results from mutations in the fragile X mental retardation 1 (FMR1) gene, which is the most prevalent monogenic cause of ASD.1 This gene encodes fragile X mental retardation protein (FMRP), an RNA binding protein that regulates expression of a select set of genes. The focus of this review is to understand the effects of Fmr1 deletion in the auditory pathway, because these effects lie at the heart of the auditory hypersensitivity seen in FXS and likely contribute significantly to communication and other neurological deficits.
A large portion of the recent work discussed here has taken advantage of rodent models of FXS (Fmr1 knockout mice and rats) and investigated structural, functional, and behavioral phenotypes at multiple stages of central auditory processing. Other studies have examined neuroanatomical and physiological abnormalities of human subjects with FXS. Additionally, the avian auditory brainstem has been used to investigate the FMRP regulation of auditory circuits that encode low frequency sounds, which are essential for acoustic communication in humans.
The significance of studying auditory processing in FXS is twofold. On the one hand, FMRP has the potential to influence a multitude of cellular processes at multiple stages of development. The diversity of FMRP function is of special significance in the auditory system, which carries out unique functions that require exceptionally fast and precise synapses. These requirements are fulfilled with specializations in axon terminations, dendritic branching, and synaptic and intrinsic properties of auditory neurons. Investigation of the roles for FMRP in each of these specializations will advance our understanding of auditory system development and function. Indeed, FMRP is found to be expressed throughout the auditory system and an array of distinct phenotypes has emerged in auditory circuits following FMRP deficiency that may account for auditory hypersensitivity and abnormal processing. At the same time, these findings highlight the auditory system as a powerful model system to study the FMRP function and FXS neuropathology. The wealth of knowledge on connections and response properties in the healthy brain allows alterations to be relatively easy to characterize, thereby allowing for an in-depth analysis of the underlying etiologies that lead to problems with sensory processing.2 Finally, the ability to stimulate neural responses in the auditory system on demand and the fact that many of these responses are independent of cognitive abilities or active participation opens the possibility to use these responses as biomarkers to characterize the effects of potential therapeutic approaches.
2 |. FXS AND BIOLOGICAL ROLES OF FMRP
Fragile X syndrome is characterized by many similar symptoms to autism, namely, hyperactivity, anxiety, seizures, learning difficulties, and poor language development (reviewed in3,4). Most cases of FXS are caused by a trinucleotide repeat disorder, in which an increase in the numbers of a CGG motif in the 5′ UTR of the Fmr1 gene (>200 repeats) leads to transcriptional silencing and the consequent loss of its product, FMRP. As a monogenetic disorder, FXS has come to be at the frontier of developing treatments for neurodevelopmental disorders. Loss of FMRP underlies the etiology of FXS, and most drug development has centered around rescuing specific biological effects caused by loss of FMRP. Evidence supporting the importance of FMRP in FXS includes (i) FMRP protein level is associated in a dose-dependent manner with brain anatomy, sensory function, autistic behaviors, and cognitive function of individuals with FXS,5–8 (ii) genetically reintroducing FMRP rescues cellular and behavioral deficits in Fmr1 knockout mice,9 and (iii) point mutations in the Fmr1 coding sequence lead to FXS symptoms similar to those of CGG expansion.10,11 Thus, examining FMRP knockouts presents a suitable avenue for exploring FXS etiology.
FMRP regulates many aspects of RNA biology from RNA transport, stability, to mRNA translation.12,13 The best-described biological role of FMRP in neurons is to bind a subset of mRNAs and to suppress their translation.14,15 Appropriate stimulation of neurons can reverse this suppression, most likely by altering the phosphorylation state of FMRP, leading to increased synthesis of the protein productsof these mRNAs. There are on the order of ~1000 mRNAs that bind FMRP and these are termed targets of FMRP.16,17 Thus, one central phenotype of Fmr1 knockout animals is an increase in levels of some of these proteins, together with a lack of activity-dependent translation. This role for FMRP is commonly termed its canonical role. However, only a limited number of FMRP targets have been validated so far in specific circuits and FMRP binds to different sets of RNA targets in an age-dependent and cell type or brain region specific manner. As evidence, strikingly distinct sets of proteins exhibit altered expression in Fmr1 knockout mice between the hippocampus and neocortex of adult brains,18 and between the developing and adult neocortex.19 In addition to RNA binding, FMRP directly binds proteins that control other aspects of neuronal activity. These include proteins required for RNA editing, RNA splicing, cytoskeletal rearrangement, regulation of protein kinases, as well as direct binding to ion channels that determine neuronal excitability.20 In some cases, it is possible to link auditory phenotypes in Fmr1 knockout animals to changes in mRNA targets or to other biological actions of FMRP. Indeed, key findings in FXS animal models include disturbances in circuit development, imbalanced inhibitory, and excitatory neuronal circuits, and altered synaptic plasticity.21–23
3 |. FMRP REGULATES THE DEVELOPMENT OF SYNAPTIC TRANSMISSION IN THE AUDITORY BRAINSTEM
The auditory brainstem is where fundamental steps of auditory processing take place, including binaural computation for sound localization. Sounds arriving at our two ears from various locations along the azimuth create discrete interaural time and level differences (ITDs and ILDs).24 These two binaural cues are first computed in the brainstem. In mammals, the major auditory nuclei involved in this process include the ventral cochlear nucleus (VCN), the lateral, ventral, and medial nucleus of the trapezoid body (LNTB, VNTB, and MNTB), and the medial and lateral superior olive (MSO and LSO; Figure 1). In reptiles and birds, nucleus magnocellularis (NM), and nucleus laminaris (NL) are structurally and functionally similar to the mammalian VCN and MSO, respectively.
FIGURE 1.

Sound localization circuits. Sound localization information is integrated in the auditory brainstem using interaural time and level differences (ITDs and ILDs). A-B, The ITD circuit is structurally and functionally comparable between birds (A) and mammals (B). The circuit is composed of spherical bushy cells in NM and VCN and fusiform bipolar cells in NL and MSO. The NL also receives an inhibitory input from the superior olive nucleus (SON). The MSO receives inhibitory inputs from MNTB and LNTB. C, In mammals, ILDs are computed in LSO through an excitatory projection from globular bushy cells in VCN and an inhibitory projection from MNTB
Traditionally, the brainstem was thought to express low levels of FMRP25,26 and received little attention in the field of FMRP research. Recent cellular analyses, however, reported strong expression of FMRP in auditory brainstem cell groups across a number of vertebrate species.27 Quantitative analyses across the entire adult (5–6 weeks) mouse brain further confirmed that auditory neurons in the brainstem and thalamus have levels of FMRP immunoreactivity comparable to those in cortical neurons.28 It is worth noting that FMRP is also expressed in spiral ganglion neurons, which convey acoustic signals from the inner ear to the brainstem, in both birds and mammals. In contrast, many cell groups in the midbrain and hypothalamus exhibit low FMRP levels.28
In this section, we first describe the expression and function of FMRP in the ITD circuit (3.1) and then discuss how Fmr1 deletion affects the cell groups involved in ILD computation (3.2). Finally, we summarize effects of FMRP loss on auditory brainstem responses (ABRs), a noninvasive physiological output of the auditory brainstem (3.3).
3.1 |. FMRP regulates dendritic development and morphology in the ITD circuit
In the brainstem, ITD computation for low frequency sounds takes place in MSO and NL (Figure 1A,B). The MSO and NL are best developed in humans and other animal species most sensitive to low frequency sounds including reptiles, birds, some rodents such as gerbils, and primates. Dendrites of MSO and NL neurons segregate into two domains; each domain receives excitatory input from the ipsilateral or contralateral ear via VCN and NM (Figure 2A). This anatomic segregation, along with specialized synaptic and intrinsic physiology, enables MSO and NL neurons to detect ITDs in the microsecond range.29
FIGURE 2.

FMRP deficiency leads to altered cytoarchitecture in MSO. A, Schematic drawing of the normal organization of MSO. B, FMRP expression in the human MSO. Empty and solid arrowheads point to cell bodies and dendritic branches, respectively. C-D, Altered organization of MSO in Fmr1 knockout rats (D) as compared to wild type (C). In Fmr1 knockout animals, MSO somata were smaller, more round/oval, and more vertically oriented (arrows). Abbreviation: VCN, ventral cochlear nucleus; MSO, medial superior olive
Strong FMRP expression is detected in the VCN-MSO circuit across taxa, including gerbils,27 rats,30 and humans31 Figure 2B). Similarly, almost all neurons of NM and NL in alligator and chicken brainstems contain exceptionally high levels of FMRP.27 Perhaps surprisingly, postmortem human brains were found to have more variation of FMRP expression depending on the cell type.31 In the human VCN (2 males, 5 females; average age 79 years), the vast majority (85%–95%) of bushy cells and octopus cells are FMRP immunoreactive, while only about 68% of stellate neurons express FMRP. It is not clear whether this difference results from technical variations associated with postmortem tissue processing of human brains or reflects varied degrees of FMRP regulation in functionally distinct human VCN cell types.
Strong FMRP expression in normal developing MSO neurons is consistent with the remarkable changes seen in FMRP-deficient MSO. A morphological study of the human MSO was conducted in an age-matched (29–32 years of age, all males) series of subjects (one neurotypical subject, one subject with ASD, and one subject with FXS).32 MSO neurons were significantly smaller in ASD and FXS. In fact, MSO neurons in the FXS subject were significantly smaller than both the control and ASD subjects, suggesting a direct link between FMRP and MSO development, although additional FXS patients are certainly needed for investigation. In control subjects, the MSO is composed of fusiform and stellate neurons, with only a small population of round/oval neurons. In the FXS patient, the MSO contained significantly more round/oval neurons, reflecting an immaturity of MSO neurons in this subject. Finally, in control subjects, individual MSO neurons are arranged with their long axis perpendicular to the dorsoventral axis of the brainstem. This orientation of neurons is lost in both ASD and FXS patients, implicating disrupted dendritic branching in MSO. Although only one human FXS subject has been examined so far, very similar cellular changes have been detected in the MSO of a Fmr1 knockout rat strain (30; Figure 2C,D), strongly supporting the notion that these MSO changes are common phenotypes in FXS.
These morphological studies in humans and rats, along with reports of altered dendritic arborization and their dynamics in nonauditory neuronal cell types in Fmr1 knock-out mice,33–37 warranted further studies of the role of FMRP in regulating dendrites in the VCN-MSO circuit. High-resolution imaging studies demonstrated intense dendritic localization of FMRP, particularly in dendritic branch points and distal endings, in NL (chicken and alligator) and MSO (gerbil and human).27 In the chicken NL, the branching points are thought to be a site for initiating activity-dependent dendritic reorganization.38 A proteomic study specifically targeting the chicken NL identified a number of dendritic proteins whose mRNAs are potential FMRP targets.39 One of these is RhoC, a member of the Rho family of GTPases that is involved in developmental regulation of neuronal dendrites,40 and that has been validated as an RNA target of FMRP.39 Together, these results support a role of FMRP in dendritic development and regulation of MSO and NL neurons.
FMRP regulation of dendritic development has been studied in the chicken NM.41 In NM and VCN, presynaptic auditory axons form large axosomatic endbulb synapses on cell bodies of postsynaptic bushy neurons. Normally, bushy neurons in NM grow extensive dendrites at early stages and retract these dendrites when endbulbs begin to form. Following FMRP knockdown with an shRNA approach, NM neurons exhibit a remarkable delay in branch retraction, failing to provide necessary somatic surface for timely formation and growth of large endbulbs. Importantly, these structural changes are coupled to functional changes in neurotransmission, specifically smaller amplitudes and slower kinetics of spontaneous and evoked excitatory postsynaptic currents (eEPSCs).
In summary, FMRP is localized in high concentrations in neuronal dendrites that are specialized for ITD computation in the brainstem. FMRP deficiency disrupts dendritic maturation, which negatively affects the development of synaptic connectivity and integration. Sound localization plays a crucial role in our ability to separate multiple simultaneous sound sources into distinct auditory streams (reviewed in 42). Disrupted dendritic function in the ITD circuit may, therefore, impair hearing in noise environments in individuals with FXS.
3.2 |. Fmr1 deletion disrupts the balance between excitation and inhibition in the ILD circuit
Interaural level differences, also known as interaural intensity differences (IIDs), are encoded by neurons in LSO for high frequency sounds (Figure 1C). The balance of glutamatergic excitation from VCN and glycinergic inhibition from MNTB allows LSO neurons to compute the ILD, which is used to determine the location of the sound source. Disturbances in the ratio of excitation to inhibition (E/I) in the brain, generally resulting in hyperexcitability, are well described in FXS and ASD.21,22,43–45 These alterations in E/I balance may account for the auditory hypersensitivity and impaired sound location in noisy environments reported in individuals with FXS and ASD.46–48 Neuroanatomical and physiological studies of Fmr1 knockout rodents have uncovered a number of cellular alterations that potentially contribute to a disrupted E/I balance in the ILD circuit.
3.2.1 |. Synaptic distribution is altered but neurotransmission is preserved in the MNTB
MNTB neurons receive glutamatergic excitation from the VCN through a giant synapse called the calyx of Held, and glycinergic and GABAergic inhibition from collateral projections and VNTB.49,50 The GABAergic component declines through adult-hood resulting in mostly glycinergic inhibition in the adult51–53). The glycinergic component of inhibition is fast and large, capable of following rapid stimulus trains (several 100 Hz) that match and suppress firing of MNTB neurons despite the extremely large calyceal excitatory input.53,54 Disruptions to glutamatergic, GABAergic or glycinergic presynaptic inputs to MNTB could have profound effects on ILD encoding.
Immunocytochemical studies of various cellular and synaptic markers have suggested that excitatory and inhibitory inputs to MNTB may be altered, but the direction of this alteration is not yet clear. A study using Fmr1 knock-out rats revealed that the MNTB contains significantly fewer terminals immunoreactive for calretinin, a marker for calyx terminals from VCN.30 Two studies have reported presynaptic alterations in inhibitory inputs to MNTB in Fmr1 knock-out mice.55,56 McCullagh et al56 showed that the number of GABAergic and glycinergic inhibitory inputs to the MNTB is decreased, as indicated by glutamate decarboxylase (GAD67) and the glycine transporter 2 (GlyT2) antibody staining.56 Interestingly, these changes are specific to the tonotopic location within the nucleus, with lateral MNTB showing decreased GABAergic inhibition while medial MNTB showing decreased glycinergic inhibition. In contrast, Rotschafer et al55 suggested strengthened inhibition by showing increased immunoreactivity for the vesicular GABA transporter (VGAT), a marker for both GABAergic and glycinergic terminal boutons.55 This discrepancy could be explained by different markers for inhibition (VGAT vs GlyT2 and GAD67), differences in mouse strain (FVB vs B6 background), or location/tonotopic specific changes in MNTB. These immunocyto-chemical studies do not, however, resolve whether FMRP loss induces a net change in the ratio of excitatory and inhibitory inputs to MNTB.
Direct in vitro slice recordings of synaptic transmission in MNTB of Fmr1 knockout mice suggest that changes in synaptic excitation are subtle.57 The maximal synaptic strength and kinetics of the glutamatergic input remain unchanged but the way the synapse responds to afferent stimulation with gradually increasing intensity becomes altered. In wild-type neurons, a typical all-or-none response pattern of evoked eEPSCs is observed in the majority of cells. Proportionally fewer Fmr1 knockout than wild-type neurons showed this pattern. In Fmr1 knockout animals, a smaller proportion of calyxes in the MNTB display simple morphology,58 which is associated with synaptic depression.59 Additionally, in vivo juxtacellular recordings detected only minor changes in excitatory spiking activity.58 In vitro slice physiology on the inhibitory inputs has also revealed no significant changes in most of the parameters examined for both glycinergic and GABAergic transmission.57 The discrepancy between anatomy and physiology suggests that the anatomical changes in the presynaptic terminals may not be sufficient to lead to significant functional changes. Alternatively, the electrophysiological changes may be attenuated by multiple anatomical alterations that balance out the overall effect on neurotransmission (for example, a decrease in GABA synthesis and an increase in GABA transport). A lack of change in synaptic strength at inhibitory synapses, measured by electrophysiological techniques, despite clear anatomical changes, has been also observed in other brain systems (eg,60,61).
One of the core mechanisms that have been proposed to account for the deficits in FXS in some parts of the nervous system is the “mGluR (metabotropic glutamate receptor) theory,” specifically that group I mGluR activity is exaggerated.62 While multiple transmitter systems may regulate auditory neurons, no significant differences have been reported in mGluR-mediated neuromodulation of the inhibitory inputs to MNTB.63 Activation of group I mGluRs affected the inhibitory transmission in the same manner in both wild-type and Fmr1 knockout neurons, enhancing spontaneous glycine release but suppressing the evoked inhibitory postsynaptic currents (elPSCs), and suppressing the evoked GABAergic IPSCs without changing the spontaneous GABA release. These observations in MNTB contrast the more dramatic changes observed in other brain regions, supporting the view that the effects of FMRP on neural functions vary across brain regions, and potentially across development.
3.2.2 |. Ion channels are regulated in a different way in the MNTB
Hyperexcitability in a circuit can result from changes in the intrinsic excitability of neurons, as well as changes in synaptic E/I ratio. Direct recordings of the firing patterns of MNTB neurons in acute slice preparations have shown that loss of FMRP renders these neurons hyperexcitable. In wild-type mice, the normal response of MNTB neurons to a sustained depolarization is to fire only a single action potential at the onset of the depolarization. In MNTB neurons from Fmr1 knockout animals, however, the same depolarization results in repetitive firing throughout the current pulse64 (Figure 3A). Such changes in the intrinsic excitability of neurons produced by loss of FMRP must reflect changes in the numbers and types of ion channels expressed in neurons. This can be accomplished by at least two quite distinct mechanisms. The first is by changes in the rates of translation of mRNAs that encode channels.65,66 The second is by altering the amplitude, gating or trafficking of the channel subunits that normally bind FMRP directly.
FIGURE 3.

MNTB neurons from Fmr1 knockout mice are hyperexcitable. A, Current-clamp recordings of action potentials in MNTB neurons from wild-type and Fmr1 knockout mice in response to hyperpolarizing and depolarizing current pulses (modified from 64). B, Bar graphs showing quantification of Kv3.1 immunoreactivity in MNTB in silence or after 30 minutes of exposure to physiological sound stimulation. C, Mean current-voltage relations for high-threshold Kv3.1 currents in patch clamp recordings of neurons in lateral and medial aspects of MNTB. The tonotopic organization of currents present in wild-type neurons is absent in neurons from Fmr1 knockout mice (B and C modified from 78). WT, wild type; KO, knockout
Ion channels are relatively minor cellular constituents compared to cytoplasmic and organellar proteins, and of the approximately 90 genes that encode Na+, Ca2+, or K+ channel subunits, only very few of their mRNAs have been shown to bind FMRP.16 A surprisingly high proportion of those that do bind FMRP are, however, expressed in neurons of the auditory system. Potassium channels that play major roles in regulating the intrinsic excitability of auditory brainstem neurons and whose mRNAs are targets of FMRP include the Kv3.1, Kv3.3, Kv1.2, Kv11.3, and KNa1.1 channels.67–75 The sodium channel Nav1.676 and the calcium channels Cav2.1, Cav2.2, and Cav 2.377 are also expressed in MNTB and their mRNAs are targets of FMRP.16 These links between FMRP and its channel targets that directly determine intrinsic excitability have allowed analyses of how loss of FMRP contributes to changes in firing patterns such as those of Figure 3.
One clear example of how a change in the rate of synthesis of an ion channel caused by loss of FMRP disrupts auditory processing is provided by the Kv3.1 channel in MNTB neurons, as well as in the VCN. Messenger RNA for Kv3.1 was one of the very first mRNAs found to bind to FMRP.17,78 This channel is absolutely required for neurons to fire at the very high rates required for phase-locking to physiological sound stimuli (up to 800 Hz).72 Kv3.1 is normally expressed in a gradient along the tonotopic axis of MNTB, with highest levels in neurons that respond optimally to high-frequency sounds.79,80 This gradient is absent and overall levels of this Kv3.1 protein and Kv3.1 currents are significantly higher in mice lacking FMRP (Figure 3B,C) .64,78 Moreover, levels of Kv3.1 in VCN and MNTB are increased by physiological increases in auditory stimulation in vivo.81 The ability of auditory stimulation to alter the already high level of Kv3.1 protein in these nuclei is abolished in Fmr1 knockout mice.78 These findings are consistent with a role for FMRP in the control of sound-induced translation of Kv3.1 mRNA, and with the enhanced firing rate of MTNB neurons in Fmr1 knockout mice.
A second mechanism by which FMRP regulates neuronal excitability is through direct protein-protein interactions with ion channels subunits.67,82–87 In each case, the interaction with FMRP alters the amplitude, kinetic behavior, or trafficking of the channel. Binding of FMRP to channels was first demonstrated for KNa1.1 channels, also termed Slack channels,67,82 and has also been documented for Kv1.287 and Cav2.2,85 all of which are expressed in MNTB. The two potassium channels, Kv1.2 and KNa1.1, that are regulated in this way play a very different function in the excitability of auditory brainstem neurons from that of the Kv3.1 channel. Both channels activate at negative membrane potentials close to the resting potential and contribute to the so-called low-threshold potassium currents. These are required for the high degree of temporal accuracy with which principal neurons of MNTB lock their action potentials to incoming stimuli.68,75 In both cases, the direct binding of the channel subunit to FMRP promotes channel opening, increasing current amplitude.67,82,87 Accordingly, the amplitude of the low-threshold potassium currents is reduced in MNTB neurons from Fmr1 knockout mice, promoting the abnormal firing patterns shown in Figure 3.64 Novel drugs that alter the voltage-dependence of Kv3.1 channels have been shown to rectify these abnormal firing patterns by increasing low-threshold currents and decreasing high-threshold currents in Fmr1 knockout animals.64 These agents, therefore, represent a potential new therapy for auditory hypersensitivity in FXS patients
Loss of FMRP affects the levels and activity of several other types of ion channels both by translational mechanisms and by direct protein-protein interactions.65 These include KCa1.1 (BK), Kv4.2, and HCN channels, which shape the excitability of many types of neurons in the auditory system, but their roles in auditory processing in FXS have not been investigated. Because FXS is a neurodevelopmental disorder, it is likely that there exist compensatory mechanisms that act over time to alter the excitability and synaptic transmission in the patients and in animal models of the disease.58
3.2.3 |. Synaptic distribution is altered and excitation is enhanced in the LSO
In LSO, alterations in synaptic E/I balance directly translate into a measurable shift in ILD sensitivity. There is evidence of increases in both excitatory and inhibitory inputs as indicated by a larger area of LSO containing the vesicular glutamate transporter (VGLUT1 or 2) and VGAT.55,88 Consistent with this, in vitro electrophysiological recordings from LSO neurons in acute brain slices of Fmr1 knockout mice showed that maximal EPSCs evoked by stimulating the ipsilateral incoming fibers from VCN are more than twice as large in mature Fmr1 knockout compared to wild-type animals (Figure 4A,B). This disproportionate increase in EPSCs occurs in the first week after hearing onset (Figure 4C). This increase is paralleled by a large increase in spontaneous EPSC frequency with no change in amplitude, and a similar increase in the number of synaptic endings labeled with VGLUT1 and VGLUT2.88 Inhibitory synaptic transmission from MNTB to LSO is mainly unaltered.56,88 These results suggest that LSO neurons of Fmr1 knockout mice receive more synaptic inputs and input fibers from VCN, whereas the strength of each individual synapse is unaffected. One possible mechanism may be that the loss of FMRP disturbs the developmental pruning of excitatory inputs to LSO which in wild-type animals results in a sharpening of frequency tuning in these neurons.89,90
FIGURE 4.

Enhanced excitation, altered E/I balance and broadened frequency tuning in LSO neurons of Fmr1 knockout mice. A, Schematic of patch-clamp recordings from LSO neurons in acute brain slices. B, Examples of excitatory postsynaptic currents (EPSCs) evoked by stimulation of AVCN-LSO fibers with different intensities in wild-type (black) and Fmr1 knockout mice (red). C, Averaged EPSC amplitudes evoked by maximal stimulation for different developmental stages (P8 to P33) in wild-type (black) and Fmr1 knockout mice (red). D, Averaged spike poststimulus time histograms of wild-type and knockout LSO neurons in response to monaural, ipsilateral sound stimulation. E, Schematic of ILD function in wild-type and Fmr1 knockout mice. Circles represent points of half-maximal inhibition. F, Frequency response areas of two representative LSO neurons, each patch showing the total number of spikes of four repetitions. Q-values as a measure for frequency tuning sharpness, expressing the bandwidth of the tuning curve 10 and 30 dB above threshold. Smaller Q10 and Q30 values indicate broader tuning in Fmr1 knockout animals. Modified from 88 with permission
Single unit recordings from LSO of anesthetized Fmr1 knockout mice show specific changes in their spike responses to monaural and binaural acoustic stimulation. The number of spikes in response to stimulation at each neuron’s characteristic frequency is increased and more neurons respond throughout the stimulus presentation88 (Figure 4D). This is consistent with the larger number of excitatory input fibers and more excitatory synapses as observed in the in vitro experiments. Binaural stimulation at the characteristic frequency with different ILDs reveal that the E/I balance in LSO neurons in Fmr1 knockout mice is shifted. On average, a louder stimulus at the contralateral, inhibitory ear is needed to cause complete suppression of spikes evoked by ipsilateral sound stimulation (Figure 4E). In addition, the slope of the ILD function, a measurement for how many decibels the sound at the contralateral ear has to change to go from no spiking to maximal spiking, was shallower. Shallower ILD slopes are considered to provide less accurate information on the azimuthal location of a sound.24 Whether Fmr1 knockout animals exhibit sound localization deficits needs to be tested in future experiments. Moreover, generating frequency response maps, where neurons are stimulated with a large range of different frequencies and intensities, show that frequency tuning of LSO neurons is broadened in Fmr1 knockout compared to wild-type animals (Figure 4F).
In summary, current electrophysiological and immunostaining studies indicate that, despite clear changes in morphology and intrinsic excitability at the level of the VCN and MNTB, overall changes in the balance of excitation and inhibition in Fmr1 knockout animals only become fully evident at the LSO where synaptic excitation is enhanced while the inhibitory input remains largely unchanged in strength. The disturbed E/I balance in LSO neurons may thus underlie broadened frequency tuning as well as shifted ILD responses in Fmr1 knockout animals.
3.3 |. FMRP loss affects ABR measurements in FXS and Fmr1 knockout mice
Noninvasive measures of brainstem activity can be performed in both humans and animal models. ABRs are electrophysiological representations of the population activity of auditory structures in the peripheral and central nervous system.91 Each wave in an ABR corresponds to specific structures along the ascending auditory tract, with some differences between humans and rodents. Generally, the waves of the ABR correspond to the ascending auditory pathway through the areas of the brainstem responsible for sound localization (reviewed in 92,93).
Whether FMRP loss affects human ABRs is controversial. Early studies on patients with FXS showed significantly delayed wave peaks and prolonged interpeak latencies (IPLs; Table 1). Among the changes consistently detected, the absolute latency of peak III is delayed and the I-III IPL is prolonged.96,97,99 Prolonged I-III IPL could be explained by a delay in the transmission of the auditory signal from the periphery to the brainstem nuclei, or a delay in the activation of the superior olivary complex itself. In these studies, the participants were not screened for sensorineural hearing loss and, because individuals with FXS are prone to otitis media, presumably had a wide range of peripheral hearing function.104,105 It is thus possible that the detected ABR alterations may have resulted indirectly from impaired middle ear function. Indeed, more recent studies from FXS individuals without middle ear infection did not find any differences in the absolute peak latencies and IPLs of ABRs.102,103,106 However, one should be careful in attempting to interpret this result as implying that ABRs and FMRP expression are independent. Because more than half of FXS children have otitis media,105 individuals without this peripheral pathology present only a specific population of FXS who are probably less sensitive to FMRP loss in both the periphery and the central nervous system. Other work compared children with FXS, with or without receiving sedation, during ABR testing and argued that differences seen in ABR testing across studies may be caused by the administration of sedatives.107 However, this study may have over-sampled from high-functional patients who may have been more tolerant of the ABR test.
TABLE 1.
ABR measurements in FXS
| Study | Participants | Abnormal ABR/BAEP? | Results |
|---|---|---|---|
| Wisniewski et al94 | n = 6 All male Mean age = 13.3 years |
No | Normal ABRs; methodology was vague |
| Gillberg et al95 | n = 10 All male Mean age unknown* |
6 of 7 were abnormal | Longer latencies; ABR methodology was vague |
| Ferri et al96 | n = 8 Males and females Mean age = 13.8 years |
Yes | Delayed peak III; Prolonged I-III IPL; Shorter III-V IPL |
| Ferri et al97; Ferri98 | n = 10 All male Mean age = 18.4 years |
Yes | Delayed peak III; Prolonged I-III IPL; Shorter III-V IPL |
| Arinami et al99; Arinami100 | n = 12 All male Mean age = 32.2 years |
Yes | Longer peak V latencies; increased I-V and III-V IPL |
| Wisniewski et al101 | n = 12 All male Mean age unknown** |
5 of 12 were abnormal | Ambiguous late waves (peaks III-V) prolonged peak III-V IPL |
| Miezejeski et al102 | n = 13 All male Mean age = 16.5 years |
No | 5 of 13 individuals with FXS sedated*** during ABR testing; Prolonged ABR latencies attributed to sedation |
| Roberts et al103 | n = 23 All male Mean age = 11 years |
No | No difference in FXS absolute or interpeak latencies |
7 participants were sampled from a pool of 10 FXS patients, mean age = 10.9 years.
12 participants were sampled from a pool of 62 FXS patients, mean age = 23.1 +/− 14.3 years.
Agents of sedation: chloral hydrate, thiopental, fentanyl citrate, diazepam, or droperidol. Sedation lasted less than 1 h.
Studies with mice are not confounded by some of the variables that affect human studies. Several ABR alterations have been identified by comparing Fmr1 knockout mice and age-matched wild types under anesthesia. Similar to human studies, peak latencies appear to be a subject of FMRP loss in FVB mice,108 but not in B6 mice.55,64 Interestingly, although there is no report of altered ABR wave amplitudes in human FXS, the amplitudes of several ABR peaks are altered in Fmr1 knockout mice, including decreased peak I and increased peak IV, suggesting impaired peripheral and central auditory systems. As the methodology for collecting ABRs can be quite variable, future work using similar experimental protocols on mice and humans may be useful for further characterization of the auditory brainstem phenotype in FXS.
4 |. FMRP LOSS ALTERS AUDITORY CORTICAL ACTIVITY
Consequences of Fmr1 loss at the cortical level have been primarily studied using physiological recordings at the single cell and population levels. These studies have made three main contributions to our understanding of sensory phenotypes in FXS. First, auditory cortex exhibits increased sound evoked responses, and one potential mechanism for this involves changes in levels of matrix metalloprotease-9 (MMP-9), a protein whose mRNA is a target of FMRP. This finding points to a potential therapeutic avenue that targets MMP-9 for treating FXS. Second, deficits in cortical processing are generated early in development. Third, humans with FXS and Fmr1 knockout mice exhibit remarkably similar changes in their electroencephalogram (EEG), which may prove a useful biomarker in FXS translational studies.
Studies in humans with FXS reveal a number of electrophysiological differences in auditory cortex compared to control participants.2 Increased activation of left hemispheric circuitry, including superior temporal gyrus, was observed in FXS subjects during an auditory temporal discrimination task.46 FXS subjects also show reduced synchronization of neural responses to time-varying signals, suggesting impairments in temporal processing.109 Rojas et al110 showed that tone-evoked responses measured using magnetic fields are higher in the auditory cortex of individuals with FXS.110 EEG recordings revealed increased gamma frequency band power in resting state EEG of FXS patients.111 Auditory event related potential (ERP) studies report abnormally high amplitudes of the N1 wave in response to tones and reduced habituation to repeated sound in FXS.110,112–115 These data indicate a noisy resting state of auditory cortex in FXS that may lead to abnormal amplitude and synchronization of evoked responses.
Electroencephalogram recordings from the auditory cortex of the Fmr1 knockout mouse show remarkably similar phenotypes to those seen in humans.116,117 The gamma frequency band power is increased in resting state EEG in auditory cortex of Fmr1 knockout mice. The N1 amplitude of ERP is elevated and shows reduced habituation to repeated sounds. The auditory cortex shows a reduced ability to synchronize responses to time-varying signals. In fact, almost every EEG phenotype that have been measured in both Fmr1 knockout mice and humans with FXS has shown a similar change in both species, indicating a potential use of these deficits as biomarkers to facilitate the translation pipeline. Moreover, EEG deficits in Fmr1 knockout mice are present from early in development.118 At the single neuron level, Rotschafer and Razak119 showed that adult mouse cortical neurons produce a stronger response to brief tones in Fmr1 knockout mice compared to wild-type mice.119 Developmental studies further demonstrated that the auditory cortex develops hyper-responsiveness between P14 and P21, and that this is maintained in adulthood.118 In addition, the adult knockout neurons showed broader frequency tuning (similar to LSO - see above) and reduced selectivity for frequency modulated (FM) sweeps suggesting abnormal spectrotemporal processing.119
In addition to potential changes in synaptic transmission and ion channel regulation similar to those described for the auditory brainstem, there is evidence that an alteration in MMP-9 dependent regulation of perineuronal nets (PNNs) may contribute to hyperactivity of the auditory cortex, as well as perhaps lower brainstem structures. PNNs are extracellular matrix components that are often associated with parvalbumin (PV)-expressing cortical cells and regulate their development and functions.121–124 MMP-9 is a secreted endo-peptidase that regulates PNN formation and organization.125 FMRP negatively regulates MMP-9 translation and MMP-9 levels in neurons, including those in the auditory cortex, are elevated in FXS.120,126–128 Consistently, the auditory cortex exhibits delayed development of PNNs and PV cells in Fmr1 knockout mice.120 Genetic reduction of MMP-9 in Fmr1 knockout mice restored the formation of PNNs around PV cells and rescued spontaneous and sound-driven responses to normal level in the auditory cortex.120 Together, these studies demonstrate that elevated MMP-9 levels contribute to the development of auditory processing deficits by influencing the development of PNNs and PV cells in the auditory cortex of Fmr1 knockout mice (Figure 5). Whether altered synaptic transmission and ion channel dysregulation as described in the auditory brainstem is also present in the auditory cortex requires further investigation.
FIGURE 5.
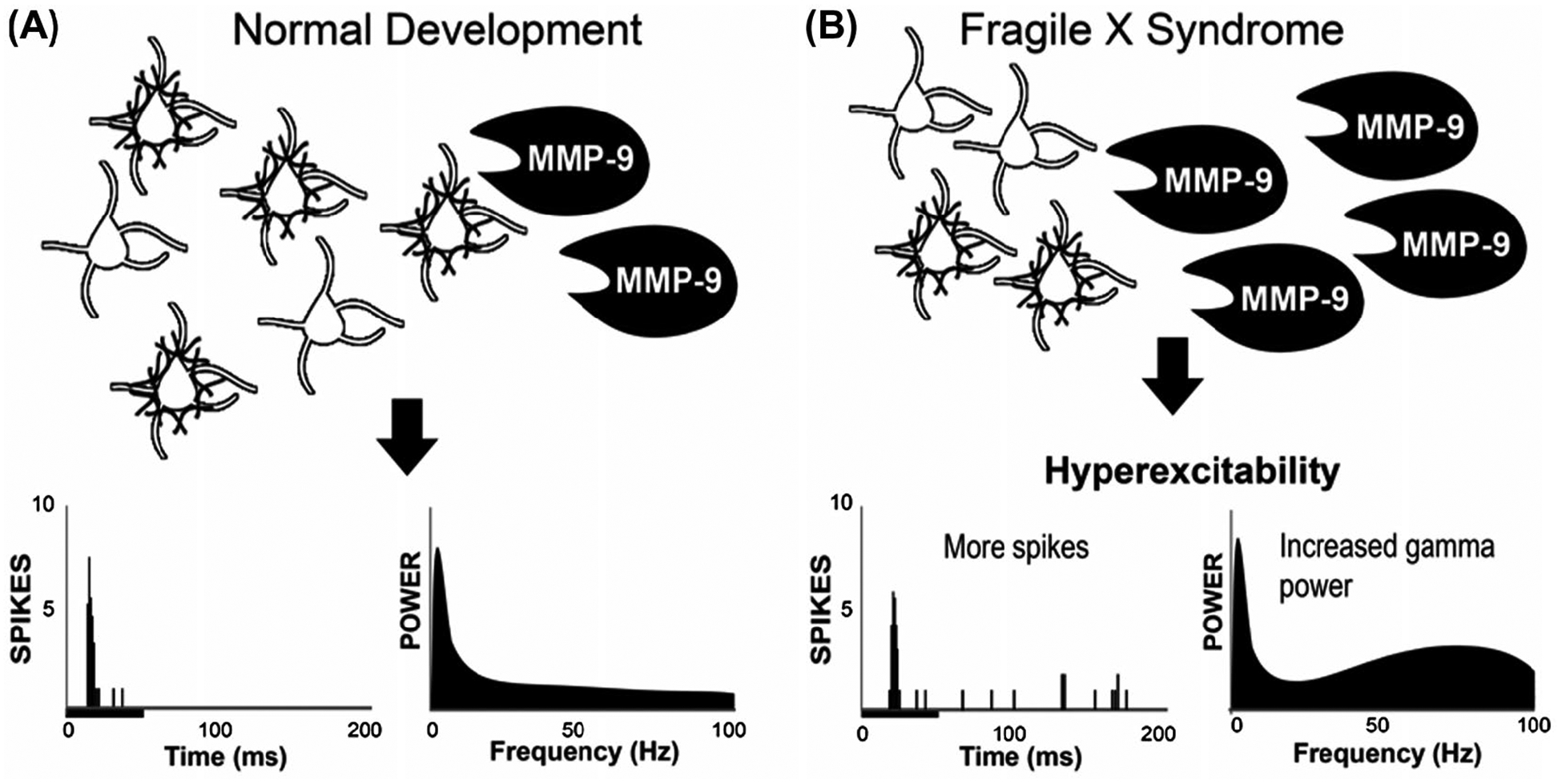
Mechanisms underlying cortical hyper-responsiveness in FXS involve local circuit deficits. A, In normal development, MMP-9 provides a certain level of control over PNN integrity around PV cells leading to normal balance between inhibition and excitation, auditory cortical responses as shown below with the poststimulus time histogram and power spectral density plots in the lower left panels. B, In FXS, the MMP-9 level is increased leading to greater breakdown of PNN around PV cells leading to increased spiking and increased resting EEG gamma band power. MMP-9/PV/PNN defects are present in global Fmr1 knockout mice, as well as mice in which FMRP is removed only from forebrain excitatory neurons. This suggests that local PV neuron functional deficits can lead to cortical hyper-responsiveness. Moreover, even though FMRP was affected only in excitatory neurons, deficits were observed at the network level involving PV cells and PNN that surround them. Image modified from 120
Given that FMRP is expressed across multiple auditory system nuclei, the question arises as to whether abnormal cortical responses reflect local circuit dysfunction. This is a complex issue because of the nature of ascending and descending connections in the auditory system. To begin to evaluate the relative contribution of subcortical inputs and local cortical circuits to observed changes in cortical recordings, a conditional knockout in which FMRP was deleted specifically from forebrain excitatory neurons using the Nex1 promoter was generated.129 Similar to global knockout animals, this conditional knockout strain exhibits elevated gamma frequency band power in EEGs as well as delayed development of PNN and PV cells, indicating that these deficits arise locally in the cortex. However, the deficit in temporal synchronization of responses to time varying stimuli was absent when FMRP expression is preserved at subcortical levels, demonstrating that the change following global Fmr1 knockout is a secondary consequence of altered subcortical inputs. Together, these observations suggest that FMRP deficiency at both subcortical and cortical levels contribute to the physiological deficits recorded from the auditory cortex.
5 |. AUDITION-ASSOCIATED BEHAVIORAL CHANGES IN FXS
Three main groups of auditory-associated behavioral phenotypes have been described in Fmr1 knockout rodents: (i) alterations to the acoustic startle response (ASR) and/or prepulse inhibition (PPI) of the ASR, (ii) increased audiogenic seizure susceptibility, and (iii) impaired auditory-cued fear conditioning.
5.1 |. Altered ASR and PPI
Deletion of Fmr1 impacts acoustic processing in both rodents and humans, as evaluated by altered ASR and PPI.130 ASR is a full body reflexive response elicited by a loud sound stimulus. PPI is thought to occur when a weak nonstartling sound presented immediately before a startling sound that suppresses the ASR. ASR provides a robust and high throughput assay for measuring sound sensitivity, but it is difficult to differentiate changes to sound perception from generalized hyperactivity or motor abnormalities.131,132 Additionally, ASR only occurs for high-intensity sounds, which limits its sensitivity for identifying perceptual abnormalities at moderate sound levels. On the contrary, PPI can be used to assess behavioral responses to softer stimuli that do not elicit a startle response themselves. Enhanced or reduced PPI indicates increased or decreased sensitivity to a sound stimulus and implicates sensorimotor gating disruptions that are specific to the auditory system.
In contrast to the variability in human measurements of the ABR, studies of ASR and PP1 in humans have given clear-cut results. Two clinical studies have compared ASR and PPI responses between individuals with FXS and control subjects.130,133 Both studies agree on unchanged threshold and magnitude of the baseline ASR but a reduced PPI in FXS individuals. Interestingly, studies of Fmr1 knockout mice show mixed results, perhaps because of the very different hearing range of humans and mice. Many studies found reduced ASR to startle sounds at the level of 90–120 dB, along with enhanced PPI, as compared to age-matched wild-type mice.130,134–145 Other studies reported unaltered, enhanced ASR, or reduced ppi.134,138,139,142 Potential contributors to these discrepancies include, but are not limited to, the genetic background,140,146 sex (147; but also see137), age,145 and the sound level for triggering ASR.140 Particularly, strain-specific differences have been reported in a variety of behavioral phenotypes following Fmr1 deletion142 and it is known that different mouse strains exhibit varying degrees of ASR in wild-type mouse strains.148 Despite the variability in ASR and PPI phenotypes, ASR changes in Fmr1 knockout mice are developmentally regulated145 and can be rescued with reintroduction of the Fmr1 gene,141 supporting a direct involvement of Fmr1 gene in these auditory-mediated behaviors. Because their hearing range overlaps with that of humans, it is likely that Fmr1 rats will prove a better model for studying these auditory phonotypes.
5.2 |. Increased audiogenic seizure susceptibility
A high proportion of FXS patients have childhood seizures, which typically abate with development.149 In Fmr1 knockout mice, a continuous loud sound lasting longer than 30 seconds leads to audiogenic seizures (AGS).136,150 AGS are generalized convulsive seizures characterized by wild running, loss of righting reflex, generalized tonic-clonic seizing and, in some cases, death of the animal. As the seizure phenotype is robust, it is frequently used as a marker for testing drug efficacy in mouse models of FXS. Indeed, many therapeutics for FXS have been shown to reduce or abolish AGS in Fmr1 knockout mice (metformin:151; CTEP:152; Lovastatin:153,154; Baclofen:155; PAK inhibitor:156). Similar to ASR and PPI, there are strain-specific differences in both susceptibility and developmental timing of AGS, with the most robust seizure phenotype seen in Fmr1 knockout mice on the FVB background.136,150 Animals from the C57BL/6 background are most susceptible to seizures during a restricted developmental window around postnatal day 21–25.137 Determining the contribution of genetic background to audiogenic seizure susceptibility in Fmr1 knockout mice could potentially help explain epilepsy variability seen across individuals with FXS.149
Cell type specific Fmr1 deletion demonstrates that lack of FMRP in subcortical regions is sufficient to cause AGS. Moreover, restoration of FMRP expression solely in the inferior colliculus is sufficient to prevent AGS in the global knockout mouse strain.157 At the molecular level, generation of AGS likely involves the common signal pathways underlying other FXS phenotypes, because the frequency of AGS in Fmr1 knockout mice is reduced by correcting mGluR5 signaling143,158 or ERK1/2 activity.159,160 Although the AGS in Fmr1 knockout mice are frequently compared to epilepsy in FXS individuals, it is not clear that the underlying seizures are similar in humans and mice.149 Moreover, the relationship between AGS and sound tolerance issues in FXS individuals has not been established, presenting questions for future research.
5.3 |. Perceptual learning and decision making
Recent studies have provided evidence for perceptual learning or discrimination deficits in Fmr1 knockout mice in both the tactile161,162 and visual domains.163 Although fewer studies have examined auditory perceptual learning in FXS models, there is evidence for impaired auditory-cued fear conditioning in Fmr1 knockout mice.164,165 It is not clear, however, whether this impairment is more cognitive than perceptual in nature, because Fmr1 knockout mice exhibit impairment on a variety of associative learning paradigms that are not dependent on auditory cues.166 Examination of auditory learning and/or discrimination in Fmr1 knockout mice has the potential to shed light onto the mechanistic basis of sound sensitivity and processing deficits in FXS, as well as fuel development of behavioral paradigms that can be directly translated to human studies.
6 |. CONCLUSIONS AND FUTURE DIRECTIONS
The studies of the auditory system in FXS in humans and animal models represent one of the most detailed examinations across a sensory system in an ASD. The results discussed here demonstrate that FMRP deficiency affects the auditory system at multiple stages along the ascending pathway, starting with the spiral ganglion and very first nuclei in the brainstem and extending all the way to auditory cortex. This wide-spread involvement strongly supports the notion that FXS is not purely a cognitive condition but alters fundamental sensory information processing that is required for a subject to perceive, separate, and localize sounds. Interestingly, striking changes in morphology or intrinsic neuronal excitability caused directly by loss of FMRP may be compensated by other mechanisms, such that changes in overall synaptic strength may be little changed at lower levels but become progressively clearer at higher levels. In some cases, studies of auditory neurons have also revealed new potential therapeutic strategies for hypersensitivity to sounds in FXS, including treatments that alter the activity of potassium channels or metalloproteases.
Fmr1 knockout rodents have provided numerous insights into specific cellular and molecular changes that contribute to the auditory problems of FXS patients. Similar genetic approaches are likely to provide even more insights in the future. It is important to note that although Fmr1 knockout mice recapture some phenotypes of the auditory system of human FXS, large variations and even completely opposite changes are frequently encountered across mouse studies as well as between mouse and human studies. In part, these problems may arise because some of the observed changes are the result of compensatory neuronal plasticity rather than true FMRP-dependent biological pathways, and may therefore, differ with age, sex, and genetic background. More systematic future studies of the role of FMRP at different developmental states in multiple auditory nuclei may, therefore, resolve some of these issues.167
Finally, it may be possible to “borrow” useful approaches in other fields for studying FMRP regulation of auditory processing. For example, hearing loss often results in auditory perceptual disruptions, including hyperacusis, where moderate-intensity “everyday” sounds are perceived as intolerably loud or aversive.168 While sound hypersensitivity due to hearing loss or FMRP deficiency are likely to be mechanistically distinct, there are well-developed assays for assessing sound sensitivity following hearing loss that can be applied to FXS and ASD models. Potential approaches include operant conditioning paradigms that assess perceptual decision making,169–171 which likely engage a more extensive range of auditory structures than startle-based measures, or innate place-preference paradigms that reflect the aversive nature of sounds without extensive training or conditioning.172,173
ACKNOWLEDGMENTS
We would like to acknowledge our funding sources: NIH NIDCD 3T32DC01228005S1, FRAXA (Elizabeth A. McCullagh); Deutsche Forschungsgemeinschaft SFB665 (Ursula Koch); NIH NIDCD R01DC01919 & NS102239, FRAXA (Leonard K. Kaczmarek); NIHNIDCD R01DC010796 (Karina Cramer); NIH NIDCD R01DC017924 (Achim Klug); NIH U54 HD082008, DOD PR140683, FRAXA (Jonathan Lovelace and Khaleel Razak); NIH NIDCD R01DC016054 (Yong Lu); NIH NIDCD R01DC13074 & R21DC17267, United States-Israel Binational Science Foundation (Yuan Wang); NIH NIDCD F32DC01516001A1, SFARI (Benjamin D. Auerbach).
Funding information
HHS | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD), Grant/Award Number: 3T32DC01228005S1, R01DC01919, NS102239, R01DC010796, R01DC017924, R01DC016054, R01DC13074, R21DC17267 and F32DC01516001A1; FRAXA Research Foundation (FRAXA); Deutsche Forschungsgemeinschaft (DFG), Grant/Award Number: SFB665; HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Grant/Award Number: U54 HD082008; DOD | United States Army | MEDCOM | Congressionally Directed Medical Research Programs (CDMRP), Grant/Award Number: PR140683; United States-Israel Binational Science Foundation; SFARI
Abbreviations:
- ASD
autism spectrum disorder
- AGS
audiogenic seizures
- ASR
acoustic startle response
- E/I
ratio of excitation to inhibition
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- Fmr1
non-human mammalian fragile X mental retardation 1 gene
- FMR1
human fragile X mental retardation 1 gene
- FMRP
Fragile X mental retardation protein
- FXS
Fragile X syndrome
- GlyT2
glycine transporter 2
- ITD
interaural time difference
- IID/ILD
interaural intensity/level difference
- IPL
interpeak latency
- LNTB
lateral nucleus of the trapezoid body
- LSO
lateral superior olive
- mGluR
metabotropic glutamate receptor
- MMP
matrix metalloprotease
- MNTB
medial nucleus of the trapezoid body
- MSO
medial superior olive
- NL
nucleus laminaris
- NM
nucleus magnocellularis
- PNN
perineuronal nets
- PPI
prepulse inhibition
- PV
parvalbumin
- SON
superior olivary nucleus
- VCN
ventral cochlear nucleus
- VGAT
vesicular GABA transporter
- VGLUT
vesicular glutamate transporter
- VNTB
ventral nucleus of the trapezoid body
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Crawford DC, Acuna JM, Sherman SL. FMR1 and the Fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rais M, Binder DK, Razak KA, Ethell IM. Sensory processing phenotypes in Fragile X syndrome. ASN Neuro. 2018;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu M, Han Y, Dy ABC, et al. Autism symptoms in Fragile X syndrome. J Child Neurol. 2017;32:903–909. [DOI] [PubMed] [Google Scholar]
- 4.Rajaratnam A, Shergill J, Salcedo-Arellano M, Saldarriaga W, Duan X, Hagerman R. Fragile X syndrome and Fragile X-associated disorders. F1000Research. 2017;6:2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gothelf D, Furfaro JA, Hoeft F, et al. Neuroanatomy of Fragile X syndrome is associated with aberrant behavior and the Fragile X mental retardation protein (FMRP). Ann Neurol. 2008;63:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon H, Menon V, Eliez S, et al. Functional neuroanatomy of visuospatial working memory in Fragile X syndrome: relation to behavioral and molecular measures. AJP. 2001;158:1040–1051. [DOI] [PubMed] [Google Scholar]
- 7.Loesch DZ, Bui QM, Dissanayake C, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in Fragile X. Neurosci Biobehav Rev. 2007;31:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in Fragile X. Mental Retardation Dev Disabilities Res Rev. 2004;10:31–41. [DOI] [PubMed] [Google Scholar]
- 9.Gholizadeh S, Arsenault J, Xuan ICY, Pacey LK, Hampson DR. Reduced phenotypic severity following Adeno-associated virus-mediated Fmr1 gene delivery in Fragile X mice. Neuropsychopharmacology. 2014;39:3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boulle K, Verkerk AJMH, Reyniers E, et al. A point mutation in the FMR-1 gene associated with Fragile X mental retardation. Nat Genet. 1993;3:31–35. [DOI] [PubMed] [Google Scholar]
- 11.Okray Z, de Esch CE, Van Esch H, et al. A novel Fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function. EMBO Mol Med. 2015;7:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of Fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. [DOI] [PubMed] [Google Scholar]
- 13.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for Fragile X syndrome. Nat Neurosci. 2013;16:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee A, Ifrim MF, Valdez AN, Raj N, Bassell GJ. Aberrant RNA translation in fragile X syndrome: from FMRP mechanisms to emerging therapeutic strategies. Brain Res. 2018;1693:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnell JC, Van Driesche SJ, Zhang C, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Zhang Y, Zhan S, et al. Proteomic profiling of brain and testis reveals the diverse changes in ribosomal proteins in fmr1 knockout mice. Neuroscience. 2018;371:469–483. [DOI] [PubMed] [Google Scholar]
- 19.Tang B, Wang T, Wan H, et al. Fmr1 deficiency promotes age-dependent alterations in the cortical synaptic proteome. PNAS. 2015;112:E4697–E4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis JK, Broadie K. Multifarious functions of the Fragile X mental retardation protein. Trends Genet. 2017;33:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contractor A, Klyachko VA, Portera-Cailliau C. Altered neuronal and circuit excitability in Fragile X syndrome. Neuron. 2015;87:699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of Fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of Fragile X mental retardation. PNAS. 2002;99:7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. [DOI] [PubMed] [Google Scholar]
- 25.Hinds HL, Ashley CT, Sutcliffe JS, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in Fragile X syndrome. Nat Genet. 1993;3:36–43. [DOI] [PubMed] [Google Scholar]
- 26.Zangenehpour S, Cornish KM, Chaudhuri A. Whole-brain expression analysis of FMRP in adult monkey and its relationship to cognitive deficits in Fragile X syndrome. Brain Res. 2009;1264:76–84. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Sakano H, Beebe K, et al. Intense and specialized dendritic localization of the Fragile X mental retardation protein in binaural brainstem neurons: a comparative study in the alligator, chicken, gerbil, and human: FMRP localization in NL/MSO dendrites. J Comp Neurol. 2014;522:2107–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zorio DAR, Jackson CM, Liu Y, Rubel EW, Wang Y. Cellular distribution of the Fragile X mental retardation protein in the mouse brain: Fmrp distribution in the mouse brain. J Comp Neurol. 2017;525:818–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature. 1998;393:268–272. [DOI] [PubMed] [Google Scholar]
- 30.Ruby K, Falvey K, Kulesza RJ. Abnormal neuronal morphology and neurochemistry in the auditory brainstem of Fmr1 knockout rats. Neuroscience. 2015;303:285–298. [DOI] [PubMed] [Google Scholar]
- 31.Beebe K, Wang Y, Kulesza R. Distribution of Fragile X mental retardation protein in the human auditory brainstem. Neuroscience. 2014;273:79–91. [DOI] [PubMed] [Google Scholar]
- 32.Kulesza RJ, Mangunay K. Morphological features of the medial superior olive in autism. Brain Res. 2008;1200:132–137. [DOI] [PubMed] [Google Scholar]
- 33.Galvez R, Smith RL, Greenough WT. Olfactory bulb mitral cell dendritic pruning abnormalities in a mouse model of the Fragile-X mental retardation syndrome: further support for FMRP’s involvement in dendritic development. Dev Brain Res. 2005;157:214–216. [DOI] [PubMed] [Google Scholar]
- 34.Galvez R, Gopal AR, Greenough WT. Somatosensory cortical barrel dendritic abnormalities in a mouse model of the Fragile X mental retardation syndrome. Brain Res. 2003;971:83–89. [DOI] [PubMed] [Google Scholar]
- 35.Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the Fragile X mental retardation syndrome. Am J Med Genet A. 2005;135A:155–160. [DOI] [PubMed] [Google Scholar]
- 36.Restivo L, Ferrari F, Passino E, et al. Enriched environment promotes behavioral and morphological recovery in a mouse model for the Fragile X syndrome. PNAS. 2005;102:11557–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas CC, Combe CL, Dyar KA, Inglis FM. Modest alterations in patterns of motor neuron dendrite morphology in the Fmr1 knockout mouse model for Fragile X. Int J Dev Neurosci. 2008;26:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Rubel EW. In vivo reversible regulation of dendritic patterning by afferent input in bipolar auditory neurons. J Neurosci. 2012;32:11495–11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakano H, Zorio DAR, Wang X, et al. Proteomic analyses of nucleus laminaris identified candidate targets of the Fragile X mental retardation protein: SAKANO et al. J Comp Neurol. 2017;525:3341–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govek E-E. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zorio DAR, Schecterson L, Lu Y, Wang Y. Postsynaptic FMRP regulates synaptogenesis in vivo in the developing cochlear nucleus. J Neurosci. 2018;38:6445–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darwin CJ. Listening to speech in the presence of other sounds. Philos Trans R Soc London B: Biol Sci. 2008;363:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the Fragile X syndrome amygdala. J Neurophysiol. 2014;112:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall SS, Walter E, Sherman E, Hoeft F, Reiss AL. The neural basis of auditory temporal discrimination in girls with Fragile X syndrome. J Neurodev Dis. 2009;1:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visser E, Zwiers MP, Kan CC, Hoekstra L, van Opstal AJ, Buitelaar JK. Atypical vertical sound localization and sound-onset sensitivity in people with autism spectrum disorders. J Psychiatry Neurosci. 2013;38:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teder-Sälejärvi WA, Pierce KL, Courchesne E, Hillyard SA. Auditory spatial localization and attention deficits in autistic adults. Brain Res Cogn Brain Res. 2005;23:221–234. [DOI] [PubMed] [Google Scholar]
- 49.Dondzillo A, Thompson JA, Klug A. Recurrent inhibition to the medial nucleus of the trapezoid body in the Mongolian Gerbil (Meriones Unguiculatus). PLoS ONE. 2016;11:e0160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albrecht O, Dondzillo A, Mayer F, Thompson JA, Klug A. Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse. Front Neural Circuits. 2014;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. [DOI] [PubMed] [Google Scholar]
- 53.Awatramani GB, Turecek R, Trussell LO. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol. 2005;93:819–828. [DOI] [PubMed] [Google Scholar]
- 54.Mayer F, Albrecht O, Dondzillo A, Klug A. Glycinergic inhibition to the medial nucleus of the trapezoid body shows prominent facilitation and can sustain high levels of ongoing activity. J Neurophysiol. 2014;112:2901–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rotschafer SE, Marshak S, Cramer KS. Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS ONE. 2015;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCullagh EA, Salcedo E, Huntsman MM, Klug A. Tonotopic alterations in inhibitory input to the medial nucleus of the trapezoid body in a mouse model of Fragile X syndrome. J Comp Neurol. 2017;262:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y Subtle differences in synaptic transmission in medial nucleus of trapezoid body neurons between wild-type and Fmr1 knockout mice. Brain Res. 2019;1717:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang T, de Kok L, Willemsen R, Elgersma Y, Borst JGG. In vivo synaptic transmission and morphology in mouse models of tuberous sclerosis, Fragile X syndrome, neurofibromatosis type 1, and costello syndrome. Front Cell Neurosci. 2015;9:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grande G, Wang L-Y. Morphological and functional continuum underlying heterogeneity in the spiking fidelity at the calyx of held synapse in vitro. J Neurosci. 2011;31:13386–13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curia G, Papouin T, Séguéla P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of Fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in Fragile X syndrome. J Neurophysiol. 2011;106:2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bear MF, Huber KM, Warren ST. The mGluR theory of Fragile X mental retardation. Trends Neurosci. 2004;27:370–377. [DOI] [PubMed] [Google Scholar]
- 63.Curry RJ, Peng K, Lu Y. Neurotransmitter- and release-mode-specific modulation of inhibitory transmission by group I metabotropic glutamate receptors in central auditory neurons of the mouse. J Neurosci. 2018;38:8187–8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Hassar L, Song L, Winston TJT, et al. Modulators of Kv3 potassium channels rescue the auditory function of Fragile X mice. J Neurosci. 2019;39:4797–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frick A, Ginger M, El-Hassar L, Kaczmarek LK. Chapter 16 - ion channel dysfunction and FXS In Willemsen R, Kooy RF, eds. Fragile X Syndrome. Cambridge, Massachusetts: Academic Press; 2017:323–340. [Google Scholar]
- 66.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of Fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. [DOI] [PubMed] [Google Scholar]
- 67.Brown MR, Kronengold J, Gazula V-R, et al. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang B, Desai R, Kaczmarek LK. Slack and slick KNa channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardman RM, Forsythe ID. Ether-à-go-go -related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons: Dubito ERGo cogito; cogito ERGo sum. J Physiol. 2009;587:2487–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol. 2003;548:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in Kcnal -null mice: an electrophysiological study in vivo. J Neurosci. 2003;23:9199–9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macica CM, von Hehn CAA, Wang L-Y, et al. Modulation of the Kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J Neurosci. 2003;23: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song P, Yang Y, Barnes-Davies M, et al. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nat Neurosci. 2005;8:1335–1342. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Zhang X-F, Fleming MR, et al. Kv3.3 channels bind Hax-1 and Arp2/3 to assemble a stable local actin network that regulates channel gating. Cell. 2016;165:434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leao RM. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci. 2005;25:3724–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tozer AJB, Forsythe ID, Steinert JR. Nitric oxide signalling augments neuronal voltage-gated L-type (CaV1) and P/Q-type (CaV2.1) channels in the mouse medial nucleus of the trapezoid Body. PLoS ONE. 2012;7:e32256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30:10263–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W, Kaczmarek LK, Perney TM. Localization of two high-threshold potassium channel subunits in the rat central auditory system. J Comp Neurol. 2001;437:196–218. [DOI] [PubMed] [Google Scholar]
- 80.von Hehn CAA. Loss of Kv3.1 tonotopicity and alterations in cAMP response element-binding protein signaling in central auditory neurons of hearing impaired mice. J Neurosci. 2004;24:1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strumbos JG, Polley DB, Kaczmarek LK. Specific and rapid effects of acoustic stimulation on the tonotopic distribution of Kv3.1b potassium channels in the adult rat. Neuroscience. 2010;167:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Brown MR, Hyland C, et al. Regulation of neuronal excitability by interaction of fragile x mental retardation protein with slack potassium channels. J Neurosci. 2012;32:15318–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng P-Y, Rotman Z, Blundon JA, et al. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng P-Y, Carlin D, Oh YM, et al. Voltage-independent SK-channel dysfunction causes neuronal hyperexcitability in the hippocampus of Fmr1 knock-out mice. J Neurosci. 2019;39:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun. 2014;5:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myrick LK, Deng P-Y, Hashimoto H, et al. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci. 2015;112:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y-M, Arsenault J, Bah A, et al. Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain. Mol Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia-Pino E, Gessele N, Koch U. Enhanced excitatory connectivity and disturbed sound processing in the auditory brainstem of Fragile X mice. J Neurosci. 2017;37:7403–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanes D, Rubel E. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanes DH, Merickel M, Rubel EW. Evidence for an alteration of the tonotopic map in the gerbil cochlea during development. J Comp Neurol. 1989;279:436–444. [DOI] [PubMed] [Google Scholar]
- 91.Jewett DL, Romano MN, Williston JS. Human auditory evoked potentials: possible brain stem components detected on the scalp. Science. 1970;167:1517–1518. [DOI] [PubMed] [Google Scholar]
- 92.Moller AR. Neural mechanisms of BAEP. Electroencephalogr Clin Neurophysiol Suppl. 1999;49:27–35. [PubMed] [Google Scholar]
- 93.Biacabe B, Chevallier JM, Avan P, Bonfils P. Functional anatomy of auditory brainstem nuclei: application to the anatomical basis of brainstem auditory evoked potentials. Auris Nasus Larynx. 2001;28:85–94. [DOI] [PubMed] [Google Scholar]
- 94.Wisniewski KE, French JH, Fernando S, et al. Fragile X syndrome: associated neurological abnormalities and developmental disabilities. Ann Neurol. 1985;18:665–669. [DOI] [PubMed] [Google Scholar]
- 95.Gillberg C, Persson E, Wahlström J. The autism-Fragile-X syndrome (AFRAX): a population-based study of ten boys. J Mental Deficiency Res. 1986;30:27–39. [DOI] [PubMed] [Google Scholar]
- 96.Ferri R, Bergonzi P, Colognola R, et al. Brainstem auditory evoked potentials in subjects with mental retardation and different karyotypes In: Gallai V, ed. Maturation of the CNS and evoked potentials. Amsterdam, The Netherlands: Elsevier; 1986:369–374. [Google Scholar]
- 97.Ferri R, Colognola R, Falsone S, et al. Brainstem auditory and visual evoked potentials in subjects with Fragile-X mental retardation syndrome In: Barber C, Blum T, eds. Evoked Potentials III. The Third International Evoked Potentials Symposium London: Butterworths; 1987:167–169. [Google Scholar]
- 98.Ferri R Brain-stem auditory evoked potentials in Fragile X syndrome. Am J Hum Genet. 1989;45:977–978. [PMC free article] [PubMed] [Google Scholar]
- 99.Arinami T, Sato M, Nakajima S, Kondo I. Auditory brainstem responses in the Fragile X syndrome. Am J Hum Genet. 1988;43:46–51. [PMC free article] [PubMed] [Google Scholar]
- 100.Arinami T Reply to Dr. Ferri. Am J Human Genet. 1989;45: 978–979. [Google Scholar]
- 101.Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet Part A. 1991;38:476–480. [DOI] [PubMed] [Google Scholar]
- 102.Miezejeski CM, Heaney G, Belser R, Brown WT, Jenkins EC, Sersen EA. Longer brainstem auditory evoked response latencies of individuals with Fragile X syndrome related to sedation. Am J Med Genet Part A. 1997;74:167–171. [DOI] [PubMed] [Google Scholar]
- 103.Roberts J, Hennon EA, Anderson K, et al. Auditory brainstem responses in young males with Fragile X syndrome. J Speech Lang Hear. Res 2005;48:494–500. [DOI] [PubMed] [Google Scholar]
- 104.Badran HS, Abulnasr KM, Nasser SAEH. Effect of recurrent otitis media on language profile in children with Fragile X syndrome. Clin Med Insights: Ear, Nose Throat. 2013;6:CMENT. S11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hagerman RJ, Altshul-Stark D, McBogg P. Recurrent otitis media in the Fragile X syndrome. JAMA Pediatrics. 1987;141:184–187. [DOI] [PubMed] [Google Scholar]
- 106.Wisniewski KE, French JH, Fernando S, et al. Fragile X syndrome: associated neurological abnormalities and developmental disabilities. Ann Neurol. 1985;18:665–669. [DOI] [PubMed] [Google Scholar]
- 107.Miezejeski CM, Heaney G, Belser R, Brown WT, Jenkins EC, Sersen EA. Longer brainstem auditory evoked response latencies of individuals with Fragile X syndrome related to sedation. Am J Med Genet A. 1997;74:167–171. [DOI] [PubMed] [Google Scholar]
- 108.Kim H, Gibboni R, Kirkhart C, Bao S. Impaired critical period plasticity in primary auditory cortex of Fragile X model mice. J Neurosci. 2013;33:15686–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ethridge LE, White SP, Mosconi MW, et al. Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in Fragile X syndrome. Mol Autism. 2017;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rojas DC, Benkers TL, Rogers SJ, Teale PD, Reite ML, Hagerman RJ. Auditory evoked magnetic fields in adults with Fragile X syndrome. NeuroReport. 2001;12:2573–2576. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, Ethridge LE, Mosconi MW, et al. A resting EEG study of neocortical hyperexcitability and altered functional connectivity in Fragile X syndrome. J Neurodevelopmental Disorders. 2017;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castrén M, Pääkkönen A, Tarkka IM, Ryynänen M, Partanen J. Augmentation of auditory N1 in children with Fragile X syndrome. Brain Topogr. 2003;15:165–171. [DOI] [PubMed] [Google Scholar]
- 113.Ethridge LE, White SP, Mosconi MW, Wang J, Byerly MJ, Sweeney JA. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X syndrome. Transl Psychiatry. 2016;6:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneider A, Leigh MJ, Adams P, et al. Electrocortical changes associated with minocycline treatment in Fragile X syndrome. JPsychopharmacol. 2013;27:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van der Molen MJW, Van der Molen MW, Ridderinkhof KR, Hamel BCJ, Curfs LMG, Ramakers GJA. Auditory change detection in Fragile X syndrome males: a brain potential study. Clin Neurophysiol. 2012;123:1309–1318. [DOI] [PubMed] [Google Scholar]
- 116.Lovelace JW, Wen TH, Reinhard S, et al. Matrix metallopro-teinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X syndrome. Neurobiol Dis. 2016;89:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lovelace JW, Ethell IM, Binder DK, Razak KA. Translation-relevant EEG phenotypes in a mouse model of Fragile X syndrome. Neurobiol Dis. 2018;115:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen TH, Lovelace JW, Ethell IM, Binder DK, Razak KA. Developmental changes in EEG phenotypes in a mouse model of Fragile X syndrome. Neuroscience. 2019;398:126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rotschafer S, Razak K. Altered auditory processing in a mouse model of Fragile X syndrome. Brain Res. 2013;1506:12–24. [DOI] [PubMed] [Google Scholar]
- 120.Wen TH, Afroz S, Reinhard SM, et al. Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cereb Cortex. 2018;28:3951–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro. 2016;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Härtig W, Singer A, Grosche J, Brauer K, Ottersen OP, Brückner G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res. 2001;899:123–133. [DOI] [PubMed] [Google Scholar]
- 123.Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37:1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. [DOI] [PubMed] [Google Scholar]
- 125.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. [DOI] [PubMed] [Google Scholar]
- 126.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the Fragile X mouse model. J Med Genet. 2009;46:94–102. [DOI] [PubMed] [Google Scholar]
- 127.Gkogkas CG, Khoutorsky A, Cao R, et al. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses Fragile X syndrome-like phenotypes. Cell Rep. 2014;9:1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of Fragile X syndrome in a mouse model. J Neurosci. 2014;34:9867–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lovelace JW, Rais M, Palacios AR, et al. Deletion of Fmr1 from forebrain excitatory neurons triggers abnormal cellular, EEG and behavioral phenotypes in the auditory cortex of a mouse model of Fragile X syndrome. Cerebral Cortex. 2019; pii: bhz141 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frankland PW, Wang Y, Rosner B, et al. Sensorimotor gating abnormalities in young males with Fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. [DOI] [PubMed] [Google Scholar]
- 131.Lauer AM, Behrens D, Klump G. Acoustic startle modification as a tool for evaluating auditory function of the mouse: progress, pitfalls, and potential. Neurosci Biobehav Rev. 2017;77:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knudson IM, Melcher JR. Elevated acoustic startle responses in humans: relationship to reduced loudness discomfort level, but not self-report of hyperacusis. J Assoc Res Otolaryngol. 2016;17:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hessl D, Berry-Kravis E, Cordeiro L, et al. Prepulse inhibition in Fragile X syndrome: feasibility, reliability, and implications for treatment. Am J Med Genet. 2009;150B:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arsenault J, Gholizadeh S, Niibori Y, et al. FMRP expression levels in mouse central nervous system neurons determine behavioral phenotype. Hum Gene Ther. 2016;27:982–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9:562–574. [DOI] [PubMed] [Google Scholar]
- 136.Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043−−1050. [DOI] [PubMed] [Google Scholar]
- 137.Ding Q, Sethna F, Wang H. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav Brain Res. 2014;271:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kokash J, Alderson EM, Reinhard SM, et al. Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res. 2019;1719:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCullagh EA, Poleg S, Greene NT, Huntsman MM, Tollin DJ, Klug A. Characterization of auditory and binaural spatial hearing in a Fragile X Syndrome mouse model. bioRxiv. 2020; 7:pii: ENEUR0.0300–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of Fragile X syndrome. Brain Res. 2002;927:8–17. [DOI] [PubMed] [Google Scholar]
- 141.Paylor R, Yuva-Paylor LA, Nelson DL, Spencer CM. Reversal of sensorimotor gating abnormalities in Fmr1 knockout mice carrying a human Fmr1 transgene. Behav Neurosci. 2008;122:1371–1377. [DOI] [PubMed] [Google Scholar]
- 142.Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1984–1994. [DOI] [PubMed] [Google Scholar]
- 143.Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for Fragile X syndrome. Psychopharmacology. 2012;219:47–58. [DOI] [PubMed] [Google Scholar]
- 144.Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of Fragile X syndrome (FXS). Behav Brain Res. 2012;228:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yun S-W, Platholi J, Flaherty MS, Fu W, Kottmann AH, Toth M. Fmrp is required for the establishment of the startle response during the critical period of auditory development. Brain Res. 2006;1110:159–165. [DOI] [PubMed] [Google Scholar]
- 146.Errijgers V, Fransen E, D’hooge R, De deyn PP, Frank kooy R. Effect of genetic background on acoustic startle response in Fragile X knockout mice. Genet Res. 2008;90:341–345. [DOI] [PubMed] [Google Scholar]
- 147.Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135:999–1009. [DOI] [PubMed] [Google Scholar]
- 148.Bullock AE, Slobe BS, Vázquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–1360. [DOI] [PubMed] [Google Scholar]
- 149.Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in Fragile X syndrome: characteristics and co-morbid diagnoses. Am J Intellect Dev Disabil. 2010;115:461–472. [DOI] [PubMed] [Google Scholar]
- 150.Musumeci SA, Bosco P, Calabrese G, et al. Audiogenic seizures susceptibility in transgenic mice with Fragile X syndrome. Epilepsia. 2000;41:19–23. [DOI] [PubMed] [Google Scholar]
- 151.Gantois I, Khoutorsky A, Popic J, et al. Metformin ameliorates core deficits in a mouse model of Fragile X syndrome. Nat Med. 2017;23:674–677. [DOI] [PubMed] [Google Scholar]
- 152.Michalon A, Sidorov M, Ballard TM, et al. Chronic pharmacological mGlu5 inhibition corrects Fragile X in adult mice. Neuron. 2012;74:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Muscas M, Louros SR, Osterweil EK. Lovastatin, not simvastatin, corrects core phenotypes in the Fragile X mouse model. eneuro. 2019;6:ENEUR0.0097–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Osterweil EK, Chuang S-C, Chubykin AA, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of Fragile X syndrome. Neuron. 2013;77:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pacey LKK, Heximer SP, Hampson DR. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of Fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 2009;76: 18–24. [DOI] [PubMed] [Google Scholar]
- 156.Dolan BM, Duron SG, Campbell DA, et al. Rescue of Fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci. 2013;110:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gonzalez D, Tomasek M, Hays S, et al. Audiogenic seizures in the Fmr1 knockout mouse are induced by Fmr1 deletion in subcortical, vGlut2-expressing excitatory neurons and require deletion in the Inferior Colliculus. J Neurosci. 2019;39:9852–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dölen G, Osterweil E, Rao BSS, et al. Correction of Fragile X syndrome in Mice. Neuron. 2007;56:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of Fragile X Syndrome. J Neurosci. 2010;30:15616–15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sawicka K, Pyronneau A, Chao M, Bennett MVL, Zukin RS. Elevated ERK/p90 ribosomal S6 kinase activity underlies audiogenic seizure susceptibility in Fragile X mice. Proc Natl Acad Sci. 2016;113:E6290–E6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arnett MT, Herman DH, McGee AW. Deficits in tactile learning in a mouse model of Fragile X syndrome. PLoS ONE. 2014;9:e109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.He CX, Cantu DA, Mantri SS, Zeiger WA, Goel A, Portera-Cailliau C. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J Neurosci. 2017;37:6475–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goel A, Cantu DA, Guilfoyle J, et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat Neurosci. 2018;21:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao M-G. Deficits in trace fear memory and long-term potentiation in a mouse model for Fragile X syndrome. J Neurosci. 2005;25:7385–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Neuwirth LS, Volpe NP, Ng S, et al. Taurine recovers mice emotional learning and memory disruptions associated with Fragile x syndrome in context fear and auditory cued-conditioning. Adv Exp Med Biol. 2015;803:425–438. [DOI] [PubMed] [Google Scholar]
- 166.Santos AR, Kanellopoulos AK, Bagni C. Learning and behavioral deficits associated with the absence of the Fragile X mental retardation protein: what a fly and mouse model can teach us. Learn Memory. 2014;21:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Antoine MW, Langberg T, Schnepel P, Feldman DE. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. 2019;101:648–661.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hyperacusis Baguley D.. J R Soc Med. 2003;96:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Auerbach BD, Radziwon K, Salvi R. Testing the central gain model: loudness growth correlates with central auditory gain enhancement in a rodent model of hyperacusis. Neuroscience. 2019;407:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lauer AM, Dooling R. Evidence of hyperacusis in canaries with permanent hereditary high-frequency hearing loss. Sem Hear. 2007;28:319–326. [Google Scholar]
- 171.Zhang C, Flowers E, Li J-X, Wang Q, Sun W. Loudness perception affected by high doses of salicylate-a behavioral model of hyperacusis. Behav Brain Res. 2014;271:16–22. [DOI] [PubMed] [Google Scholar]
- 172.Manohar S, Spoth J, Radziwon K, Auerbach BD, Salvi R. Noise-induced hearing loss induces loudness intolerance in a rat Active Sound Avoidance Paradigm (ASAP). Hear Res. 2017;353: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Flores EN, Duggan A, Madathany T, et al. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol. 2015;25:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]


