Abstract
Purpose
We determined the progression of visual function, macular structure, and quality of life in patients with regressed proliferative diabetic retinopathy (PDR) after panretinal photocoagulation (PRP).
Methods
In this prospective study, 22 patients who underwent PRP for PDR and 11 age-matched control participants underwent examinations at baseline and after 5 years. Visual acuity, contrast sensitivity, reading acuity, frequency doubling perimetry, Humphrey field analyzer, and dark adaptation were measured. The Low Luminance Questionnaire and National Eye Institute Vision Function Questionnaire-25 were administered. Macular spectral-domain optical coherence tomography was taken.
Results
After 5 years, patients who had previously undergone PRP for PDR (18.4 ± 7.9 years previously) showed significant deterioration in contrast sensitivity, reading acuity, frequency doubling perimetry 24-2 pattern standard deviation, and Humphrey field analyzer 10-2 foveal sensitivity, which were equivalent to age-related decreases in control participants. They revealed no further impairment in vision-related activities on questionnaires. In contrast with controls, their maculas showed pathologic disorganization of the retinal layers, especially the nerve fiber layer, which were thicker and constituted a greater proportion of the overall retinal thickness than the norm and associated with impaired vision.
Conclusions
Patients with treated PDR had age-related decreases in vision, but stable quality of life. Prior injuries from the diabetes and, possibly, laser treatment led to substantial disruption in the retinal structure, which may explain the loss of vision.
Translational Relevance
Despite PRP treatment, patients with regressed PDR had pathologic progression of the nerve fiber layer; further investigation may identify a new therapeutic target to reverse the visual deficits.
Keywords: diabetic retinopathy, panretinal photocoagulation, visual function, patient-reported outcome, retinal structure
Introduction
Proliferative diabetic retinopathy (PDR) is one of the leading causes of severe vision loss in the world, estimated to affect as many as 10 million people worldwide by 2030.1–4 The standard of treatment for PDR has been panretinal photocoagulation (PRP), which induces tissue coagulation in the peripheral retina through laser burns and reduces its overall ischemic drive.5,6 As a result, PRP decreases the risk of severe vision loss in eyes with PDR up to 2 years after treatment.7 However, PRP also damages the peripheral retina, leading to loss of peripheral visual field, impaired night vision, and decreased visual acuity and contrast sensitivity, along with edematous change in the central retinal.8–12 Thus, it is important to understand the long-term consequences of PRP treating PDR.
Concurrently, landmark studies in the past decade have demonstrated the ability to partially restore vision via gene therapy in persons who were essentially blind owing to Leber congenital amaurosis.13,14 The ability to restore function in persons who have only light perception raises the prospect of improving vision in persons who are less impaired. For example, patients who have received PRP for PDR share features found, in eyes with photoreceptor dystrophies, notably pigmentary atrophy and hyperplasia in the laser scars, vascular attenuation and optic disk pallor, yet they are often able to read and drive. To develop new therapies, it is important to understand the natural progression of PDR after PRP.
The Diabetic Retinopathy Clinical Research Network (DRCR.net) recently published the 5-year outcomes of PRP for PDR, which reported gradual loss of visual field from the baseline though visual acuity remained stable after treatment.15 However, the long-term outcome in people who received PRP for PDR beyond the 5-year mark is not well-documented. In this study, we evaluated the long-term outcomes in patients with regressed PDR beyond the 5-year mark to identify changes in visual function, retinal structure, and quality of life after treatment. Notably, we also followed healthy volunteers to determine whether these changes were associated with the normal aging process. These results provide important new data on the natural history of vision in patients with regressed PDR, which may aid the development of future therapies to improve their vision and overall quality of life.
Methods
This prospective longitudinal study was conducted at the University of Michigan W. K. Kellogg Eye Center between August 2012 and September 2018 with the approval from University of Michigan Medical School Institutional Review Board and adhered to the tenets of Declaration of Helsinki and Health Insurance Portability and Accountability Act.
Patient Enrollment and Follow-up Evaluation
This follow-up study was an extension of the report published by Boynton et al.,16 which described the inclusion and exclusion criteria of the participants and were briefly outlined here. The study enrolled two groups of participants: adults with diabetes who had received PRP for PDR (post-PRP) and age-matched healthy adults. (1) Post-PRP participants were older than 18 years old, had received PRP at least 6 months before recruitment, and had no diabetic macular edema. (2) Age-matched controls were older than 18 years of age and had normal vision. In each participant, the eye with better visual acuity was chosen as the study eye if both eyes were eligible.
The study eye received a baseline assessment and then a follow-up assessment after 5 years. In each assessment, the study eye received a comprehensive ophthalmologic examination to evaluate visual function and retinal structure.16 First, best-corrected visual acuity was measured with electronic visual acuity tester with Snellen chart. Reading acuity was measured with the Minnesota Low Vision Reading Test. Contrast sensitivity was examined using the Pelli-Robson contrast sensitivity chart (Haag-Streit USA, Mason, OH). A frequency doubling perimetry (FDP) 24-2 full threshold visual field was conducted on the Matrix perimeter (Carl Zeiss Meditec, Dublin, CA). Photopic central 10-2 Swedish Interactive Thresholding Algorithm standard and peripheral 60-4 threshold visual field were performed on a Humphrey field analyzer (HFA) (II-750; Carl Zeiss Meditec, Dublin, CA). Dark adaptation speed was determined using the AdaptDx dark adaptometer (MacuLogix, Hummelstown, PA) after bleaching the study eye with a 5.8 × 104 scotopic cd/m2-second flash.
Fundus photography was performed with an Optos camera (Optos, Dunfermline, UK), and spectral domain optical coherence tomography (OCT) was performed with a Spectralis spectral domain OCT (Heidelberg Engineering, Heidelberg, Germany). Spectral domain OCT 20° × 20° volume scans (97 sections, 512 A-scans in each B-scan, and 3.87-micron axial resolution) of the macula were obtained. Macular thickness was measured in three concentric rings: fovea (with a 1-mm diameter), parafovea (an inner ring with an inner diameter of 1 mm and an outer diameter of 3 mm), and perifovea (an outer ring with an inner diameter of 3 mm and an outer diameter of 5 mm). The retinal thickness was measured with previously validated Duke Optical Coherence Tomography Retinal Analysis Program software, using a semiautomatic approach. First, the boundaries for nerve fiber layer (NFL), ganglion cell/inner plexiform layer (GC/IPL), inner nuclear layer, outer plexiform/outer nuclear layer, inner segment/outer segment (IS/OS), and retinal pigmented epithelium were delineated automatically. Then, expert graders (LK, SF) carefully reviewed and made adjustments, when necessary, to improve segmentation accuracy.17,18
Although not included in the initial report, the National Eye Institute Visual Field Questionnaire-25 and the Low Luminance Questionnaire (LLQ) were administered at the baseline and follow-up visits.16 The National Eye Institute Visual Field Questionnaire-25 is divided into 12 subscales: general health, general vision, near vision, distance vision, driving, peripheral vision, color vision, ocular pain, and vision-specific tasks such as role difficulties, dependency, social functioning, and mental health. The LLQ is composed of 6 subscales: driving, extreme lighting conditions, mobility, emotional distress, general dim lighting, and peripheral vision. Both questionnaires were scored using the recommended method instructed by the developers. The subscale scores were calculated by summation of relevant scores and then transformed into a scale of 0 to 100, where higher scores indicate better function or well-being. For each questionnaire, composite scores were calculated by combining all subscale scores.19,20
Statistical Analysis
Data analysis was performed using SPSS Statistics (version 25.0; SPSS, Inc, Chicago, IL). The distribution of each retinal layer was calculated by dividing the thickness of each layer with the total retinal thickness. Categorical variables were compared between groups using chi-square or Fischer's exact tests. Continuous variables were expressed as mean and standard deviation. The Shapiro-Wilk test was used to check for normal distribution. Mann-Whitney U tests were used to compared continuous variables between groups. Wilcoxon sign-rank tests was used to compare between the baseline and follow-up visits. Spearman's rank-order correlation tests assessed associations between two variables, which the statistical significance was defined as a P value of less than 0.01 to reduce the chance of type I errors given that a large number of variables were compared. Otherwise, statistical significance was defined as a P value of less than 0.05.
Results
Among the original 45 participants, 33 completed the second evaluation after 5 years. The cohort included 11 healthy adults (control group) and 22 adults who had received PRP for PDR (post-PRP group) (Table 1). At the follow-up, post-PRP patients had received laser treatments, ranging from 5.6 to 37.0 years ago with a median of 16.1 years ago. Of note, 21 patients received bilateral PRP, and only 1 patient received additional laser between the visits. Generally, the laser scars spread across the mid-peripheral and peripheral regions, sparing the macula within the temporal arcades. Despite regression of PDR on clinical assessment, these diabetic patients had higher level of hemoglobin A1c than the control participants (7.8 ± 1.3 vs. 5.6 ± 0.3; P < 0.005). Otherwise, there was no significant difference in age, gender ratio, or body mass index between the groups.
Table 1.
Characteristics of Returned Participants at the Baseline and Follow-up Visits
| Control | Post-PRP PDR | |||
|---|---|---|---|---|
| Characteristics | Baseline | Follow-up | Baseline | Follow-up |
| Sex | ||||
| Female | 5 (45) | 5 (45) | 6 (27) | 6 (27) |
| Male | 6 (55) | 6 (55) | 16 (73) | 16 (73) |
| Diabetes type | ||||
| T1DM | 15 (68) | 15 (68) | ||
| T2DM | 7 (32) | 7 (32) | ||
| Age (years) | 58.9 ± 15.2 | 63.7 ± 15.1 | 60.3 ± 14.6 | 65.0 ± 14.5 |
| Diabetes duration (years) | 36.1 ± 11.2 | 40.6 ± 10.8 | ||
| Years since PRP | 13.9 ± 11.2 | 18.6 ± 8.4 | ||
| HbA1c (%) | 5.6 ± 0.3 | 5.6 ± 0.3 | 7.59 ± 1.09 | 7.84 ± 1.28 |
| BMI (kg/m2) | 28.5 ± 8.8 | 28.6 ± 9.1 | 31.2 ± 6.1 | 30.2 ± 7.1 |
BMI, body mass index; HbA1c, hemoglobin A1c; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Values are expressed as number (%) or mean ± SD. Participants were followed up after 5 years.
Visual Function Outcomes
We investigated the changes in vision between enrollment time and after 5 years. The post-PRP patients generally had more visual impairments over time (Table 2). First, Pelli-Robson contrast threshold was 0.13 unit lower (P = 0.012). Second, reading acuity, which measures the smallest font participants can read accurately, deteriorated by more than two lines (P = 0.001). Third, FDP 24-2 showed a greater variability in field patterns, as measured by pattern standard deviation, which was 0.68 dB higher (P = 0.020). Fourth, HFA 10-2 foveal sensitivity was decreased by 1.76 dB (P = 0.039). There was no statically significant change in visual acuity, HFA 60-4, and dark adaptation.
Table 2.
Comparison of Visual Function and Patient-Reported Outcomes
| Control | Post-PRP PDR | Follow-up Minus Baselinea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessments | Baseline | Follow-up | P value | Baseline | Follow-up | P value | Control | Post-PRP PDR | P value |
| Visual acuity (logMAR) | –0.07 ± 0.09 | –0.03 ± 0.10 | 0.866 | 0.18 ± 0.21 | 0.20 ± 0.28 | 0.580 | 0.04 ± 0.07 | 0.02 ± 0.18 | 0.866 |
| Pelli-Robson contrast sensitivity (log) | 1.76 ± 0.14 | 1.64 ± 0.09 | 0.070 | 1.44 ± 0.17 | 1.31 ± 0.21 | 0.012 | –0.12 ± 0.18 | –0.14 ± 0.22 | 0.853 |
| Reading acuity (logMAR) | –0.04 ± 0.05 | 0.19 ± 0.20 | < 0.001 | 0.23 ± 0.32 | 0.41 ± 0.22 | < 0.001 | 0.23 ± 0.19 | 0.18 ± 0.20 | 0.515 |
| FDP 24-2 (dB) | |||||||||
| MD | 0.73 ± 2.76 | 0.91 ± 1.84 | 0.820 | –7.20 ± 5.12 | –8.48 ± 6.23 | 0.371 | 0.18 ± 2.53 | –0.98 ± 4.76 | 0.459 |
| PSD | 2.53 ± 0.32 | 2.97 ± 0.75 | 0.019 | 5.74 ± 1.89 | 6.42 ± 1.78 | 0.020 | 0.44 ± 0.53 | –0.49 ± 5.72 | 0.317 |
| FS | 29.55 ± 5.72 | 30.00 ± 4.63 | 0.541 | 22.41 ± 1.89 | 23.86 ± 5.33 | 0.272 | 0.45 ± 4.78 | 1.07 ± 5.67 | 0.925 |
| HFA 10-2 (dB) | |||||||||
| MD | –0.04 ± 0.80 | –1.18 ± 1.50 | 0.009 | –4.08 ± 2.94 | –5.00 ± 3.19 | 0.170 | –1.14 ± 1.09 | –0.92 ± 3.07 | 0.370 |
| PSD | 1.25 ± 0.58 | 1.20 ± 0.24 | 0.750 | 3.02 ± 2.20 | 3.19 ± 2.88 | 0.728 | –0.05 ± 0.48 | 0.17 ± 2.96 | 0.852 |
| FS | 36.50 ± 2.64 | 34.60 ± 2.41 | 0.012 | 30.38 ± 6.05 | 28.62 ± 5.38 | 0.039 | –1.90 ± 1.91 | –1.76 ± 6.76 | 0.950 |
| HFA 60-4 total threshold (dB) | 1149 ± 117 | 1042 ± 173 | 0.027 | 230 ± 203 | 201 ± 209 | 0.572 | –97.92 ± 133.61 | –17.43 ± 112.43 | 0.108 |
| Dark adaptation (min) | 8.85 ± 1.69 | 9.01 ± 4.51 | 0.706 | 13.54 ± 5.75 | 15.14 ± 8.83 | 0.619 | 0.71 ± 0.71 | 1.59 ± 6.48 | 0.977 |
| LLQ composite score | 97.5 ± 2.4 | 93.7 ± 5.9 | 0.102 | 62.7 ± 22.1 | 62.0 ± 20.9 | 0.819 | –3.77 ± 6.96 | –0.63 ± 12.47 | 0.447 |
| NEI-VFQ composite score | 97.8 ± 1.4 | 94.7 ± 5.0 | 0.067 | 77.7 ± 15.5 | 76.6 ± 18.5 | 0.618 | –3.06 ± 4.94 | –1.14 ± 10.32 | 0.567 |
FS, foveal sensitivity; MD, mean deviation; PSD, pattern standard deviation.
After 5 years, diabetic patients treated with PRP had age-related decreases in vision, which were comparable with those seen in the control participants. These patients also had stable quality of life despite poor vision.
Compared the extent of vision loss and change in patient-reported outcomes over time between the control and diabetic patients.
Next, we compared the progression of visual function over time between the control and post-PRP groups to determine whether these changes were age related (Table 2). In general, post-PRP patients had similar decreases in visual function as the age-matched healthy adults. These findings suggest that patients with regressed PDR have deterioration of vision as they age, and these changes are age appropriate instead of signs of disease progression or adverse effects from the laser scars.
Patient-reported Outcomes
We also assessed vision-related quality of life using LLQ and National Eye Institute Visual Field Questionnaire-25 (Table 2 and Supplementary Table S1 for the results of patient-reported outcomes). The post-PRP patients generally reported lower scores on both questionnaires than the control participants. However, these patients did not report significant decreases in their quality of life after 5 years, despite reduced vision. In contrast, control participants revealed significantly more trouble with extreme lighting conditions (e.g., moving from bright day light to a dark room or vice versa; P = 0.043) and general vision (P = 0.025). Then, we compared the changes in patient-reported outcomes over time between the control and diabetic participants. We found that the self-reported improvements in extreme lighting subscale on the LLQ among the post-PRP patients was significantly different from that of the control participants (3.25 ± 11.83 vs. –9.66 ± 13.80; P = 0.010). These results provide evidence of resiliency and functional adaptation to poor vision in patients with regressed PDR.
Macular Structure
We evaluated the thickness changes in each retinal layer, which remained significantly different between the control and post-PRP participants even after 5 years (Table 3). Generally, eyes that received PRP had thicker NFL while thinner IS/OS in the fovea, parafovea, and perifovea (all P < 0.05). In addition, these diabetic eyes showed redistribution of retinal layers (Fig. 1 and see Supplementary Table S2 for relative retinal thickness). For example, in the fovea, NFL was consisted of 7.59% of the overall thickness in the diabetic eyes, whereas in the control eyes it only constituted 6.17% (P = 0.048). In the diabetic eyes, inner nuclear layer also constituted a greater proportion than the norm while IS/OS constituted a smaller proportion (P = 0.020 and P = 0.036, respectively). In the parafovea and perifovea, there were also significant changes in the proportion of NFL, GC/IPL, outer plexiform/outer nuclear layer, IS/OS, and/or retinal pigmented epithelium (all P ≤ 0.04).
Table 3.
Comparison of Retinal Layer Thickness
| Control | Post-PRP PDR | ||||||
|---|---|---|---|---|---|---|---|
| Thickness (µm) | Baseline | Follow-up | P value | Baseline | Follow-up | P value | P value* |
| Fovea | |||||||
| NFL | 18.84 ± 1.15 | 17.65 ± 0.68 | 0.003 | 20.25 ± 4.79 | 20.88 ± 4.98 | 0.502 | 0.006 |
| GC/IPL | 39.57 ± 13.52 | 39.94 ± 13.43 | 0.477 | 40.28 ± 11.34 | 36.70 ± 13.22 | 0.018 | 0.515 |
| INL | 23.39 ± 6.84 | 23.39 ± 7.69 | 0.635 | 29.20 ± 6.24 | 30.12 ± 11.57 | 0.606 | 0.092 |
| OP/ONL | 139.55 ± 10.74 | 138.86 ± 9.22 | 0.386 | 141.24 ± 25.52 | 135.76 ± 28.06 | 0.072 | 0.642 |
| IS/OS | 38.39 ± 1.96 | 37.47 ± 1.55 | 0.075 | 32.80 ± 9.28 | 32.30 ± 9.41 | 0.139 | 0.006 |
| RPE | 31.03 ± 3.28 | 30.12 ± 4.18 | 0.075 | 28.58 ± 3.83 | 28.51 ± 3.75 | 0.911 | 0.273 |
| Parafovea | |||||||
| NFL | 27.66 ± 1.77 | 27.56 ± 1.80 | 0.722 | 33.87 ± 5.98 | 33.29 ± 5.66 | 0.445 | <0.001 |
| GC/IPL | 93.67 ± 8.14 | 91.75 ± 7.08 | 0.013 | 86.79 ± 12.00 | 83.63 ± 12.89 | 0.005 | 0.062 |
| INL | 39.92 ± 3.86 | 39.79 ± 4.60 | 0.722 | 39.05 ± 5.87 | 38.97 ± 5.84 | 0.733 | 0.688 |
| OP/ONL | 118.28 ± 4.88 | 117.50 ± 4.93 | 0.033 | 123.60 ± 15.46 | 122.77 ± 14.46 | 0.592 | 0.134 |
| IS/OS | 33.31 ± 1.98 | 33.17 ± 2.03 | 0.657 | 30.30 ± 5.79 | 29.70 ± 6.02 | 0.088 | 0.026 |
| RPE | 30.66 ± 2.96 | 29.87 ± 3.03 | 0.155 | 26.63 ± 3.20 | 26.86 ± 3.22 | 0.783 | 0.015 |
| Perifovea | |||||||
| NFL | 41.46 ± 5.17 | 40.73 ± 5.08 | 0.213 | 52.17 ± 9.50 | 50.90 ± 9.63 | 0.200 | <0.001 |
| GC/IPL | 64.05 ± 5.08 | 63.12 ± 4.67 | 0.182 | 67.12 ± 7.25 | 66.10 ± 7.15 | 0.322 | 0.222 |
| INL | 30.44 ± 2.53 | 30.19 ± 2.69 | 0.328 | 31.40 ± 5.09 | 31.59 ± 5.39 | 0.884 | 0.895 |
| OP/ONL | 95.96 ± 6.85 | 95.24 ± 6.80 | 0.033 | 103.37 ± 10.71 | 103.84 ± 10.12 | 0.709 | 0.009 |
| IS/OS | 34.02 ± 2.96 | 34.10 ± 1.67 | 0.722 | 29.64 ± 4.85 | 29.16 ± 5.08 | 0.131 | <0.001 |
| RPE | 27.46 ± 4.63 | 26.72 ± 3.83 | 0.374 | 25.48 ± 2.94 | 25.41 ± 4.08 | 0.783 | 0.384 |
INL, inner nuclear layer; OP/ONL, outer plexiform/outer nuclear layer; RPE, retinal pigmented epithelium.
Even after 5 years, diabetic patients treated with PRP still had pathologic changes in the NFL and IS/OS. They also showed progressive thinning of GC/IPL over time.
Compared the results at the follow-up visit between control and diabetic patients.
Figure 1.
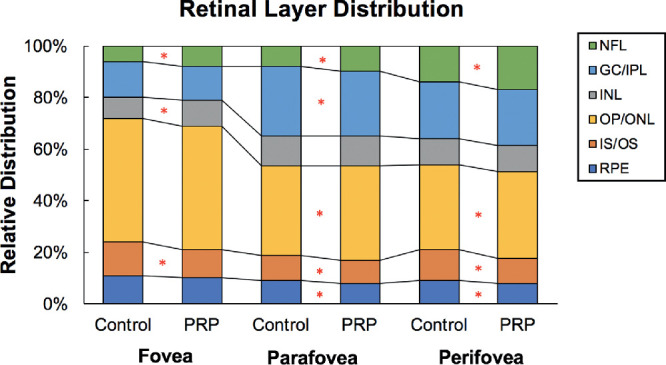
Retinal layer distributions in the macula at the follow-up visit. The graph shows the relative distribution of retinal layers in the diabetic eyes treated with PRP and control eyes. In general, diabetic eyes had redistribution of retinal layer thickness, which may be pathologic. *The comparison between the control and diabetic groups was statistically significant (P < 0.05). INL, inner nuclear layer; OP/ONL, outer plexiform/outer nuclear layer; RPE, retinal pigmented epithelium.
In addition, the foveal and parafoveal GC/IPL of the diabetic eyes became thinner after 5 years (P = 0.018 and P = 0.005) (Table 3). Likewise, there were significant reductions in the proportion of GC/IPL in these regions as well (–1.00% in fovea, P = 0.016; –0.60% in parafovea, P = 0.020) after 5 years. In contrast, the foveal NFL distribution increased by 0.54% (P = 0.039) in the diabetic eyes. There was no significant change in other retinal layers.
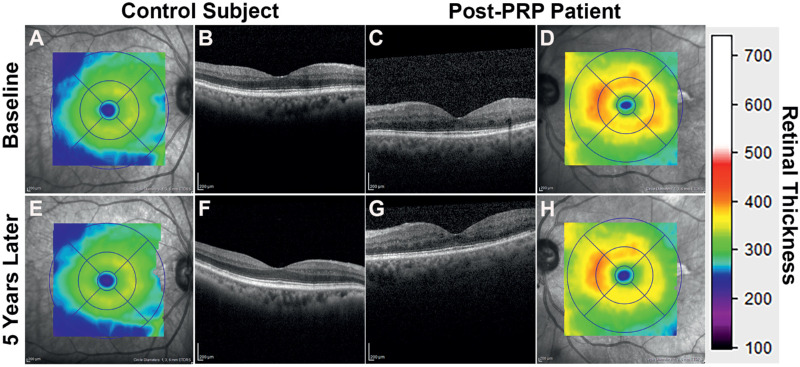
Next, we compared the changes in thickness over time in the control and diabetic eyes. Although there were minimal changes in the overall retinal thickness, we found significant changes in some of the specific retinal layers (Figs. 2A-H). Unlike the foveal region of the control eyes, the diabetic eyes showed a slight thickening in NFL (–1.19 ± 1.06 µm vs. 0.64 ± 4.32 µm; P = 0.012) while significant thinning in the GC/IPL (–3.59 ± 6.55 µm vs. 0.36 ± 2.48 µm; P = 0.018) after 5 years. There was no significant difference in the other layers. These changes suggest that the inner retinal layers change at a rate different from the natural progression.
Figure 2.
After 5 years, a post-PRP patient (a 69-year-old man with a BCVA of 20/25 and had PRP 9 years ago) and a healthy control participant (a 77-year-old man with a BCVA of 20/25) had minimal changes in their overall retinal thickness and general topology.
Furthermore, we showed that greater distribution of foveal NFL was associated with declines in reading acuity (in LogMAR), FDP 24-2 mean deviation, and HFA 10-2 mean deviation (r = 0.474, r = –0.665, and r = -0.557, respectively; P ≤ 0.01). In addition, increased proportions of NFL in the parafovea and perifovea were correlated with reduced FDP 24-2 mean deviation (r = –0.637 and r = –0.587, respectively; P ≤ 0.01). There was no significant correlation between NFL and visual acuity or contrast sensitivity. These findings suggest that increased distribution of NFL is linked to impaired vision in patients who were treated with PRP.
Discussion
In this prospective study, we found progressive deterioration of visual function and retinal thickness in patients who had regressed PDR after PRP, most of whom had received laser treatments more than a decade earlier. Over 5 years, they showed further decreases in central and peripheral vision compared with baseline, but these progressions were comparable with the changes found with normal aging in people without diabetes. These patients also reported minimal changes in their vision-related quality of life despite poor vision. Yet, their eyes still had progressive changes in the organization of the retinal structure, which was associated with visual impairment. This information is important for future efforts to restore vision in people with treated diabetic retinopathy, as proposed recently by the Juvenile Diabetes Research Foundation.21
In our study, patients who underwent PRP experienced significant declines in measures of central vision, including reading acuity, contrast sensitivity, and visual field after 5 years, even though they had regressed PDR on fundus examination. Likewise, in a recent clinical trial that followed the 5-year outcome of PRP versus intravitreous ranibizumab for PDR, Gross et al.15 reported progressive loss of visual field among the PRP-treated eyes at the 5-year visit compared with baseline. In comparison, the ranibizumab group had similar visual acuity, although lower incidences of diabetic macular edema and vitrectomy and less reduction of visual field as the PRP group, supporting the intravitreous injection as a viable alternative for treating PDR. Of note, regular follow-up is an important criterion when managing patients with ranibizumab because its effect is generally temporary and good long-term outcomes require scheduled injections. Clinicians need to consider the risk of loss to follow-up in their patient population when treating with injection alone.
When evaluating vision, it is also important to consider the declines associated with normal aging.20,22–25 In our study, we found that post-PRP patients had a similar degree of vision loss as the control participants at the 5-year follow-up. Hence, the progressive loss of vision in regressed PDR is likely due to aging and less likely attributed to disease progression or residual effects from the laser injury. In other words, these patients could greatly benefit from therapies that slow down the natural progression of vision loss related to aging.
Next, based on the responses from patient-reported outcomes, we also found that patients with regressed PDR can adapt to their poor vision. We observed that, after 5 years, some patients reported subjective improvement in vision-related activities such as navigating between bright day light and a dimly lit corridor, managing their emotional distress, and adapting to their restricted peripheral vision. In contrast, control participants generally reported worsening performance in their vision-specific activities. They had more difficulties with moving around in dim lighting, loss of peripheral vision, and declining driving ability and confidence. Owsley et al.26 also found that adults 60 years of age and older even with normal macular health generally reported notable declines in their vision-related quality of life after 3 years, especially with seeing at night and under low luminance condition.
Despite both control and diabetic participants experiencing age-related deterioration in vision, only patients with diabetes paradoxically reported subjective improvement and adaptation in their daily life, which was not seen in individuals without diabetes. Dissociations of changes in visual function and quality of life responses indicate that factors other than vision also affect people's quality of life. Trillo and Dickninson27 reported that, in people with low vision, nonvisual factors, such as physical and mental health, were better indicators of quality of life than visual factors, such as contrast sensitivity and visual acuity. Another study found that positive coping strategies were associated with better quality of life in people with long-term visual impairment, which suggested that counseling and training in positive coping mechanisms could be part of their care.28 Currently, there is no established treatment to restore vision in patients with regressed PDR. However, they may benefit from other interventions, whether psychological or social supports, to optimize their quality of life.
In terms of retinal morphology, we found that, even after 5 years, there were minimal changes in each retinal layer of the macula, except the GC/IPL. The GC/IPL was thinner in the fovea and parafovea, but relatively unchanged in the perifovea. Then compared with the progression of healthy aging eyes, these eyes also showed pathologic hypertrophy of the NFL and atrophy of the GC/IPL. However, thinning of the GC layer usually corresponds with a loss of NFL because of the axonal loss from the injured GCs.29 Lee et al.30 showed that both GC layer and NFL were thinner in diabetic eyes that received PRP at least 3 years ago as compared with diabetic eyes before laser treatment and the healthy eyes. Specifically, Lee et al.31 found that the peripapillary NFL thickness initially increased after PRP, but then gradually decreased over time and then became thinner than the baseline thickness at the end of 2 years. In our study, however, most patients received PRP more than 10 years ago and were afflicted by diabetes for many more years. The pathologic thickening of NFL may be a maladaptive response after prolonged injuries from diabetes and may be related to the laser treatment.
Furthermore, unlike the healthy eyes, the NFL of the diabetic eyes constituted a greater proportion of the total retinal thickness, which corresponded with abnormal thickening of the NFL and associated with reduced central vision. In other words, these retinal nerve fibers were likely malfunctional, and pathologic modifications may explain some of the vision impairments. One explanation is that the apparent thickening of the NFL is due to retinal fibrosis. Histopathologic studies have demonstrated that formation of fibrotic tissues was associated with neovascularization during PDR stage, and some studies suggested that Müller cells, astrocytes, microglia may contribute to this fibrotic process.32–35 Fibrosis might also occur at the site of photocoagulation and contribute to loss of function.36 One of the most feared complications associated with fibrovascular proliferative tissue was tractional retinal detachment, separating the neurosensory retina from the retinal pigment epithelium, leading to severe vision loss if left untreated.37 Even with successful reattachment after vitrectomy, most eyes never returned to their baseline vision, suggesting irreversible damage to the neurovascular components despite gross restoration of the retinal architecture.38 Another possibility is that microscopic swelling within the nerve fiber mimics the appearance of thickened NFL. These maladaptations could impede visual pathway signaling and lead to impaired functionality. Nevertheless, future studies should further investigate the mechanisms of pathologic changes in the NFL, which may identify new therapeutic targets to improve vision in patients with diabetic retinopathy.
There are some limitations to consider when interpreting the results of this study. First, despite careful manual correction of automatically delineated retinal boundary layers by expert graders, some pathologic features, including epiretinal membrane, and patient-specific factors, such as media opacity and eye movement, could affect the accuracy of segmentation. Second, the study had a small sample size of 30 patients who had PRP for PDR and 15 healthy adults. Then, after 5 years, only 22 diabetic patients and 11 healthy controls were reexamined. Limited by the small sample size, we could not examine the interaction effects of multiple retinal layers on vision. We hypothesize that vision impairments are likely the product of modifications in multiple retinal layers. Hence, a large study in the future could congruently examine multiple layers to explain the changes in vision outcomes in these patients.
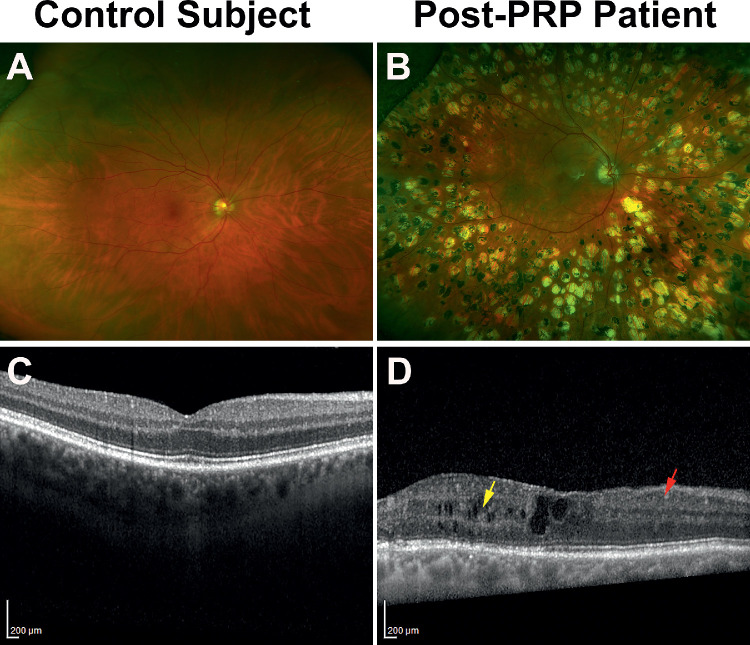
A larger study could also investigate the effect of other pathologic features associated with diabetic retinopathy, such as disorganization of the retinal inner layer and retinal ischemia, which were found in some retinas of people with diabetes (Figs. 3A-D). Sun et al.39 suggested that disorganization of the retinal inner layers could indicate the cellular injuries within the inner retina, affecting bipolar, amacrine, or horizontal cells, disrupting the transmission of visual information from the photoreceptors to the GCs. Recent studies revealed the presence of disorganization of retinal inner layer was correlated with a loss of visual acuity, contrast sensitivity, and visual field and severity of macular capillary nonperfusion.39–42 Similarly, the extent of retinal ischemia in patients with diabetes corresponded with the severity of vision loss and development of macular edema.43–45 The advent of OCT angiography further elucidated the consequence of vascular pathology in diabetic retinopathy. Several OCT angiography studies showed that loss of peripapillary vascular density was associated with reduction in retinal NFL thickness, the enlargement of foveal avascular zone was corresponded to disruption in the photoreceptor layer, and loss of macular vasculature was linked to loss of GCs and progressive retinal neurodegeneration.46–49
Figure 3.
Fundus and OCT images of a control participant and a diabetic patient. The diabetic retina had significant laser scars surrounding the macula (B) and showed evidence of pathologic modifications of the retinal layers (D), such as signs of disorganization of retinal inner layers (DRIL; red arrow) and cystoid changes (yellow arrow).
Strengths of this study include comprehensive examination of multiple aspects of visual function, which had not previously been evaluated in a longitudinal study of patients treated with PRP for PDR. In addition, we included healthy participants, which allowed assessments of age-related loss in vision, retinal thickness, and quality of life. The results ultimately add to the existing knowledge about the long-term outcomes in patients with regressed PDR.
In this study, most patients with regressed PDR had initially received PRP more than a decade previously. Despite poor baseline vision, they only had age-related declines in visual function after 5 years. However, their macula showed reorganization of retinal layers, especially NFL, which may explain some of the vision impairments despite regressed retinopathy. These patients also reported stable quality of life and demonstrated the ability to adapt to their new baseline. These findings support the usefulness of PRP for PDR and its long-term benefits in preserving vision after regression of PDR.5,7,15 In addition, after treatment and regression of PDR, patients can expect subtle deterioration of visual function related to aging. Looking forward, although no current treatments can restore normal vision in these patients, we believe that future regenerative therapies could restore retinal nerve fibers, photoreceptors, and blood vessels, which are injured by diabetes and laser treatment.31,50–52 Thus, this study provides a new understanding of the natural history of treated PDR that may enable future therapies to improve their vision and quality of life.
Supplementary Material
Acknowledgments
The authors thank Robin Ali, Adrienne Chen, and Michael Flannagan, who reviewed this article and provided helpful suggestions.
Supported by Research to Prevent Blindness, The Taubman Medical Research Institute and NIDDK Summer Research Program (P30DK020572), P30EY005722, R01 EY20582, R01 EY022691, and R24 DK08284.
Disclosure: X.D. Chen, None; A. Omari, None; M. Hwang, None; L. Kwark, None; N. Dakki, None; S. Farsiu, Duke (P); T.W. Gardner, Zebra Biologics (F)
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Al-Rubeaan K. Type 2 diabetes mellitus red zone. Int J Diabetes Mellitus. 2010; 2: 1–2. [Google Scholar]
- 3. Solomon SD, Chew E, Duh EJ, et al.. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40: 412–418. doi: 10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong DS, Aiello L, Gardner TW, et al.. Retinopathy in diabetes. Diabetes Care. 2004; 27(Suppl 1): s84–s87. [DOI] [PubMed] [Google Scholar]
- 5. Singh R, Stone T. 2018 Global Trends in Retina Survey. Chicago, IL: American Society of Retina Specialists; 2018. [Google Scholar]
- 6. Alasil T, Waheed NK. Pan retinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Curr Opin Ophthalmol. 2014; 25: 164–170. doi: 10.1097/ICU.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 7. Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1976; 81: 383, 396. [DOI] [PubMed] [Google Scholar]
- 8. McDonald HR, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1985; 92: 388–393. doi: 10.1016/S0161-6420(85)34016-2 [DOI] [PubMed] [Google Scholar]
- 9. Chew EY, Ferris FL, Csaky KG, et al.. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology. 2003; 110: 1683–1689. doi: 10.1016/S0161-6420(03)00579-7 [DOI] [PubMed] [Google Scholar]
- 10. Preti RC, Ramirez LMV, Monteiro MLR, Carra MK, Pelayes DE, Takahashi WY. Contrast sensitivity evaluation in high risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: a randomised clinical trial. Br J Ophthalmol. 2013; 97: 885–889. doi: 10.1136/bjophthalmol-2012-302675 [DOI] [PubMed] [Google Scholar]
- 11. Blankenship GW. Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology. 1991; 98: 125–128. [DOI] [PubMed] [Google Scholar]
- 12. Soman M, Ganekal S, Nair U, Nair K. Effect of panretinal photocoagulation on macular morphology and thickness in eyes with proliferative diabetic retinopathy without clinically significant macular edema. Clin Ophthalmol. 2012; 6: 2013–2017. doi: 10.2147/OPTH.S37340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bainbridge JWB, Smith AJ, Barker SS, et al.. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008; 358(21): 2231–2239. doi: 10.1056/NEJMoa0802268 [DOI] [PubMed] [Google Scholar]
- 14. Bainbridge JWB, Mehat MS, Sundaram V, et al.. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015; 372: 1887–1897. doi: 10.1056/NEJMoa1414221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gross JG, Glassman AR, Liu D, et al.. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018; 136: 1138–1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015; 122: 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010; 18: 19413–19428. doi: 10.1364/OE.18.019413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JY, Chiu SJ, Srinivasan PP, et al.. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by Cirrus and Spectralis systems. Invest Ophthalmol Vis Sci. 2013; 54: 7595–7602. doi: 10.1167/iovs.13-11762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mangione CM, Lee PP, Gutierrez PR, et al.. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 20. Owsley C, McGwin G, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 528–535. doi: 10.1167/iovs.05-1222 [DOI] [PubMed] [Google Scholar]
- 21. JDRF and The Mary Tyler Moore & S. Robert Levine, MD Charitable Foundation Launch Research Moonshot to Restore Vision in People with Diabetes-Related Eye Disease. JDRF. Available at: www.jdrf.org/press-releases/jdrf-and-the-mary-tyler-moore-s-robert-levine-md-charitable-foundation-launch-research-moonshot-to-restore-vision-in-people-with-diabetes-related-eye-disease/ Accessed January 5, 2019.
- 22. Gardiner SK, Johnson CA, Spry PGD. Normal age-related sensitivity loss for a variety of visual functions throughout the visual field. Optom Vis Sci. 2006; 83: 438–443. doi: 10.1097/01.opx.0000225108.13284.fc [DOI] [PubMed] [Google Scholar]
- 23. Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001; 78: 436–441. [DOI] [PubMed] [Google Scholar]
- 24. Haas A, Flammer J, Schneider U. Influence of age on the visual fields of normal subjects. Am J Ophthalmol. 1986; 101: 199–203. [DOI] [PubMed] [Google Scholar]
- 25. Ross JE, Clarke DD, Bron AJ. Effect of age on contrast sensitivity function: uniocular and binocular findings. Br J Ophthalmol. 1985; 69: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owsley C, McGwin G. Vision-targeted health related quality of life in older adults: patient-reported visibility problems in low luminance activities are more likely to decline than daytime activities. BMC Ophthalmol. 2016; 16: 92. doi: 10.1186/s12886-016-0274-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hernandez Trillo A, Dickinson CM. The impact of visual and nonvisual factors on quality of life and adaptation in adults with visual impairment: quality of life in low vision. InvestOphthalmolVisSci. 2012; 53: 4234–4241. doi: 10.1167/iovs.12-9580 [DOI] [PubMed] [Google Scholar]
- 28. Rai P, Rohatgi J, Dhaliwal U. Coping strategy in persons with low vision or blindness – an exploratory study. Indian J Ophthalmol. 2019; 67: 669–676. doi: 10.4103/ijo.IJO_1655_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Dijk HW, Verbraak FD, Kok PHB, et al.. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010; 51: 3660–3665. doi: 10.1167/iovs.09-5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H-J, Kang T, Kwak B-S, Jo Y-J, Kim J-Y. Long-term effect of panretinal photocoagulation on spectral domain optical coherence tomography measurements in diabetic retinopathy. Current Eye Res. 2017; 42: 1169–1173. doi: 10.1080/02713683.2017.1280510 [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Kwag J, Lee H, Jo Y, Kim J. The longitudinal changes of retinal nerve fiber layer thickness after panretinal photocoagulation in diabetic retinopathy patients. Retina. 2013; 33: 188–193. doi: 10.1097/IAE.0b013e318261a710 [DOI] [PubMed] [Google Scholar]
- 32. Dobree JH. Proliferative diabetic retinopathy. Br J Ophthalmol. 1964; 48: 637. doi: 10.1136/bjo.48.12.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMeel J. Diabetic retinopathy: fibrotic proliferation and retinal detachment. Trans Am Ophthalmol Soc. 1971; 69: 440–493. [PMC free article] [PubMed] [Google Scholar]
- 34. Guidry C. The role of Müller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005; 24: 75–86. doi: 10.1016/j.preteyeres.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 35. Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007; 117: 576–586. doi: 10.1172/JCI31030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guyer DR, D'Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992; 113: 652–656. doi: 10.1016/s0002-9394(14)74789-0 [DOI] [PubMed] [Google Scholar]
- 37. Yang C-M, Su P-Y, Yeh P-T, Chen M-S. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: clinical manifestations and surgical outcome. Can J Ophthalmol. 2008; 43: 192–198. doi: 10.3129/i08-007 [DOI] [PubMed] [Google Scholar]
- 38. Storey PP, Ter-Zakarian A, Philander SA, et al.. Visual and anatomical outcomes after diabetic traction and traction-rhegmatogenous retinal detachment repair. Retina (Philadelphia, Pa). 2018; 38: 1913–1919. doi: 10.1097/IAE.0000000000001793 [DOI] [PubMed] [Google Scholar]
- 39. Sun JK, Lin MM, Lammer J, et al.. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014; 132: 1309–1316. doi: 10.1001/jamaophthalmol.2014.2350 [DOI] [PubMed] [Google Scholar]
- 40. Nicholson L, Ramu J, Triantafyllopoulou I, et al.. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin ExpOphthalmol. 2015; 43: 735–741. doi: 10.1111/ceo.12557 [DOI] [PubMed] [Google Scholar]
- 41. Sun JK, Radwan SH, Soliman AZ, et al.. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015; 64: 2560–2570. doi: 10.2337/db14-0782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joltikov KA, de Castro VM, Davila JR, et al.. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. InvestOphthalmolVisSci. 2017; 58: BIO277–BIO290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bell JA, Feldon SE. Retinal microangiopathy. Correlation of OCTOPUS perimetry with fluorescein angiography. Arch Ophthalmol. 1984; 102: 1294–1298. doi: 10.1001/archopht.1984.01040031044020 [DOI] [PubMed] [Google Scholar]
- 44. Sim DA, Keane PA, Rajendram R, et al.. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014; 158: 144–153.e1. doi: 10.1016/j.ajo.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 45. Wessel MM, Nair N, Aaker GD, Ehrlich JR, D'Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012; 96: 694–698. doi: 10.1136/bjophthalmol-2011-300774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu L, Wang Y, Liu HX, Gao J. Peripapillary region perfusion and retinal nerve fiber layer thickness abnormalities in diabetic retinopathy assessed by OCT angiography. Transl Vis Sci Technol. 2019; 8: 14. doi: 10.1167/tvst.8.4.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scarinci F, Nesper PL, Fawzi AA. Deep Retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016; 168: 129–138. doi: 10.1016/j.ajo.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim K, Kim ES, Kim DG, Yu S-Y. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: longitudinal study using OCT angiography. Acta Diabetol. 2019; 56: 1275–1282. doi: 10.1007/s00592-019-01395-6 [DOI] [PubMed] [Google Scholar]
- 49. Samara WA, Shahlaee A, Adam MK, et al.. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017; 124: 235–244. doi: 10.1016/j.ophtha.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 50. Bavinger JC, Dunbar GE, Stem MS, et al.. The effects of diabetic retinopathy and pan-retinal photocoagulation on photoreceptor cell function as assessed by dark adaptometry. Invest Ophthalmol Vis Sci. 2016; 57: 208–217. doi: 10.1167/iovs.15-17281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Dijk HW, Kok PHB, Garvin M, et al.. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009; 50: 3404–3409. doi: 10.1167/iovs.08-3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kriechbaum K, Bolz M, Deak GG, Prager S, Scholda C, Schmidt-Erfurth U. High-resolution imaging of the human retina in vivo after scatter photocoagulation treatment using a semiautomated laser system. Ophthalmology. 2010; 117: 545–551. doi: 10.1016/j.ophtha.2009.07.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.