Abstract
Background
Chronotropic incompetence (CI) is common in heart failure with preserved ejection fraction (HFpEF) and is associated with impaired aerobic capacity. We investigated the integrity of cardiac β–receptor responsiveness, an important mechanism involved in exertional increases in HR, in HFpEF and control subjects.
Methods
Thirteen carefully screened HFpEF patients and 13 senior controls underwent exercise testing and graded isoproterenol infusion (ISO) to quantify cardiac β–receptor mediated HR responses. To limit autonomic neural influences on HR during ISO, dexmedetomidine and glycopyrrolate were given. ISO doses were increased incrementally until HR increased by 30 bpm. Plasma levels of ISO at each increment were measured by liquid chromatography with electrochemical detection and plotted against HR.
Results
Peak VO2 and HR (117 ± 15 vs 156 ± 15 bpm; p<0.001) were lower in HFpEF than senior controls. Cardiac beta-receptor sensitivity was lower in HFpEF compared to controls (0.156 ± 0.133 vs 0.254 ± 0.166 bpm/[ISO ng/ml]; p<0.001). Seven of 13 HFpEF subjects had β-receptor sensitivity similar to senior controls but still had lower peak HRs (122 ± 14 vs 156 ± 15 bpm; p<0.001).
Conclusions
Contrary to our hypothesis, HFpEF patients displayed impaired cardiac β–receptor sensitivity compared to senior controls. In the 7 out of 13 HFpEF patients with age-appropriate β–receptor sensitivity, peak HR remained low suggesting impaired sinus node β-receptor function may not fully account for low exercise HR response. Rather in some HFpEF patients, CI might reflect premature cessation of exercise prior to maximal sinus node activation.
Clinical Trial Registration
ClinicalTrials.gov; Unique Identifier: NCT02524145
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for nearly 50% of heart failure diagnoses.1 One of the hallmarks of HFpEF is severe exercise intolerance, with peak VO2 decreased to values similar to those with heart failure with reduced ejection fraction (HFrEF). Chronotropic incompetence, an inability to increase heart rate, may be a factor in exercise intolerance by limiting increases in cardiac output. While the definition varies, approximately 30–50% of HFpEF patients are thought to have chronotropic incompetence manifested by a lower than predicted maximal HR during symptom limited exercise.2–4
Because increases in heart rate constitute an important component of the cardiovascular response to exercise, several studies have linked chronotropic incompetence with low peak VO2 in HFpEF and even advocated implantation of rate-responsive pacemakers to improve exercise tolerance.5 In addition to lower peak VO2, decreased heart rate reserve is an important prognostic marker and has been associated with increased risk of adverse clinical events.6
The mechanisms responsible for impaired chronotropic responsiveness in HFpEF remain unclear. It is difficult to differentiate the relationship between lack of heart rate response and poor exercise tolerance and whether there is truly a functional limitation to further sinus node stimulation. Alternatively, patients may stop exercising prematurely prior to maximal skeletal muscle activation due to excessive dyspnea and fatigue, driven primarily by rapid increases in pulmonary pressures from a stiffened left ventricle, in which case sinus node function should be preserved. We therefore tested sinus node function in HFpEF patients and senior controls by measuring intrinsic heart rate and heart rate responses to beta-adrenoceptor stimulation. We hypothesized that there would be no differences between groups and that despite lower peak exercise heart rate, HFpEF patients would have similar intrinsic heart rates and β-receptor responsiveness to graded isoproterenol (ISO) infusion.
Methods
The authors will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher upon reasonable request for purposes of reproducing the results or replicating the procedure.
Study recruitment
HFpEF patients were recruited from a university cardiology clinic. The Institutional Review Boards (IRB) of the University of Texas Southwestern Medical Center and Texas Health Resources approved all study procedures. This trial was registered on ClinicalTrials.gov ( NCT02524145) and was overseen by an independent data safety and monitoring board. All the subjects gave written informed consent to IRB-approved protocols before participating in research procedures. Subjects were invited to participate if they: 1) were older than 60 years of age; 2) had been hospitalized previously for heart failure; 3) had evidence of pulmonary congestion by chest x-ray or elevated cardiac filling pressures (pulmonary capillary wedge or left ventricular end-diastolic pressures > 16 mmHg); and 4) LV ejection fraction > 50%. HFpEF subjects were excluded for body mass index > 40 kg/m2, eGFR < 30 ml/min/m2, severe chronic obstructive pulmonary disease (COPD), chronic atrial fibrillation, constrictive or restrictive cardiomyopathy, severe valvular disease or history of valvular surgery, if they were unable to perform exercise testing.
Control subjects were recruited from the Dallas Heart Study, a population-based cohort of over 6,000 individuals, enriched by a random sampling of employees of Texas Health Resources, a large healthcare provider in the Dallas-Fort Worth metroplex, as previously described. 7 Control subjects were excluded if they had a history of hypertension or elevated 24-hour ambulatory blood pressure > 140/90 mmHg (Oscar 2, Suntech Medical), had a history of cardiovascular disease, diabetes, COPD, former or current cigarette smokers or had a body mass index (BMI) > 30 kg/m2. HFpEF and controls underwent screening maximal exercise stress echocardiography prior to enrollment and were excluded if they had evidence of coronary ischemia by ECG and echocardiography. AV-nodal agents (e.g. non-dihydropyridine calcium channel blockers, beta-adrenoceptor blockers) were held for at least five half-lives prior to testing days. A small group of young, healthy, recreationally active controls were recruited to define normal chronotropic responses to isoproterenol infusion. These young controls did not undergo maximal exercise testing.
Exercise Testing
A modified Astrand-Saltin incremental treadmill protocol was used to determine peak exercise capacity and peak exercise heart rate. Both HFpEF and senior controls performed two lower intensity exercise workloads to quantify VO2 and heart rate at sub-maximal exercise. Measures of ventilatory gas exchange were made by use of the Douglas bag technique.8 Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100), and ventilatory volume was measured by a Tissot spirometer. Maximum oxygen uptake was defined as the highest oxygen uptake measured from at least a 30 second Douglas bag. Cardiac output was measured using a modified acetylene gas re-breathing technique.9
Sinus node function
Sinus node function was quantified by the stimulation of cardiac β-receptors with increasing doses of intravenous ISO and measured as resultant increases in heart rate to directly measured ISO concentrations in plasma. All subjects were tested in a fasted state in the morning to limit diurnal influence on resting heart rate. To limit neural influences on sinus node function during ISO infusion, either from reflex tachycardia from potential vasodilation due to β−2 stimulation or activation of arterial baroreceptors due to increased stroke volume, dexmedetomidine (a central α−2 adrenoceptor agonist) followed by glycopyrrolate (a muscarinic cholinergic antagonist) were used to “denervate” the sinus node from extra-cardiac autonomic influence.10 Infusions were administered through a triple lumen catheter placed in an antecubital or brachial vein. Heart rate (ECG) and finger cuff blood pressure (Nexfin, BMEye, Amsterdam, The Netherlands) were monitored continuously. An anesthesiologist was present during drug infusion to monitor for excessive sedation from dexmedetomidine. Oxygen saturations, respiratory rate, and end-tidal CO2 levels were monitored continuously. A baseline blood sample was drawn after 20 minutes of supine rest prior to administration of any drugs.
Dexmedetomidine was given as a bolus (0.225 μg/kg) intravenously followed by an infusion at 0.5–0.7 μg/kg/hr for 20 minutes. The effectiveness of blockade of reflexive sympathetic noradrenergic outflows was determined by abolition of the late phase IIb recovery blood pressure during the Valsalva maneuver (30 mmHg for 20 seconds). Glycopyrrolate was then administered intravenously as a bolus (10 μg/kg) followed by a maintenance infusion at 7 μg/kg/hr. The effectiveness of muscarinic blockade was confirmed by a blunting of baroreflex-cardiovagal increases in heart rate <10 bpm) during phase IIb through the release of the Valsalva maneuver. The persistence of cardiac autonomic blockade during ISO infusion was confirmed by measuring plasma norepinephrine levels at each stage of the infusion.
After inhibiting cardiac autonomic reflexes, ISO was infused intravenously continuously, with dosage increased every 6 minutes until heart rate increased by 30 beats per minute above the heart rate for each subject post-autonomic inhibition. The ISO infusion began at 3.5 ng/kg/min and was then increased to 7, 14, and up 35 ng/kg/min until the heart rate target was met.11 Subjects were monitored for 15 minutes after all study drugs were stopped to ensure heart rate and blood pressure returned to baseline. ISO was dosed to ideal body weight12 to avoid large differences in dosing due to differences in body mass between groups.
At the end of each ISO infusion stage, blood was drawn from an antecubital vein on the arm opposite to the one used for infusion of the study drugs. Samples were centrifuged and plasma (frozen) sent to the National Institutes of Health for assays of plasma catecholamines as described previously.13 Plasma ISO levels were plotted against HR and the slope of this relationship was used to determine sinus node function and β-receptor responsiveness.
Statistical Analysis
Statistical analysis was performed using commercially available software (SAS version 9.2, SAS Institute). All reported variables are presented as means with standard deviations unless otherwise noted. Analysis assumptions were carefully evaluated, and results were robust to analyses with square root, rank, or log transformations. However, results were similar to untransformed analysis, therefore results based on raw data analysis are reported. A p value less than 0.05 was considered statistically significant. ANOVA was used to compare group differences between HFpEF, senior controls, and young healthy volunteer subjects. Because the peak ISO infusion rate was based on the individual’s heart rate response, Fisher’s exact test was used to determine group differences in achieving the peak ISO dose of 35 ng/kg/min. Continuous end points from baseline to the 14 ng/kg/min dose were compared between groups by using mixed-effects model repeated-measures analysis. The repeated-measures models included the intervention group factor (control versus HFpEF), plasma ISO level, and a group×ISO level interaction term.
Results
Subjects characteristics and exercise testing results
Baseline demographic data for HFpEF patients and senior control subjects are listed in table 1. HFpEF patients had lower tissue Doppler relaxation velocities (e’ 5.7 ± 1.5 vs 7.8 ± 1.3 cm/s; p=−0.001) and higher E/e’ ratios (14.2 ± 3.8 vs 8.9 ± 3.2; p=−0.008). There were no differences in E/A ratio. (Supplemental table 1) Approximately half of the HFpEF patients were diabetic. There were no significant differences in age and sex between groups. Peak VO2 was lower in HFpEF than senior controls both in absolute (1.32 ± 0.31 vs 1.73 ± 0.43 L/min; p=0.013) and relative (13.5 ± 2.2 vs 22.9 ± 4.0 ml/kg/min; p<0.001) values. Peak respiratory exchange ratio (RER), blood lactate levels, and maximal ventilation were all significantly higher in senior controls than in HFpEF patients. Respiratory rate at the end of exercise in HFpEF patients was 37 ± 6 breaths/minute suggesting symptom limiting cessation of effort. Peak heart rate was lower in HFpEF by nearly 40 bpm (117 ± 15 vs 156 ± 15; p<0.001). Heart rate response to sub-maximal exercise was also blunted with lower heart rates at similar aerobic power compared to senior controls (Figure 1). Only 5 out of 13 (38%) HFpEF patients achieved a heart rate greater than 80% predicted compared to all 13 out of 13 the senior controls who did. Exercise performance variables were similar to those obtained during screening maximal exercise test to exclude provocable ischemia. (Supplemental table 2) HFpEF patients performed slightly better during the second maximal exercise testing session compared to their screening test with higher peak heart rate (117 vs 108 bpm) and higher VO2 (13.5 vs 12.9 ml/kg/min).
Table 1:
Demographic and Exercise Performance Data
| HFpEF (n=13) | Senior Controls (n=13) | p value | |
|---|---|---|---|
| Age (yr) | 68 ± 6 | 71 ± 4 | 0.13 |
| Women, n (%) | 7 (54%) | 6, (46%) | 1.0 |
| Body Mass Index (kg/m2) | 34.6 ± 4.1 | 27.1 ± 3.9 | <0.001 |
| Weight (kg) | 99.1 ± 17.0 | 77.5 ± 11.7 | <0.001 |
| Diabetes mellitus, n (%) | 7 (54%) | 0 (0%) | <0.001 |
| Medications | |||
| ACEi/ARB, n (%) | 5 (38%) | 0 (0%) | |
| Beta-blocker, n (%) | 12 (92%) | 0 (0%) | |
| Calcium-channel blocker, n (%) | 5 (38%) | 0 (0%) | |
| Loop diuretic, n (%) | 11 (85%) | 0 (0%) | |
| Exercise variables | |||
| Peak heart rate (bpm) | 117 ± 15 | 156 ± 15 | <0.001 |
| % Predicted heart rate | 77 ± 9 | 102 ± 10 | <0.001 |
| Peak stroke volume (ml/m2) | 45.2 ± 8.7 | 42.8 ± 9.1 | 0.49 |
| Peak cardiac output (L/min/ m2) | 5.2 ± 0.9 | 6.8 ± 1.1 | 0.001 |
| Peak VO2 (L/min) | 1.32 ± 0.31 | 1.73 ± 0.43 | 0.013 |
| Peak VO2 (ml/kg/min) | 13.5 ± 2.2 | 22.9 ± 4.0 | <0.001 |
| Peak AVO2 difference (ml/dL) | 11.7 ± 2.0 | 13.7 ± 2.0 | 0.022 |
| Respiratory Exchange Ratio (RER) | 1.03 ± 0.10 | 1.12 ± 0.08 | 0.021 |
| Peak Lactate (mmol/L) | 3.6 ± 1.7 | 5.9 ± 1.8 | 0.007 |
| Peak Ventilation (L/min) | 53.6 ± 12.6 | 73.9 ± 21.5 | 0.012 |
Figure 1:
Differences in heart rate response as a function of increasing metabolic work in HFpEF and control subjects. Heart rate responsiveness to increasing exercise intensities is blunted in patients with HFpEF compared to senior controls. Error bars represent standard error.
Sinus Node Function
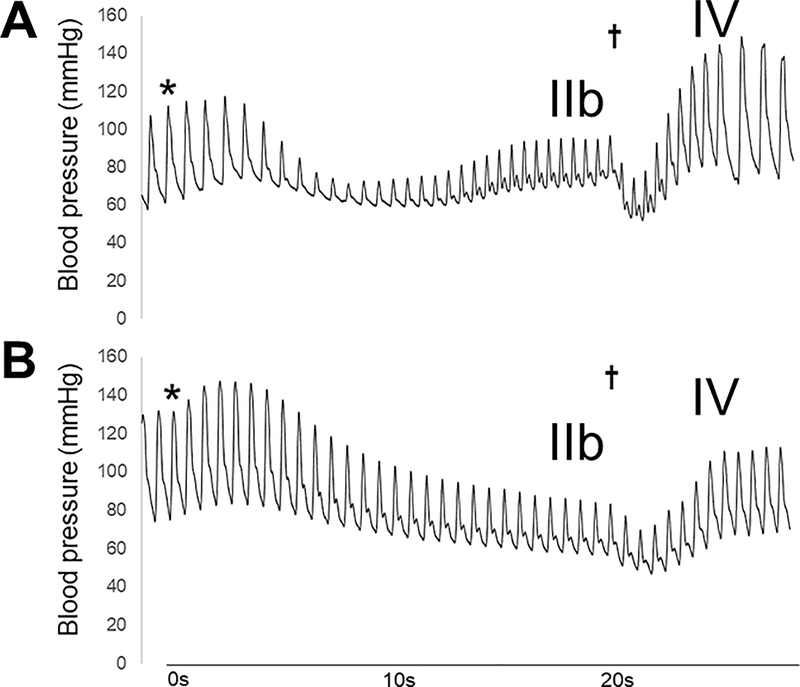
All subjects underwent dexmedetomidine and glycopyrrolate infusion to inhibit cardiac autonomic reflexes prior to ISO infusion. Figures 2 and 3 show the efficacy of sympathetic and parasympathetic blockade. Blood pressure recovery during phase IIb and blood pressure overshoot during phase IV after release of the Valsalva maneuver were both absent, indicating sympathetic inhibition (Figure 2). Heart rate acceleration just prior to release of the maneuver was attenuated, demonstrating inhibition of the reduction in parasympathetic tone normally responsible for cardio-acceleration during the Valsalva maneuver (Figure 3A).
Figure 2:
Representative example of continuous blood pressure associated with the Valsalva maneuver before (A) and after (B) autonomic blockade in the same individual. The asterisks align with start of the Valsalva and the cross denotes release of Valsalva. After autonomic blockade, pressure recovery is abolished during phase IIb with attenuation of phase IV blood pressure overshoot upon release of Valsalva.
Figure 3:
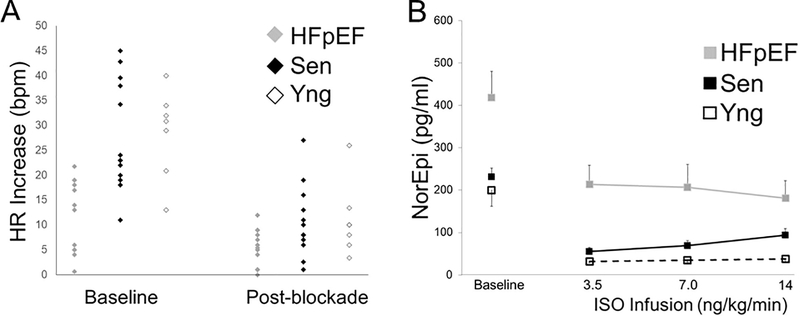
Panel A: Change in heart rate just prior to the onset of Valsalva to phase III release before (baseline) and during autonomic blockade in HFpEF, senior and young controls. After autonomic blockade, all groups demonstrated blunted (<10 bpm) rise in heart rate signifying decreased cardiac parasympathetic activity. Panel B: Plasma norepinephrine (NE) levels during isoproterenol infusion. HFpEF patients had higher baseline NE levels compared to young and senior controls (418 ± 223 vs 231 ± 75 vs 200 ± 101 pg/ml; p = 0.009) but had similar relative drop after blockade (p = 0.74). NE levels remained steady for all groups (p = 0.88) during ISO infusion reflecting stable and persistent withdrawal of sympathetic noradrenergic outflows. Both young and senior controls had NE levels below 100 pg/ml after autonomic blockade, consistent with sympathetic withdrawal. Error bars are standard error of mean.
Baseline NE levels were highest in HFpEF subjects compared to senior and young controls (HFpEF vs senior vs young controls: 418 ± 223 vs 231 ± 75 vs 200 ± 101 pg/ml; p=0.009). After autonomic blockade, both senior and young controls had plasma NE levels below 100 pg/ml, levels similar to patients with pure autonomic failure.14 NE levels in HFpEF post-blockade were higher than either control group but the absolute response to autonomic blockade was not statistically different (group*stage p=0.74; group p<0.001, stage p<0.001). Levels of NE remained stable throughout the ISO infusion (group*stage p=0.88; group p<0.001, stage p=0.99) indicating withdrawal of sympathetic noradrenergic outflows (Figure 3B).
After autonomic blockade, heart rate (75 ± 14 vs 73 ± 9 bpm, p=NS) and blood pressure (MAP 88 ± 15 vs 92 ± 13 mmHg, p=0.46) were similar between the HFpEF and senior controls. At the 3.5, 7.0, and 14 ng/kg/min ISO infusion rates, plasma ISO levels were similar between the HFpEF and age-matched controls (group*stage p=0.12; group p=0.11, stage p<0.001).
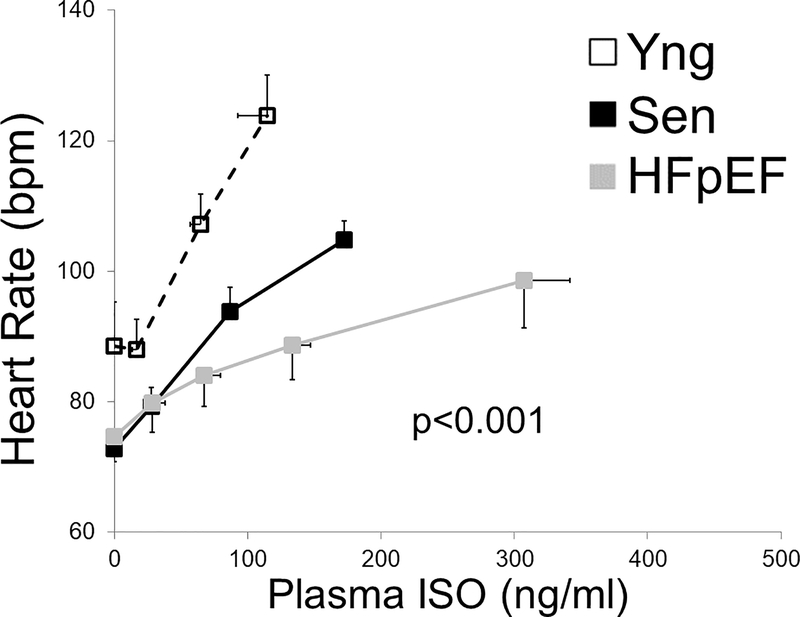
More HFpEF patients than senior controls required escalation of the ISO infusion rate to 35 ng/kg/min (8 out 13 vs 1/13; p=0.01 by Fisher’s exact test) to achieve the pre-specified increase in heart of 30 bpm. Figure 4 shows the heart rate response as a function of plasma ISO concentration. Young subjects had the highest heart rate response to increasing plasma ISO while HFpEF subjects had the lowest (group*ISO levels p<0.001; group p<0.001, ISO levels <0.001). Senior subjects ISO sensitivity was higher than HFpEF but lower than young controls consistent with age related declines in cardiac β-receptor function.15 Four out of the 13 HFpEF subjects (31%) were unable to reach the heart rate target despite reaching the maximal protocol isoproterenol infusion rate of 35 ng/kg/min.
Figure 4:
Change in heart rate as a function of plasma isoproterenol (ISO) levels in HFpEF, senior and young controls. HFpEF subjects had the lowest change in heart rate with increasing levels of plasma isoproterenol (p<0.001). Error bars are standard error of the mean.
Relationship of Sinus Node Dysfunction and Maximal Exercise Heart Rate
The sinus node β-receptor sensitivity was defined as the slope of the heart rate and plasma ISO relationship and used to dichotomize HFpEF subjects as having either blunted or age appropriate sinus β-receptor responsiveness defined as a β-receptor sensitivity greater than the lowest senior control (Table 2). AV-nodal agent usage was similar in the two groups. As expected by design for this analysis, the heart rate/plasma ISO slopes of the HFpEF subjects with preserved slopes were similar to healthy controls (0.247 ± 0.111 vs 0.254 ± 0.166 bpm/(ng/kg/min); p=0.92). HFpEF patients with blunted β-receptor function had slightly lower peak HR compared to those with preserved β-receptor function (111 ± 16 vs 122 ± 14 bpm; p=0.2) suggesting some contribution of sinus node responsiveness to peak exercise HR in HFpEF patients. In contrast, peak heart rates in HFpEF subjects with preserved β-receptor function were still lower than senior controls (122 ± 14 vs 156 ± 15 bpm; p<0.001). An exploratory analysis was performed to identify factors associated with impaired sinus node function (Table 3). Although not reaching statistical significance, the strongest predictors of sinus node β-receptor dysfunction among HFpEF subjects were baseline plasma NE levels (standardized beta −0.39, p=0.18) and peak exercise heart rate (standardized beta 0.36, p=0.22).
Table 2:
Clinical Characteristics by Beta-Receptor Function in HFpEF
| Blunted | Preserved | p value | |
|---|---|---|---|
| Subjects, n | 6 | 7 | |
| Sinus β-receptor sensitivity (bpm/(ngISO/kg/min)) | 0.050 ± 0.050 | 0.247 ± 0.111 | |
| Age (years) | 68 ± 6 | 67 ± 6 | 0.77 |
| Women, n (%) | 3 (50%) | 4 (57%) | 0.59 |
| Diabetes mellitus, n (%) | 4 (67%) | 3 (43%) | 0.43 |
| Norepinephrine Baseline | 488 ± 232 | 359 ± 214 | 0.32 |
| Peak Heart Rate (bpm) | 111 ± 16 | 122 ± 14 | 0.20 |
| % Predicted heart rate | 75 ± 13 | 80 ± 8 | 0.41 |
| Peak VO2 (ml/min/kg) | 13.7 ± 2.4 | 13.3 ± 2.3 | 0.77 |
| Peak Stroke Volume (ml) | 100 ± 18 | 94 ± 19 | 0.59 |
| Peak AVO2 difference (%) | 11.6 ± 2.8 | 11.8 ± 1.4 | 0.87 |
| Peak RER | 1.06 ± 0.08 | 1.01 ± 0.11 | 0.34 |
| Peak lactate (mmol/L) | 3.4 ± 1.1 | 3.7 ± 2.0 | 0.75 |
Group characteristics in HFpEF subjects with blunted or preserved sinus node function.
Table 3:
Univariate Predictors of β-receptor Sensitivity in HFpEF
| Standardized Beta Coefficient | p value | |
|---|---|---|
| Norepinephrine Baseline | −0.39 | 0.18 |
| Peak Heart Rate (bpm) | 0.36 | 0.22 |
| Diabetes | 0..27 | 0.38 |
| Peak VO2 | −0.051 | 0.88 |
Discussion
The main findings in this study were that, contrary to our hypothesis HFpEF patients had blunted heart rate responses to increasing plasma ISO concentrations indicating decreased sinus node β-adrenoceptor responsiveness. Approximately half of HFpEF patients though, had relatively preserved sinus node function but still had blunted increases in exercise heart rate, suggesting that impaired sinus node β-receptor function may not fully account for the low peak exercise heart rate response. Our findings provide new evidence regarding the mechanisms involved in chronotropic incompetence in HFpEF and the role for apparent sinus node dysfunction.
Exercise intolerance is a common symptom in patients with HFpEF. While several altered hemodynamic parameters are manifest with exercise, a pooled meta-analysis of HFpEF exercise studies found the strongest associations with lower peak VO2 were reduced chronotropic reserve and exaggerated increase in pulmonary capillary wedge pressure.16 In healthy subjects, heart rate increases nearly 2–3 fold with maximal exercise, which is largely responsible for the 3–4 fold increase in cardiac output. Because of the large contribution of increasing heart rate to increased cardiac output during exercise, impairments in heart rate reserve can lead to lower peak VO2 and exertional intolerance. Two clinical trials have attempted to address whether increases in exercise heart rate via rate-adaptive pacing improves peak VO2 and functional capacity in HFpEF. The first trial – RESET5, was stopped early due to low enrollment while the second – RAPID-HF NCT02145351 is ongoing.
It is not clear whether the blunted increase in HR in HFpEF patients is the cause of the reduced exercise intolerance, or rather secondary to premature cessation of exercise for other reasons. The etiology and mechanisms underlying chronotropic incompetence in HFpEF are not well understood. Heart rate responses to exercise depend on autonomic outflows (“central command”), reflex responses to skeletal muscle activation (the “exercise pressor reflex”), systemic hemodynamic changes, sinus node function, parasympathetic withdrawal and beta-adrenoceptor responsiveness. Pre-clinical models suggest central command may be heightened in models of heart failure and hypertension, which may lead to heightened exercise pressor responses.17, 18 Few studies have assessed these pathways in HFpEF. Our study provides new insight into sinus node function and adrenoreceptor responsiveness in HFpEF and their contribution to chronotropic incompetence.
Sinus node function
Intrinsic heart rate, or the heart rate free of autonomic influences was identical between the HFpEF and age equivalent senior control groups. Resting intrinsic sinus node function therefore seems to be unaffected in HFpEF. This finding is in contrast to HFrEF where previous studies suggest changes in sinus node morphology in addition to prolongation of the intrinsic sinus cycle lengths.19
β-receptor sensitivity
Previous studies utilizing 123I-metaiodobenzylguanidine (123I-MIBG) cardiac neuroimaging have shown correlations between faster “washout” rates and lower peak exercise heart rate.20 Faster 123I-MIBG washout has also been correlated with worsened diastolic function and NYHA functional status.21 These results suggest that sympathetic signaling to the myocardium is not only preserved but heightened and suggests β-receptor desensitization rather than autonomic failure is the primary culprit for decreased heart rate reserve. These physiologic changes mirror those observed in patients with HFrEF where chronic elevations in circulating NE levels lead to down regulation of β-receptor concentrations and function.22 Our finding that HFpEF patients with blunted β-receptor sensitivity have higher plasma NE levels is consistent with this concept. Patients with blunted sensitivity achieved slightly lower peak HR compared to patients with preserved receptor sensitivity suggesting impaired sinus node responsiveness may account for some degree of lower peak HR in these HFpEF patients. The presence of preserved sinus node responsiveness however did not alleviate chronotropic incompetence. Peak HR in patients with preserved receptor function were still lower than controls by ~ 35 bpm.
In the context of normal intrinsic heart rates and nearly half of HFpEF subjects having age appropriate β-receptor sensitivities despite low peak exercise heart rates, our findings raise the possibility of an alternative explanation for apparent chronotropic incompetence – that patients stop exercise prior to reaching maximal metabolic work. Ventilatory and metabolic data from the exercise testing supports this possibility that HFpEF patients stop exercising prematurely prior to maximal activation of exercising skeletal muscle mass for reasons other than a true limitation of cardiac output. Peak minute ventilation,and lactate were significantly lower in HFpEF compared to senior controls, findings similar to other reports of HFpEF exercise studies.23, 24 In addition, HFpEF patients achieved a peak AVO2 extraction that was approximately 15% lower than controls, suggesting premature cessation of exercise and effort prior to maximal skeletal muscle activation. While these parameters are effort-dependent, all subjects were pushed to maximal, exhaustive effort on two separate days with similar cardiopulmonary testing results. Although RER was lower than controls, RER is an unreliable marker of peak effort and can vary widely at maximal exercise.25 Other markers of symptom limiting exercise (e.g. peak respiratory rate) were elevated.
Although we did not perform right heart catheterization during exercise in the current study population, one possible mechanism leading to a premature termination of effort is a rapidly rising pulmonary capillary wedge pressure (PCWP). If PCWP could be acutely lowered with a reduction in the sensation of extreme dyspnea, HFpEF patients might be able to exercise to higher intensities and achieve higher peak heart rates. To date, no trial has tested whether lowering cardiac filling pressures leads to improved exercise performance and peak VO2. Studies using inhaled nitrite have shown reductions in PCWP but did not test whether patients could achieve higher exercise intensities.26
Study strengths and limitations
The main strength of the study was measuring plasma ISO levels rather than relying on infusion rates. This approach minimized individual differences in plasma ISO concentrations and “overdosing” HFpEF subjects due to differences in body mass. Furthermore, the establishment of autonomic blockade ensured that the responses to ISO were a direct function of β-receptor function, and not masked by autonomic reflex adjustments. The primary limitation of this study reflects the rigorous selection criteria for HFpEF and excluded conditions that could potentiate chronotropic incompetence, namely chronic kidney disease severity greater than stage 4 and individuals with pre-existing conduction disease (e.g. LBBB). Although we studied a relatively small number of subjects, the differences we observed between HFpEF and senior controls in measures of chronotropic incompetence and β-receptor sensitivity were robust and highly statistically significant. During autonomic blockade, HFpEF patients had persistently elevated NE levels and may not have had effective suppression of noradrenergic outflows. Inadequate blockade would bias towards the null hypothesis with higher adrenergic outflow causing increased heart rate response from isoproterenol induced vasodilation. Lastly, we were underpowered to identify determinants of reduced β-receptor sensitivity within HFpEF subjects and these findings should be interpreted with caution.
Conclusion
Cardiac β–receptor responsiveness in HFpEF patients is impaired compared to senior controls while intrinsic heart rate is normal. Despite this group difference, about half of HFpEF subjects have normal β–receptor sensitivity, yet peak exercise heart rate remained blunted suggesting that impaired sinus node β-receptor function does not account for low peak exercise heart rate response. Rather, premature cessation of exercise and not deficient β-receptor responsiveness may account for lower peak heart rate. Future studies targeting factors responsible for premature cessation of exercise, specifically lowering cardiac filling pressures, may reduce apparent chronotropic incompetence and improve exercise tolerance.
Supplementary Material
What is new?
Exercise intolerance and low peak VO2 are common in patients with heart failure with preserved ejection fraction and thought to be due to chronotropic incompetence – an inability to increase heart rate to match increases in metabolic stress. We investigated the integrity of cardiac β–receptor responsiveness, an important mechanism involved in exertional increases in HR. HFpEF patients had reduced cardiac β–receptor responsiveness compared to controls suggesting impaired β–receptor function may contribute to chronotropic incompetence in some HFpEF patients.
What are the clinical implications?
Chronotropic incompetence is common in HFpEF. It is not clear whether the blunted increase in HR in HFpEF patients is the cause of the reduced exercise intolerance, or rather secondary to premature cessation of exercise for other reasons. Our study findings suggest some HFpEF patients have reduced β–receptor responsiveness which may contribute to lower peak exercise heart rate. Not all HFpEF displayed abnormal β–receptor function raising the possibility that chronotropic incompetence may not reflect sinus node dysfunction per se but rather alternative causes for exercise intolerance.
Acknowledgments
We would like to thank Valeant Pharmaceuticals for providing isoproterenol free of cost.
Sources of Funding
This project was supported by the National Institutes of Health (Grant RO1 AG17479). The research reported here was supported in part by the Division of Intramural Research, National Institute of Neurological Disorders and Stroke.
Abbreviations
- HFpEF
heart failure with preserved ejection fraction
- ISO
isoproterenol
Footnotes
Disclosures
The authors have no conflicts to disclose.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL and Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 2.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I and Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circulation Heart failure. 2010;3:29–34. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC and Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass DA, Kitzman DW and Alvarez GE. The restoration of chronotropic competence in heart failure patients with normal ejection fraction (RESET) study: rationale and design. J Card Fail. 2010;16:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh S, Shishido T, Kutsuzawa D, Arimoto T, Netsu S, Funayama A, Ishino M, Niizeki T, Nishiyama S, Takahashi H, Miyashita T, Miyamoto T, Nitobe J, Watanabe T and Kubota I. Iodine-123-metaiodobenzylguanidine imaging can predict future cardiac events in heart failure patients with preserved ejection fraction. Ann Nucl Med. 2010;24:679–86. [DOI] [PubMed] [Google Scholar]
- 7.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM and Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 8.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D and Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–805. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG and Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol. 2007;103:867–74. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins BW, Hesse C, Sviggum HP, Nicholson WT, Moyer TP, Joyner MJ and Eisenach JH. Alternative to ganglionic blockade with anticholinergic and alpha-2 receptor agents. Clin Auton Res. 2007;17:77–84. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Zimlichman R, Stull R and Keiser HR. Plasma catecholamine and hemodynamic responses during isoproterenol infusions in humans. Clin Pharmacol Ther. 1986;40:233–8. [DOI] [PubMed] [Google Scholar]
- 12.Riddle W YM, Remmers JE, deGroot W. Graphical analysis of patient performance in the pulmonary function laboratory. Proc Annu Symp Comput Appl Med Care. 1980;1:282–290. [Google Scholar]
- 13.Holmes C, Eisenhofer G and Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–8. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D and Autonomic Disorders C. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol. 2017;81:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D and Bonaduce D. beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J and Berry JD. Relative Impairments in Hemodynamic Exercise Reserve Parameters in Heart Failure With Preserved Ejection Fraction: A Study-Level Pooled Analysis. JACC Heart failure. 2018;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koba S, Xing J, Sinoway LI and Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. American journal of physiology Heart and circulatory physiology. 2008;294:H311–21. [DOI] [PubMed] [Google Scholar]
- 18.Liang N, Mitchell JH, Smith SA and Mizuno M. Exaggerated sympathetic and cardiovascular responses to stimulation of the mesencephalic locomotor region in spontaneously hypertensive rats. American journal of physiology Heart and circulatory physiology. 2016;310:H123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders P, Kistler PM, Morton JB, Spence SJ and Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. [DOI] [PubMed] [Google Scholar]
- 20.Messias LR, Messias AC, de Miranda SM, Wiefels CC, Ferreira AG, Santos LM, Teixeira JA, Marostica E and Mesquita CT. Abnormal adrenergic activation is the major determinant of reduced functional capacity in heart failure with preserved ejection fraction. Int J Cardiol. 2016;203:900–2. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura M, Yamamoto K, Takeda Y, Takeda Y, Dohmori T, Ogata M, Kondo H, Suzuki S and Fukutomi T. The relationship between variables of 123-I-metaiodobenzylguanidine cardiac imaging and clinical status of the patients with diastolic heart failure. Int J Cardiol. 2006;113:223–8. [DOI] [PubMed] [Google Scholar]
- 22.Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF and Hartley LH. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–23. [DOI] [PubMed] [Google Scholar]
- 23.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS and Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circulation Heart failure. 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD and Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole DC, Wilkerson DP and Jones AM. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. European journal of applied physiology. 2008;102:403–10. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Melenovsky V and Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circulation research. 2016;119:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.