To the Editor:
Metastatic solid tumors remain challenging to diagnose4 and cure.1–3 We present the case of a child with an undifferentiated, metastatic malignancy showing rhabdoid and neuroendocrine features exhibiting a partial response to carboplatin, etoposide phosphate and Atezolizumab.
A 7 year old male presented with a large retroperitoneal mass with liver metastases and gastric outlet obstruction. Pathology revealed rhabdoid histology with uniform cells, focal nuclear pleomorphism, and large central nucleoli though expression of INI-1 and BRG1 were retained, making a diagnosis of malignant rhabdoid tumor unlikely.5 The tumor expressed epithelial and mesenchymal markers including diffuse positivity for cytokeratin Cam 5.2, and focal staining for cytokeratin AE1/AE3, pancytokeratin, and vimentin. Patchy positive staining for synaptophysin and chromogranin implied features of neuroendocrine differentiation.6 Beta-catenin staining was membranous only excluding a primary liver tumor and negative trypsin staining indicated an unlikely origin from pancreatic acinar cells. Staining was negative for desmin, MyoD1, S100-protein, ALK-1, SMA, CD134, CD117, CD30, Arginase, WT1, SOX10, OCT3/4, Myf-4, EMA, PGP 9.5, alpha-fetoprotein, and alpha-1-antitrypsin excluding most sarcomas and primary liver tumors. FISH was negative for CIC, NUT and EWS rearrangement. A diagnosis of an undifferentiated high-grade neoplasm with rhabdoid and focal neuroendocrine features was rendered.
Based on the retroperitoneal primary as well as tumor imaging, there was suspicion for pancreatic origin, and we decided to treat as a pancreatic carcinoma with rhabdoid phenotype which has been previously described, with similar histopathology.7–9 A pediatric protocol was developed based on National Comprehensive Cancer Network guidelines as well as several studies in adults reporting good response rates to platinum-based agents combined with etoposide.10–13 Chemotherapy was administered in four 21-day cycles and included etoposide phosphate (100mg/m2) given on days 1–3 and carboplatin (5 Area Under the Curve) on day 1. The tumor expressed PD-L1, so a PD-L1 antibody, Atezolizumab, was given on the first day of each cycle (15mg/kg).14 Following 4 cycles of chemotherapy, the patient continued maintenance Atezolizumab every 21 days. Palliative radiation was administered to the primary tumor and liver metastases. The regimen was well tolerated; expected side effects including myelosuppression and electrolyte derangements were observed. The patient also experienced coagulopathy and hyperbilirubemia which were presumed secondary to progressive liver dysfunction rather than a chemotherapy side effect.
Aftertwo cycles of chemotherapy, the gastric outlet obstruction resolved and imaging showed interval decrease in size of the primary tumor (15 × 8cm, previously 16.4 × 10cm) and liver metastases (Fig. 1A and 1B). Following this partial response, the child returned home for nearly two months. As described in the adult literature, this patient had a significant but not sustained response. Five months after initial diagnosis the child’s disease progressed, and an invasive fungal infection precluded further systemic chemotherapy. His overall survival was almost 6 months.
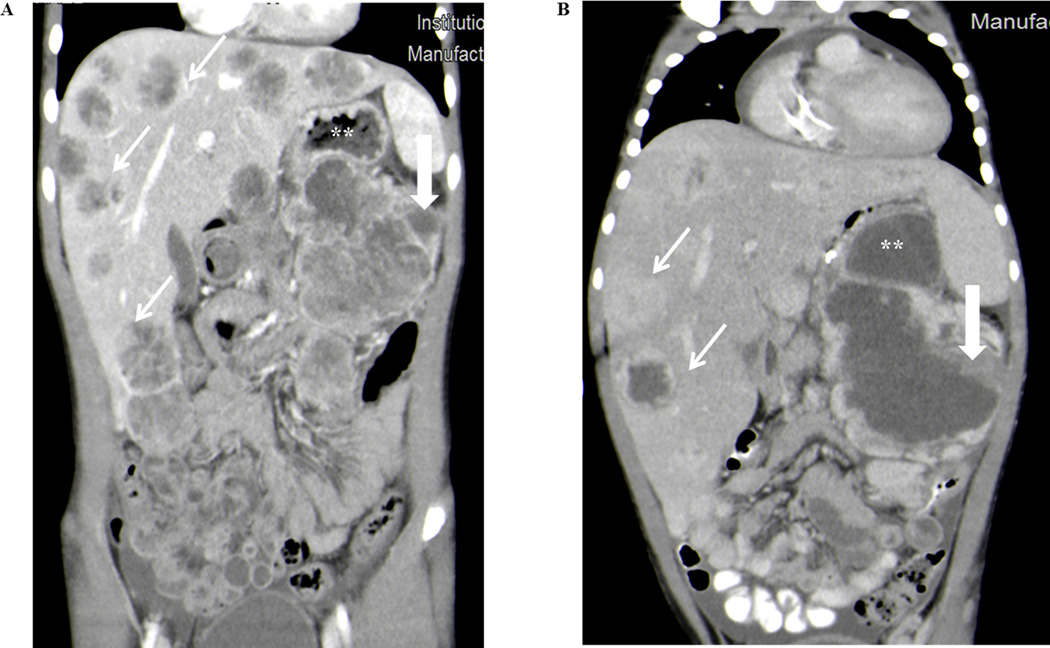
FIGURE 1.
Patient imaging demonstrated a marked tumor response to therapy. (A) CT scan prior to initiation of treatment with multiple liver masses (open white arrows) and large retroperitoneal mass (closed white arrow) compressing stomach (**). (B) CT scan showing marked decrease in tumor burden in both the liver metastases (open white arrows) and retroperitoneal tumor (closed white arrow) following two cycles of chemotherapy with carboplatin, etoposide phosphate and Atezulizumab. The gastric obstruction was also improved (**).
Undifferentiated tumors with rhabdoid phenotype remain challenging to classify and treat. Our experience highlights a rare subset of pancreatic neuroendocrine tumors that display rhabdoid morphology. The treatment regimen described in combination with palliative radiation was well tolerated, resulted in partial disease response and improved quality of life.
ACKNOWLEDGEMENTS
This project was made possible by generous support of National Cancer Institute of the National Institutes of Health under Award Number T32CA183926 (AW) and Supported in part by the National Cancer Institute Award Number K08CA234225 (GW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors would like to thank Emily Waite and Martin Heslin for their contributions to the diagnosis and treatment plan for the patient.
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors acknowledge no conflicts of interest.
REFERENCES
- 1.Ceschel S, Casotto V, Valsecchi MG, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer 2006;47:560–6. [DOI] [PubMed] [Google Scholar]
- 2.Perkins SM, Shinohara ET, DeWees T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One 2014;9:e100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 4.Plotsky C, Jaffe R, Curley J, Blatt J. A retrospective review of undifferentiated malignancy in childhood. Am J Pediatr Hematol Oncol 1990;12:141–6. [DOI] [PubMed] [Google Scholar]
- 5.Hoot AC, Russo P, Judkins AR, Perlman EJ, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol 2004;28:1485–91. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, Chen ZE, Wang HL. Utility of immunohistochemistry in the pancreatobiliary tract. Arch Pathol Lab Med 2015;139:24–38. [DOI] [PubMed] [Google Scholar]
- 7.Agaimy A, Haller F, Frohnauer J, et al. Pancreatic undifferentiated rhabdoid carcinoma: KRAS alterations and SMARCB1 expression status define two subtypes. Mod Pathol 2015;28:248–60. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T, Aishima S, Fujino M, et al. Neuroendocrine tumor of the pancreas with rhabdoid feature. Virchows Arch 2018;473:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Montiel FW, Suster S. Neuroendocrine carcinomas of the pancreas with ‘Rhabdoid’ features. Am J Surg Pathol 2003;27:642–9. [DOI] [PubMed] [Google Scholar]
- 10.Hainsworth JD, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol 2006;24:3548–54. [DOI] [PubMed] [Google Scholar]
- 11.Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227–32. [DOI] [PubMed] [Google Scholar]
- 12.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157–76. [DOI] [PubMed] [Google Scholar]
- 13.Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol 2009;36:52–9. [DOI] [PubMed] [Google Scholar]
- 14.Geoerger B, Karski EE, Zwaan M, et al. A phase I/II study of atezolizumab in pediatric and young adult patients with refractory/relapsed solid tumors (iMATRIX-Atezolizumab). Journal of Clinical Oncology 2017;35:10524-. [Google Scholar]