Abstract
Insulin-like growth factor (IGF) binding protein 2 (IGFBP2) was discovered and identified as an IGF system regulator, controlling the distribution, function, and activity of IGFs in the pericellular space. IGFBP2 is a developmentally regulated gene that is highly expressed in embryonic and fetal tissues and markedly decreases after birth. Studies over the last decades have shown that in solid tumors, IGFBP2 is upregulated and promotes several key oncogenic processes, such as epithelial-to-mesenchymal transition, cellular migration, invasion, angiogenesis, stemness, transcriptional activation, and epigenetic programming via signaling that is often independent of IGFs. Growing evidence indicates that aberrant expression of IGFBP2 in cancer acts as a hub of an oncogenic network, integrating multiple cancer signaling pathways and serving as a potential therapeutic target for cancer treatment.
Introduction
Tumorigenesis is a highly complicated process involving multiple genetic and epigenetic changes controlled by diverse pathways. Over the past decade, integrated genome-wide screens of DNA copy number and gene expression have facilitated the discovery of amplified and overexpressed genes in human cancers, which has greatly expanded our knowledge of the altered genomic landscapes that promote human neoplasia [1, 2]. Among candidate cancer markers, one such gene, IGFBP2, is expressed aberrantly in such a way as to promote initiation and progression of many different tumor types.
Emerging as a comprehensive tumor biomarker, IGFBP2 is found to be highly expressed in a broad range of cancers, including glioma [3–9], pancreatic [10, 11], ovarian [12–17], breast [18–21], cervical [22], melanoma [23, 24], colorectal adenoma [25] and carcinoma [26–30], leukemia [31–37], prostate [38–41], lung [42–46], liver [47–49], gastric [50–52], bladder [53], head and neck [54], rhabdomyosarcoma [55], synovial sarcoma [56], adrenocortical tumor [57, 58], nonseminomatous germ cell cancer [59], Wilms’ tumor [60], and salivary adenoid cystic carcinoma [61]. Moreover, serving as a valuable biomarker, IGFBP2 predicts an unfavorable prognosis [4, 7, 9, 10, 14, 23, 32, 44, 57, 62–67], and confers increasing malignant status [30, 34, 58, 68–72]. Intriguingly, as a secreted protein initially characterized as a pericellular modulator of insulin-like growth factors I (IGF-I) and II (IGF-II), IGFBP2 mediates IGF-independent tumorigenic program through the involvement of an intracellular and nuclear regulatory network. Emerging evidence points toward IGFBP2 playing a crucial role in tumorigenesis via modulation of several hallmarks of cancer, particularly employing intracellular tumorigenic signal transduction [73, 74]. In this article, we review the tumorigenic role of IGFBP2 in cancer and the underlying regulatory mechanisms.
Functional domains of IGFBP2
Overview of domains in IGFBP2
The primary structure of IGFBP2 was determined by sequence analysis of a cloned cDNA from a human fetal liver cDNA library; the mature 289 amino acid (aa) protein has a predicted molecular weight of 31 Kd [75]. In general, the six high-affinity IGFBPs are comprised of three major domains of approximately equivalent length: An N-terminal domain and a C-terminal domain joined by an L-domain (Fig. 1). The N-terminal and C-terminal domains are both highly conserved and cysteine-rich, while the L-domain is characterized as a nonconserved linker region, which is sensitized to proteases [76, 77]. The important subdomains, or functional motifs, within each domain of IGFBP2 contribute to multiple biological actions (Table 1).
Fig. 1. Domain organization of human IGFBP2 protein.
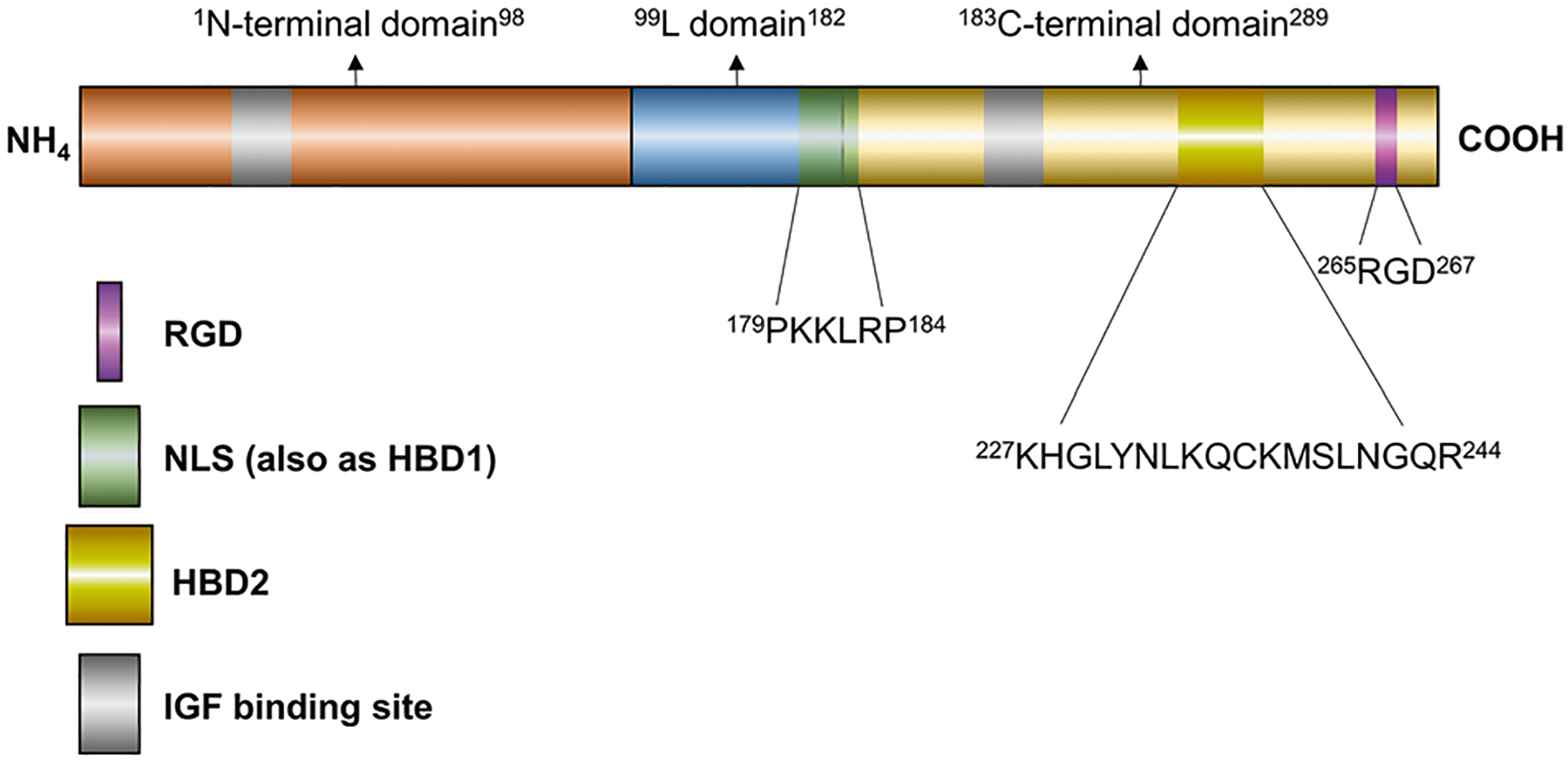
Mature IGFBP2 commonly comprises three major domains: N-terminal domain and C-terminal domain joined by an L-domain. N-terminal: Involved in the binding with IGFs. L-domain: the NLS motif is mainly within the L-domain and guarantees nuclear translocation. C-terminal domain: RGD motif, HBD2 motif, and an IGF-binding domain are located in C-terminal domain. Amino acid sequence numbering is based on human IGFBP2.
Table 1.
Overview of IGFBP2 domains.
| Domains | Motifs | Sequence of motifs | Functions | References |
|---|---|---|---|---|
| N-terminal domain | IGF-binding site | IGF-I and -II binding | [81] | |
| L-domain | NLS (also as HBD1) | 179pkklrp184 | GAG recognition, IGFBP2 nuclear translocation, Interaction with RPTPβ | [74, 91, 93, 97] |
| C-terminal domain | RGD | 265rgd267 | Interaction with IIp45 and Integrins | [99–101] |
| HBD2 | 227khglynlkqckmslngqr244 | Binding to GAGs in a pH-dependent manner | [79] | |
| IGF-binding site | IGF-I and -II binding | [81, 85] |
The N-terminal domain consists of aa residues 1–98 of the mature full-length IGFBP2 protein, and the C-terminal domain contains 107 residues spanning around aa 183–289 [78, 79]. IGFBP2 has 18 conserved cysteines, 12 of which are organized into six disulfide bonds in the N-terminal domain. Three additional disulfide bridges are located within the C-terminal domain; IGFBP2 lacks interdomain bonds [80]. The high content of cysteine within small regions suggests a highly conserved structural composition, which is important for functional integrity, and indeed both the N- and C-terminal domains independently bind to IGFs [81–84]. Moreover, the IGFBP2 C-terminal fragment plays a key role in binding with IGFs and has slower dissociation kinetics when compared with N-terminal fragment [81, 85].
A role for the L-domain
The IGFBP L-domain is a variable region linking the N- and C- terminal domains and contains a region for proteolytic cleavage by proteases such as MMPs, PAPP-A and cathepsin-D [76, 86]. Proteolysis of IGFBP2 is recognized as the predominant mechanism to reduce the binding affinity of IGFBP2 for IGF-II, and has been shown to play a role in increasing bioavailability of IGF-II in malignancy [87, 88]. Apart from containing a target region for proteolysis, the L-domain contains potential posttranslational modification sites for most of the IGFBPs [76, 77, 89]. The L-domain within IGFBP2 comprises 85 aa residues; however, there has been inconclusive evidence regarding post-translational alteration, although several predicted sites may be phosphorylated [76, 89].
Most IGFBPs contain putative heparin-binding domains (HBD) [90]—consensus sequences for glycosaminoglycan (GAG) recognition that have a determined structure as XBBXBX and XBBBXXBX, where B stands for a basic residue and X for a hydropathic residue [91]. Within the L-domain of mature IGFBP2, an HBD sequence is located at residues 179PKKLRP184 (HBD1) in an XBBXBX pattern that is unique to IGFBP2 compared with other IGFBPs [76, 92]. Multiple studies have demonstrated the IGFBP2 HBD1 interactions occurring on cell surface and extra-cellular matrix (ECM), and HBD1 is required for IGFBP2-mediated cell proliferation, migration, and invasion [93–96]. In the rat olfactory bulb, IGFBP2 has been shown to interact with the GAG component of membrane proteoglycans. In vitro, IGFBP2 binds to chondroitin-4 and −6-sulfate, keratan sulfate, heparin, and the proteoglycan aggrecan [93]. Apart from recognition of GAG, the HBD1 sequence within the Linker region also plays a role in binding cell surface receptor protein tyrosine phosphatase β (RPTPβ), and mediates RPTPβ downstream activity [97].
As an IGF modulator, IGFBP2 is commonly associated with functional activities in the pericellular space [76, 94, 98, 99]. Using cell fractionation and confocal microscopy, however, IGFBP2 detection was confirmed in the nuclei of common cancer cells, implicating a role for nuclear regulation [74]. These studies from Azar et al. showed that nuclear translocation of IGFBP2 in cancer cells relies on classical nuclear import mechanisms, primarily in an importin-α dependent manner, through a nuclear localization signal (NLS) sequence within the IGFBP2 linker domain. Further bioinformatics analysis of the IGFBP2 protein sequence with PSORT II identified the NLS sequence at 179PKKLRPP185, which completely overlaps the HBD1 motif, although the nuclear translocation of IGFBP2 occurs independent of HBD1 motif function [74].
Multiple functions of the C-terminal domain
The C-terminal domain of IGFBP2 (C-BP-2) is implicated in a wide range of functions [79, 99–101]. Mature human IGFBP2, but not IGFBP3–6, possesses an RGD motif (Arg-Gly-Asp) at position 265–267 in the C-domain [99, 101]. The IGFBP2 RGD region mediates bimolecular interaction between IGFBP2 and cell membrane integrins, which is required for downstream oncogenic signaling transduction [69, 98, 102, 103]. Although the RGD motif was implicated in biologic regulation of IGFBP2, C-BP-2 (but not the RGD domain alone) was reported to be essential for supporting hematopoietic stem cell activity [104]. Interestingly, the invasion inhibitory protein 45 (IIp45 or MIIP; which also contains an RGD motif) was identified as an IGFBP2-binding protein that can interact with C-BP-2 and attenuate IGFBP2-stimulated glioma invasion [101]. In addition, C-BP-2 was reported to induce β-catenin nuclear activity and tumor growth via interaction with integrin in glioma [105].
A second HBD domain, HBD2, was identified as a pH-dependent heparin-binding site in C-BP-2, with protonation of two histidine residues (His228 and His271, also located in C-BP-2) being responsible for the acidity-dependent HBD2 binding to GAGs [79]. As a common feature of the tumor microenvironment, extracellular acidification suggests that IGFBP2 might accumulate preferentially in the tumor ECM, and may participate in the regulation of tumor biological behaviors that are unique from activities in normal, neutral pH, tissues.
It is worth noting that the complexity of IGFBP activities in the regulation of the IGF system, both locally in tissues and in circulation, may contribute to difficulties in discerning between IGF-dependent and IGF-independent IGFBP actions. This is especially true in systems where one or more components of both groups are expressed. As such, we attempt to provide an accurate view of IGFBP2 actions based on published mechanisms.
IGFBP2 in embryonic development
IGF-I and -II promote cellular mitosis and differentiation, and have been implicated in fetal and placental growth. During the preimplantation period, IGF-I plays an important role as its supplementation can enhance cell proliferation, mitogenesis, and regulate apoptosis [106]. Meanwhile, IGFII is known to modulate fetal growth in vivo, and a mutated IGF-II gene resulted in a growth deficiency of both the embryo and the placenta [107, 108]. IGFs, together with the IGF receptors and IGFBPs, comprise the complete IGF system, which is essential for development of the early embryo. IGFBP2 expression is high in populations of rapidly dividing cells, and in regions directly neighboring areas of cell growth and differentiation, suggesting an important role in the development of fetal tissues (Fig. 2) [109, 110].
Fig. 2. Diagram of IGFBP2 functions in embryogenesis and tumorigenesis.
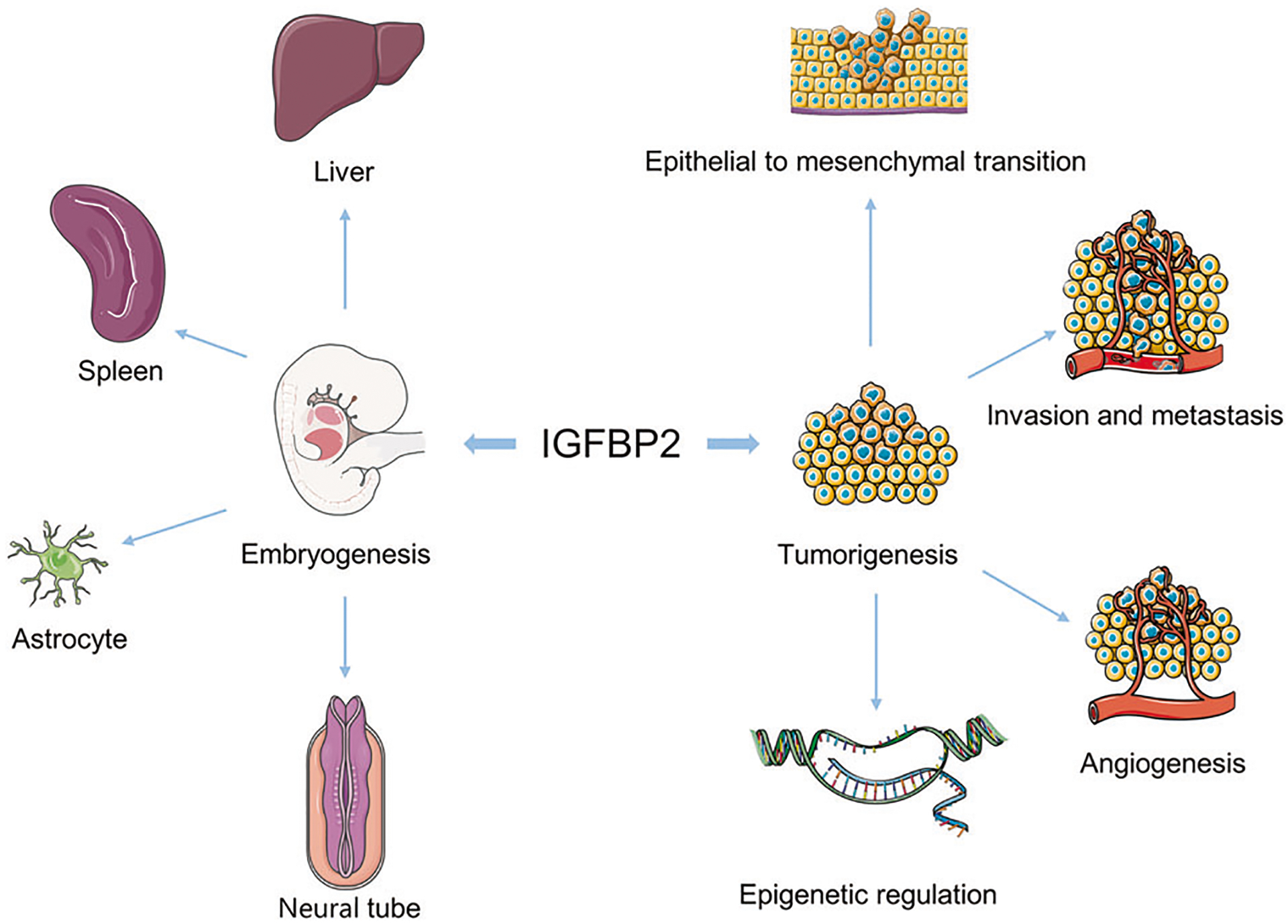
IGFBP2 is predominantly expressed in fetal tissues that are highly proliferative, such as the early postimplantation epiblast and ventricular zone of rostral neuroepithelium, the apical ectodermal ridge, nascent astroglia, and the progenitors of spleen and liver cells. Aberrant expression of IGFBP2 is found in many types of human cancer and is implicated in multiple diverse oncogenic events.
IGFBP2 is predominantly expressed in fetal tissues that are highly proliferative, such as the early postimplantation epiblast, the ventricular zone of rostral neuroepithelium [109, 111], the apical ectodermal ridge [112], and also by progenitor cells for tissues such as the spleen [109] and liver [113]. In the rat, IGFBP2 expression was shown to be present in neocortex before birth in a pattern consistent with expression by newborn astroglia, while in postnatal development expression is greatly reduced [114]. However, in central nervous system (CNS) tumors, IGFBP2 becomes reactivated in the most aggressive stage of glioma, and is consistently overexpressed in glioblastoma [3]. It is proposed that cancer cells possess traits similar to those of embryonic stem (ES) cells, as salient parallels exist between ES cells and tumor cells, such as the self-renewal capacity of ES cells and the high proliferative capacity of tumor cells [115]. Sharing the same enrichment patterns of gene sets, the ES cell signature is also present in poorly differentiated tumors like glioblastoma and bladder carcinoma [115]. IGFBP2 is one such gene that is highly expressed in both ES cells and poorly differentiated malignancy. Characterized as the most typified example of the adult stem cell, hematopoietic stem cells (HSCs) reside in a complex environment, and among the factors that contribute to the repopulation of HSCs, IGFBP2 was reported to support HSC survival and cycling [116]. Consistently, among multiple different cancer types, the level of IGFBP2 appears to be low in well-differentiated tumors and high in poorly differentiated tumors [3, 117].
Oncogenic role of IGFBP2 in multiple cancer types
Aberrant expression patterns of IGFBP2 have been detected in many types of human cancers and broadly associate with a poor prognosis. Accordingly, increasing evidence implicates IGFBP2 with oncogenic events in multiple cancer types. A case for IGFBP2 involvement in tumorigenesis is discussed below focusing on four different types of malignancies. We summarize the potential tumorigenic programs governed by IGFBP2 in different cancer types in Table 2, and schematically illustrate involvement of IGFBP2 in embryogenesis and tumorigenesis in Fig. 2.
Table 2.
IGFBP2 regulates tumorigenesis in multiple cancer types.
| Cancer types | Potential pathway | Description | References |
|---|---|---|---|
| Glioma | integrin/ILK/NF-κB | Interacting with integrin and stimulating downstream ILK/NF-κB signaling | [68] |
| EGFR/STAT3 | Facilitating EGFR nuclear signaling accumulation to potentiate STAT3 transactivation activities | [73] | |
| integrin/FAK/β-catenin | Increasing nuclear activity of β-catenin via integrin/FAK induced inactivation of GSK3β | [105] | |
| integrin/ERK | Promoting ERK phosphorylation and nuclear translocation | [122] | |
| LSD1/H3K9 and H3K27 | Decreasing the methylation level via upregulating LSD1 | [126] | |
| FAK/ERK/SP1/CD144 | Enhancing CD144 expression via the FAK/ERK/SP1 pathway | [151] | |
| Breast cancer | PTEN/p21 | Counteracting the antitumor effect by PTEN with an increase of p21 | [102] |
| β-catenin/Wnt | Activating the β-catenin/Wnt pathway to exert pro-tumorigenic function | [129] | |
| IGF-I/IGF-IR | Mediating endothelial recruitment through IGF-I/IGF-IR | [148] | |
| Colorectal cancer | L1/ezrin/NF-kB | Mediated by the L1/ezrin/NF-kB network and contributes to tumorigensis | [30] |
| Pancreatic cancer | PTEN/Akt/IKKb/p65 | Facilitating nuclear translocation of P65 in a PI3K/Akt/IKKb pathway dependent manner | [134] |
| Prostate cancer | hTERT, telomerase | Upregulating the expression of hTERT and activating the activation of telomerase | [70] |
| MAPK, PI3K | Activating MAPK and PI3K signaling to conduct proliferation | [70] | |
| Integrin/PTEN | Interacting with integrin to increase the phosphorylation of PTEN | [136] |
Glioma
Insulin-like growth factor-binding protein 2 (IGFBP2) is increasingly recognized as a glioma oncogene, emerging as a target for therapeutic intervention [118, 119]. As a candidate biomarker, aberrant expression of IGFBP2 was detected in high-grade gliomas and identified as one of nine genes in a signature associated with poor prognosis [3, 120, 121]. Numerous studies have solidified the potential role of IGFBP2 in glioma initiation [68, 73, 101, 118, 122, 123]. For example, in the replication-competent avian leukemia virus splice acceptor (RCAS) model, neither activated K-Ras nor Akt alone is sufficient to induce glioma formation, while co-delivery of K-Ras and Akt results in astrocytoma genesis with an incidence at 18% [124, 125]. Similarly, the combination of K-Ras and IGFBP2 also leads to astrocytoma formation, with an incidence of 17.4%, suggesting that IGFBP2 and Akt likely lie in converging pathways that are required for gliomagenesis [123].
Platelet-derived growth factor β (PDGFB) is thought to be a driving event for the development of oligodendroglioma [124]. PDGF signaling increases the proliferation rate of glial precursors and blocks their differentiation toward oligodendrocytes and astrocytes [124]. In the PDGFB-driven RCAS model, co-delivery of IGFBP2 was found to enhance malignancy, resulting in the progression of oligodendroglioma to anaplastic oligodendroglioma in 37.9% of the co-injected mice, while also increasing overall tumor incidence from 90.9% to 96.6% [68, 123]. In a new in vivo glioma model with highly expressed PDGFB and expression levels of IGFBP2 controlled using doxycycline, IGFBP2 was shown to promote glioma progression in a time-dependent manner [126]. IGFBP2 was able to drive high-grade glioma (HGG) progression only in the early stages of tumor initiation upon co-delivery with PDGFB, while being unable to promote HGG progression in an established PDGFB-induced tumor, suggesting a pivotal role of IGFBP2 at the tumor initiation stage [126].
β-catenin was reported to mediate biological processes that are required for cancer initiation and progression [127]. IGFBP2 was shown to act as an upstream regulator of the β-catenin pathway in glioma and contributed to increased tumor growth in vivo [105]. This study demonstrated that IGFBP2, through C-terminal domain binding to glioma cell surface integrin, activates FAK, leading to the phosphorylation and inactivation of GSK3β and resulting in increased nuclear activity from stabilized levels of β-catenin [105].
Breast cancer
In an immunohistochemical study of 120 breast specimens, IGFBP2 was detected in breast tumor tissues and showed particularly high levels in neoplastic cells of invasive ductal carcinoma, while being undetectable in normal breast glandular cells or in hyperplasia [19]. In addition, a clinicopathologic study using tissue microarrays and immunohistochemistry revealed an elevated expression level of IGFBP2 in T1 stage breast cancer compared with benign lesions, suggesting that IGFBP2 fosters the development of metastasis, and may serve as a useful marker in breast cancer to predict lymph node metastasis [18].
In human breast cancer cells, IGF-II and IGFBP2 differentially regulate phosphatase and tensin homolog (PTEN) [102]. Acting as a tumor suppressor, PTEN has been shown to suppress the functions of many growth factors, among which IGF-II is most prevalent [128]. Intriguingly, PTEN expression is also stimulated by IGF-II, forming a feedback loop that impairs the response of MCF-7 breast cancer cells to high doses of IGF-II [102]. IGFBP2, when free from IGF-II, has been shown to suppress the antitumor effect of PTEN, and appears to block IGF-II-induced feedback on PTEN, which is then associated with increased p21-mediated pro-tumorigenic function [102].
Recently, a transcriptome analysis of breast cancer showed that IGFBP2 governs pro-tumorigenic cell cycle and Wnt signaling pathways [129]. As a strong indicator of aberrant Wnt pathway activation in breast cancer, β-catenin was shown to be regulated by IGFBP2, with a high lymph node metastasis correlation [129].
Prostate cancer
Prostate carcinoma is a common cancer in males and is among the leading causes of death in elderly men. Elevated levels of plasma IGFBP2 are found in prostate cancer patients [39, 41], and increased expression levels of IGFBP2 are detected during prostate disease progression from benign prostatic hyperplasia (BPH) to metastatic prostate cancer [130]. Increasing evidence over the past 25 years has associated elevated levels of circulating IGFBP2 with malignant prostate disease. IGFBP2 was among the most consistently overexpressed genes in hormone-refractory CWR22R (human prostate cancer transplanted into nude mice) prostate cancer xenografts as detected using a cDNA microarray platform. Immunohistochemical analysis of tissue microarrays also illustrates that IGFBP2 is overexpressed in 100% of the hormone-refractory clinical tumors and in 36% of the primary tumors, but not in benign prostatic specimens [39]. In addition, a recent study from the Prostate Cancer Prevention Trial identified a 55% increased risk of low-grade prostate cancer in patients with higher serum IGFBP2 compared with patients with lower serum IGFBP2 [131].
LAPC-4 is an androgen-dependent prostate cancer cell line. IGFBP2 was found to have a potent stimulatory effect on growth of LAPC-4 prostate cancer cells, which was more pronounced in the absence of androgens, indicating that IGFBP2 may interact with the androgen receptor system [70]. In LAPC-4 and DU145 prostate cancer cells, delivery of IGFBP2 upregulated the expression of hTERT, the regulatory subunit of telomerase that is directly associated with telomerase expression level and is believed to play a role in cell immortality [70]. Moreover, the MAP-kinase and PI3-kinase pathways are thought to be the major regulators of the IGFBP2- induced pro-carcinogenic effect on prostate cancer, as IGFBP2 growth stimulation is completely blocked by MAP-kinase inhibitors and partially blocked by PI3-kinase inhibitors [70].
Gastrointestinal cancer
Pancreatic ductal adenocarcinoma (PDAC) is currently ranked as the fourth most common cause of cancer-related death. Discovery of diagnostic biomarkers is a research priority for the improved management of PDAC. Quantitative proteomic profiling has identified aberrant expression of IGFBP2 in the pancreatic juice and tumor tissue of pancreatic cancer patients [132, 133]. IGFBP2 has also been identified as a compensatory biomarker alongside carbohydrate antigen 19–9 (CA19–9) in the diagnosis of early-stage pancreatic cancer, and also appears to be elevated in risk diseases for development of pancreatic malignancy [11]. Serum IGFBP2 can also serve as a diagnostic bio-marker for PDAC [10]. Clinical findings and cell modeling in vivo have confirmed that high levels of IGFBP2 associate with higher risk of peri-pancreas lymph node metastasis, which is an independent prognostic factor in PDAC [134]. Moreover, IGFBP2 was found to be a critical regulator of the epithelial-to-mesenchymal transition (EMT) axis, and exhibited oncogenic activities in an animal model of PDAC [134].
More clearly, elevated plasma or serum IGFBP2 expression level in colorectal cancer (CRC) is regarded as a valuable prognostic biomarker [26–30]. In a study of 92 colorectal cancer patients compared with controls, serum IGFBP2 was markedly elevated in patients with Dukes B, Dukes C, and advanced disease, with a significant trend from early to advanced disease. In addition, IGFBP2 concentrations were positively correlated with tumor size [28]. L1, a neuronal cell adhesion receptor of the immunoglobulin-like protein family, was detected in a subpopulation of cells at the invasive front of CRC tissue, and was found to form a molecular complex with IGFBP2. In L1-mediated signaling, increased expression of IGFBP2 was regulated by an ezrin/NF-κB-mediated transactivation of the IGFBP2 gene promoter. Furthermore, IGFBP2 overexpression was capable of reproducing all the properties of L1 overexpression, including enhanced motility, growth in the absence of serum, and increased tumor-igenesis and liver metastasis [30].
Regulatory Network of IGFBP2 in Tumorigenesis
As set forth, aberrant expression of IGFBP2 is detected in many malignancies and is tightly related to an increasingly malignant status, pointing to a potential involvement of IGFBP2 in tumor initiation. Acting as a molecular point of signal convergence, IGFBP2 forms a complex regulatory network to exert tumor-promoting functions in tumors (Fig. 3). The major components comprising the IGFBP2 regulatory network involved in tumorigenesis are discussed below.
Fig. 3. Schematic diagram of the oncogenic network governed by IGFBP2.
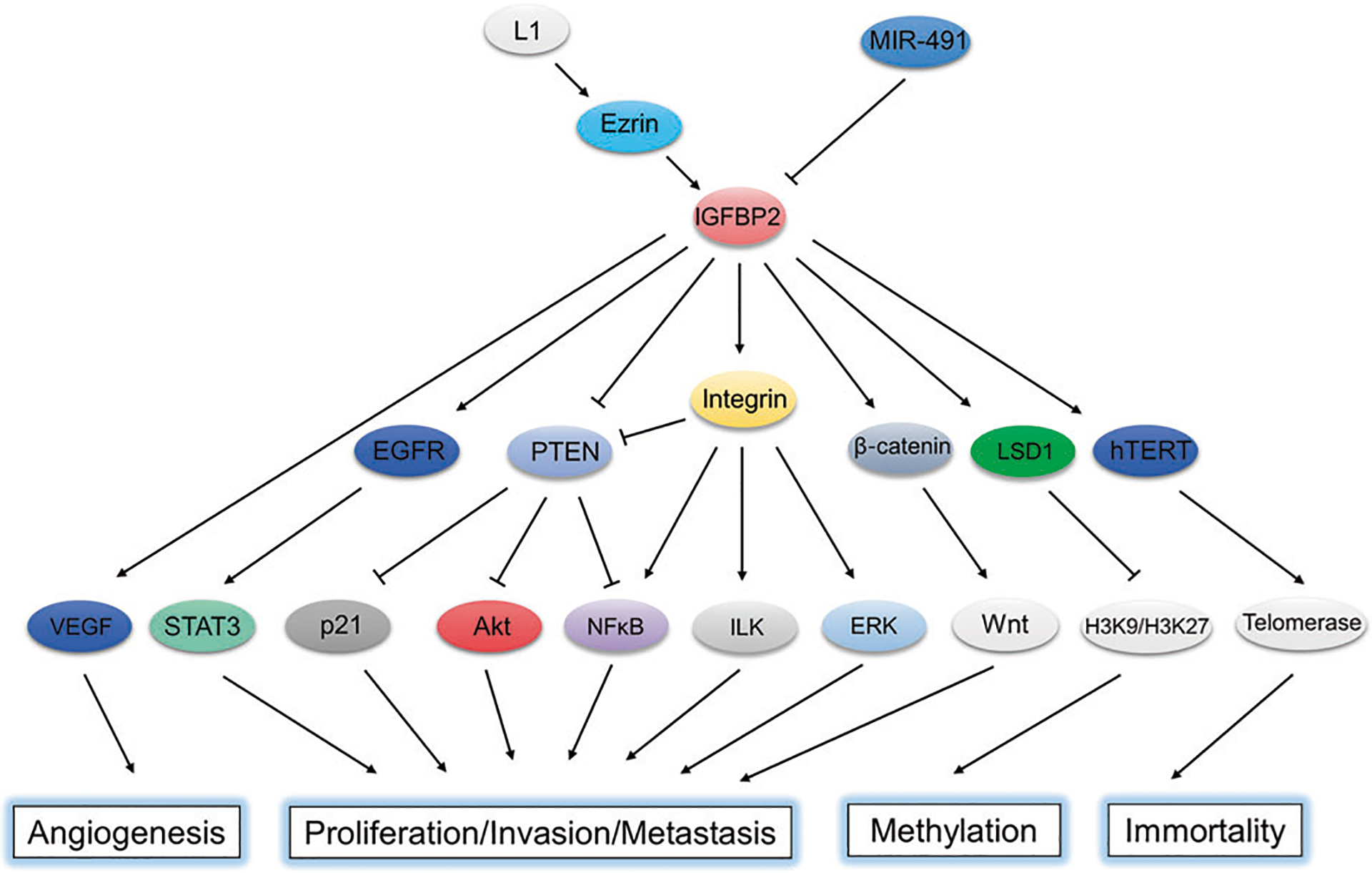
IGFBP2 governs an oncogenic network involved in the proliferation, invasion, metastasis, angiogenesis, methylation and immortality of tumors.
Interaction partners of IGFBP2 in tumorigenesis
Integrins
The tripeptide integrin recognition sequence, RGD, is present in the C-terminal domain of IGFBP2, and is the minimum requirement for the IGFBP2/integrin interaction. In recent years, cell adhesion receptors of the integrin family have been found to regulate a variety of functions that are crucial to tumor initiation, progression and metastasis. Although integrins alone lack the ability to transform cells, a number of studies have revealed that several integrins are required by oncogenes or receptor tyrosine kinases to initiate tumor growth [135].
Among the integrin family, expression of integrin β in various cell types is involved in malignant progression, and crosstalk between IGFBP2 and integrin β1 is implicated in tumorigenesis [68, 122, 136]. Exogenous IGFBP2, which can interact with membrane-located integrins, has been shown to have an IGF-I-independent proliferative effect on prostate cancer DU145 cells that was markedly inhibited by a peptide targeting the RGD motif, or by a β1-integrin receptor-specific blocking antibody, indicating a role for IGFBP2 interaction with integrin β1 subunit in tumor cell growth [136]. Similarly, in studies modeling glioblastoma, IGFBP2 expression was tightly linked to genes enriched in the integrin and integrin-linked kinase (ILK) pathways, and IGFBP2 governed downstream pathways via integrin activation. Moreover, studies have shown that IGFBP2 promotes ERK phosphorylation and nuclear translocation in an integrin-dependent manner, and the inhibition of ERK abrogates exogenous IGFBP2-induced proliferation and cell cycle progression [68]. Intriguingly, another study showed that endogenous IGFBP2 overexpression in glioma cells affected invasion but not proliferation, whilst exogenous delivery of IGFBP2 potentiated glioma cell growth, indicating that IGFBP2/integrin interaction may be integral to tumorigenesis [122].
PTEN
PTEN is an important negative regulator of the cell-survival signaling pathway initiated by PI3K and is frequently disrupted in cancer [137, 138]. IGFBP2 is considered to be a candidate biomarker for PTEN status and PI3K/Akt pathway activation, and crosstalk between IGFBP2/PTEN is involved in oncogenic processes of multiple cancer types [21, 97, 102, 118, 134, 136, 139]. A gene signature for loss of PTEN function was identified in glioblastoma tissue samples and in prostate cancer xenografts through expression profiling; the signature comprised genes involved in various pathways already implicated in tumor formation. Among these signature gene alterations, the most salient was an increase in IGFBP2 mRNA [118]. Consistent with the human tumor findings, IGFBP2 protein expression was substantially higher in isogenic mouse embryonic fibro-blasts (MEFs) isolated from PTEN knockout mice, indicating that IGFBP2 expression is antagonized by the PTEN tumor suppressor [118]. In addition, in a xenograft model, serum levels of human IGFBP2 were elevated in mice bearing PTEN-null tumors, but not PTEN wild-type tumors, indicating IGFBP2 serum levels may serve as clinical bio-markers of PTEN status [118]. Similarly, PTEN loss is also reported to be significantly associated with high levels of IGFBP2 expression in triple-negative breast cancer [21].
Alternatively, IGFBP2 has been demonstrated to suppress PTEN. IGFBP2 acts as a mitogen for vascular smooth muscle cells (VSMC), and has been shown to inhibit PTEN activity via enhanced PTEN tyrosine phosphorylation through dimerization with RPTPβ [97]. In addition, upon interaction with integrin β1, IGFBP2 mediates a consequent increase in PTEN phosphorylation in prostate cancer, which leads to inhibition of PTEN activation [136].
Interestingly, IGF-II appears to induce an increase of PTEN, and IGFBP2 is reported to block feedback of IGF-II on PTEN, which eventually abrogates the PTEN antitumor effect in breast cancer [102]. In pancreatic cancer, IGFBP2 has been shown to suppress the expression level of PTEN protein, whereas PTEN transcript levels do not change, indicating that the regulation is at a post-transcriptional level [134].
NF-κB
Nuclear factor of κB (NF-κB) is a sequence-specific transcription factor that consists of different protein dimers and binds a common sequence motif known as the κB site. NF-κB target genes have been most extensively studied for their involvement in regulating cell proliferation, apoptosis and cell migration [140]. As a major transcription factor, NF-κB controls a variety of cancer-related genes, and enrichment of NF-κB targeted genes was observed in glioma and PDAC when stably expressing IGFBP2 [68, 134]. RELA (also known as p65) is responsible for most of the NF-κB transcriptional activity [141]. Higher levels of nuclear enrichment of p65 and p50 has been detected in wild-type IGFBP2 cells, compared with low levels in parental and RGE mutant (a mutant form of IGFBP2 that cannot bind integrin) cells [68]. As mentioned above, co-delivery of PDGFB/IGFBP2 significantly contributes to an increase in HGDG incidence [123]. However, co-injecting a mutant form of IκBα (inhibiting NF-κB activation by retaining it in the cytoplasm) with PDGFB and IGFBP2 robustly prevents glioma progression in the RCAS/Ntv-a glial-specific transgenic mouse model, suggesting that IGFBP2 requires active or nuclear NF-κB to induce progression [68].
PI3K/Akt signaling has been implicated in the IGFBP2/ NF-κB regulatory network [134]. NF-κB contains an NLS motif at its carboxyl terminus that is recognized and functionally inhibited by IκB proteins. The IκB proteins that trap NF-κB dimers in the cytoplasm can be phosphorylated at a site comprised of two conserved serines (SS). Phosphorylation at this site targets the IκBs for ubiquitin-dependent degradation, and eventually releases the RELA subunit [141]. IGFBP2 has been shown to phosphorylate IκB via PI3K/Akt signaling activation, resulting in the nuclear translocation of RELA [134].
Nuclear regulation via EGFR-STAT3
As a pleiotropic oncogenic protein, IGFBP2 has both extracellular and intracellular functions (Fig. 4). Increasing evidence has shown that IGFBP2 governs a nuclear regulatory network that is tightly associated with tumor progression. Aside from extracellular modulation of IGFs, IGFBP2 was shown to be present within peri/nuclear fractions [142, 143]. Recently, a classical nuclear localization signal (cNLS) sequence at 179PKKLRPP185 within the IGFBP2 linker domain was detected, and mutation of this site abolished IGFBP2 nuclear import. Consistently, as a transcriptional enhancer of the VEGF gene, IGFBP2 with NLS mutation failed to promote transcriptional activation of VEGF and induce angiogenesis in vivo, confirming that nuclear IGFBP2 is the key for VEGF transcriptional activation of [74].
Fig. 4. Mode of signal transduction of IGFBP2 in tumorigenesis.
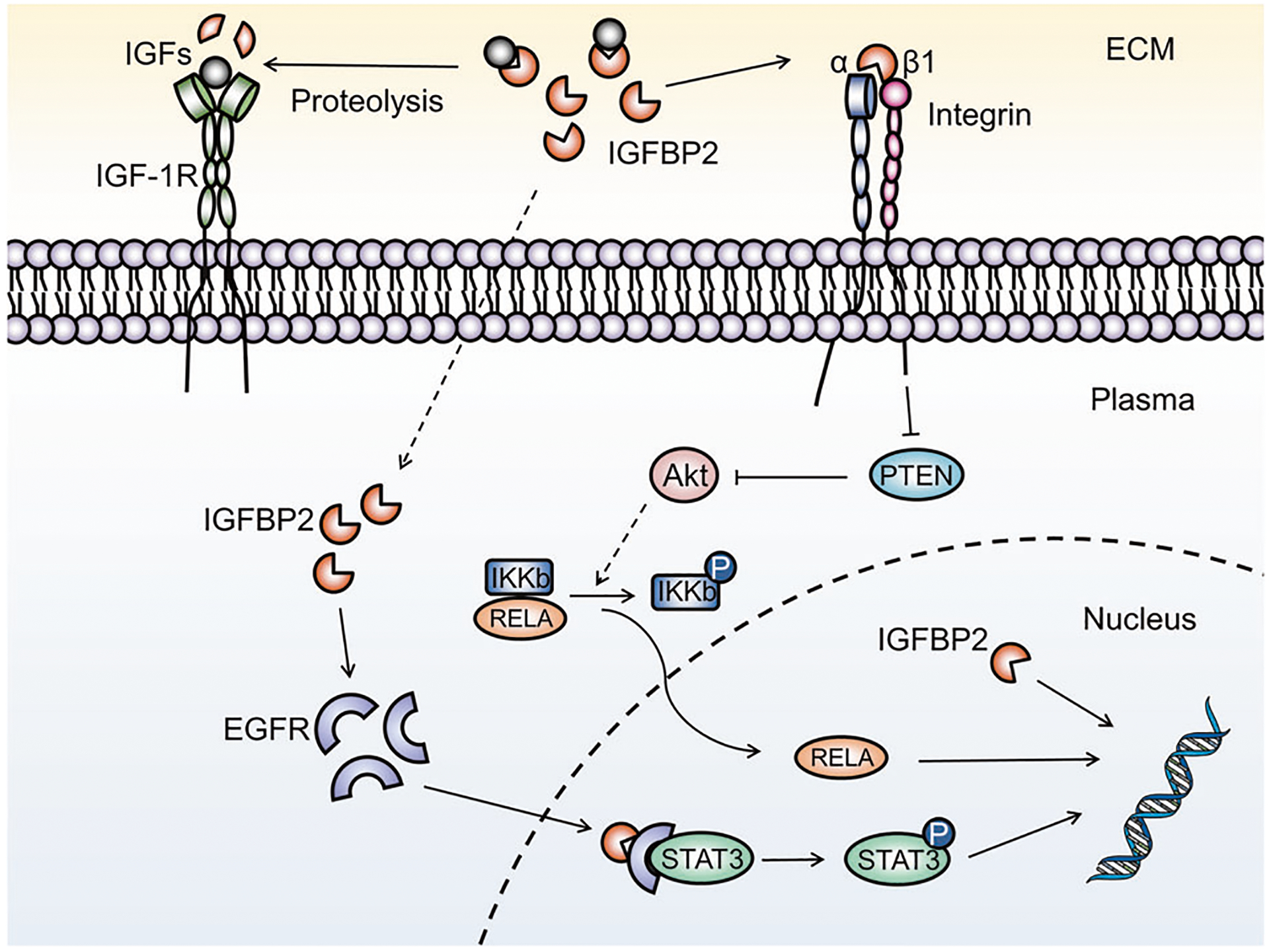
IGFBP2 is detected in the ECM, plasma and nucleus. Extracellular IGFBP2 can modulate intracellular activity via interaction with integrin, by translocation through the cell membrane, and by contributing to IGF-dependent actions. PTEN acts as a checkpoint in IGFBP2 cascade signal transduction. IGFBP2 potentiates EGFR and RELA nuclear accumulation, with resultant activation of oncogenic pathways. IGFBP2 can also function as a transcriptional enhancer, directly targeting oncogene promoters.
IGFBP2 also participates in the regulation of important transcription factors. IGFBP2 has been reported to promote STAT3 transactivation activities via activation of the nuclear EGFR signaling pathway [73]. In the clinical glioma tissues, immunohistochemical results show a clear correlation between IGFBP2 and STAT3 phosphorylation and nuclear co-localization of IGFBP2 and EGFR. In the IGFBP2/EGFR/STAT3 network, IGFBP2 forms a complex with EGFR, and nuclear translocation of IGFBP2 is required for IGFBP2-mediated EGFR nuclear accumulation and eventual augmentation of the nuclear EGFR signal contributes to STAT3 transactivation activities [73]. Moreover, inhibition of IGFBP2 using a neutralizing antibody leads to an impairment of IGFBP2-associated phosphorylation of key members of the EGFR/STAT3 signaling pathway [126].
Oncogenic processes regulated by IGFBP2
Epithelial to mesenchymal transition
EMT is thought to be a fundamental embryonic process in which polarized epithelial cells convert into motile mesenchymal cells [144]. Abnormal reactivation of EMT in malignant disease can fuel both tumor initiation and meta-static spread [144], and pathological EMT features are observed during tumorigenesis [145].
IGFBP2 acts as a pivotal regulator of the EMT axis in PDAC and esophageal adenocarcinoma (EAC) [134, 146]. Both cancers tend to be asymptomatic and patients thus present at later stages. Moreover, high IGFBP2 levels are expressed in subsets of both EAC and PDAC patients, and tend to associate with poorer outcomes. In PDAC, IGFBP2 was reported as a key inducer of EMT, with both clinical and in vivo findings indicating that highly expressed IGFBP2 is a driver of lymph node metastasis. Moreover, IGFBP2 was shown to mediate EMT through activation of the NF-κB-associated pathway, and a model of IGFBP2-mediated regulation of the PTEN/Akt/IKKb/p65 axis in EMT has been proposed [134] (Fig. 4). In the IGFBP2-activated NF-kB network, IGFBP2 first activates PI3K/Akt by inactivating PTEN, which is an essential step for the phosphorylation of IKKβ and subsequent nuclear translocation of RELA (P65). Nuclear p65 enrichment leads to EMT initiation [134].
Role in angiogenesis
Angiogenesis, the generation of new blood vessels, is commonly deregulated in malignant tumors. Aberrant angiogenesis provides nutrients and oxygen to tumor cells, ultimately facilitating uncontrolled proliferation and malignant progression [147]. Numerous studies indicate a crucial role of IGFBP2 in pathologic angiogenesis [72, 74, 143, 148, 149].
In human neuroblastoma, IGFBP2 overexpression activates a pattern of gene sets involved in proliferation, migration and angiogenesis, among which the mRNA of vascular endothelial growth factor (VEGF) shows a 2-fold up-regulation [143]. Similarly, coexpression of IGFBP2 and VEGF is found in pseudopalisading cells surrounding tumor necrosis in glioma, suggesting IGFBP2 involvement in tumor angiogenesis [149]. Using a xenograft model of angiogenesis, studies demonstrated that nuclear IGFBP2 accounts for pathologic angiogenesis via enhancement of VEGF promoter transcriptional activity and subsequent proangiogenic activity [74, 143]. In melanoma, IGFBP2 has been found to induce angiogenesis via extracellular interaction with αVβ3 integrin and subsequent activation of the PI3K/AKT pathway [72].
Endothelial cell migration is essential to angiogenesis [150]. In a breast cancer metastasis model, IGFBP2 secreted by the metastatic cells has been reported to initiate angio-genesis via recruiting endothelia [148]. Secreted IGFBP2 modulates extracellular IGF-I and subsequently activates IGF-IR on endothelial cells, ultimately enhancing endothelial chemotaxis toward metastatic cells [148].
It is known that tumor cells display the ability to drive tumor perfusion via vasculogenic mimicry (VM), an alternative fluid-conducting channel independent of endothelial cell angiogenesis. Recent studies have shown that elevated IGFBP2 expression is detected in VM positive glioma samples, and IGFBP2 promotes VM formation in vitro and in vivo via activation of CD144 and MMP2 [151]. Mechanistically, IGFBP2 activates the FAK/ERK pathway via interaction with integrin α5 and β1 subunits, which subsequently leads to augmentation of CD144 via activation of transcription factor SP1 [151].
Epigenetic regulation
DNA methylation often becomes deregulated in cancer cells [152]. IGFBP2 has been reported to have an association with DNA methylation status [126, 153, 154]. Low expression levels of IGFBP2 are linked to a generalized hypermethylation phenotype and improved survival in glioma [153]. Promoter DNA methylation profiling identified a distinct subset of glioblastoma that displays hyper-methylation at a number of loci, indicating the existence of a glioma-CpG island methylator phenotype (G-CIMP) [155]. Protein array data from the Cancer Genome Atlas GBM cohort showed that IGFBP2 levels are significantly decreased in G-CIMP+ cases [154]. Consistently, treatment of recombinant human IGFBP2 upregulates the level of epigenetic factor lysine-specific demethylase 1 (LSD1/ KDM1A), resulting in a decrease in the levels of methylated histones (H3K9 and H3K27), which have been identified as direct targets of LSD1, further suggesting a role for IGFBP2 in epigenetic regulation [126].
Role in stemness
HSCs are multipotent stem cells that give rise to all lymphoid, myeloid, and erythroid cells when undergoing differentiation. A number of studies have identified several growth factors and secreted proteins that support the repopulation of HSCs. Among these secreted proteins, IGFBP2 was found to be an extrinsic factor that supports ex vivo expansion of HSCs [104, 156, 157]. Mechanistically, HSCs from IGFBP2-null mice have decreased expression of the antiapoptotic molecule Bcl-2, and increased levels of multiple cell cycle inhibitors, including p21, p19, p16, p57, and PTEN [104]. Moreover, the C-terminus of extrinsic IGFBP2, and not the RGD domain, is essential for sustaining HSC activity and the effect of extraneous IGFBP2 on HSCs is independent of IGF-IR-mediated signaling [104].
Basal cell carcinoma (BCC) skin tumors develop due to the aberrant expansion of epidermal basal cells in the proliferative compartment of the skin [158]. In BCC, IGFBP2 has been shown to regulate epidermal progenitor cell expansion, and blocking IGFBP2 expression reduces the Hedgehog-mediated expansion of epidermal progenitor cells [158]. In glioma, IGFBP2 has been found to be overexpressed within the stem cell compartment of GBMs, acting as an essential factor for the clonal expansion and proliferative properties of glioma stem cells [159].
Potential role in immune regulation
Growing evidence implicates IGFBP2 in immune modulation. As a circulating biomarker for several cancers, IGFBP2 can be secreted by tumor cells and contributes to shaping the tumor microenvironment.
Microglia and macrophages comprise the resident immune population within the brain. Activation of the microglia/macrophage population, which also constitutes the major immune cell composition within brain tumors, contributes to glioma proliferation [160, 161]. In the human CNS, accumulation of IGFBP2 is observed in activated microglia/macrophages [162]. Consistently, in a panel of 50 human gliomas of varying malignancy, IGFBP2 immunoreactivity is detected in both glioma cells and macrophages/ microglia, and these IGFBP2-positive macrophages/micro-glial and glioma cells accumulate in the immediate vicinity of focal areas of necrosis [163], indicating a potential correlation between IGFBP2-positive macrophages/microglia and glioma cells in necrosis areas. In addition, activation of the NF-κB pathway, which has been shown to be a downstream target of IGFBP2, has been confirmed to induce cancer-related inflammation in colitis-associated cancer, and this process determines the balance between the protumor and antitumor properties of macrophages [164]. Recently, bioinformatics analysis of gene expression profiles from 2447 glioma samples demonstrated an association of IGFBP2 with immunosuppressive activities and with well-characterized immunosuppressive biomarkers such as CHI3L1, TNFRSF1A, LGALS1, TIMP1, VEGFA, ANXA1, and LGALS3 in GBM [119]. In summary, increasing evidence links IGFBP2 to cancer-related immune responses. Further studies are required to address the underlying mechanisms.
Therapeutic implication
Given its key role as a tumorigenesis network hub that integrates multiple cancer signaling pathways, IGFBP2 emerges as a promising therapeutic target in cancer. OGX-225, an antisense oligonucleotide targeting IGFBP2, was first used in a study in breast cancer. Both in vitro and in vivo, OGX-225 resulted in a decrease in IGFBP2 expression level and abolished the associated aggressive phenotype of MDA-MB-231 cells that constitutively over-express IGFBP2 [20]. In glioma, inhibition of IGFBP2 using a neutralizing antibody resulted in impairment of IGFBP2-associated oncogenic signaling pathways, as measured by the abolished phosphorylation of members in the EGFR/STAT3 signaling pathway and suppression of epigenetic factors LSD1 [126]. In addition, a human single chain recombinant monoclonal antibody that was developed against IGFBP2 showed an inhibitory effect on GBM invasiveness [165]. This single chain recombinant monoclonal antibody was designed to target IGFBP2 at its carboxy terminal domain, disassociating the IGFBP2-cell surface interaction to inhibit IGFBP2-induced migration and invasion of glioma cells [165].
IGFBP2 is aberrantly expressed in breast cancer and contributes to uncontrolled proliferation and poor prognosis as previously set forth. Stimulating immune eradication of IGFBP2-overexpressing breast cancer cells has been recognized as a potential immunomodulation strategy [166]. In breast cancer patients, immunity of IGFBP2–specific IgG antibody is almost tenfold the level of nonmalignant control patients. Moreover, IGFBP2 peptide-specific T cells have been shown to respond to IGFBP2 protein; IGFBP2 peptide vaccination significantly inhibits tumor growth in the neu transgenic mouse model [166]. A compounding factor in immunization against self-tumor antigens is the induction of T-regulatory cells, which weakens the proliferation of Type I T cells that are required for potent antitumor immunity. To solve this problem, the idea of selection of particular portions of a tumor antigen emerges, which specifically stimulates type I T cells, but not type II cells. In IGFBP2 vaccine, to improve efficacy, immunosuppressive epitopes from the C-terminus were removed, and the remaining N-terminus vaccine showed potent antitumor activity [167].
MicroRNA (miR) mimics and sponges, short non-coding RNAs that can alter gene expression by post-transcriptional regulation, are being assessed for their utility as therapeutic agents. Both mature products of miR-491 (miR-491–5p and −3p) have been shown to have tumor suppressor functions in multiple cancer types [168, 169]. Both miR-491–5p and −3p show an inverse correlation with expression of IGFBP2 in GBM and are demonstrated to be downregulated in tumors compared with the normal brain [170]. Furthermore, decreased expression of miR-491–3p secondary to genomic deletion is a key event in the generation of elevated IGFBP2 expression via the target site on IGFBP2 3′-UTR. Thus, miR-491–5p and miR-491–3p represent a new class of therapeutic agents that directly target IGFBP2 to exert an antiproliferative effect on glioblastoma cells [170].
Conclusions
The ongoing development of knowledge about IGFBP2 and elucidation of its roles in cancer has led to a conceptual change for this oncogene, progressing from an IGFs system regulator to an independent tumorigenic factor. Nevertheless, the detailed mechanism underlying its tumorigenic function and immune regulation still needs to be clarified. IGFBP2 has been found to participate in oncogenic processes in multiple cancer types while utilizing multiple regulatory mechanisms, indicating that IGFBP2-associated oncogenes may have a specific activation status in different malignancies. In addition, the multiple functional domains of IGFBP2 suggest a spatial regulation of IGFBP2 tumor biology: different regulatory mechanisms operating in the extracellular, intracellular, and nuclear environments. The complicated regulatory patterns seen in human tumors underscores the need for detailed understanding of the IGFBP2-driven oncogenic network, which is necessary for the future clinical applications. In this review, we provide an overview of the most relevant modes of crosstalk and cooperativity between IGFBP2 and other signaling molecules during tumorigenesis. Oncogenic signaling pathways governed by IGFBP2 are functionally enriched in proliferation, invasion, metastasis, angiogenesis, and epigenetic regulation motifs. Functioning as the hub of the oncogenic network, IGFBP2 integrates a series of signaling pathways that contribute to tumor initiation and progression. Among the members of the IGFBP2-associated oncogene network, IGFBP2 itself modulates the distribution, function, and activity of IGFs in the pericellular space. Integrin functions as a receptor for collecting IGFBP2 extracellular signals. PTEN, a tumor suppressor, acts as a checkpoint in IGFBP2 cascade signal transduction. STAT3 and NFκB are two major downstream transcriptional factors of IGFBP2 that direct tumorigenic intracellular signaling. Finally, nuclear IGFBP2 can also function as a tumor enhancer by directly targeting multiple oncogene promoters.
Through a large number of detailed investigations, IGFBP2 has been shown to be a crucial tumorigenic factor and a viable therapeutic target in multiple cancers. However, even as a strong, clinically relevant oncogene, there is currently no available inhibitor directly targeting IGFBP2 so far. As an alternative therapeutic strategy, multiple interacting proteins, such as integrin, STAT3, NF-κB, and other molecules that are significantly involved in IGFBP2-driven tumorigenesis, can serve as promising druggable targets for those tumors with high IGFBP2 expression, with possible therapeutic strategies including targeting the IGFBP2-associated effectors in combination as a primary treatment or as an adjuvant to current standard therapies. Undoubtedly, a detailed understanding of IGFBP2-regulated signaling will be crucial to the identification of pathways that are preferentially mediated by IGFBP2 in different cancer types, and pharmacological manipulation based on this knowledge holds promise for revolutionizing outcome for patients with IGFBP2-driven tumors.
Acknowledgements
We would like to thank all of the investigators who have contributed to the study of IGFBP2 in cancer that inspired this review article. This work was supported in part by the NCI Cancer Center Support Grant to the Comprehensive Cancer Center of Wake Forest University Health Sciences (P30CA012197). WZ is supported by the Hanes and Willis Professorship in Cancer and a Fellowship from the National Foundation for Cancer Research. XY and TL are supported by the National Natural Science Foundation of China (No. 81872063).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. A census of amplified and overexpressed human cancer genes. Nat Rev Cancer. 2010;10:59–64. [DOI] [PubMed] [Google Scholar]
- 3.Fuller GN, Rhee CH, Hess KR, Caskey LS, Wang R, Bruner JM, et al. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: a revelation by parallel gene expression profiling. Cancer Res. 1999;59:4228–32. [PubMed] [Google Scholar]
- 4.McDonald KL, O’Sullivan MG, Parkinson JF, Shaw JM, Payne CA, Brewer, et al. IQGAP1 and IGFBP2: valuable biomarkers for determining prognosis in glioma patients. J Neuropathol Exp Neurol. 2007;66:405–417. [DOI] [PubMed] [Google Scholar]
- 5.Higgins RJ, Dickinson PJ, LeCouteur RA, Bollen AW, Wang H, Wang H, et al. Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol. 2010;98:49–55. [DOI] [PubMed] [Google Scholar]
- 6.Scrideli CA, Carlotti CG, Mata JF, Neder L, Machado HR, Oba-Sinjo SM, et al. Prognostic significance of co-overexpression of the EGFR/IGFBP-2/HIF-2A genes in astrocytomas. J Neurooncol. 2007;83:233–9. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Jiang T, Zhou K, Xu L, Chen B, Li G, et al. Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro Oncol. 2009;11:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rickman DS, Bobek MP, Misek DE, Kuick R, Blaivas M, Kurnit DM, et al. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001;61:6885–91. [PubMed] [Google Scholar]
- 9.Marucci G, Morandi L, Magrini E, Farnedi A, Franceschi E, Miglio R, et al. Gene expression profiling in glioblastoma and immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch. 2008;453:599–609. [DOI] [PubMed] [Google Scholar]
- 10.Kendrick ZW, Firpo MA, Repko RC, Scaife CL, Adler DG, Boucher KM, et al. Serum IGFBP2 and MSLN as diagnostic and prognostic biomarkers for pancreatic cancer. HPB. 2014; 16:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneyama T, Ohtsuki S, Honda K, Kobayashi M, Iwasaki M, Uchida Y, et al. Identification of IGFBP2 and IGFBP3 as compensatory biomarkers for CA19–9 in early-stage pancreatic cancer using a combination of antibody-based and LC-MS/MS-based proteomics. PLoS One. 2016;11:e0161009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS. Elevated serum insulin-like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab. 1997;82:2308–13. [DOI] [PubMed] [Google Scholar]
- 13.Kanety H, Kattan M, Goldberg I, Kopolovic J, Ravia J, Menczer J, et al. Increased insulin-like growth factor binding protein-2 (IGFBP-2) gene expression and protein production lead to high IGFBP-2 content in malignant ovarian cyst fluid. Br J Cancer. 1996;73:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemisto A, et al. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer. 2005;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron-Hay S, Boyle F, Ferrier A, Scott C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res. 2004;10:1796–806. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol. 2006;19:1149–56. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster JM, Dressman HK, Whitaker RS, Havrilesky L, Gray J, Marks JR, et al. Gene expression patterns that characterize advanced stage serous ovarian cancers. J Soc Gynecol Investig. 2004;11:51–59. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Arun BK, Wang H, Fuller GN, Zhang W, Middleton LP, et al. IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J 2008;14:261–7. [DOI] [PubMed] [Google Scholar]
- 19.Busund LT, Richardsen E, Busund R, Ukkonen T, Bjørnsen T, Busch C, et al. Significant expression of IGFBP2 in breast cancer compared with benign lesions. J Clin Pathol. 2005;58:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So AI, Levitt RJ, Eigl B, Fazli L, Muramaki M, Leung S, et al. Insulin-like growth factor binding protein-2 is a novel therapeutic target associated with breast cancer. Clin Cancer Res. 2008;14:6944–54. [DOI] [PubMed] [Google Scholar]
- 21.Dean SJ, Perks CM, Holly JM, Bhoo-Pathy N, Looi LM, Mohammed NA, et al. Loss of PTEN expression is associated with IGFBP2 expression, younger age, and late stage in triple-negative breast cancer. Am J Clin Pathol. 2014;141:323–33. [DOI] [PubMed] [Google Scholar]
- 22.Kim YW, Bae SM, Kim YW, Park DC, Lee KH, Liu HB, et al. Target-based molecular signature characteristics of cervical adenocarcinoma and squamous cell carcinoma. Int J Oncol. 2013;43:539–47. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Shen SS, Wang H, Diwan AH, Zhang W, Fuller GN, et al. Expression of insulin-like growth factor-binding protein 2 in melanocytic lesions. J Cutan Pathol. 2003;30:599–605. [DOI] [PubMed] [Google Scholar]
- 24.Olney RC, Anhalt H, Neely EK, Wilson DM. A quantitative assay for IGF-I and IGF binding protein mRNAs: expression in malignant melanoma cells. Mol Cell Endocrinol. 1995;110:213–23. [DOI] [PubMed] [Google Scholar]
- 25.Renehan AG, Painter JE, O’Halloran D, Atkin WS, Potten CS, O’Dwyer ST, et al. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000;85:3402–8. [DOI] [PubMed] [Google Scholar]
- 26.Ladd JJ, Busald T, Johnson MM, Zhang Q, Pitteri SJ, Wang H, et al. Increased plasma levels of the APC-interacting protein MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of color-ectal cancer in women. Cancer Prev Res. 2012;5:655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liou JM, Shun CT, Liang JT, Chiu HM, Chen MJ, Chen CC, et al. Plasma insulin-like growth factor-binding protein-2 levels as diagnostic and prognostic biomarker of colorectal cancer. J Clin Endocrinol Metab. 2010;95:1717–25. [DOI] [PubMed] [Google Scholar]
- 28.Renehan AG, Jones J, Potten CS, Shalet SM, O’Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.el AF, Garrouste F, Remacle-Bonnet M, Sastre B, Pommier G. Alterations in serum levels of insulin-like growth factors and insulin-like growth-factor-binding proteins in patients with colorectal cancer. Int J Cancer. 1994;57:491–7. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shmuel A, Shvab A, Gavert N, Brabletz T, Ben-Ze’ev A. Global analysis of L1-transcriptomes identified IGFBP-2 as a target of ezrin and NF-κB signaling that promotes colon cancer progression. Oncogene. 2013;32:3220–30. [DOI] [PubMed] [Google Scholar]
- 31.Kühnl A, Kaiser M, Neumann M, Fransecky L, Heesch S, Radmacher M, et al. High expression of IGFBP2 is associated with chemoresistance in adult acute myeloid leukemia. Leuk Res. 2011;35:1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller HL, Oh Y, Lehrnbecher T, Blum WF, Rosenfeld RG. Insulin-like growth factor-binding protein-2 concentrations in cerebrospinal fluid and serum of children with malignant solid tumors or acute leukemia. J Clin Endocrinol Metab. 1994;79:428–434. [DOI] [PubMed] [Google Scholar]
- 33.Dawczynski K, Steinbach D, Wittig S, Pfaffendorf N, Kauf E, Zintl F. Expression of components of the IGF axis in childhood acute myelogenous leukemia. Pediatr Blood Cancer. 2008;50:24–28. [DOI] [PubMed] [Google Scholar]
- 34.Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I, -II and IGFBP-2, −3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transpl. 2003;32:411–5. [DOI] [PubMed] [Google Scholar]
- 35.Argüelles B, Barrios V, Pozo J, Muñoz MT, Argente J. Modifications of growth velocity and the insulin-like growth factor system in children with acute lymphoblastic leukemia: a long-itudinal study. J Clin Endocrinol Metab. 2000;85:4087–92. [DOI] [PubMed] [Google Scholar]
- 36.Vorwerk P, Mohnike K, Wex H, Röhl FW, Zimmermann M, Blum WF, et al. Insulin-like growth factor binding protein-2 at diagnosis of childhood acute lymphoblastic leukemia and the prediction of relapse risk. J Clin Endocrinol Metab. 2005; 90:3022–7. [DOI] [PubMed] [Google Scholar]
- 37.Crofton PM, Ahmed SF, Wade JC, Elmlinger MW, Ranke MB, Kelnar CJ, et al. Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Pediatr Res. 2000;48:490–6. [DOI] [PubMed] [Google Scholar]
- 38.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, et al. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77:229–33. [DOI] [PubMed] [Google Scholar]
- 39.Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, et al. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 1999;91:1758–64. [DOI] [PubMed] [Google Scholar]
- 40.Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–5. [DOI] [PubMed] [Google Scholar]
- 41.Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, et al. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and −3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20:833–41. [DOI] [PubMed] [Google Scholar]
- 42.Lee DY, Kim SJ, Lee YC. Serum insulin-like growth factor (IGF)-I and IGF-binding proteins in lung cancer patients. J Korean Med Sci. 1999;14:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaques G, Kiefer P, Schöneberger HJ, Wegmann B, Kaiser U, Brandscheid D, et al. Differential expression of insulin-like growth factor binding proteins in human non-small cell lung cancer cell lines. Eur J Cancer. 1992;28A:1899–904. [DOI] [PubMed] [Google Scholar]
- 44.Guo C, Lu H, Gao W, Wang L, Lu K, Wu S, et al. Insulin-like growth factor binding protein-2 level is increased in blood of lung cancer patients and associated with poor survival. PLoS One. 2013;8:e74973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migita T, Narita T, Asaka R, Miyagi E, Nagano H, Nomura K, et al. Role of insulin-like growth factor binding protein 2 in lung adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3. Am J Pathol. 2010;176:1756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu CJ, Wang CL, Wang CI, Chen CD, Dan YM, Wu CC, et al. Comprehensive proteome analysis of malignant pleural effusion for lung cancer biomarker discovery by using multidimensional protein identification technology. J Proteome Res. 2011;10:4671–82. [DOI] [PubMed] [Google Scholar]
- 47.Ranke MB, Maier KP, Schweizer R, Stadler B, Schleicher S, Elmlinger MW, et al. Pilot study of elevated levels of insulin-like growth factor-binding protein-2 as indicators of hepatocellular carcinoma. Horm Res. 2003;60:174–80. [DOI] [PubMed] [Google Scholar]
- 48.Lee CF, Ling ZQ, Zhao T, Lee KR. Distinct expression patterns in hepatitis B virus- and hepatitis C virus-infected hepatocellular carcinoma. World J Gastroenterol. 2008;14:6072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Q, Mao YQ, Jiang WD, Chen YR, Huang RY, Zhou XB, et al. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One. 2012;7:e46851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Huang W, Chen J, Zhou X, Lu Z, Zhou H. Expression of IGFBP2 in gastric carcinoma and relationship with clinicopathologic parameters and cell proliferation. Dig Dis Sci. 2007;52:248–53. [DOI] [PubMed] [Google Scholar]
- 51.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–63. [DOI] [PubMed] [Google Scholar]
- 52.Shi LH, Zhu XQ, Zhao GH, Xia YB, Zhang YS. Expression of insulin-like growth factor binding protein-2 in gastric carcinoma and its relationship with cell proliferation. World J Gastroenterol. 2006;12:6285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyake H, Hara I, Yamanaka K, Muramaki M, Gleave M, Eto H. Introduction of insulin-like growth factor binding protein-2 gene into human bladder cancer cells enhances their metastatic potential. Oncol Rep. 2005;13:341–5. [PubMed] [Google Scholar]
- 54.Matuschek C, Rudoy M, Peiper M, Gerber PA, Hoff NP, Buhren BA, et al. Do insulin-like growth factor associated proteins qualify as a tumor marker? Results of a prospective study in 163 cancer patients. Eur J Med Res. 2011;16:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tombolan L, Orso F, Guzzardo V, Casara S, Zin A, Bonora M, et al. High IGFBP2 expression correlates with tumor severity in pediatric rhabdomyosarcoma. Am J Pathol. 2011;179:2611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allander SV, Illei PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, et al. Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol. 2002;161:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boulle N, Baudin E, Gicquel C, Logié A, Bertherat J, Penfornis A, et al. Evaluation of plasma insulin-like growth factor binding protein-2 as a marker for adrenocortical tumors. Eur J Endocrinol. 2001;144:29–36. [DOI] [PubMed] [Google Scholar]
- 58.Boulle N, Logié A, Gicquel C, Perin L, Le BY. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713–20. [DOI] [PubMed] [Google Scholar]
- 59.Fottner C, Sattarova S, Hoffmann K, Spöttl G, Weber MM. Elevated serum levels of IGF-binding protein 2 in patients with non-seminomatous germ cell cancer: correlation with tumor markers alpha-fetoprotein and human chorionic gonadotropin. Eur J Endocrinol. 2008;159:317–27. [DOI] [PubMed] [Google Scholar]
- 60.Zumkeller W, Schwander J, Mitchell CD, Morrell DJ, Schofield PN, Preece MA. Insulin-like growth factor (IGF)-I, -II and IGF binding protein-2 (IGFBP-2) in the plasma of children with Wilms’ tumour. Eur J Cancer. 1993;29A:1973–7. [DOI] [PubMed] [Google Scholar]
- 61.Yao X, Wang Y, Duan Y, Zhang Q, Li P, Jin R, et al. IGFBP2 promotes salivary adenoid cystic carcinoma metastasis by activating the NF-κB/ZEB1 signaling pathway. Cancer Lett. 2018;432:38–46. [DOI] [PubMed] [Google Scholar]
- 62.Gállego PJ, Paris S, Idbaih A, Dehais C, Laigle-Donadey F, Navarro S, et al. Diagnostic and prognostic value of preoperative combined GFAP, IGFBP-2, and YKL-40 plasma levels in patients with glioblastoma. Cancer. 2014;120:3972–80. [DOI] [PubMed] [Google Scholar]
- 63.Probst-Hensch NM, Steiner JH, Schraml P, Varga Z, Zürrer-Härdi U, Storz M, et al. IGFBP2 and IGFBP3 protein expressions in human breast cancer: association with hormonal factors and obesity. Clin Cancer Res. 2010;16:1025–32. [DOI] [PubMed] [Google Scholar]
- 64.Biernacka KM, Uzoh CC, Zeng L, Persad RA, Bahl A, Gillatt D, et al. Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat Cancer. 2013;20:741–51. [DOI] [PubMed] [Google Scholar]
- 65.Planque C, Kulasingam V, Smith CR, Reckamp K, Goodglick L, Diamandis EP. Identification of five candidate lung cancer bio-markers by proteomics analysis of conditioned media of four lung cancer cell lines. Mol Cell Proteom. 2009;8:2746–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu H, Wang L, Gao W, Meng J, Dai B, Wu S, et al. IGFBP2/ FAK pathway is causally associated with dasatinib resistance in non-small cell lung cancer cells. Mol Cancer Ther. 2013;12:2864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Ying X, Han S, Wang J, Zhou X, Bai E, et al. Autoantibodies against insulin-like growth factor‑binding protein-2 as a serological biomarker in the diagnosis of lung cancer. Int J Oncol. 2013;42:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes KM, Annala M, Chua CY, Dunlap SM, Liu Y, Hugen N, et al. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-κB network. Proc Natl Acad Sci USA. 2012;109:3475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foulstone EJ, Zeng L, Perks CM, Holly JM. Insulin-like growth factor binding protein 2 (IGFBP-2) promotes growth and survival of breast epithelial cells: novel regulation of the estrogen receptor. Endocrinology. 2013;154:1780–93. [DOI] [PubMed] [Google Scholar]
- 70.Moore MG, Wetterau LA, Francis MJ, Peehl DM, Cohen P. Novel stimulatory role for insulin-like growth factor binding protein-2 in prostate cancer cells. Int J Cancer. 2003;105:14–19. [DOI] [PubMed] [Google Scholar]
- 71.Hoeflich A, Fettscher O, Lahm H, Blum WF, Kolb HJ, Engel-hardt D, et al. Overexpression of insulin-like growth factor-binding protein-2 results in increased tumorigenic potential in Y-1 adrenocortical tumor cells. Cancer Res. 2000;60:834–8. [PubMed] [Google Scholar]
- 72.Das SK, Bhutia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, et al. MDA-9/syntenin and IGFBP-2 promote angio-genesis in human melanoma. Cancer Res. 2013;73:844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chua CY, Liu Y, Granberg KJ, Hu L, Haapasalo H, Annala MJ, et al. IGFBP2 potentiates nuclear EGFR-STAT3 signaling. Oncogene. 2016;35:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Onco-gene. 2014;33:578–88. [DOI] [PubMed] [Google Scholar]
- 75.Binkert C, Landwehr J, Mary JL, Schwander J, Heinrich G. Cloning, sequence analysis and expression of a cDNA encoding a novel insulin-like growth factor binding protein (IGFBP-2). EMBO J. 1989;8:2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. [DOI] [PubMed] [Google Scholar]
- 77.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999; 20:761–87. [DOI] [PubMed] [Google Scholar]
- 78.Galea CA, Mobli M, McNeil KA, Mulhern TD, Wallace JC, King GF, et al. Insulin-like growth factor binding protein-2: NMR analysis and structural characterization of the N-terminal domain. Biochimie. 2012;94:608–16. [DOI] [PubMed] [Google Scholar]
- 79.Kuang Z, Yao S, Keizer DW, Wang CC, Bach LA, Forbes BE, et al. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2). J Mol Biol. 2006;364:690–704. [DOI] [PubMed] [Google Scholar]
- 80.Forbes BE, Turner D, Hodge SJ, McNeil KA, Forsberg G, Wallace JC. Localization of an insulin-like growth factor (IGF) binding site of bovine IGF binding protein-2 using disulfide mapping and deletion mutation analysis of the C-terminal domain. J Biol Chem. 1998;273:4647–52. [DOI] [PubMed] [Google Scholar]
- 81.Carrick FE, Forbes BE, Wallace JC. BIAcore analysis of bovine insulin-like growth factor (IGF)-binding protein-2 identifies major IGF binding site determinants in both the amino- and carboxyl-terminal domains. J Biol Chem. 2001;276:27120–8. [DOI] [PubMed] [Google Scholar]
- 82.Hobba GD, Löthgren A, Holmberg E, Forbes BE, Francis GL, Wallace JC. Alanine screening mutagenesis establishes tyrosine 60 of bovine insulin-like growth factor binding protein-2 as a determinant of insulin-like growth factor binding. J Biol Chem. 1998;273:19691–8. [DOI] [PubMed] [Google Scholar]
- 83.Wang JF, Hampton B, Mehlman T, Burgess WH, Rechler MM. Isolation of a biologically active fragment from the carboxy terminus of the fetal rat binding protein for insulin-like growth factors. Biochem Biophys Res Commun. 1988;157:718–26. [DOI] [PubMed] [Google Scholar]
- 84.Ho PJ, Baxter RC. Characterization of truncated insulin-like growth factor-binding protein-2 in human milk. Endocrinology. 1997;138:3811–8. [DOI] [PubMed] [Google Scholar]
- 85.Carrick FE, Hinds MG, McNeil KA, Wallace JC, Forbes BE, Norton RS. Interaction of insulin-like growth factor (IGF)-I and -II with IGF binding protein-2: mapping the binding surfaces by nuclear magnetic resonance. J Mol Endocrinol. 2005;34:685–98. [DOI] [PubMed] [Google Scholar]
- 86.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–81. [DOI] [PubMed] [Google Scholar]
- 87.Rorive S, Berton A, D’haene N, Takacs CN, Debeir O, Decaestecker C, et al. Matrix metalloproteinase-9 interplays with the IGFBP2-IGFII complex to promote cell growth and motility in astrocytomas. Glia. 2008;56:1679–90. [DOI] [PubMed] [Google Scholar]
- 88.Menouny M, Binoux M, Babajko S. Role of insulin-like growth factor binding protein-2 and its limited proteolysis in neuroblastoma cell proliferation: modulation by transforming growth factor-beta and retinoic acid. Endocrinology. 1997;138:683–90. [DOI] [PubMed] [Google Scholar]
- 89.Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 1997;128:1–5. [DOI] [PubMed] [Google Scholar]
- 90.Bach LA, Headey SJ, Norton RS. IGF-binding proteins–the pieces are falling into place. Trends Endocrinol Metab. 2005;16:228–34. [DOI] [PubMed] [Google Scholar]
- 91.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. [DOI] [PubMed] [Google Scholar]
- 92.Russo VC, Azar WJ, Yau SW, Sabin MA, Werther GA. IGFBP-2: the dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015;26:329–46. [DOI] [PubMed] [Google Scholar]
- 93.Russo VC, Bach LA, Fosang AJ, Baker NL, Werther GA. Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb. Endocrinology. 1997;138:4858–67. [DOI] [PubMed] [Google Scholar]
- 94.Russo VC, Rekaris G, Baker NL, Bach LA, Werther GA. Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells. Endocrinology. 1999;140:3082–90. [DOI] [PubMed] [Google Scholar]
- 95.Arai T, Busby W, Clemmons DR. Binding of insulin-like growth factor (IGF) I or II to IGF-binding protein-2 enables it to bind to heparin and extracellular matrix. Endocrinology. 1996;137:4571–5. [DOI] [PubMed] [Google Scholar]
- 96.Russo VC, Schütt BS, Andaloro E, Ymer SI, Hoeflich A, Ranke MB, et al. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology. 2005;146:4445–55. [DOI] [PubMed] [Google Scholar]
- 97.Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32:4116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, et al. Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with alphavbeta3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res. 2004;64:977–84. [DOI] [PubMed] [Google Scholar]
- 99.Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32: 859–68. [DOI] [PubMed] [Google Scholar]
- 100.Jones JI, Gockerman A, Busby WH, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90: 10553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song SW, Fuller GN, Khan A, Kong S, Shen W, Taylor E, et al. IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci USA. 2003;100:13970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26:5966–72. [DOI] [PubMed] [Google Scholar]
- 103.Frommer KW, Reichenmiller K, Schutt BS, Hoeflich A, Ranke MB, Dodt G, et al. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol. 2006;37:13–23. [DOI] [PubMed] [Google Scholar]
- 104.Huynh H, Zheng J, Umikawa M, Zhang C, Silvany R, Iizuka S, et al. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood. 2011;118:3236–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patil SS, Gokulnath P, Bashir M, Shwetha SD, Jaiswal J, Shastry AH, et al. Insulin-like growth factor binding protein-2 regulates β-catenin signaling pathway in glioma cells and contributes to poor patient prognosis. Neuro Oncol. 2016;18:1487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adashi EY. Intraovarian regulation: the proposed role of insulin-like growth factors. Ann N. Y Acad Sci 1993;687:10–12. [DOI] [PubMed] [Google Scholar]
- 107.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. [DOI] [PubMed] [Google Scholar]
- 108.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–59. [DOI] [PubMed] [Google Scholar]
- 109.Wood TL, Streck RD, Pintar JE. Expression of the IGFBP-2 gene in post-implantation rat embryos. Development. 1992;114:59–66. [DOI] [PubMed] [Google Scholar]
- 110.Green BN, Jones SB, Streck RD, Wood TL, Rotwein P, Pintar JE. Distinct expression patterns of insulin-like growth factor binding proteins 2 and 5 during fetal and postnatal development. Endocrinology. 1994;134:954–62. [DOI] [PubMed] [Google Scholar]
- 111.Pintar JE, Wood TL, Streck RD, Havton L, Rogler L, Hsu MS. Expression of IGF-II, the IGF-II/mannose-6-phosphate receptor and IGFBP-2 during rat embryogenesis. Adv Exp Med Biol. 1991;293:325–33. [DOI] [PubMed] [Google Scholar]
- 112.van Kleffens M, Groffen C, Lindenbergh-Kortleve DJ, van Neck JW, González-Parra S, Dits N, et al. The IGF system during fetal-placental development of the mouse. Mol Cell Endocrinol. 1998;140:129–35. [DOI] [PubMed] [Google Scholar]
- 113.Streck RD, Pintar JE. The embryonic pattern of rat insulin-like growth factor-I gene expression suggests a role in induction and early growth of the liver. Endocrinology. 1992;131:2030–2. [DOI] [PubMed] [Google Scholar]
- 114.Lee WH, Michels KM, Bondy CA. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: correlation with insulin-like growth factors I and II. Neuroscience. 1993;53:251–65. [DOI] [PubMed] [Google Scholar]
- 115.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. [DOI] [PubMed] [Google Scholar]
- 117.Akmal SN, Yun K, MacLay J, Higami Y, Ikeda T. Insulin-like growth factor 2 and insulin-like growth factor binding protein 2 expression in hepatoblastoma. Hum Pathol. 1995;26:846–51. [DOI] [PubMed] [Google Scholar]
- 118.Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci USA. 2007;104:5563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai J, Chen Q, Cui Y, Dong J, Chen M, Wu P, et al. Immune heterogeneity and clinicopathologic characterization of IGFBP2 in 2447 glioma samples. Oncoimmunology. 2018;7:e1426516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han S, Li Z, Master LM, Master ZW, Wu A. Exogenous IGFBP-2 promotes proliferation, invasion, and chemoresistance to temozolomide in glioma cells via the integrin β1-ERK pathway. Br J Cancer. 2014;111:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dunlap SM, Celestino J, Wang H, Jiang R, Holland EC, Fuller GN, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci USA. 2007;104:11736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holland EC, Li Y, Celestino J, Dai C, Schaefer L, Sawaya RA, et al. Astrocytes give rise to oligodendrogliomas and astrocytomas after gene transfer of polyoma virus middle T antigen in vivo. Am J Pathol. 2000;157:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phillips LM, Zhou X, Cogdell DE, Chua CY, Huisinga A, Hess KR, et al. Glioma progression is mediated by an addiction to aberrant IGFBP2 expression and can be blocked using anti-IGFBP2 strategies. J Pathol. 2016;239:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moorehead RA, Hojilla CV, De Belle I, Wood GA, Fata JE, Adamson ED, et al. Insulin-like growth factor-II regulates PTEN expression in the mammary gland. J Biol Chem. 2003;278:50422–7. [DOI] [PubMed] [Google Scholar]
- 129.Sehgal P, Kumar N, Praveen KVR, Patil S, Bhattacharya A, Vijaya KM, et al. Regulation of protumorigenic pathways by insulin like growth factor binding protein2 and its association along with β-catenin in breast cancer lymph node metastasis. Mol Cancer. 2013;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miyake H, Pollak M, Gleave ME. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res. 2000;60:3058–64. [PubMed] [Google Scholar]
- 131.Neuhouser ML, Platz EA, Till C, Tangen CM, Goodman PJ, Kristal A, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Prev Res. 2013;6:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen R, Pan S, Yi EC, Donohoe S, Bronner MP, Potter JD, et al. Quantitative proteomic profiling of pancreatic cancer juice. Proteomics. 2006;6:3871–3879. [DOI] [PubMed] [Google Scholar]
- 133.Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, Lane Z, et al. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteom. 2007;6:1331–1342. [DOI] [PubMed] [Google Scholar]
- 134.Gao S, Sun Y, Zhang X, Hu L, Liu Y, Chua CY, et al. IGFBP2 activates the NF-κB pathway to drive epithelial-mesenchymal transition and invasive character in pancreatic ductal adeno-carcinoma. Cancer Res. 2016;76:6543–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Uzoh CC, Holly JM, Biernacka KM, Persad RA, Bahl A, Gillatt D, et al. Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br J Cancer. 2011;104:1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. [DOI] [PubMed] [Google Scholar]
- 138.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. [DOI] [PubMed] [Google Scholar]
- 139.Levitt RJ, Georgescu MM, Pollak M. PTEN-induction in U251 glioma cells decreases the expression of insulin-like growth factor binding protein-2. Biochem Biophys Res Commun. 2005;336:1056–61. [DOI] [PubMed] [Google Scholar]
- 140.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. [DOI] [PubMed] [Google Scholar]
- 141.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002; 2:301–10. [DOI] [PubMed] [Google Scholar]
- 142.Hoeflich A, Reisinger R, Schuett BS, Elmlinger MW, Russo VC, Vargas GA, et al. Peri/nuclear localization of intact insulin-like growth factor binding protein-2 and a distinct carboxyl-terminal IGFBP-2 fragment in vivo. Biochem Biophys Res Commun. 2004;324:705–10. [DOI] [PubMed] [Google Scholar]
- 143.Azar WJ, Azar SH, Higgins S, Hu JF, Hoffman AR, Newgreen DF, et al. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology. 2011;152:3332–42. [DOI] [PubMed] [Google Scholar]
- 144.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–94. [DOI] [PubMed] [Google Scholar]
- 145.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nat Rev Cancer. 2007;7:415–28. [DOI] [PubMed] [Google Scholar]
- 146.Myers AL, Lin L, Nancarrow DJ, Wang Z, Ferrer-Torres D, Thomas DG, et al. IGFBP2 modulates the chemoresistant phenotype in esophageal adenocarcinoma. Oncotarget. 2015;6:25897–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 148.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–4. [DOI] [PubMed] [Google Scholar]
- 149.Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–25. [PubMed] [Google Scholar]
- 150.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, Li F, Yang YT, Xu XD, Chen JS, Chen TL. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene. 2018;11:1815–31. [DOI] [PubMed] [Google Scholar]
- 152.Laffaire J, Everhard S, Idbaih A, Crinière E, Marie Y, de Reyniès A, et al. Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro Oncol. 2011;13:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng S, Houseman EA, Morrison Z, Wrensch MR, Patoka JS, Ramos C, et al. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol. 2011;13:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huynh H, Iizuka S, Kaba M, Kirak O, Zheng J, Lodish HF, et al. Insulin-like growth factor-binding protein 2 secreted by a tumorigenic cell line supports ex vivo expansion of mouse hematopoietic stem cells. Stem Cells. 2008;26:1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/ SCID transplantation. Blood. 2008;111:3415–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Villani RM, Adolphe C, Palmer J, Waters MJ, Wainwright BJ. Patched1 inhibits epidermal progenitor cell expansion and basal cell carcinoma formation by limiting Igfbp2 activity. Cancer Prev Res. 2010;3:1222–1234. [DOI] [PubMed] [Google Scholar]
- 159.Hsieh D, Hsieh A, Stea B, Ellsworth R. IGFBP2 promotes glioma tumor stem cell expansion and survival. Biochem Biophys Res Commun. 2010;397:367–372. [DOI] [PubMed] [Google Scholar]
- 160.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31:326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chesik D, De Keyser J, Wilczak N. Involvement of insulin-like growth factor binding protein-2 in activated microglia as assessed in post mortem human brain. Neurosci Lett. 2004;362:14–6. [DOI] [PubMed] [Google Scholar]
- 163.Elmlinger MW, Deininger MH, Schuett BS, Meyermann R, Duffner F, Grote EH, et al. In vivo expression of insulin-like growth factor-binding protein-2 in human gliomas increases with the tumor grade. Endocrinology. 2001;142:1652–8. [DOI] [PubMed] [Google Scholar]
- 164.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 165.Patil SS, Railkar R, Swain M, Atreya HS, Dighe RR, Kondaiah P. Novel anti IGFBP2 single chain variable fragment inhibits glioma cell migration and invasion. J Neurooncol. 2015; 123:225–35. [DOI] [PubMed] [Google Scholar]
- 166.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res. 2008; 68:8400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cecil DL, Holt GE, Park KH, Gad E, Rastetter L, Childs J, et al. Elimination of IL-10-inducing T-helper epitopes from an IGFBP-2 vaccine ensures potent antitumor activity. Cancer Res. 2014;74:2710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakano H, Miyazawa T, Kinoshita K, Yamada Y, Yoshida T. Functional screening identifies a microRNA, miR-491 that induces apoptosis by targeting Bcl-X(L) in colorectal cancer cells. Int J Cancer. 2010;127:1072–80. [DOI] [PubMed] [Google Scholar]
- 169.Zhao Y, Qi X, Chen J, Wei W, Yu C, Yan H, et al. The miR-491–3p/Sp3/ABCB1 axis attenuates multidrug resistance of hepatocellular carcinoma. Cancer Lett. 2017;408:102–11. [DOI] [PubMed] [Google Scholar]
- 170.Li X, Liu Y, Granberg KJ, Wang Q, Moore LM, Ji P, et al. Two mature products of MIR-491 coordinate to suppress key cancer hallmarks in glioblastoma. Oncogene. 2015;34:1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]


