Abstract
The clinically approved Fondaparinux (Arixtra) has been used for the treatment of deep vein thrombosis (DVT) and acute pulmonary embolism (PE) since 2002, and considered to be better than the low molecular-weight heparin (LMWH) in terms of anticoagulation response, duration of action and biosafety. However, the synthetic methods developed for its manufacture in the past years are relatively complicated thus restricting its extensive use. We reported here a potentially scalable and programmable one-pot synthesis of Fondaparinux using the [1,2,2] strategy and designed thioglycosides with well-defined reactivity as building blocks.
Graphical Abstract

Fondaparinux 1, a synthetic pentasaccharide with the brand name Arixtra, is a heparin-based anti-coagulant which has been used for the treatment of deep vein thrombosis (DVT) and acute pulmonary embolism (PE) since 2002. Two types of heparins, namely, high molecular weight heparin (HMWH) and low molecular weight heparin (LMWH) have been used as injectable anticoagulants which bind to antithrombin III (AT) and exhibit selective inhibition of factor Xa and thrombin in blood clotting cascade.1 However, active monitoring is required for the patients administrated with heparins as serious complications like heparin-induced thrombocytopenia bleeding may occur. The sulfate-containing synthetic pentasaccharide 1 with the sequence d-GlcNS6S-α-(1,4)-d-GlcA-β-(1,4)-d-GlcNS3,6S-α-(1,4)-l-IdoA2S-α-(1,4)-d-GlcNS6S-OMe was identified as AT-binding sequence2 and later was introduced to the market in 2002 with a trade name “Fondaparinux (Arixtra)”(Figure 1).3 Fondaparinux was shown to have faster anticoagulation response, higher and more predictable anti-Xa activity, longer half-life and duration of action, lower risk of heparin-induced thrombocytopenia (HIT), and better biosafety than LMWH, making it a more acceptable anti-coagulant.4 In addition, the contamination in naturally occurring heparins that caused several deaths5 in 2008 led to the increasing clinical use of Fondaparinux as an alternative and perhaps better anti-coagulant.
Figure 1:

Structure of Fondaparinux.
For the treatment of DVT and acute PE, the recommended dose of 1 ranges from 5mg-10mg/daily based on the body weight. However, the high-cost treatment (ranging from $600-1400 in the USA), mainly due to its complicated and high-cost manufacturing process, has limited the availability of Fondaparinux.
Thus, development of an efficient and cost-effective synthesis of 1 is highly desirable to meet the clinical demand. The synthesis of Fondaparinux is very challenging due to the difficulty in the regio- and stereoselective glycosylation between the glucosamine, glucuronic acid and iduronic acid building blocks and the strategic installation of OSO3− and NHSO3− groups. In particular, the 1,2-cis or α-glycosylation between a glucosamine and an uronic acid building block without the formation of the unwanted β-isomer as well as improvement of the overall yield in a shortest possible synthetic route represents a major challenge. In the past years, many groups, including that of Petitou,6 Lin,7 Hung,8 Wang,9 Qin,10 Manikowski,11 and Ding12 have reported the synthesis of Fondaparinux, but the procedures still encounter problems of long stepwise process, non-stereoselective glycosylation and low yield and efficiency. Zhao and co-workers recently reported a pre-activation based iterative one-pot synthesis of Fondaparinux with less than 40% yield.13 We thought the method of programmable one-pot synthesis of oligosaccharides using designed thioglycoside building blocks with defined relative reactivity values (RRVs) developed by us14, 15 could be useful for the practical synthesis of 1.
The concept of RRV is based on the quantitative determination of the reactivity of a thioglycoside donor with methanol as compared to the reactivity of the thioglycoside donor of per-acetyl mannose. RRV is measured using HPLC to determine the amount of leaving group released and the starting donor left in the reaction time course. With the RRV of various thioglycoside building blocks (BBLs) available, one can design a computer software to guide the selection of appropriate BBLs with well-differentiated RRVs for the one-pot assembly of oligosaccharides. We developed the first computer program, “Optimer” in 199914 as a database search tool for the rapid one-pot assembly of large numbers of linear and branched complex oligosaccharides including N-glycans15 and glycosaminoglycans.16 In 2018, we reported an upgraded version of this software, namely, Auto CHO with a library of 150 building blocks (BBLs) with experimentally measured RRVs and 50,000 BBLs with predicted RRVs by machine learning (including those with RRV predicted by chemical shifts by NMR)14 to diversify the applicability of the software for the synthesis of oligosaccharides. To use either “Optimer” or “Auto CHO” software, the user needs to input the desired oligosaccharide structure then the software will generate one or more synthetic routes based on the RRVs of the BBLs needed for the synthesis of the oligosaccharide as output. Once the user chooses a specific synthetic route from the output, BBLs are required to be synthesized in the laboratory and then one-pot synthesis can be performed by sequential addition of BBLs starting from the most reactive from the non-reducing end unit toward the less reactive, least reactive and so on in the reducing end. The one-pot strategy was successfully applied to the synthesis of heparin-like oligosaccharides16a,b and the heparin-based anticoagulant Idraparinux.16c
The building blocks used in this one-pot strategy allow differential removal of the protecting groups for the regioselective introduction of sulfate groups to evaluate their role in biological functions. Following this strategy, we report here an efficient and scalable programmable one-pot synthesis of Fondaparinux 1 using the [1,2,2] strategy and designed thioglycosides (2, 10 and 18) as building blocks.
All the building blocks are readily attainable from commercially available monosaccharides. The synthetic design involves the use of our established programmable one-pot method to conduct highly α-selective glycosylation using TBDPS and Ac groups at O6 and late stage introduction of the acidic functionalities (glucuronic and Iduronic). For the selective installation of the 3-SO3− group, we masked the C3-hydroxyl group (C3-OH) with an orthogonal protecting group, namely, 2-napthyl ether (Nap). The synthesis of 2-azido thioglycoside donor 2 was achieved from D-glucosamine hydrochloride using our previous reported procedure (Supporting Information, Scheme S1).16b The RRVs of the newly synthesized building blocks were measured by HPLC analysis in a competition assay with a reference thioglycoside donor of known RRV (Supporting Information).14,15
The synthesis of disaccharide 10 involved the glycosylation between glycosyl trichloroacetimidate 317 and thioglycoside acceptor 418 in the presence of TMSOTf to generate 5 in 96% yield. Zémplen de-acetylation gave 6 and O-benzylation of 2′,3′-OH led to the formation of 7 in 80% yield. Removal of the 2-Nap protecting group using DDQ19 furnished disaccharide 8 with free hydroxyl group at C3 in 84% yield. Removal of the silyl protecting group under F− source (HF-Py) followed by protection of 3,6-OH as acetyl ester using Ac2O/py led to 9 in 90% yield. Hydrolysis of the 4′,6′-O-benzylidene acetal using 80 % AcOH-H2O produced the crude dihydroxy derivative for the selective oxidation of the primary hydroxyl group to carboxylic acid using TEMPO/BAIB and subsequent esterification with MeI/KHCO3 to give disaccharide acceptor 10 in 58 % yield (Scheme 1).
Scheme 1.


Synthesis of d-Glc-β-(1→4)-d-GlcN3 Disaccharide Building Block
For the synthesis of Ido-GlcN3 disaccharide derivatives (17 & 18), we used commercially available diacetone glucose 11 which was converted to α-l-idopyranoside 12 using known procedures.20 The 2-OH group of 12 was protected both as benzoyl (Bz) and acetyl ester (Ac) to generate 1316b and 14, in 80% and 95% yield, respectively. NIS/TMSOTf-Mediated glycosylation of 13 with α-methyl acceptor 1916b generated disaccharide 15 in 94% yield. The 4′,6′-O-benzylidene acetal was hydrolyzed using 80% AcOH and the crude dihydroxy derivative was treated with TEMPO/BAIB to oxidize the primary hydroxyl group to acid and subsequent esterification of the acid with MeI/KHCO3 generated L-iduronic acid-containing disaccharide acceptor 17 in 58% yield (Scheme 2). It is noted that we have also reported the synthesis of 17 using a different synthetic route.16b
Scheme 2.

Synthesis of l-Ido-α-(1→4)-d-GlcN3 Disaccharide Building Block
We measured the RRVs of glycosyl donors (2 and 10),14,15 and found that the RRV of 2 was 132.0 whereas for 10 it was 2.14. After synthesizing all the required building blocks (2, 10 and 17), we attempted the programmable one-pot synthesis of the protected pentasaccharide 20. However, the yield was only 26%. To improve the yield of the one-pot synthesis, we changed the disaccharide acceptor 17 to 18, in which the 2-OH is protected as acetyl ester. The 2-O-acetyl protected donor 14 was coupled with α-methyl acceptor 19 in the presence of NIS/TMSOTf to generate disaccharide 16 in 95% yield. Hydrolysis of the 4′,6′-O-benzylidene acetal, TEMPO/BAIB oxidation of primary alcohol to acid and subsequent esterification of the acid using MeI/KHCO3 generated 18 (56%) which was then used in the one-pot synthesis of protected Fondaparinux 21 in 50 % yield (Scheme 3). The concept of RRV is based on the measurement of the reactivity of thioglycoside donor. Both 17 & 18 are glycosyl acceptors without leaving group, so the RRV were not measured and assigned to “zero”. Thus, it was difficult to foresee the lower yield (26%) of the pentasaccharide 20 using 2-O-Bz protected disaccharide 17 (Scheme 3) and higher yield of 21 (50%) using 2-OAc containing disaccharide 18.
Scheme 3.

One-pot Synthesis of Protected Fondaparinux
We used the protected pentasaccharides 20 and 21 for differential deprotection and chemical sulfation. The silyl group (TBDPS) in 20 and 21 was removed using HF-Py to generate compounds 22 and 23, in 89% and 91% yield, respectively. Protection group free O-sulfated pentasaccharide 24 was obtained in three steps sequences. Saponification of 22 and 23 using LiOH/H2O221 in the presence of NaOH/MeOH hydrolyzed all ester functional groups. For the installation of the OSO3− groups, it was further treated with excess SO3-Et3N followed by unmasking all O-benzyl groups and reduction of N3 to amine under catalytic hydrogenation with Pd (OH)2/C to give 24 in 82% yield. The selective N-sulfation was performed at pH 9.5 with SO3-Py, and the pH of the reaction was controlled by slow addition of 1M NaOH(aq) from time to time. The crude product was passed through size-exclusion (Sephadex G-25) and ion-exchange (Dowex 50WX8Na+) columns to furnish Fondaparinux 1 (Scheme 4). The reported NMR and mass spectrometry data are well matched with the reported.8 All newly synthesized derivatives were characterized by 1H, 13C NMR spectra and high-resolution mass spectra (HRMS). 1JC-H Coupling constants were measured from the 2D-NMR to determine the α- and β-linkages between the building blocks (Supporting Information).
Scheme 4.
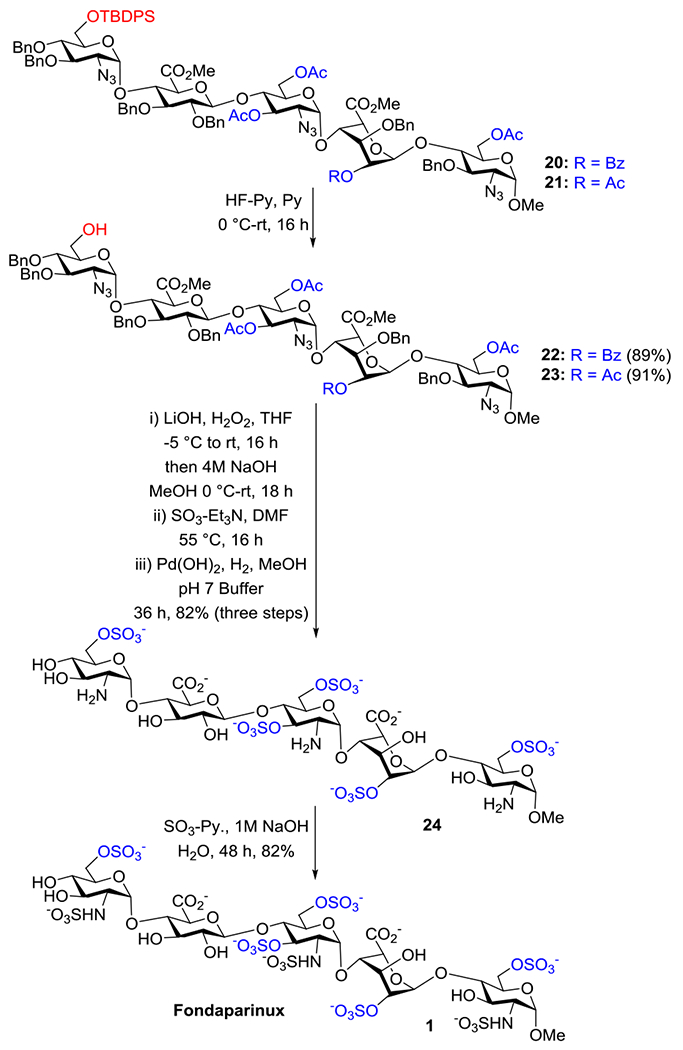
Synthesis of Fondaparinux
In conclusion, we have developed a programmable one-pot synthesis of the clinically important anticoagulant Fondaparinux using designed thioglycoside building blocks with well-defined RRVs for α-selective glycosylation guided by silyl ether and acetyl ester functionality at O6 in the one-pot sequence. The introduction of 3-OSO3− was performed with the aid of 2-napthyl ether (Nap). The carefully selected orthogonal protecting groups which can be differentially deprotected and readily accessible thioglycoside building blocks in the one-pot synthesis effectively reduce the number of synthetic steps and eliminate the multiple purification steps. In addition, the advantage of programmable approach is to allow a pre-evaluation of the building blocks to be used in a one-pot manner. The total synthesis was accomplished in 22 longest linear route with 4.2% overall yield from diacetone glucose which is a very significant improvement compare to previously reported synthetic methodologies. The protected pentasaccharide was synthesized for more than 200 mg and can be performed in gram scales. The synthetic route reported here is scalable and should be useful for the synthesis of Fondaparinux and closely related structures decorated with regiodefined O-and N-sulfation.16b
Supplementary Material
ACKNOWLEDGMENT
This work is supported by the NSF (CHE-1664283) and the NIH (AI-130227).
ABBREVIATIONS
- DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
- NIS
N-Iodosuccinimide
- TEMPO
2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl
- BAIB
Diacetoxyiodo benzene
- RRV
Relative Reactivity Values
- BBLs
Building blocks
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supporting Information [Experimental and purification procedures, characterization data (1H and 13C (ppm) and high-resolution mass spectra). Copies of 1H and 13C spectra are also provided for all newly synthesized compounds.
REFERENCES
- 1.Verstraete M Pharmacotherapeutic aspects of unfractionated and low molecular weight heparins. Drugs. 1990, 40, 498. [DOI] [PubMed] [Google Scholar]
- 2.(a) Petitou M; van Boeckel CAA A Synthetic Antithrombin III Binding Pentasaccharide is Now a Drug! What Comes Next? Angew. Chem. Int. Ed 2004, 43, 3118. [DOI] [PubMed] [Google Scholar]; (b) Petitou M; Duchaussoy P; Driguez P; Jaurand G; He J; Lormeau J; Boeckel CAA; Van Herbert J First Synthetic Carbohydrates with the Full Anticoagulant Properties of Heparin. Angew. Chem., Int. Ed 1998, 37, 3009. [DOI] [PubMed] [Google Scholar]
- 3.(a) Bauer KA; Hawkins DW; Peters PC; Petitou M; Herbert JM; van Boeckel CAA; Meuleman DG Fondaparinux, a Synthetic Pentasaccharide: The First in a New Class of Antithrombotic Agents - the Selective Factor Xa Inhibitors. Cardiovasc. Drug Rev. 2002, 20, 37. [DOI] [PubMed] [Google Scholar]; (b) petitou M; Duchaussoy P; Herbert J-M; Duc G; Hajji ME; Branellec J-F; Donat F; Necciari J; Cariou R; Bouthier J; Garrigou E The Synthetic pentasaccharide fondaparinux: first in the class of antithrombotic agents that selectively inhibit coagulation factor Xa. Semin Thromb Hemost. 2002, 28, 393. [DOI] [PubMed] [Google Scholar]
- 4.(a) Nagler M; Haslauer M; Wuillemin WA Fondaparinux –Data on Efficacy and Safety in Special Situations. Thromb. Res 2012, 129, 407. [DOI] [PubMed] [Google Scholar]; (b) Leentjens J; Peters M; Esselink AC; Smulders Y; Kramers C Initial Anticoagulation in patients with pulmonary embolism: thrombolysis, unfractionated heparin, LMWH, fondaparinux, or DOACs? Br. J. Clin. Pharmacol 2017, 83, 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kumar A; Talwar A; Farley JF; Muzumdar J; Schommer JC; Balkrishnan R; Wu W Fondaparinux Sodium Compared With Low-Molecular-Weight Heparins for Perioperative Surgical Thromboprophylaxis: A Systematic Review and Meta-analysis J. Am. Heart Assoc 2019, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blossom DB; Kallen AJ; Patel PR; Elward A; Robinson L; Gao G; Langer R; Perkins KM; Jaeger JL; Kurkjian KM; Jones M; Schillie SF; Shehab N; Ketterer D; Venkataraman G; Kishimoto TK; Shriver Z; McMahon AW; Austen KF;Kozlowski S; Srinivasan A; Turabelidze G; Gould CV; Arduino MJ; Sasisekharan RN Outbreak of Adverse Reactions Associated with Contaminated Heparin. N. Engl. J. Med 2008, 359, 2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Duchaussoy P; Lei PS; Petitou M; Sinäy P; Lormeau JC; Choay J first total synthesis of the antithrombin III binding site of porcine mucosa heparin, Bioorg. Med. Chem. Lett, 1991, 1, 99 [Google Scholar]; (b) Petitou M; Derrien GJM; Duchaussoy P; Choay J A new, highly potent, heparin-like pentasaccharide fragment containing a glucose residue instead of a glucosamine. Bioorg. Med. Chem. Lett, 1991, 1, 95. [Google Scholar]
- 7.Lin F; Lian G; Zhou Y Synthesis of Fondaparinux: Modular Synthesis Investigation for Heparin Synthesis. Carbohydr. Res 2013, 371, 32. [DOI] [PubMed] [Google Scholar]
- 8.Chang C-H; Lico LS; Huang T-Y; Lin S-Y; Chang C-L; Arco SD; Hung S-C Synthesis of the Heparin-Based Anticoagulant Drug Fondaparinux. Angew. Chem., Int. Ed 2014, 53, 9876. [DOI] [PubMed] [Google Scholar]
- 9.Li T; Ye H; Cao X; Wang J; Liu Y; Zhou L; Liu Q; Wang W; Shen J; Zhao W; Wang P Total Synthesis of Anticoagulant Pentasaccharide Fondaparinux. ChemMedChem 2014, 9, 1071. [DOI] [PubMed] [Google Scholar]
- 10.Dai X; Liu W; Zhou Q; Cheng C; Yang C; Wang S; Zhang M; Tang P; Song H; Zhang D; Qin Y Formal Synthesis of Anticoagulant Drug Fondaparinux Sodium. J. Org. Chem 2016, 81, 162. [DOI] [PubMed] [Google Scholar]
- 11.(a) Manikowski A; Koziol A; Czajkowska-Wojciechowska E An Alternative Route for Fondaparinux Sodium Synthesis Via Selective Hydrogenations and Sulfation of Appropriate Pentasaccharides. Carbohydr. Res 2012, 361, 155. [DOI] [PubMed] [Google Scholar]; (b) Koziol A; Lendzion-Paluch A; Manikowski A A Fast and Effective Hydrogenation Process of Protected Pentasaccharide: A Key Step in the Synthesis of Fondaparinux Sodium. Org. Process Res. Dev 2013, 17, 869. [Google Scholar]
- 12.Ding Y; Prasad CVNSV; Bai H; Wang B Efficient and practical synthesis of Fondaparinux. Bioorg. Med. Chem. Lett, 2017, 27, 2424. [DOI] [PubMed] [Google Scholar]
- 13.Jin H; Chen Q; Zhang Y-Y Hao K-F; Zhang G-Q; Zhao W Preactivation-based, iterative one-pot synthesis of anticoagulant pentasaccharide fondaparinux sodium. Org. Chem. Front, 2019, 6, 3116. [Google Scholar]
- 14.(a) Zhang Z; Ollmann IR; Ye X-S; Wischnat R; Baasov T; Wong C-H Programmable One-Pot Oligosaccharide Synthesis. J. Am. Chem. Soc 1999, 121, 734 [Google Scholar]; (b) Cheng C-W; Zhou Y; Pan W-H; Dey S; Wu C-Y; Hsu W-L; Wong C-H Hierarchical and programmable one-pot synthesis of oligosaccharides. Nat. Commun 2018, 9, 5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Lee J-C; Greenberg WA; Wong C-H Programmable reactivity-based one-pot oligosaccharide synthesis. Nat. Protoc 2006, 1, 3143. [DOI] [PubMed] [Google Scholar]; (b) Hsu C-H; Hung S-C; Wu C-Y; Wong C-H Toward automated oligosaccharide synthesis. Angew. Chem., Int. Ed 2011, 50, 11872. [DOI] [PubMed] [Google Scholar]; (c) Cheng C-W; Wu C-Y; Hsu W-L; Wong C-H Programmable One-Pot Synthesis of Oligosaccharides. Biochemistry. 10.1021/acs.biochem.9b00613 [DOI] [PubMed] [Google Scholar]; (d) Lo H-J; Krasnova L; Dey S; Cheng T; Liu H; Tsai T-I; Wu KB; Wu C-Y; Wong C-H Synthesis of Sialidase-Resistant Oligosaccharide and Antibody Glycoform Containing α2,6-Linked 3F-Neu5Ac. J. Am. Chem. Soc 2019, 141, 6484. [DOI] [PubMed] [Google Scholar]; (d) Ting C-Y; Lin Y-W; Wu C-Y; Wong C-H Design of disaccharide modules for a programmable one-pot synthesis of building blocks with LacNAc repeating units for asymmetric N-glycans. Asian J. Org. Chem, 2017, 6, 1800. [Google Scholar]
- 16.(a) Polat T; Wong C-H Anomeric Reactivity-Based One-Pot Synthesis of Heparin-Like Oligosaccharides. J. Am. Chem. Soc 2007, 129, 12795. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dey S; Wong C-H Programmable one-pot synthesis of heparin pentasaccharides enabling access to regiodefined sulfate derivatives. Chem. Sci 2018, 9, 6685. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dey S; Lo H-J; Wong C-H An Efficient Modular One-Pot Synthesis of Heparin-Based Anticoagulant Idraparinux. J. Am. Chem. Soc 2019, 141, 10309. [DOI] [PubMed] [Google Scholar]
- 17.Weiler S; Schmidt RR A versatile strategy for the synthesis of complex type N-Glycans: Synthesis of diantennary and bisected diantennary oligosaccharides. Tetrahedron Lett. 1998, 39, 2299. [Google Scholar]
- 18.Singh L; Lam A; Premraj R; Seifert J Synthesis of a metabolite of an anti-angiogenic lead candidate based on a D-glucosamine motif. Tetrahedron Lett. 2008, 49, 4854. [Google Scholar]
- 19.Xia J; Abbas SA; Locke RD; Piskorz CF; Alderfer JL; Matta KL Use of 1,2-dichloro-4,5-dicyanoquinone (DDQ) for cleavage of the 2-naphthymethyl (NAP) group. Tetrahedron Lett. 2000, 41, 169. [Google Scholar]
- 20.Tatai J; Osztrovszky G; Kajtár-Peredy M; Fügedi P An efficient synthesis of L-idose and L-iduronic acid thioglycosides and their use for the synthesis of heparin oligosaccharides. Carbohydr. Res, 2008, 343, 596. [DOI] [PubMed] [Google Scholar]
- 21.(a) Wu X-A; Ying P; Liu J-Y; Shen H-S; Chen Y; He L Lithium Chloride–Assisted Selective Hydrolysis of Methyl Esters Under Microwave Irradiation. Synth. Commun 2009, 39, 3459 [Google Scholar]; (b) Evans DA; Britton TC; Ellman JA; Contrasteric Carboximide hydrolysis with lithium hydroperoxide. Tetrahedron Lett. 1987, 28, 6141 [Google Scholar]; (c) Beutner GL; Cohen BM; Delmonte AJ; Dixon DD; Fraunhoffer KJ; Galce AW; Lo E; Stevens JM; Vanyo D; Wilbert C Revisiting the cleavage of Evans Oxazolidinones with LiOH/H2O2 Org. Process Res. Dev 2019, 23, 1378. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


