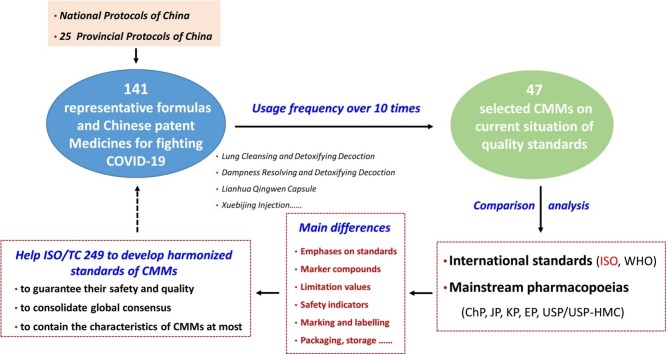
Graphical abstract
Keywords: Coronavirus disease 2019, Traditional Chinese medicine, Chinese Materia Medica, Quality standards, ISO/TC 249
Abstract
Purpose
Traditional Chinese medicine (TCM) has fully engaged and played an essential role in the prevention and treatment of Coronavirus Disease 2019 (COVID-19). This study compares relevant standards on high-frequent Chinese Materia Medicia (CMM) used in this pandemic aiming at reaching a global consensus and ensuring the use of Chinese medicines safely.
Methods
141 representative Chinese formulas and Chinese Patent Medicines from the National Protocol and the most of Provincial Protocols for controlling COVID-19 in China have been collected to statistical analyze the composition and characteristics of CMM. Among them, the domestic and international standards of 47 varieties with the frequency usage over 10 times were selected to compare their quality requirements in the mainstream pharmacopoeias and international standards.
Results
The quality requirements of used CMM for fighting COVID-19 on the terms of overall quality control, marker compounds, and safety indicators showed different patterns in these mainstream pharmacopoeias and international standards. The uniformed and scientific quality standards of CMM were urgently needed to promote global acceptation and trade.
Conclusions
These findings will provide evidence for building unified quality and safety standards that can adapt to the characteristics of CMM and promote international trade, and also will be stated that it is of the highest priority for ISO/TC 249 to formulate high-quality standards that consolidate international consensus to ensure quality and safety of the urgently needed CMM.
1. Introduction
Since the outbreak of Coronavirus Disease 2019 (COVID-19) in China in December 2019, in the absence of a specific Western medicine and vaccine, traditional Chinese medicine (TCM) has played an essential role in COVID-19 prevention and treatment in China. From the third trial version onwards of the seventh edition of Diagnosis and Treatment Protocol for COVID-19 (the National Protocol) issued by the General Office of National Health Commission of China and State Administration of TCM, it has been clarified that COVID-19 falls under the category of "pestilences" in TCM. In accordance with the treatment principle of abidance by individuality, locality and seasons and the National Protocol, China's provinces (autonomous regions and municipalities directly under the Central Government) have formulated and released regional-specific TCM treatment and prevention protocols based on regional characteristics (the Provincial Protocol) [1,2]. With the full play of the characteristics and advantages of TCM, integrated Chinese and Western treatment has achieved satisfactory result [[3], [4], [5], [6]].
It is widely acknowledged that TCM plays an important role and is one of the merits in the epidemic prevention and control. A press conference held on the effectiveness of TCM in the treatment of COVID-19 cases in Wuhan reported that clinical observation showed that TCM has proven to be effective in the treatment of over 90 % of all confirmed COVID-19 cases on the Chinese mainland, said a TCM official on March 24, 2020. Among these confirmed cases, 74,187 were given TCM products, accounting for 91.5 % of the total [7]. It was found that TCM helped to relieve the symptoms, reduce the progression from mild to severe and critical, increase recovery rate, decrease mortality rate, and bring the convalescent to normal body functions [[8], [9], [10], [11]]. Meanwhile, we noticed that China has adopted the methods of integrating traditional Chinese medicine with western medicine which led to a better outcome of this pandemic.
As Chinese Materia Medica (CMM) played a key role in this pandemic, its standards on quality and safety are attracting more attentions from the public. ISO/TC 249 has accumulated rich experience in developing international standards on CMM and relevant monographs have been recorded in national pharmacopoeias which enable CMM to be used globally. In this study, we aimed at analyzing the above-mentioned standards on CMM to recognize the current challenges in the process of their development.
2. Materials and methods
141 representative formulas and Chinese Patent Medicines from the National Protocol (the third trial version onwards of the seventh edition) and the main 25 Provincial Protocol of China [1,2] were selected and the usage frequency of each herbal medicine was counted. There are 47 varieties of CMM with the usage frequency over 10 times. Their botanical origins, morphological identification, and qualitative identification, quantification of marker compounds, heavy metals and residual pesticides in the following mainstream pharmacopoeias and international standards were summarized and compared,
Chinese Pharmacopoeia (ChP),
The Japanese Pharmacopoeia (JP),
The Korean Pharmacopoeia (KP),
European Pharmacopoeia (EP),
U.S. Pharmacopoeia (USP),
U.S. Pharmacopoeia-Herbal Medicine Compendium (USP-HMC),
WHO monographs on selected medicinal plants, and
ISO standards.
3. Results and discussions
3.1. Statistical analysis of Chinese Materia Medica used in the Chinese formulas and Chinese Patent Medicines for COVID-19 prevention and treatment
There are 231 varieties of CMM used in the 141 representative formulas and Chinese Patent Medicines, covering botanical medicines, mineral medicines, and animal medicines. Among them, botanical medicines account for 83.55 % (193 varieties), mineral medicines for 8.66 % (20 varieties) and animal medicines for 7.79 % (18 varieties), and the number varieties types between mineral medicines and animal medicines is close to each other. The CMM used in the formulas over 10 times account for 20.35 % (47 varieties), including 45 varieties of botanical medicines, one variety of mineral medicine, and one variety of animal medicine (Table 1 ).
Table 1.
The Chinese Materia Medica of 141 representative formulas and Chinese Patent Medicines for COVID-19 prevention and treatment (usage frequency≥10 times).
| Rank | Botanical/mineral/animal Name | Pharmaceutical Name | Chinese Name | English Name | Frequency of use (times) |
|---|---|---|---|---|---|
| 1 | Glycyrrhiza uralensis Root and Rhizome/ Glycyrrhiza inflata Root and Rhizome/ Glycyrrhiza glabra Root and Rhizome | Glycyrrhizae Radix et Rhizoma | 甘草 | Licorice Root | 66 |
| 2 | Prunus armeniaca var. ansu Seed/ Prunus sibirica Seed/ Prunus mandshurica Seed/ Prunus armeniaca Seed | Armeniacae Semen Amarum | 苦杏仁 | Bitter Apricot Seed | 43 |
| 3 | Gypsum | Gypsum Fibrosum | 石膏 | Plaster | 36 |
| 4 | Scutellaria baicalensis Root | Scutellariae Radix | 黄芩 | Baical Skullcap Root | 35 |
| 5 | Forsythia suspensa Fruit | Forsythiae Fructus | 连翘 | Weeping Forsythia Capsule | 34 |
| 6 | Poria cocos Sclerotium | Poria | 茯苓 | Indian Bread | 33 |
| 7 | Ephedra sinica Stem/ Ephedra intermedia Stem/ Ephedra equisetina Stem | Ephedrae Herba | 麻黄 | Ephedra | 33 |
| 8 | Citrus reticulata Pericarp | Citri Reticulatae Pericarpium | 陈皮 | Dried Tangerine Peel | 31 |
| 9 | Pogostemon cablin Herb | Pogostemonis Herba | 广藿香 | Cablin Patchouli Herb | 31 |
| 10 | Lonicera japonica Flower | Lonicerae Japonicae Flos | 金银花 | Japanese Honeysuckle Flower | 30 |
| 11 | Platycodon grandiflorum Root | Platycodonis Radix | 桔梗 | Platycodon Root | 28 |
| 12 | Pinellia ternata Tuber | Pinelliae Rhizoma | 半夏 | Pinellia Tuber | 28 |
| 13 | Magnolia officinalis Bark/ Magnolia officinalis var. biloba Bark | Magnoliae Officinalis Cortex | 厚朴 | Officinal Magnolia Bark | 26 |
| 14 | Ophiopogon japonicus Root Tuber | Ophiopogonis Radix | 麦冬 | Dwarf Lilyturf Tuber | 23 |
| 15 | Atractylodes macrocephala Rhizome | Atractylodis Macrocephalae Rhizoma | 白术 | Largehead atractylodes Rhizome | 23 |
| 16 | Atractylodes chinense Rhizome/ Atractylodes lancea Rhizome | Atractylodis Rhizoma | 苍术 | Atractylodes Rhizome | 22 |
| 17 | Rheum palmatum Root and Rhizome/ Rheum tanguticum Root and Rhizome/ Rheum officinale Root and Rhizome | Rhei Radix et Rhizoma | 大黄 | Rhurbarb | 21 |
| 18 | Astragalus membranaceus var. mongholicus Root/ Astragalus membranaceus Root | Astragli Radix | 黄芪 | Milkvetch Root | 20 |
| 19 | Phragmites communis Trin. Rhizome | Phragmitis Rhizoma | 芦根 | Reed Rhizome/ Rhizome Phragmitis | 19 |
| 20 | Panax ginseng Root | Ginseng Radix et Rhizoma | 人参 | Ginseng | 19 |
| 21 | Descurainia sophia Seed/ Lepidium apetalum Seed | Descurainiae Semen/ Lepidii Semen | 葶苈子 | Pepperweed Seed/ Tansymustard Seed | 17 |
| 22 | Schisandra chinensis Fruit | Schisandrae Chinensis Fructus | 五味子 | Chinese Magnoliavine Fruit | 17 |
| 23 | Anemarrhena asphodeloides Rhizome | Anemarrhenae Rhizoma | 知母 | Common Anemarrhena Rhizome | 15 |
| 24 | Paeonia lactiflora Root/ Paeonia veitchii Root | Paeoniae Radix Rubra | 赤芍 | Red Peony Root | 15 |
| 25 | Trichosanthes kirilowii Seed/ Trichosanthes rosthornii Seed | Trichosanthis Semen | 瓜蒌子 | Snakegound Seed | 15 |
| 26 | Bupleurum chinense Root/ Bupleurum scorzonerifolium Root | Bupleuri Radix | 柴胡 | Chinese Thorowrax Root | 14 |
| 27 | Prunus persica Seed/ Prunus davidiana Seed | Persicae Semen | 桃仁 | Peach Seed | 14 |
| 28 | Rehmannia glutinosa Root | Rehmanniae Radix | 地黄 | Rehmannia Root | 14 |
| 29 | Pseudostellaria heterophylla Root | Pseudostellariae Radix | 太子参 | Heterophylly Falsestarwort Root | 13 |
| 30 | Morus alba Bark | Mori Cortex | 桑白皮 | Mulberry Root Bark | 13 |
| 31 | Amomum tsao-ko Fruit | Tsaoko Fructus | 草果 | Caoguo | 13 |
| 32 | Areca catechu Seed | Arecae Semen | 槟榔 | Areca Seed | 13 |
| 33 | Coix lacrymajobi var. mayuen Kernel | Coicis Semen | 薏苡仁 | Coix Seed | 13 |
| 34 | Artemisia Annua Herb | Artemisiae Annuae Herba | 青蒿 | Sweet Wormwood Herb | 12 |
| 35 | Cornus officinalis Sarcocarp | Corni Fructus | 山茱萸 | Asiatic Cornelian Cherry Fruit | 12 |
| 36 | Perilla frutescens Leaf | Perillae Folium | 紫苏叶 | Perilla Leaf | 11 |
| 37 | Processed Aconitum carmichaeli Daughter Root | Aconiti Lateralis Radix Praeparata | 附子 | Prepared Common Monkshood Daughter Root | 11 |
| 38 | Codonopsis pilosula Root/ Codonopsis pilosula var. modesta Root/ Codonopsis tangshen Root | Codonopsis Radix | 党参 | Tangshen | 10 |
| 39 | Lophatherum gracile Stem and Leaf | Lophatheri Herba | 淡竹叶 | Lophatherum Herb | 10 |
| 40 | Saposhnikovia divaricata Root | Saposhnikoviae Radix | 防风 | Divaricate Saposhnikovia Root | 10 |
| 41 | Zingiber officinale Rhizome | Zingiberis Rhizoma | 生姜 | Zingiber | 10 |
| 42 | Picrorhiza scrophulariiflora Rhizome | Picrorhizae Rhizoma | 胡黄连 | Figwortflower Picrohiza Rhizome | 10 |
| 43 | Gardenia jasminoides Fruit | Gardeniae Fructus | 栀子 | Cape Jasmine Fruit | 10 |
| 44 | Fritillaria thunbergii Bulb | Fritillariae Thunbergii Bulbus | 浙贝母 | Thunberg Fritillary Bulb | 10 |
| 45 | Mentha haplocalyx Herb | Menthae Haplocalycis Herb | 薄荷 | Peppermint | 10 |
| 46 | Notopterygium incisum Rhizome and Root/ Notopterygium franchetii Rhizome and Root | Notopteryqii Rhizoma et Radix | 羌活 | Incised Notopterygium Rhizome or Root | 10 |
| 47 | Bubalus bubalis Cornu | Bubali Cornu | 水牛角 | Buffalo Horn | 10 |
The commonly used CMM are Glycyrrhizae Radix et Rhizoma (gān căo), Armeniacae Semen Amarum (xìng rén), Gypsum Fibrosum (shí gāo), Scutellariae Radix (huáng qín), Forsythiae Fructus (lián qiào), Ephedrae Herba (má huáng), Lonicerae Japonicae Flos (jīn yín huā), Atractylodis Macrocephalae Rhizoma (bái zhú), etc. The formulas for COVID-19 have been put forward combined with TCM theory and clinical practice, and the commonly used pairs of CMM are Ephedrae Herba-Armeniacae Semen Amarum (má huáng-xìng rén), Ephedrae Herba-Gypsum Fibrosum (má huáng-shí gāo), Agastachis Herba-Magnoliae Officinalis Cortex (huò xiāng-hòu pò), Lonicerae Japonicae Flos-Forsythiae Fructus (jīn yín huā-lián qiào), Lepidii Semen-Gypsum Fibrosum (tíng lì zĭ-shí gāo).
3.2. Current situations of quality standards for CMM and comparative analysis
The quality standards of CMM is a key technical link for CMM to break the barriers of international trade and gain access to international markets. Under the framework of the globalization of CMM, with the ultimate goal to gain access to international markets, it is indispensable to promote internationalization of CMM by formulating a scientific, advanced and applicable system for quality standards with international recognition to effectively ensure efficacy and safety of CMM.
The current quality standards of the above selected 47 varieties of CMM in the mainstream pharmacopoeias and international standards are shown as follows.
3.2.1. Situation of the standards
3.2.1.1. Situation of standards collected by the mainstream pharmacopoeias [[12], [13], [14], [15], [16], [17]]
All varieties of used CMM have corresponding quality standards in ChP, with more than 85 % collected in JP and KP but with fewer description in EP and USP/ USP-HMC for 31.91 % and 25.53 %, respectively (Table 2 ).
Table 2.
The status of the collection of standards for 47 varieties of Chinese Materia Medica (usage frequency ≥ 10 times) in the mainstream pharmacopoeias and international organizations.
| Rank | Pharmaceutical Name | ChP | JP | KP | EP | USP | ISO | WHO |
|---|---|---|---|---|---|---|---|---|
| 1 | Glycyrrhizae Radix et Rhizoma | √ | √ | √ | √ | √ | √a | √ |
| 2 | Armeniacae Semen Amarum | √ | √ | √ | - | √ | - | √ |
| 3 | Gypsum Fibrosum | √ | √ | √ | - | √ | - | - |
| 4 | Scutellariae Radix | √ | √ | √ | √ | √ | √ | √ |
| 5 | Forsythiae Fructus | √ | √ | √ | - | √ | - | - |
| 6 | Poria | √ | √ | √ | √ | - | - | - |
| 7 | Ephedrae Herba | √ | √ | √ | √ | - | - | √ |
| 8 | Citri Reticulatae Pericarpium | √ | √ | √ | - | √ | - | - |
| 9 | Pogostemonis Herba | √ | √ | √ | - | - | - | - |
| 10 | Lonicerae Japonicae Flos | √ | √ | √ | - | √ | √ | - |
| 11 | Platycodonis Radix | √ | √ | √ | - | - | √a | √ |
| 12 | Pinelliae Rhizoma | √ | √ | √ | - | - | √a | - |
| 13 | Magnoliae Officinalis Cortex | √ | √ | √ | - | - | - | √ |
| 14 | Ophiopogonis Radix | √ | √ | √ | - | - | - | - |
| 15 | Atractylodis Macrocephalae Rhizoma | √ | √ | √ | √ | - | - | - |
| 16 | Atractylodis Rhizoma | √ | √ | √ | √ | - | - | - |
| 17 | Rhei Radix et Rhizoma | √ | √ | √ | √ | - | √a | √ |
| 18 | Astragli Radix | √ | √ | √ | √ | - | √ | √ |
| 19 | Phragmitis Rhizoma | √ | - | - | - | - | - | - |
| 20 | Ginseng Radix et Rhizoma | √ | √ | √ | √ | √ | √a | √ |
| 21 | Descurainiae Semen/ Lepidii Semen | √ | - | - | - | - | - | |
| 22 | Schisandrae Chinensis Fructus | √ | √ | √ | √ | √ | √ | √ |
| 23 | Anemarrhenae Rhizoma | √ | √ | √ | √ | - | - | - |
| 24 | Paeoniae Radix Rubra | √ | √ | √ | √ | √ | - | - |
| 25 | Trichosanthis Semen | √ | √ | √ | - | - | - | - |
| 26 | Bupleuri Radix | √ | √ | √ | - | - | √ | √ |
| 27 | Persicae Semen | √ | √ | √ | - | - | - | - |
| 28 | Rehmanniae Radix | √ | √ | √ | - | √ | √a | √ |
| 29 | Pseudostellariae Radix | √ | - | - | - | - | - | - |
| 30 | Mori Cortex | √ | √ | √ | - | - | - | - |
| 31 | Tsaoko Fructus | √ | √ | √ | - | - | - | - |
| 32 | Arecae Semen | √ | √ | √ | - | - | √ | - |
| 33 | Coicis Semen | √ | √ | √ | √ | √ | - | - |
| 34 | Artemisiae Annuae Herba | √ | - | - | - | - | - | - |
| 35 | Corni Fructus | √ | √ | √ | - | - | - | - |
| 36 | Perillae Folium | √ | √ | √ | - | - | √ | - |
| 37 | Aconiti Lateralis Radix Praeparata | √ | √ | √ | - | - | √ | - |
| 38 | Codonopsis Radix | √ | √ | √ | √ | - | - | - |
| 39 | Lophatheri Herba | √ | - | - | - | - | - | - |
| 40 | Saposhnikoviae Radix | √ | √ | √ | - | - | √a | - |
| 41 | Zingiberis Rhizoma | √ | √ | √ | - | - | - | √ |
| 42 | Picrorhizae Rhizoma | √ | √ | √ | - | - | √a | √ |
| 43 | Gardeniae Fructus | √ | √ | √ | √ | - | - | - |
| 44 | Fritillariae Thunbergii Bulbus | √ | √ | √ | - | - | - | - |
| 45 | Menthae Haplocalycis Herb | √ | √ | √ | - | - | - | - |
| 46 | Notopteryqii Rhizoma et Radix | √ | √ | - | - | - | - | - |
| 47 | Bubali Cornu | √ | - | - | - | - | - | - |
| Coverage ratio of standards for the 47 varieties (%) | 100.00 | 87.23 | 85.11 | 31.91 | 25.53 | 30.04 | 29.79 | |
These CMM as new work item proposal (NWIP) have been submitted into ISO/TC 249 platform, and they have not been officially approved as new projects.
3.2.1.2. Situation of standards collected by WHO monographs and ISO [18,19]
There are 14 varieties included in WHO monographs on selected medicinal plants, and 16 varieties included in ISO international standards. For ISO standards, 2 varieties of CMM, Lonicerae Japonicae Flos and Scutellariae Radix have been officially published, and 14 varieties are under development stage (Table 3 ).
Table 3.
The list of 47 varieties of Chinese Materia Medica with usage frequency over10 times included by ISO/TC 249 platform.
| No. | English Name | Chinese Name | Sponsor Countries | Current Stage |
|---|---|---|---|---|
| 1 | ISO 21317:2019 Traditional Chinese medicine—Lonicera japonica flower | 中医药—金银花 | China | Published |
| 2 | ISO 22988:2020Traditional Chinese medicine—Astragalus mongholicus root | 中医药—蒙古黄芪 | China | |
| 3 | ISO 23,962 Traditional Chinese Medicine—Processed Aconitum carmichaeliilateral root | 中医药—附子 | China | Draft international Standard (DIS) |
| 4 | ISO 22,585 Traditional Chinese Medicine—Codonopisispilosula root | 中医药—党参 | China | Working Draft (WD) |
| 5 | ISO 23,965 Traditional Chinese Medicine—Bupleurum chinense and Bupleurum scorzonrifoliumroot | 中医药—柴胡 | China | |
| 6 | ISO 4564 Traditional Chinese Medicine—Scutellariabaicalensis Georgi root | 中医药—黄芩 | China | |
| 7 | Traditional Chinese Medicine—Panax ginseng root | 中医药—人参 | China | New Work Item Proposal (NWIP) |
| 8 | Traditional Chinese medicine—Glycyrrhiza uralensis root and rhizome | 中医药—甘草 | China | |
| 9 | Traditional Chinese medicine—Pinelliaternata tuber | 中医药—半夏 | China | |
| 10 | Traditional Chinese medicine—Rehmanniaglutinosa root | 中医药—地黄 | China | |
| 11 | Traditional Chinese Medicine—Arecae Semen | 中医药—槟榔 | China | |
| 12 | Traditional Chinese Medicine—Saposhnikoviadivaricata root and rhizome | 中医药—防风 | China | |
| 13 | Traditional Chinese Medicine— Platycodon grandiflorum root | 中医药—桔梗 | Republic of Korea | |
| 14 | Traditional Chinese medicine— Schisandra chinensis fruit | 中医药—五味子 | Republic of Korea | |
| 15 | Traditional Chinese Medicine —Rheum root and rhizome | 中医药—大黄 | Germany | |
| 16 | Traditional Chinese Medicine—Coptis rhizome | 中医药—黄连 | Canada |
3.2.1.3. Situation of standards collected by the mainstream pharmacopoeias and international standards
The standard of Scutellariae Radix is the only CMM that has been recorded in ChP, JP, KP, EP, USP, ISO and WHO monograph. The standards of Glycyrrhizae Radix et Rhizoma, Ginseng Radix et Rhizoma, and Schisandrae Chinensis Fructus have been recorded in ChP, JP, KP, EP, USP and WHO other than ISO.
3.2.2. Comparative analysis of the standards and current challenges
Based on the characteristics of CMM, the standards of the mainstream pharmacopoeias and international standards mainly focus on the safety issues of CMM, such as botanical origins, morphological identification, and qualitative identification, quantification of marker compounds, heavy metals and residual pesticides [[11], [12], [13], [14], [15], [16], [17], [18]]. However, the mainstream pharmacopoeias and international standards have different emphases on standards in terms of overall quality control of CMM, selection of marker compounds, safety issues and others. Taking Scutellariae Radix as an example, the comparative analysis of the standards among the mainstream pharmacopoeias, ISO and other international standards is as follows (Table 4 ):
-
1)
Apart from the basic contents such as botanical origins, morphological features, microscopic characteristics, qualitative identification, purity test, assay, and storage, etc. of the CMM, ChP has included other factors including nature, flavor and meridian distribution, functions and indications, usage and dosage; while ISO and USP standards elaborate on packaging, transportation and labeling.
-
2)
Purity test includes moisture and total ash contents, but with differences in limit values of each standard.
-
3)
The regulations and requirements on safety indicators such as heavy metals and residual pesticides showed a different pattern in these mainstream pharmacopoeias and international standards.
-
4)
The main differences of the standards are the gaps in the selection of marker compounds and limitation regulation. The single marker compound of baicalin is used for quantitative analysis in ChP, EP, JP, ISO, and WHO standards, while apart from baicalin, multi-marker compounds are referred in KP and USP.
Table 4.
The comparative analysis of quality standards for Scutellariae Radix in the standards of the mainstream pharmacopoeias, WHO and ISO.
| Items | ChP, 2015 | JP, 17th | KP, 11th | EP, 9.0 | USP-HMC | WHO monograph | ISO | |
|---|---|---|---|---|---|---|---|---|
| Definition | √ | √ | √ | √ | √ | √ | √ | |
| Description | √ | √ | √ | √ | √ | √ | √ | |
| Identification | Microscopic | √ | √ | √ | √ | √ | √ | √ |
| TLC | Reference crude drug; Baicalin; Baicalein; Wogonin | Reference crude drug; Baicalin | Reference crude drug | Baicalin; acteoside |
Reference crude drug; Baicalin; Baicalein | Baicalin | — | |
| Loss on drying | ≤10.0 % | ≤12.0 % | ≤15.0 % | ≤12.0 % | ≤12.0 % | ≤12.0 % | ≤15.0 % | |
| Total ash | ≤6.0 % | ≤5.0 % | ≤6.0 % | ≤6.0 % | ≤6.0 % | ≤6.0 % | ≤6.0 % | |
| Acid-insoluble Ash | — | — | ≤1.0 % | ≤2.0 % | ≤1.0 % | ≤1.0 % | — | |
| Ethanol-soluble extractives | ≥40.0 % | — | ≥24.0 % | — | ≥18.0 % | ≥15.0 % | ≥26.0 % | |
| Water-soluble extractives | — | — | — | — | ≥28.0 % | ≥40.0 % | — | |
| Assay | Baicalin: ≥9.0 % | Baicalin: ≥10.0 % | ≥10.0 % (total of Baicalin, baicalein and wogonin) | Baicalin: ≥9.0 % | ≥11.0 % (total of Baicalin and wogonin-7-O-glucuronide); ≥3.50 % (total of Baicalin and wogonin) |
Baicalin: ≥9.0 % | Baicalin: ≥8.0 % | |
| Heavy metals | — | √ | √ | √ | √ | √ | √ | |
| Pesticide residuals | — | — | √ | √ | √ | √ | √ | |
| Sulfur dioxide | ≤ 150 ppm | — | ≤ 30 ppm | — | — | — | — | |
3.3. Necessity of formulating international standards for CMM and characteristics of ISO/TC 249 international standards for CMM
Pharmacopoeias are the important technical code for quality and safety management of medicines in different countries and are the legal norms and evidence for the entry to the pharmaceutical markets. Through the coordination of pharmacopoeia standards, global free trade of high-quality pharmaceutical products can be promoted. Although TCM has been widely promoted globally, due to the inherent characteristics of CMM and the sparks between traditional and modern evaluation systems, CMM has been variously identified in different countries and has difficulties in international registration [[20], [21], [22]]. The phenomena mainly result from the barriers to connect the quality standards for CMM with the mainstream markets in Europe and US, and restrictions of medical regulations and policies in different countries/regions. Major differences were identified in the classification of CMM and its products, market entry pathways, requirements of compliance with Good Manufacturing Practices; and level of evidence to demonstrate safety and efficacy based on historical use, non-clinical and clinical studies. Variations in the evaluation standards adopted by regulatory authorities pose a number of barriers and opportunities for the internationalization of TCM products [22].
It is not easy to fully understand the properties and curative effects of CMMs under the current knowledge and methodologies we have had. On the other hand, some western countries still have stipulated strict regulations on the registration of Chinese medicines before they could be sold. Such is the dilemma we meet: trying to follow the modern evidence-based approaches for registration while retaining unique characteristics of TCM. It can be highly desirable to develop ISO standards on each herbal medicine to serve as a reference during those registration processes.
Although the pharmacopoeias of different countries have a certain global impact, due to the disparities in initial motivations and target groups of the pharmacopoeias, it is difficult to apply to the standard of a specific country as supporting evidence for arbitration in international arbitration once a trade dispute occurs. Furthermore, due to the limited varieties of CMM included in the pharmacopoeias of Europe, US and other countries, or the disputes in the selection of the marker compounds, it is difficult for the existing standards to adapt to the needs of international trade of CMM.
Based on consultative and consensus-based processes, ISO/TC249 provides a platform to develop standards which represent global expert opinion across consumers, practitioners, industry, academics from universities, and government. For standards on CMMs, there are still large disparities in the levels of requirements between a range of countries and their national pharmacopoeias. Therefore, ISO/TC249 can serve the purpose of filling the gaps and supporting the appropriate use of CMMs. With a focus on the demands for the international market, ISO/TC 249 has been dedicated to filling in the blanks of the universal standards for international trade of CMM, stipulating international standards for CMM, strengthening quality control and safety requirements of CMM, and conducting a large amount of research on the detection of residual pesticides and heavy metals, etc.. In order to complete the above tasks in an organized manner, ISO/TC 249 have formulated and released ‘ISO/TR 23975: 2019 Traditional Chinese medicine–Priority list of single herbal medicines for developing standards’ [23] from the needs of international trade, the status of pharmacopoeias in different countries, the degree of safety issues of CMM, the status of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) collection, and the consensus of experts in all countries. It aims at establishing unified quality standards that can adapt to the characteristics of CMM, ensure safety and effectiveness, and are applicable to international trade. With the new challenges and suggestions that COVID-19 brings us, the commonly used CMM in COVID-19 prevention and treatment need to be prioritized more appropriate, and the international standards should be published in a timely manner. With the endorsement of ISO international standards, TCM should play its role in the prevention and treatment of major infectious diseases worldwide. By comparative studies, the data has shown that among the 47 varieties of CMM with a usage frequency over 10 times for fighting COVID-19, only 22 varieties (46.81 %) are currently included in ISO/TR 23975. In other words, there is much to be engaged for ISO international standards in this field.
3.4. Further thoughts on international standards of ISO/TC 249 in responding to major emergencies of public health
The outbreak of COVID-19 reminds us that in dealing with major emergencies of global public health, it is of great importance to transcend the borders and build a global community of shared wellbeing now. The understanding and diagnosis and treatments of TCM for diseases and health care, over hundreds of years’ application supports the efficiency and safety of Chinese medicines, and its contribution to the wellbeing should be shared by the mankind. It is an important mission of ISO/TC 249 to promote TCM contribution to the wellbeing of the mankind by stipulating international standards of high quality.
CMM have the characteristics of diversity and complexity of ingredients, which is difficult to comprehensively evaluate by the quality control and assessment mode of single ingredient of modern chemical medicines. For the key scientific problems urgently needed to be solved in the internationalization and standardization of CMM, with the focus on the methodology for comprehensive characterization of the overall composition of CMM and the establishment of quality evaluation system, the demarcation of toxic CMM between effectiveness and safety, and the formulation of the limitation range of effect-toxic ingredients [[24], [25], [26]], we need to integrate the concepts of stipulating standards of the mainstream pharmacopoeias and ISO standards, etc., and to establish a scientific quality standard system that can adapt to the characteristics of CMM and can be generally recognized by the international community. Based on the experience of fighting COVID-19, it is urgent for ISO/TC 249 to formulate high-quality standards that consolidate international consensus to ensure the quality and safety of the urgently needed CMM.
4. Conclusions
In this study, the characteristics of used CMM for prevention and treatment of COVID-19, and the quality requirements of 47 main varieties of CMM in mainstream pharmacopoeias and international standards were comprehensively analyzed and compared. These findings provide evidence for developing harmonized standards which can be relied on to guarantee the safety and quality of CMM. This study also stated that it is of the highest priority for ISO/TC 249 to formulate high-quality standards that can consolidate global consensus and contain the characteristics of CMM at most.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
This work was supported Three-year action plan for the development of TCM in Shanghai-Construction on international standardization of TCM in ISO (No. ZY (2018-2020)-GJHZ-1003); the Science and Technology Development Fund, Macao SAR (File no. 0061/2019/AGJ, 0027/2017/AMJ and 062/2017/A2); and Special project of Technical Barriers to Trade in Shanghai (No. 19TBT019).
References
- 1.General Office of the National Health and Health Commission, Office of the State Administration of Traditional Chinese Medicine. Notice on Issuing a New Coronary Virus Pneumonia Diagnosis and Treatment guidelines (Trial Version 7) [EB/OL]. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml, (accessed Mar 4, 2020, in Chinese).
- 2.Jiangsu Research Center for Chinese Medicine Development . 2020. Trend Book of Diagnosis and Treatment Protocol of Traditional Chinese Medicine for COVID-19. [EB/OL]. http://www.jstcm.com/uploadfile/20200228215356601.pdf (accessed Feb 19, 2020, in Chinese) [Google Scholar]
- 3.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese–Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am. J. Chin. Med. 2020;43:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 4.Ni L., Zhou L., Zhou M., Zhao J., Wang D.W. Combination of western medicine and chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front. Med. 2020;14:210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The State Council Information Office, The People’s Republic of China, TCM treatment effective on over 90% of COVID-19 patients on China’s mainland: Official [EB/OL], http://english.scio.gov.cn/pressroom/2020-03/24/content_75852369.htm (accessed March 24, 2020, in English).
- 8.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen Capsules, a repurposed chinese Herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D., Zhang B., J.T Lv, Sa R.N., Zhang X.M., Lin Z.J. The clinical benefits of chinese patent medicines against COVID-19 based on current evidence. Pharmacol. Res. 2020;157:104882. doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo E., Zhang D., Luo H., Liu B., Zhao K., Zhao Y., Bian Y., Wang Y. Treatment efficacy analysis of traditional chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province. China, Chin. Med. 2020;15:34. doi: 10.1186/s13020-020-00317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-New coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Pharmacopoeia Committee . Chemical Industry Press; Beijing: 2015. Chinese Pharmacopoeia, Part 1. [Google Scholar]
- 13.Japanese Pharmacopoeia Convention . 17th ed. Society of Japanese Pharmacopoeia; Tokyo: 2016. Japanese Pharmacopoeia. [Google Scholar]
- 14.Korea food and drug administration . 10th ed. Yakup Daily, Seoul; 2002. The Korean Herbal Pharmacopoeia. [Google Scholar]
- 15.European Pharmacopoeia. 9th ed. 2016. The European pharmacopoeia directorate for the quality of medicines & HealthCare of the council of Europe (EDQM) [Google Scholar]
- 16.The United States Pharmacopieial Convention . 2016. The United States Pharmacopeia (USP40-NF35), New York. [Google Scholar]
- 17.The United States Pharmacopieial Convention . 2015. United States Pharmacopoeia-Herbal Medicines Compendium 1.0. [Google Scholar]
- 18.World Health Organization (WHO): Essential medicines and health products-WHO monographs on Selected Medicinal Plants (Vol.1-Vol.3), https://www.who.int/medicines/technical_briefing/tbs/2009_traditionalmedicines_rdg_prs/en/.
- 19.The International Organization for Standardization/ Technical Committee of Traditional Chinese medicine (ISO/TC 249): https://www.iso.org/committee/598435.html.
- 20.Lin A.X., Chan G., Hu Y., Ouyang D., Ung C.O.L., Shi L., Hu H. Internationalization of traditional Chinese medicine: current international market, internationalization challenges and prospective suggestions. Chin. Med. 2018;13(9):1–6. doi: 10.1186/s13020-018-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoess W., Wiesner J. The globalization of Traditional Medicine: perspectives related to the European Union regulatory environment. Engineering. 2019;5:22–31. [Google Scholar]
- 22.Li J., Zhu J., Hu H., Harnett J.E., Lei C.I., Chau K.Y., Chan G., Ung C.O.L. Internationalization of Traditional/Complementary Medicine products: market entry as medicine. Chin. Med. 2018;13(50):1–15. doi: 10.1186/s13020-018-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISO/TR 23975 . 2019. Traditional Chinese Medicine–Priority List of Single Herbal Medicines for Developing Standards. [Google Scholar]
- 24.Wu W.Y., Yang W.Z., Hou J.J., Guo D.A. Current status and future perspective in the globalization of traditional Chinese medicines. World J. Tradit. Chin. Med. 2015;1(1):1–4. [Google Scholar]
- 25.Gao Y., Liang A., Fan X., Hu L., Hao F., Li Y. Safety research in traditional Chinese medicine: methods, applications, and outlook. Engineering. 2019;5:76–82. [Google Scholar]
- 26.Zhang T., Bai G., Han Y., Xu J., Gong S., Li Y., Zhang H., Liu C. The method of quality marker research and quality evaluation of traditional Chinese medicine based on drug properties and effect characteristics. Phytomedicine. 2018;15:204–211. doi: 10.1016/j.phymed.2018.02.009. [DOI] [PubMed] [Google Scholar]