Abstract
Background: Early detection of suspected critical patients infected with coronavirus disease 2019 (COVID-19) is very important for the treatment of patients. This study aimed to investigate the role of COVID-19 associated coagulopathy (CAC) to preview and triage. Methods and Results: A cohort study was designed from government designated COVID-19 treatment center. CAC was defined as International Society on Thrombosis and Haemostasis (ISTH) score ≥2. Data from 117 patients COVID-19 were reviewed on admission. The primary and secondary outcomes were admission to Intensive Care Unit (ICU), the use of mechanical ventilation, vital organ dysfunction, discharges of days 14, 21 and 28 from admission and hospital mortality. Among them, admission to ICU was increased progressively from 16.1% in patients with non-CAC to 42.6% in patients with CAC (P < 0.01). Likely, invasive ventilation and noninvasive ventilation were increased from 1.8%, 21.4% in patients with non-CAC to 21.3%, 52.5% in patients with CAC, respectively (P < 0.01). The incidences of acute hepatic injury and acute respiratory distress syndrome in non-CAC and CAC were 28.6% vs. 62.3%, 8.9% vs. 27.9%, respectively (P < 0.01). The discharges of days 14, 21 and 28 from admission were more in non-CAC than those of CAC (P < 0.05). Multiple logistic regression results showed that ISTH score ≥2 was obviously associated with the admission to ICU (OR 4.07, 95% CI 1.47–11.25 P = 0.007) and the use of mechanical ventilation (OR 5.54, 95% CI 2.01–15.28 P = 0.001) in patients with COVID-19. Conclusion: All results show ISTH score ≥2 is an important indicator to preview and triage for COVID-19 patients.
Keywords: Coagulopathy, Outcome, Mechanical ventilation, ISTH score, Coronavirus disease 2019
Highlights
-
•
COVID-19 patients with ISTH score ≥ 2 on admission need more admission to ICU and mechanical ventilation.
-
•
The incidence is high in acute hepatic injury and acute respiratory distress syndrome in COVID-19 patients with ISTH score ≥ 2.
-
•
The discharges of 14 days, 21 days and 28 days from admission were less in COVID-19 patients with ISTH score ≥ 2.
-
•
ISTH score ≥ 2 is an important indicator to preview and triage for COVID-19 patients on early admission.
1. Introduction
The COVID-19 has features typical of the coronavirus family and is classified in the beta coronavirus 2β lineage [1,2]. Clinical classification of COVID-19 is mainly divided into light, common, severe, and critical types [3]. How to identify the patients who may become severe on the day of admission plays an important role in clinical triage treatment. For patients with bacterial sepsis, the early recognition of quick sepsis-related organ failure assessment (qSOFA) score is often used in clinic, but the respiratory rate in the early patients with COVID-19 is usually <22 times/min, and the blood pressure is basically within normal range, thus it is difficult to identify early patients with qSOFA score.
Early detection of suspected severe patients infected with SARS-CoV-2 is very important for the treatment of patients. As we known, patients have been identified according to their respiratory rates, blood oxygen saturation or PO2/FiO2, which might delay their treatments and even increase the mortality to some extent at the early stage. Therefore, it is of great significance to further elucidate the pathophysiological mechanisms, and to seek the early indicators to estimate the severity of the patients with COVID-19. It has been generally accepted that during pathogenic microorganism infection, host immunity, inflammation, coagulation are critically involved in the development of septic complications. The results of clinical study showed that some COVID-19 could cause coagulation abnormality and progressive aggravation at the later stage, but disseminated intravascular coagulation (DIC) was rare in those cases [[2], [3], [4]].
In order to determine the abnormal coagulation at the early stage, coagulation markers, including thrombin-antithrombin complex (TAT), α2-plasmininhibitor- plasmin complex (PIC), soluble thrombomodulin (sTM), and tissue plasminogen activator-inhibitor complex (tPAIC) are used to evaluate pre-DIC clinically. However, it is difficult to detect these coagulation markers in the fever clinic when patients are novel coronavirus nucleic acid positive. For evaluation of coagulation function, clinicians usually carry out five coagulation and platelet count tests for patients. Using these indicators related to coagulation may provide a reference for early detection of coagulopathy. Persistent coagulopathy is associated with poor outcomes. Thus, this study was based on coagulation monitoring indicators, using International Society on Thrombosis and Haemostasis (ISTH) interim guidance to recognition and management of coagulopathy in COVID-19, which might be able to identify patients with coagulation activation and estimate the severity of patients [5].
As the most consistent hemostatic abnormalities with COVID-19 include mild thrombocytopenia and increased D-dimer levels, >70% of COVID-19 patients who died fulfilled the ISTH score ≥5 points, compared with only 0.6% among survivors [6]. This means that COVID-19-related DIC is rare in surviving patients especially in the early stages of COVID-19. In the present study, COVID-19 patient cohort was admitted by ISTH score and its scores between 0 and 3 points from January 14, 2020 to February 11, 2020. Thus, the COVID-19 patients were divided into non-COVID-19 associated coagulopathy (CAC) and CAC groups according to the ISTH score (2 points), then the clinical characteristics, admission to intensive care unit (ICU), the use of mechanical ventilation, the vital organ dysfunction, and discharges of 14 days, 21 days and 28 days from admission as well as hospital mortality were compared between two groups in our study.
2. Methods
2.1. Study design, setting and participants
This retrospective cohort study was designed by the investigators and performed at the Third People's Hospital of Shenzhen between January 14, 2020 and February 11, 2020. The data cutoff for the study was March 20, 2020. Written informed consent was waived in light of the urgent need to collect data. Data were obtained from 149 patients with COVID-19 hospitalized at Department of Critical Care Medicine and Infection Third Ward during the study dates. A confirmed case of COVID-19 was defined as a positive result on real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of pharyngeal swab specimens by Shenzhen center for disease prevention and control (CDC). The discharges criterion was negative two times 24 hour interval result on RT-PCR assay of pharyngeal swab specimens by Shenzhen CDC. The study analyzed de-identified data from the hospital's healthcare informatics group, which was supervised by Shenzhen Municipal Health Commission. The study protocol was approved by the Second People's Hospital of Shenzhen & First Affiliated Hospital of Shenzhen University (institutional review board [IRB] number 202003009004).
2.2. Data collection and definitions
All routinely collected vital signs and symptoms and laboratory values were extracted from the electronic health records. Data included, but were not limited to, demographic data (e.g. age, gender, body mass index [BMI]), biochemical parameters (e.g. blood cell count, liver function, kidney function, coagulation function, blood gas analysis), mechanic ventilation and comorbidities including hypertension, diabetes, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), and malignancy for severity of illness. We calculated the Acute Physiology and Chronic Health Evaluation (APACHE) II score within first 24 h of hospitalization.
Shock and acute respiratory distress syndrome (ARDS) were defined in accordance with the WHO interim guidance [7]. Acute kidney injury was defined based on Kidney Disease: Improving Global Outcomes Clinical Practice Guidelines (KDIGO) [8]. Acute cardiac dysfunction was defined as the clinical syndrome characterized by typical symptoms (e.g. breathlessness, fatigue, and ankle swelling) that may be accompanied by signs (e.g. elevated jugular venous pressure, and pulmonary crackles) caused by cardiac abnormality. Acute hepatic injury was defined as a state in which the patient's blood laboratory results met at least one of three criteria: serum total bilirubin (TBil) of 3.0 mg/dL or greater; aspartate aminotransferase (AST) of 41 IU/L or greater; alanine aminotransferase (ALT) of 41 IU/L or greater; The patients who met none of these criteria were classified as the “normal liver function” group [9].
2.3. Main measures and outcomes
CAC was defined as ISTH score more than or equal to 2 points. The primary end points were admission to an ICU and the use of mechanical ventilation. The secondary end points were vital organ function, and discharges of 14 days, 21 days and 28 days from admission as well as hospital mortality.
2.4. Statistical analysis
The categorical data were summarized as numbers and percentages, and χ2 tests or Fisher's exact test to compare between CAC and non-CAC groups. Continuous variables were expressed as the arithmetic mean and standard deviation (SD) or as the median and interquartile range, depending on whether or not they showed a Gaussian distribution. Continuous data with Gaussian distribution were compared with the Student's t-test and those with a non-Gaussian distribution, with the Wilcoxon rank-sum test. Univariable and multivariable logistic regression analysis with odds ratio (OR) and 95% confidence interval levels (95% CI) were performed to evaluate risk factors associated with admission to ICU and mechanical ventilation. The following variables were investigated as independent risk factors for mechanical ventilation: comorbid conditions, age, gender, platelet count, neutrophil lymphocyte ratio, alanine aminotransferase, aspartate transaminase, creatinine, ISTH score, and APACH II score. The same variables plus creatinine were investigated as risk factors for admission to ICU. Statistical analysis was performed using the statistical package SAS 9.4 (Windows, SAS Institute, Cary, North Carolina). P values (two-tailed) below 0.05 were considered statistically significant.
3. Results
3.1. Demographics and baseline characteristics
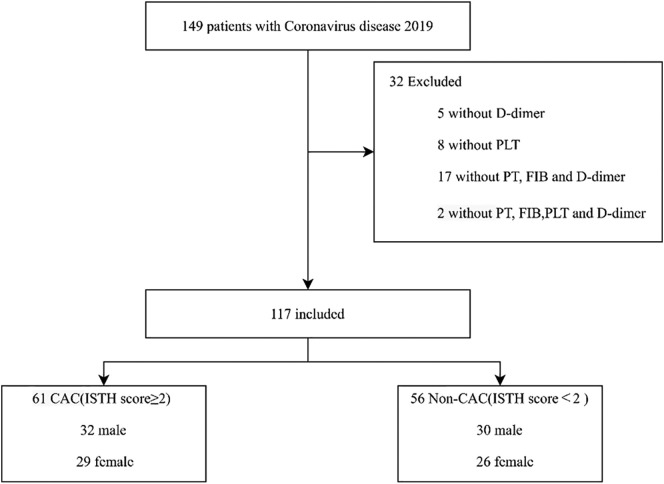
The detailed demographic and clinical profile data of all patients with COVID-19 on admission baseline were summarized in Table 1 . By February 11, 2020, clinical data were collected in 149 patients in the Department of Critical Care Medicine and Infection Third Ward with laboratory confirmed COVID-19 (Fig. 1 ). There were 5 patients without D-Dimer, 8 patients without platelets, 17 patients without prothrombin time, fibrinogen, D-Dimer and 2 patients without platelets, prothrombin time, fibrinogen and D-Dimer on admission. A total of 117 patients were enrolled in this study finally (52 males, 53%). Patient's mean age was 61.9 years. 104 (88.9%) patients had a history of exposure to the Hubei or the individuals with confirmed COVID-19. Of these patients, the mean body mass index (BMI) was 23.9, body temperature was 37.5 °C, respiration rate was 20.5 times/min, mean arterial pressure (MAP) was 97.3 mm Hg. The APACHE II was between 2 to 7 points and the ISTH score was between 0 to 3points, the distribution of ISTH scores in Fig. 2 . This patients with ISTH ≥ 2 points had more comorbidities than that of ISTH< 2 points (41.0% vs. 17.9%, P = 0.006).
Table 1.
Demographics and baseline characteristics of patients with COVID-19.
| Characteristics | Total (n = 117) | Non-CAC (n = 56) | CAC (n = 61) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.9 ± 17.9 | 60.3 ± 18.0 | 63.3 ± 17.8 | 0.29 |
| Distribution, N (%) | 0.009 | |||
| ≤60 years | 71 (60.7) | 41 (73.2) | 30 (49.2) | |
| >60 years | 46 (39.3) | 15 (26.8) | 31 (50.8) | |
| Male, N (%) | 62 (53.0) | 30 (53.6) | 32 (52.5) | 0.90 |
| BMI, mean ± SD | 23.9 ± 3.6 | 23.2 ± 3.6 | 24.5 ± 3.5 | 0.055 |
| MAP (mm Hg), mean ± SD | 97.3 ± 12.2 | 97.0 ± 12.2 | 97.6 ± 12.2 | 0.78 |
| Temperature (°C), mean ± SD | 37.5 ± 0.9 | 37.3 ± 0.8 | 37.7 ± 0.9 | 0.017 |
| Heart rate (bpm), mean ± SD | 91.9 ± 13.7 | 91.3 ± 13.6 | 92.4 ± 13.8 | 0.68 |
| Respiration rate (bpm) | 20.5 ± 1.9 | 20.1 ± 1.6 | 20.8 ± 2.1 | 0.031 |
| Classification of body temperature (°C), N (%) | ||||
| <37.3 | 55 (47.0) | 33 (58.9) | 22 (36.1) | 0.076 |
| 37.3–38.0 | 33 (28.2) | 12 (21.4) | 21 (34.4) | |
| 38.1–39.0 | 24 (20.5) | 10 (17.9) | 14 (23.0) | |
| >39.0 | 5 (4.3) | 1 (1.8) | 4 (6.6) | |
| Comorbidities, N (%) | 35 (29.9) | 10 (17.9) | 25 (41.0) | 0.006 |
| Hypertension | 23 (19.7) | 7 (12.5) | 16 (26.2) | 0.062 |
| Diabetes | 12 (10.3) | 3 (5.4) | 9 (14.8) | 0.13 |
| COPD | 3 (2.6) | 1 (1.8) | 2 (3.3) | 1.0 |
| Cerebrovascular disease | 1 (0.9) | 0 (0.0) | 1 (1.6) | 1.0 |
| Malignancy | 1 (0.9) | 0 (0.0) | 1 (1.6) | 1.0 |
| Hubei exposure, N (%) | 104 (88.9) | 51 (91.1) | 53 (86.9) | 0.56 |
| Signs and symptoms, N (%) | 107 (91.5) | 51 (91.1) | 56 (91.8) | 1.0 |
| Fever | 96 (82.1) | 43 (76.8) | 53 (86.9) | 0.16 |
| Fatigue | 30 (25.6) | 10 (17.9) | 20 (32.8) | 0.065 |
| Muscle pain | 27 (23.1) | 15 (26.8) | 12 (19.7) | 0.36 |
| Dry cough | 36 (30.8) | 17 (30.4) | 19 (31.1) | 0.93 |
| Expectoration | 29 (24.8) | 12 (21.4) | 17 (27.9) | 0.42 |
| Chest tightness | 12 (10.3) | 6 (10.7) | 6 (9.8) | 0.88 |
| Dyspnea | 4 (3.4) | 2 (3.6) | 2 (3.3) | 1.0 |
| Bellyache | 1 (0.9) | 0 (0.0) | 1 (1.6) | 1.0 |
| Diarrhea | 8 (6.8) | 3 (5.4) | 5 (8.2) | 0.72 |
| Nausea | 4 (3.4) | 2 (3.6) | 2 (3.3) | 1.0 |
| Vomit | 1 (0.9) | 1 (1.8) | 0 (0.0) | 0.48 |
| Inappetence | 8 (6.8) | 3 (5.4) | 5 (8.2) | 0.72 |
| Headache | 8 (6.8) | 5 (8.9) | 3 (4.9) | 0.48 |
| Dizziness | 9 (7.7) | 4 (7.1) | 5 (8.2) | 1.0 |
| GCS score < 15, N (%) | 1 (0.9) | 0 (0.0) | 1 (1.6) | 1.0 |
| APACHE II score, median (IQR) | 5.0 (2.0,7.0) | 4.0 (2.0,6.0) | 5.0 (4.0,7.0) | 0.022 |
Abbreviations: BMI, body mass index; MAP: mean arterial pressure; bpm: beats per minute; COPD, chronic obstructive pulmonary disease; GCS: Glasgow coma scale; APACHE II, Acute Physiology and Chronic Health Evaluation; ISTH, International Society of Thrombosis & Haemostasis; IQR, interquartile range.
Fig. 1.
Flow diagram of study subjects.
Fig. 2.
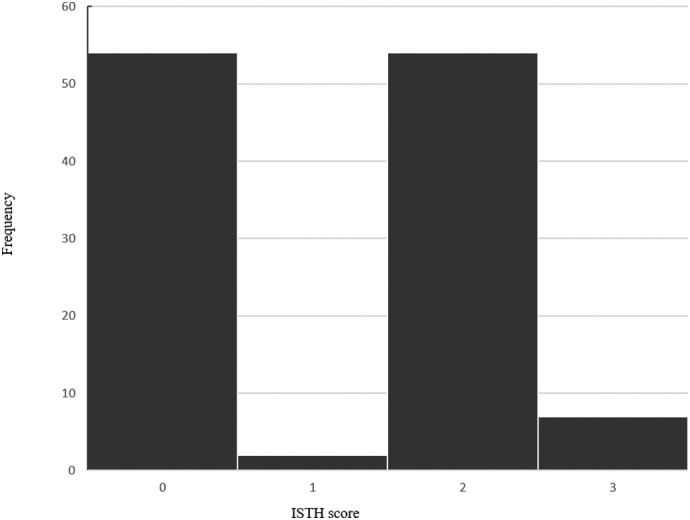
The distribution of ISTH score.
In terms of signs and symptoms, there were no significant differences between the two groups. A patient's Glasgow coma scale (GCS) was 3 points in the CAC group and the other were 15 points (P = 0.32). Notably, APACHE II score was significantly higher in the CAC group compared to the non-CAC group (CAC 5.0 [4.0, 7.0] vs. non-CAC 4.0 [2.0, 6.0], P = 0.022).
3.2. Laboratory findings and CAC score on admission
There were significant differences in levels of procalcitonin (PCT), C-reaction protein (CRP), interleukin 6 (IL-6), neurophil, lymphocyte, neutrophil to lymphocyte ratio (NLR), albumin, alanine aminotransferase, aspartate aminotransferase, pH, PO2, PO2/FiO2, D-dimer, and fibrinogen between the CAC and non-CAC groups (Table 2 ). Inflammatory indexes including PCT, CRP, and IL-6 were higher in patients with CAC (P < 0.05). In comparison to the non-CAC group, lymphocyte counts were lower, and neutrophils as well as NLR were increased in the CAC group (P < 0.01), showing that NLR in CAC patients was 3.2. D-dimer and fibrinogen levels, and ISTH scores in patients with CAC were higher than those of non-CAC patients (P < 0.01).
Table 2.
Laboratory findings of patients with COVID-19 on admission.
| Characteristics | Total (n = 117) | Non-CAC (n = 56) | CAC (n = 61) | P value |
|---|---|---|---|---|
| Inflammatory parameters | ||||
| PCT (ng/ml), median (IQR) | 0.06 (0.04, 0.08) | 0.05 (0.04, 0.07) | 0.06 (0.04, 0.09) | 0.042 |
| CRP (mg/dl), median (IQR) | 22.7 (9.0, 44.7) | 13.9 (7.8, 28.2) | 32.0 (16.2, 57.3) | <0.001 |
| IL-6 (pg/ml), median (IQR) | 19.8 (12.2, 43.1) | 19.1 (5.4, 27.0) | 22.2 (16.0, 52.1) | 0.018 |
| Blood routine tests | ||||
| WBC (1 × 109/L), median (IQR) | 4.7 (3.8, 5.9) | 4.8 ± 1.5 | 5.3 ± 2.1 | 0.11 |
| HGB (g/L), mean ± SD | 136.9 ± 15.4 | 139.0 ± 14.4 | 135.0 ± 16.2 | 0.16 |
| Neutrophils (1 × 109/L), median (IQR) | 3.0 (2.2, 3.9) | 2.6 (2.0, 3.6) | 3.2 (2.4, 4.4) | 0.014 |
| Lymphocyte (1 × 109/L), median (IQR) | 1.2 (0.9, 1.5) | 1.2 (1.0, 1.6) | 1.1 (0.8, 1.4) | 0.012 |
| Monocytes, median (IQR) | 0.4 (0.3, 0.6) | 0.5 (0.4, 0.5) | 0.4 (0.3, 0.6) | 0.17 |
| Platelets (1 × 109/L), mean ± SD | 177.0 ± 60.7 | 187.6 ± 62.0 | 167.1 ± 58.4 | 0.069 |
| NLR, median (IQR) | 2.6 (1.5, 4.1) | 2.0 (1.4, 3.1) | 3.2 (1.8, 5.2) | 0.001 |
| PLR, median (IQR) | 144.6 (116.1, 190.9) | 141.1 (116.7, 172.5) | 153.0 (114.3, 214.8) | 0.32 |
| LMR | 2.7 (2.1, 3.6) | 2.8 (2.1, 3.7) | 2.6 (2.1, 3.5) | 0.54 |
| Biochemical parameters | ||||
| Albumin (g/L), mean ± SD | 41.5 ± 3.8 | 42.8 ± 3.1 | 40.3 ± 4.0 | <0.001 |
| TBiL (μmol/L), median (IQR) | 9.9 (7.5, 13.8) | 9.9 (7.5, 13.9) | 10.5 (7.6, 13.6) | 0.98 |
| ALT (U/L), median (IQR) | 24.0 (16.2, 34.8) | 19.0 (14.2, 29.3) | 29.0 (20.0, 40.8) | 0.002 |
| AST (U/L), median (IQR) | 29.0 (23.0, 43.0) | 26.0 (21.0, 33.8) | 36.1 (27.0, 47.2) | 0.001 |
| Creatinine (μmol/L), median (IQR) | 69.0 (54.0, 83.0) | 67.7 ± 19.0 | 75.2 ± 28.9 | 0.098 |
| Creatinine >133 μmol/L, N (%) | 7 (6.0) | 2 (3.6) | 5 (8.2) | 0.44 |
| BUN (mmol/L), median (IQR) | 4.4 (3.5, 5.3) | 4.2 (3.4, 5.1) | 4.4 (3.7, 5.6) | 0.26 |
| Blood gas analysis | ||||
| pH, mean ± SD | 7.43 ± 0.03 | 7.42 ± 0.02 | 7.44 ± 0.04 | 0.002 |
| PO2, mean ± SD | 91.9 ± 27.5 | 97.2 ± 32.7 | 87.3 ± 21.5 | 0.080 |
| PCO2, mean ± SD | 37.3 ± 4.3 | 38.5 ± 3.5 | 36.2 ± 4.6 | 0.006 |
| PO2/FiO2, median (IQR) | 381.9 ± 127.6 | 423.0 ± 139.0 | 340.8 ± 101.2 | <0.001 |
| Coagulation parameters | ||||
| PT (s), mean ± SD | 12.1 ± 0.96 | 12.1 ± 0.94 | 12.1 ± 0.98 | 0.98 |
| APTT (s), mean ± SD | 37.3 ± 7.01 | 37.2 ± 4.24 | 37.4 ± 8.87 | 0.91 |
| INR, mean ± SD | 0.9 ± 0.09 | 0.9 ± 0.09 | 0.9 ± 0.09 | 0.94 |
| D-Dimer (mg/L), median (IQR) | 0.41 (0.30, 0.66) | 0.30 (0.24, 0.36) | 0.64 (0.53, 0.91) | <0.001 |
| FIB (g/L), mean ± SD | 4.29 ± 1.11 | 3.86 ± 0.88 | 4.68 ± 1.16 | <0.001 |
| ISTH score, median (range) | 2.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | 2.0 (2.0, 3.0) | <0.001 |
Abbreviations: PCT, procalcitonin; CRP, C-reaction protein; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; NLR, neutrophil/lymphocyte ratio; PLT, platelets; PLR, platelets/ lymphocyte ratio; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; PT, prothrombin time; APTT, activated partial thromboplastin time; FIB, fibrinogen. ISTH: International Society of Thrombosis & Haemostasis.
3.3. Complications and outcomes of patients with COVID-19
There were significant differences in the proportions of admission to ICU, the use of invasive ventilation and noninvasive ventilation, acute hepatic injury, and acute lung injury as well as discharges of 14 days, 21 days and 28 days from admission. There were no significant differences in incidences in shock, acute cardiac insufficiency, and acute renal injury (P > 0.05) (Table 3 ). Other main clinical outcomes, such as the proportion of admission to ICU (16.1% vs. 42.6%), acute hepatic injury (28.6% vs. 62.3%) and ARDS (8.9% vs. 27.9%), and invasive ventilation (1.8% vs. 21.3%) as well as noninvasive ventilation (21.4% vs. 52.5%) were increased in the non-CAC group than those of the CAC group (all P < 0.01). The discharges of 14 days, 21 days and 28 days from admission were 48.2% vs. 16.4%, 75.0% vs. 45.9%, 89.3% vs.72.1% in non-CAC and CAC, respectively (all P < 0.05).
Table 3.
Complications and outcomes of patients with COVID-19.
| Outcomes, N (%) | Total (n = 117) | Non-CAC (n = 56) | CAC (n = 61) | P value |
|---|---|---|---|---|
| The primary end points | ||||
| Admission to ICU | 35 (29.9) | 9 (16.1) | 26 (42.6) | 0.002 |
| Mechanical ventilation | ||||
| Invasive ventilation | 14 (12.0) | 1 (1.8) | 13 (21.3) | 0.001 |
| Noninvasive ventilation | 44 (37.6) | 12 (21.4) | 32 (52.5) | 0.001 |
| The secondary end point | ||||
| Discharge from admission | ||||
| 14 days | 37 (31.6) | 27 (48.2) | 10 (16.4) | <0.001 |
| 21 days | 70 (59.8) | 42 (75.0) | 28 (45.9) | 0.001 |
| 28 days | 94 (80.3) | 50 (89.3) | 44 (72.1) | 0.022 |
| AHI | 54 (46.2) | 16 (28.6) | 38 (62.3) | <0.001 |
| ARDS | 22 (18.8) | 5 (8.9) | 17 (27.9) | 0.009 |
| Shock | 8 (6.8) | 1 (1.8) | 7 (11.5) | 0.063 |
| ACD | 3 (2.6) | 0 (0.0) | 3 (4.9) | 0.25 |
| AKI | 5 (4.3) | 1 (1.8) | 4 (6.6) | 0.37 |
| LOS (days), median (IQR) | 17 (14, 27) | 15 (13, 22) | 23 (16, 30) | <0.001 |
| Mortality | 2 (1.7) | 0 (0.0) | 2 (3.3) | 0.25 |
Abbreviations: ICU: intensive care unit; ACD, acute cardiac dysfunction; AKI, acute kidney injury; AHI, acute hepatic injury; ARDS, acute respiratory distress syndrome; ALI, acute lung injury; MV, mechanical ventilation; ICU, intensive care unit; LOS, length of stay; IQR: interquartile range.
3.4. Risk factors associated with admission to ICU and the use of mechanical ventilation in COVID-19 patients in univariable and logistic multivariable regression models
To explore the risk factors associated with admission to ICU and the use of mechanical ventilation in COVID-19 patients, we choose age, male, BMI, platelet count, NLR, alanine aminotransferase, aspartate transaminase, and creatinine as variables from demographic data, biochemical parameters, and the others were comorbidities, ISTH scores, and APACHE II scores.
Multiple logistic regression results showed that only age > 60 (OR 5.12, 95% CI 1.96–13.89, P = 0.001), male (OR 6.47, 95% CI 2.22–18.91, P < 0.001), and ISTH score ≥2 (OR, 4.07, 95% CI 1.47–11.25 P = 0.007) were obviously associated with the admission to ICU in patient with COVID-19 (Table 4 ).
Table 4.
Risk factors associated with admission to ICU.
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age > 60 | 4.92 (2.11, 11.48) | <0.001 | 5.21 (1.96, 13.89) | 0.001 |
| Male | 4.53 (1.84, 11.17) | 0.001 | 6.47 (2.22, 18.91) | <0.001 |
| ISTH score ≥ 2 | 3.88 (1.62, 9.31) | 0.002 | 4.07 (1.47, 11.25) | 0.007 |
| Platelet counts (109/L) | 0.99 (0.98, 1) | 0.035 | N/A | |
| Neutrophil/lymphocyte ratio | 1.25 (1.07, 1.47) | 0.005 | N/A | |
| Alanine aminotransferase (U/L) | 1.01 (0.99, 1.03) | 0.34 | N/A | |
| Aspartate transaminase (U/L) | 1.05 (1.02, 1.08) | 0.001 | N/A | |
| Creatinine > 133 μmol/L | 16.76 (1.93, 145.15) | 0.011 | N/A | |
| Comorbidities | 2.79 (1.21, 6.47) | 0.017 | N/A | |
| APACHE II score | 1.28 (1.1, 1.48) | 0.001 | N/A | |
APACHE II, Acute Physiology and Chronic Health Evaluation; ISTH, International Society of Thrombosis & Haemostasis; IQR, interquartile range.
Multiple logistic regression results revealed that only age > 60 (OR 4.94, 95% CI 1.84–13.27, P = 0.0020), male (OR 8.43, 95% CI 2.86–24.85, P < 0.001), and ISTH score ≥2 (OR 5.54, 95% CI 2.01–15.28 P = 0.001) were significantly associated with the use of mechanical ventilation in patients with COVID-19 (Table 5 ).
Table 5.
Risk factors associated with the use of mechanical ventilation.
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age > 60 | 4.58 (2.06, 10.17) | <0.001 | 4.94 (1.84, 13.27) | 0.002 |
| Male | 5.19 (2.26, 11.89) | <0.001 | 8.43 (2.86, 24.85) | <0.001 |
| ISTH score ≥ 2 | 4.62 (2.05, 10.42) | <0.001 | 5.54 (2.01, 15.28) | 0.001 |
| Platelet counts (109/L) | 0.99 (0.98, 1) | 0.004 | N/A | |
| Neutrophil/lymphocyte ratio | 1.27 (1.07, 1.5) | 0.007 | N/A | |
| Alanine aminotransferase (U/L) | 1.02 (1, 1.04) | 0.082 | N/A | |
| Aspartate transaminase (U/L) | 1.05 (1.02, 1.08) | 0.001 | N/A | |
| Comorbidities | 2.42 (1.08, 5.43) | 0.032 | N/A | |
| APACHE II score | 1.24 (1.09, 1.43) | 0.002 | N/A | |
APACHE II, Acute Physiology and Chronic Health Evaluation; ISTH, International Society of Thrombosis & Haemostasis; IQR, interquartile range.
4. Discussion
Our study showed that the admission to ICU was increased progressively from 16.1% in patients with non-CAC to 42.6% in patients with CAC with COVID-19. The incidence of invasive ventilation and noninvasive ventilation, acute hepatic injury and acute respiratory distress syndrome were high in CAC than that of non-CAC group. The results showed that ISTH score ≥2 was obviously associated with the admission to ICU (OR 4.07, 95% CI 1.47–11.25 P = 0.007) and the use of mechanical ventilation (OR 5.54, 95% CI 2.01–15.28 P = 0.001) in patients with COVID-19. All results show ISTH score ≥2 is an important indicator to preview and triage for COVID-19 patients when epidemic outbreak.
COVID-19 is a systemic multi-organ injury disease caused by severe acute respiratory syndrome (SARS)-Cov-2. Similar to that of SARS [10] and Middle East respiratory syndrome (MERS) [11], it involves many basic pathological processes, such as host immune, inflammation and coagulation. Over activation of immune cells, cytokine storm and excessive oxidative stress may be the common pathophysiological mechanism(s) underlying ARDS, septic shock, multiple organ failure, and even death caused by COVID-19, SARS, and MERS [[12], [13], [14], [15]]. Critical COVID-19 characterized by refractory hypoxemia increases patient mortality because of dysregulation of host immune response. Studies showed that COVID-19 patients with severe ARDS have microthrombus [16]. The authors suggest that coagulation disorders may be involved in the pathological process of patients with critical COVID-19.
At the early stage of admission, because of high fever, water loss, and insufficient intake, patients with severe COVID-19 had insufficient capacity, low blood pressure, and high blood viscosity. Multiple organ failure caused by diffuse microvascular damage is an important cause of death in critical patients with COVID-19 [4,17]. A multicenter retrospective study of 1099 patients with COVID-19 showed that the incidence of DIC (2.9% vs. 0.1%) and the mortality (8.1% vs. 0.1%) were significantly higher than those of non-ICU patients [2]. A retrospective analysis of 21 patients with COVID-19 revealed that 71.4% of the dead patients had DIC, while the incidence of DIC in survival patients was only 0.6% [4]. Therefore, coagulation disorder and DIC are the vital causes of death in critical type with COVID-19.
COVID-19 is a systemic infectious disease mainly caused by SARS-CoV-2. The functional receptors for this newly emerged coronavirus can mediate SARS-CoV-2 S-mediated entry into cells, such as ACE2 [[18], [19], [20], [21]]. The immune system in patients with COVID-19 is over activated, thereby releasing a large number of inflammatory mediators and promoting platelet aggregation [22]. After SARS-CoV-2 infection, the immune response mediates the damage of hematopoiesis system, cell inflammatory damage, microvascular system damage, abnormal activation of coagulation system, inhibition of fibrinolysis and anticoagulation system, which eventually leads to coagulation dysfunction. Thus, COVID-19 related coagulopathy is an early manifestation in the evolution of DIC, showing a dynamic change process.
The molecular markers of coagulation and fibrinolysis can reflect the pathological process of pre-DIC, including sTM, TAT, and plasminogen activator inhibitor-1 (PAI-1), but they are detected with difficulty in fever clinic worldwide, thus it is impossible to early predict the activation of coagulation in many cases. The criterion of sepsis associated coagulation disorder (SAC) formulated in the United States only uses two indexes, i.e. international standardized ratio (INR) and platelet count. SAC criterion is optional and convenient in the emergency or basic hospitals [23], nevertheless, INR and platelets did not show obvious alterations at the early stage of patients with COVID-19 in our study. Thus, it was not suitable for the coagulation abnormality induced by COVID-19. The platelet counts in most patients with COVID-19 were in normal range or slightly increased, but the mean level was controversial between severe and no-severe patients [6,24]. Other patients, especially severe and dead patients, the platelet counts might be reduced [6,24]. Recently, 50% of patients with COVID-19 reportedly increased D-dimer contents, and FDP and D-dimer levels were significantly elevated in severe and dead patients [23]. However, fibrinogen was markedly increased at the early stage of mild patients with COVID-19, and decreased at the late stage of severe patients [3].
The coagulation dysfunction or DIC induced by COVID-19 are a dynamic process, so the sensitivity and specificity of single index for DIC diagnosis are not good. Rational use of DIC integration system is conducive to the early diagnosis, prevention, and treatment of disease. Previous studies have shown that the ISTH criterion can diagnose septic DIC within a certain range. COVID-19 is a kind of sepsis, a viral sepsis. Therefore, it may have the characteristics of sepsis. In our clinical observation, we used ISTH system and ISTH score ≥2 points to identify the coagulation dysfunction. The results showed that patients with ISTH score (≥2) had a less proportion of the discharges of 14 days, 21 days and 28 days from admission. Multiple logistic regression analysis indicated that the proportion of patients with ISTH score ≥ 2 entering ICU was 4.07 times of those with ISTH score < 2, and the proportion of patients with mechanical ventilation was 5.54 times of patients with ISTH score < 2. Therefore, using the COVID-19 associated coagulopathy scoring system can identify potential critical COVID-19 patients at early.
4.1. Limitations
Our study has several limitations. Firstly, 32 cases had incomplete documentation of laboratory testing on admission within 24 h. Secondly, because 10 patients (8critical ill patients, 2 general patients) remained in the hospital and the outcomes were unknown at the time of data cutoff, we censored the data regarding their clinical outcomes as of the time of our analysis. Thirdly, the current study is a retrospective study with limited cases, it needs to be further confirmed by large sample cohort study.
5. Conclusions
Until now, no specific indicators of patients in early admission have been recommended for COVID-19. Currently, the approach to such disease is to control the source of infection and use of personal protection precaution to reduce the risk of transmission. Early diagnosis and identifying the patients who may become critical on the day of admission are of importance for the treatment of COVID-19. Our study demonstrated that the presence of coagulopathy identifies a group of patients with COVID-19 at higher risk for admission to ICU, and mechanical ventilation as well as discharges of 14 days, 21 days and 28 days from admission. ISTH score more than or equal to 2 points is a vital indicator of patients with COVID-19 on early admission.
Author contributions
Drs R Chen, B Yu, and D Ren had full access to all of the data in the study and take responsibility for the integrity of the data;
Statistical analysis: Y Liu,
Concept and design: M Wu
Drafting of the manuscript: Y Luan and M Wu
Administrative, technical, or material support: D Ren, X Liu, L Huang, M Peng, B Yu, R Chen, Y Liu, J Li, Y Feng
Drs Y Luan, Y Liu, X Liu and D Ren contributed equally and share first authorship. Dr Y Feng contributed equally to this article
Obtained funding: Y Luan, Y Feng, Ming Wu.
Declaration of competing interest
None reported.
Acknowledgments
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81873943), Sanming Project of Medicine in Shenzhen (SZSM20162011), Science and Technology Innovation Commission of Shenzhen Municipality(JCYJ20160425103130218, JCYJ20170306091335008) and Clinical Research Project of Shenzhen Municipal Health Commission (SZLY2017007).
Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F, Tan Wenjie, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan Wei-Jie, Ni Zheng-Yi, Hu Yu, Liang Wen-Hua, Ou Chun-Quan, He Jian-Xing, Liu Lei, Shan Hong, Lei Chun-Liang, Hui David S C, Du Bin, Li Lan-Juan, Zeng Guang, Yuen Kwok-Yung, Chen Ru-Chong, Tang Chun-Li, Wang Tao, Chen Ping-Yan, Xiang Jie, Li Shi-Yue, Wang Jin-Lin, Liang Zi-Jing, Peng Yi-Xiang, Wei Li, Liu Yong, Hu Ya-Hua, Peng Peng, Wang Jian-Ming, Liu Ji-Yang, Chen Zhong, Li Gang, Zheng Zhi-Jian, Qiu Shao-Qin, Luo Jie, Ye Chang-Jiang, Zhu Shao-Yong, Zhong Nan-Shan, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Da-wei, Hu Bo, Hu Chang, Zhu Fang-fang, Liu Xing, Zhang Jing, Wang Bin-bin, Xiang Hui, Cheng Zhen-shun, Xiong Yong, Zhao Yan, Li Yi-rong, Wang Xing-huan, Peng Zhi-yong. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Ning, Li Dengju, Wang Xiong, Sun Ziyong. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thachil Jecko, Tang Ning, Gando Satoshi, Falanga Anna, Cattaneo Marco, Levi Marcel, Clark Cary, Iba Toshiaki. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/JTH.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli Behnood, Madhavan Mahesh V, Jimenez David, Chuich Taylor, Dreyfus Isaac, Driggin Elissa, Nigoghossian Caroline Der, Ageno Walter, Madjid Mohammad, Guo Yutao, Tang Liang V, Hu Yu, Giri Jay, Cushman Mary, Quéré Isabelle, Dimakakos Evangelos P, Gibson C Michael, Lippi Giuseppe, Favaloro Emmanuel J, Fareed Jawed, Caprini Joseph A, Tafur Alfonso J, Burton John R, Francese Dominic P, Wang Elizabeth Y, Falanga Anna, McLintock Claire, Hunt Beverley J, Spyropoulos Alex C, Barnes Geoffrey D, Eikelboom John W, Weinberg Ido, Schulman Sam, Carrier Marc, Piazza Gregory, Beckman Joshua A, Steg P Gabriel, Stone Gregg W, Rosenkranz Stephan, Goldhaber Samuel Z, Parikh Sahil A, Monreal Manuel, Krumholz Harlan M, Konstantinides Stavros V, Weitz Jeffrey I, Gregory Y H Lip. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. Jun 16 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. January 28, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf (opens in new tab)
- 8.Kellum John A, Lameire Norbert, Aspelin Peter, Barsoum Rashad S., Burdmann Emmanuel A, Goldstein Stuart L, Herzog Charles A, Joannidis Michael, Kribben Andreas, Levey Andrew S, MacLeod Alison M, Mehta Ravindra L, Murray Patrick T, Naicker Saraladevi, Opal Steven M, Schaefer Franz, Schetz Miet, Uchino Shigehiko. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2013;2(1):1–138. [Google Scholar]
- 9.Kobashi Haruhiko, Toshimori Junichi, Yamamoto Kazuhide. Sepsis-associated liver injury: incidence, classification and the clinical significance. Hepatol Res. 2013;43(3):255–266. doi: 10.1111/j.1872-034X.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 10.Yin Yu-dong, Wunderink Richard G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badawi Alaa, Ryoo Seung Gwan. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Hai-bo, Penninger Josef M, Li Yi-min, Zhong Nan-shan, Slutsky Arthur S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu William J, Zhao Min, Liu Ke-fang, Xu Kun, Wong Gary, Tan Wen-jie, Gao George F. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir. Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Che Xiao-yan, Di Biao, Zhao Guo-ping, Wang Ya-di, Qiu Li-wen, Hao Wei, Wang Ming, Qin Peng-zhe, Liu Yu-fei, Chan Kwok-hong, Cheng Vincent C C, Yuen Kwok-yung. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003-2004 community outbreak of SARS in Guangzhou, China. Clin. Infect. Dis. 2006;43(1):e1–e5. doi: 10.1086/504943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Vincent C C, Lau Susanna K P, Woo Patrick C Y, Yuen Kwok Yung. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann Maximilian, Verleden Stijn E, Kuehnel Mark, Haverich Axel, Welte Tobias, Laenger Florian, Vanstapel Arno, Werlein Christopher, Stark Helge, Tzankov Alexandar, Li William W, Li Vincent W, Mentzer Steven J, Jonigk Danny. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Chao-lin, Wang Ye-ming, Li Xing-wang, Ren Li-li, Zhao Jian-ping, Hu Yi, Zhang Li, Fan Guohui, Xu Jiu-yang, Gu Xiao-ying, Cheng Zhen-shun, Yu Ting, Xia Jia-an, Wei Yuan, Wu Wen-juan, Xie Xue-lei, Yin Wen, Li Hui, Liu Min, Xiao Yan, Gao Hong, Guo Li, Xie Jun-gang, Wang Guang-fa, Jiang Rong-meng, Gao Zhan-cheng, Jin Qi, Wang Jian-wei, Cao Bin. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Ren-hong, Zhang Yuan-yuan, Li Ya-ning, Xia Lu, Guo Ying-ying, Zhou Qiang. Structural basis for the recognition of the 2019-nCoV by human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Crit. Care Med. 2001;29(7 Suppl):S36–S41. doi: 10.1097/00003246-200107001-00015. [DOI] [PubMed] [Google Scholar]
- 20.Witkowski Marco, Landmesser Ulf, Rauch Ursula. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med. 2016;26(4):297–303. doi: 10.1016/j.tcm.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Varga Zsuzsanna, Flammer Andreas J, Steiger Peter, Haberecker Martina, Andermatt Rea, Zinkernagel Annelies S, Mehra Mandeep R, Schuepbach Reto A, Ruschitzka Frank, Moch Holger. Endothelial Cell Infection and Endotheliitis in COVID-19. lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefrançais Emma, Ortiz-Muñoz Guadalupe, Caudrillier Axelle, Mallavia Beñat, Liu Feng-chun, Sayah David M, Thornton Emily E, Headley Mark B, David Tovo, Coughlin Shaun R, Krummel Matthew F, Leavitt Andrew D, Passegué Emmanuelle, Looney Mark R. The lung is a site of platelet bi ogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamakawa Kazuma, Yoshimura Jumpei, Ito Takashi, Hayakawa Mineji, Hamasaki Toshimitsu, Fujimi Satoshi. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb. Haemost. 2019;119(2):203–212. doi: 10.1055/s-0038-1676610. [DOI] [PubMed] [Google Scholar]
- 24.Chen Guang, Wu Di, Guo Wei, Cao Yong, Huang Da, Wang Hong-wu, Wang Tao, Zhang Xiao-yun, Chen Hui-long, Yu Hai-jing, Zhang Xiao-ping, Zhang Min-xia, Wu Shi-ji, Song Jian-xin, Chen Tao, Han Mei-fang, Li Shu-sheng, Luo Xiao-ping, Zhao Jian-ping, Ning Qin. Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]