Abstract
Background
Several comorbidities have been associated with an increased risk of severity and mortality in coronavirus disease 2019 (COVID-19), including hypertension, diabetes, cerebrovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease.
Purpose
In this systematic review and meta-analysis, we attempted to investigate the association between heart failure (HF) and poor outcome in patients with COVID-19.
Methods
We performed a systematic literature search from PubMed, EuropePMC, SCOPUS, Cochrane Central Database, and medRxiv with the search terms, “Heart failure” and “COVID-19”. The outcome of interest was mortality and poor prognosis (defined by incidence of severe COVID-19 infection, admission to ICU, and use of ventilator) in patients with preexisting heart failure with coronavirus disease.
Results
We identified 204 potential articles from our search, and 22 duplicates were removed. After screening of the titles and abstracts of the remaining 182 articles we identified 92 potentially relevant articles. We excluded 74 studies due to the following reasons: four studies were systematic reviews, two studies were meta-analyses, three articles were literature reviews, and 65 articles did not report on the outcome of interest. Finally, we included the remaining 18 studies in our qualitative synthesis and meta-analysis. There were 21,640 patients from 18 studies. HF was associated with hospitalization in COVID19 HR was 2.37 [1.48, 3.79; p < 0.001], high heterogeneity [I2, 82%; p < 0.001]. HF was associated with a poor outcome demonstrated by an OR of 2.86 [2.07; 3.95; p < 0.001] high heterogeneity [I2, 80%; p < 0.001]. Patient with preexisting HF was associated with higher mortality OR of 3.46 [2.52, 4.75; p < 0.001] moderately high heterogeneity [I2, 77%; p < 0.001].
Conclusion
Patients with heart failure are at increased risk for hospitalization, poor outcome, and death from COVID-19. A significant difference in mortality between patients with and without heart failure was observed, patients with heart failure having a higher mortality.
Keywords: Coronavirus disease 2019, Heart failure, Poor outcomes
1. Introduction
The coronavirus disease 2019 (COVID-19) has caused significant morbidity and mortality to date, with the number of new cases and deaths continue to rise at an alarming rate [1]. Although most cases of COVID-19 are asymptomatic or mild in severity, a minority of patients experience more severe symptoms of COVID-19 along with its complications, such as acute respiratory distress syndrome (ARDS), coagulopathy, multiple organ failure (MOF), and death. A number of comorbidities have been associated with an increased risk of severity and mortality in COVID-19 patients, including hypertension, diabetes, cerebrovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease [[2], [3], [4], [5], [6]]. Individuals with preexisting cardiovascular diseases have been shown to have poor outcomes with COVID 19 [7,8]. Study from Chinese center of disease control on 72,314 cases reported a case fatality rate of 10.5% for patients with preexisting cardiovascular disease, but no specific data on mortality on heart failure [9]. Due to looming possibility of poor drug compliance and other complications related to disruption in medical services, the number of patients with new or decompensated heart failure may increase [10].
Currently, the plausible mechanisms for SARS-CoV-2 (severe acute respiratory syndrome 2) to infect human cells include binding to the angiotensin-converting enzyme 2 (ACE2) receptor and inducing hyperinflammation or cytokine storm. A high expression of ACE2 receptor is commonly found in patients with heart failure [7,8]. In this systematic review and meta-analysis, we wanted to investigate the association between heart failure with poor outcome in patients with COVID-19.
2. Methods
2.1. Search and selection criteria
We performed a systematic search of the literature on PubMed, EuropePMC, SCOPUS, Cochrane Central Database, and medRxiv for preprint studies with the search terms, “Heart Failure” and “COVID-19”. After the removal of duplicates, the abstracts were screened by two independent authors (EY and RP). Irrelevant articles were excluded. The inclusion criteria for this study were studies that studied patients with preexisting HF comorbidity with COVID-19 infection. The outcome of interest was mortality and poor prognosis (defined by incidence of severe COVID-19 infection, admission to ICU, and use of ventilator) in patients with preexisting heart failure with coronavirus disease. This systematic search conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.2. Data extraction
Data extraction was carried out by EY, RP, MAL, and IH using a standardized form containing the following details: author name, year of publication, study design, and sample size.
2.3. Statistical analysis
This meta-analysis was conducted using Review Manager (RevMan) v5.3 (Cochrane Collaboration) software. Hazard ratio (HR) and odds ratio (OR) with 95% confidence interval (CI) were used as pooled measures for dichotomous data. Heterogeneity was assessed using Inconsistency Index (I2) ranging from 0 to 100%. Statistically significant heterogeneity was defined as an I2 value above 50% or p < 0.05. The Mantel–Haenszel method was used to determine OR, and the generic inverse variance method was used to determine HR. Random effects models were implemented for analyses with significant heterogeneity. Sensitivity analyses were done to test statistical robustness of pooled results, to see whether there is significant change in pooled results by exclusion of studies, and to single out studies with high heterogeneity. Meta-analysis of prevalence was done by pooling of events of HF per total patients using the meta-analysis of proportion. Small-study effects and the risk of publication bias were assessed qualitatively using funnel plot analysis and quantitatively using regression-based Egger's test.
3. Results
We extracted 204 potential articles from our search, and 22 duplicates were removed. After the titles and abstracts of the remaining 182 articles were screened we obtained 92 potentially relevant articles. We excluded 74 studies due to the following reasons: four studies were systematic reviews, two studies were meta-analyses, three articles were literature reviews, and 65 articles did not report on the outcome of interest. Finally, we included the remaining 18 studies in our qualitative synthesis and meta-analysis. There were 21,640 patients from 18 studies. The majority of study subjects are males ranging from 54.7% to 85%, with only two studies reported male subjects being minorities, (45.1% on study by Caraballo C et al. and 44.3% on study by Ji W et al.) (Table 1 , Fig. 1 ).
Table 1.
Studies included in Meta Analysis
| Author, year | Study design | Outcome of interest | Samples | Outcome vs no outcome | Male | Overall age | HF | Outcome/total HF vs No HF | HF outcome vs no outcome | New Onset HF | HR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inciardi RM 2020 | Observational, prospective cohort | Mortality | 53 | 19 vs 34 | 85% | 68 ± 12 | 21/53 | 12/21 vs 7/32 | 12/19 vs 9/26 | N/A | N/A |
| Chen T 2020 | Retrospective | Mortality | 274 | 113 vs 161 | 62% | 62.0 (44.0–70.0) | 1/274 | 1/1 vs 112/273 | 1/113 vs 0/161 | 41/83 vs 3/94 | N/A |
| Petrilli 2020 | Retrospective | Critical illness | 2729 | 990 vs 1739 | 61.3% | 63 (51–74) | 349/2729 | 189/349 vs 801/2380 | 189/990 vs 160/1739 | N/A | HR mortality 1.77 (1.43 to 2.20) |
| Retrospective | Hospitalization | 2729 | N/A | 61.3% | 63–51-74 | N/A | N/A | N/A | N/A | 4.56 [2.59, 8.04] | |
| Yin W 2020 | Retrospective | Mortality | 112 | 52 vs 60 | 68.7% | 66.00(56.00–76.00) | 2(1.8%) | 2/2 vs 50/110 | 2/52 vs 0/60 | N/A | N/A |
| Zhou F | Retrospective | Mortality | 191 | 54 vs 137 | 62% | 56·0 (46·0–67·0) | 44 (23%) | N/A | N/A | 28/54 vs 16/137 | N/A |
| Baker KF 2020 | Retrospective | mortality | 316 | 81 vs 235 | 54.7 | 75 (60–83) | 11 | 20/45 vs 61/271 | 20/81 vs 23/210 | N/A | OR 2.67 [1.36–5.19], p = .004 |
| Cummings MJ | Retrospective | Mortality | 257 | 86/257 (33%) | 66% | 62 (51–72) | 49/257 (CVD) | N/A | N/A | N/A | Univariate 0.62 (0.33–1.17) Multivariate 0.69 (0.34–1.42) |
| Caraballo C | Retrospective | Mortality | 206 COVID positive | 34 vs 172 | 45.1% | 78 (65–87) | 36/206 | 3/36 vs 31/136 | 3/34 vs 33/172 | N/A | ??? |
| Heng GE | Retrospective | Mortality | 51 | 12 vs 39 | 72.5% | 70 (58–79) | 4/51 | 4/4 vs 35/47 | 0/20 vs 4/31 | N/A | N/A |
| Severity | 51 | 20 vs 31 | 72.5% | 70 (58–79) | 4/51 | 1/20 vs 3/31 | N/A | N/A | |||
| Garibaldi BT 2020 | Retrospective | Mortality | 747 | 113 vs 634 | 53.2% | 63 (49, 75) | 127 (15.3%) | 33/127 vs 80/634 | 33/113 vs 67/634 | N/A | N/A |
| Ebinger JE | Retrospective | Severity (ICU care) | 442 | 77 vs 365 | 58% | 52.7 ± 19.7 | 49 (11%) (prior MI or HF) | 18/49 vs 59/393 | 18/ 77 vs 31/365 | N/A | Univariate 1.72 (0.96,3.09) 0.07 Multivariate 0.56 (0.27,1.18) 0.13 |
| Liao X | Retrospective | Mortality/ventilator support | 81 | 10 vs 71 | 63% | 50.0 (39.0–65.0) | 4 (4.9% | 1/4 vs 9/77 | 1/10 vs 3/71 | N/A | N/A |
| Ji W | Retrospective | Severity | 5172 | 293 vs 4879 | 44.3% | 42 (18–100) | 217 (4.2) | 44/217 vs 249/4955 | 44/293 vs 173/4879 | N/A | (Odds ratio range 1.562–1.730) |
| Paranjpe I | Retrospective | Mortality | 1078 | 310 vs 768 | 58.1% | Dead 75 (64–85) Alive 59 (45–72) |
117/1078 | 64/117 vs 246/961 | 64/310 vs 53/768 | N/A | N/A |
| Rossi PG | Retrospective | Hospitalization | 2653 | 217 vs | 61.9% | 51–81 | 96/1075 | N/A | N/A | N/A | HR 1.6, 95% CI 1.2 to 2.1 |
| Mortality | 1292 | 217 vs 1075 | 800 (61.9%) | 51–81 | 139/1292 | 43/137 vs 174/2516 | 43/217 vs 96/1075 | N/A | HR 2.3, 95% CI 1.6 to 3.2 | ||
| Yanover C | Retrospective | Critical care/death | 4353 | 173 vs 4180 | 56.5% | 35 [22–54] | 30/4353 | 11/30 vs 162/4323 | 11/173 vs 19/4180 | N/A | N/A |
| Argenziano MG | Retrospective | ICU Care (SEVERE COVID) | 850 | 236 vs 614 | 59.6% | 63.0 (50.0–75.0) | 91/850 | N/A | 24/236 vs 67/614 | 18/236 vs 6/614 | N/A |
| Reilev M | Retrospective | Hospitalization | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 2.2 (1.7–2.9) (age adjusted) |
| Retrospective | Mortality | 2090 | 524 vs 1566 | 57% | 82 (75–89) | 218/2090 | 98/204 vs 426/1886 | 98/524 vs 120/2090 | N/A | 1.7 (1.3–2.2) (age adjusted) |
Fig. 1.
PRISMA diagram.
3.1. Risk of hospitalization in patients with preexisting heart failure
Three studies reported a statistically significant difference in the risk of hospitalization in COVID19 patients with preexisting heart failure. The pooled HR was 2.37 [1.48, 3.79; p < 0.001], with moderately high heterogeneity between studies [I2, 82%; p < 0.001]. Sensitivity analysis was performed, excluding the study by Petrilli et al., yielded HR 1.88 [1.36, 2.62; p < 0.001] with moderate heterogeneity between studies [I2, 65%; p = 0.09] (Fig. 2 ).
Fig. 2.
Meta analysis, risk of hospitalization in patients with pre-existing heart failure.
3.2. Heart failure and poor outcome
Statistically significant differences in composite poor outcomes were reported in 15 studies. HF was associated with a poor outcome demonstrated by an OR of 2.86 [2.07; 3.95; p < 0.001] with high heterogeneity between studies [I2, 80%; p < 0.001]. Sensitivity analyses were performed. Exclusion of the studies by Argenziano et al., Caraballo et al., Ji W et al., Paranjpe et al., Reilev et al., and Yanover et al. yielded an OR of 2.56 [2.17, 3.03, p < 0.001] with low heterogeneity between studies [I2, 0%; p = 0.55] (Fig. 3 ).
Fig. 3.
Meta analysis, heart failure and poor outcome.
3.3. Pooled HR of mortality in patients with coronavirus disease 2019 with preexisting heart failure
Seven studies reported a statistically significant difference in mortality between patients with COVID-19 with and without preexisting HF. The pooled HR was 1.70 [1.44, 2.02; p < 0.001] with moderate heterogeneity between studies [I2, 59%; p = 0.02]. Sensitivity analysis was performed, excluding the study by Cummings which yielded an HR of 1.70 [1.57, 1.83; p < 0.001] with a low heterogeneity between studies [I2, 9%; p = 0.36] (Fig. 4 ).
Fig. 4.
Meta analysis, pooled HR of mortality in COVID19 patients with pre-existing heart failure.
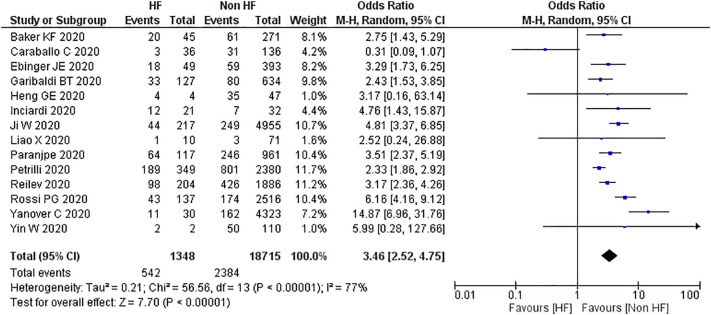
3.4. Comparison of mortality in patients with coronavirus disease 2019 accompanied with preexisting heart failure and without heart failure
Fourteen studies reported a statistically significant difference in mortality between patients with COVID-19 accompanied with and without preexisting heart failure. Pooled events yielded an OR of 3.46 [2.52, 4.75; p < 0.001] with moderately high heterogeneity between studies [I2, 77%; p < 0.001]. Sensitivity analyses were performed, excluding the studies by Caraballo et al., Ji W et al., Rossi et al., and Yanover et al. which yielded OR of 2.77 [2.40, 3.20; p < 0.001] with low heterogeneity between studies [I2, 0%; p = 0.73] (Fig. 5 ).
Fig. 5.
Meta analysis, mortality in COVID19 patients with pre-existing heart failure compared to nonheart failure patients.
3.5. Incidence of new-onset heart failure in patients with coronavirus disease 2019
Three studies reported a statistically significant difference in incidence of new-onset HF in hospitalized patients with COVID-19. Pooled results yielded an OR of 11.67 [6.96, 19.56; p < 0.001] with moderate heterogeneity between studies [I2, 44%; p0.17]. Sensitivity analysis was performed, excluding the study by Chen et al. which yielded an OR of 8.24 [4.59, 14.78; p < 0.001] with low heterogeneity between studies [I2, 0%; p < 0.96] (Fig. 6 ).
Fig. 6.
Meta analysis, incidence of new-onset heart failure in COVID19 patients.
3.6. Meta-analysis of prevalence
Sixteen studies were available for meta-analysis to determine the pooled prevalence of heart failure among patients with COVID-19; the meta-analysis revealed a pooled prevalence of heart failure of 9% (7–12%) (Fig. 7 ).
Fig. 7.
Meta prevalence of heart failure patients in study population.
3.7. Publication bias
Regression-based Egger's test showed no indication of small-study effects for Heart Failure and Poor Outcome (p = 0.852), Pooled HR of mortality in patients with coronavirus disease 2019 with preexisting heart failure (p = 0.640), and Comparison of mortality in patients with coronavirus disease 2019 accompanied with preexisting heart failure and without heart failure (p = 0.555). There was an indication of small-study effects for Risk of hospitalization in patients with preexisting heart failure (p = 0.035) and Incidence of new-onset heart failure (p < 0.001). The number of included studies were <10 for risk of hospitalization in patients with preexisting heart failure, Pooled HR of mortality in patients with coronavirus disease 2019 with preexisting heart failure, and incidence new onset heart failure, thus, the results were less reliable. Analysis using funnel plot generated asymmetrical result for Heart Failure and Poor Outcome (Fig. 8A), and Comparison of mortality in patients with coronavirus disease 2019 accompanied with preexisting heart failure and without heart failure (Fig. 8B), with Fig. 8A showing somewhat less asymmetry compared to Fig. 8B. Based on Cochrane collaboration recommendation we only performed funnel plot analysis in meta-analysis involving more than 10 studies.
Fig. 8.
A. Funnel plot analysis, heart failure and poor outcome. B. Funnel plot analysis, comparison of mortality in patients with coronavirus disease 2019 accompanied with preexisting heart failure and without heart failure.
4. Discussion
In this meta-analysis, we observed an association between HF and poor outcome in patients with COVID-19, a higher risk of death and hospitalization in those with preexisting HF, and higher mortality in those with preexisting HF compared to patients without HF.
As with other types of pneumonia, preexisting cardiovascular diseases, including HF, are risk factors for poor prognosis during and after the course of pneumonia [11]. The effect of an infective organism on the myocardium can cause non-ischemic cardiac injury. The systemic inflammatory response, which is particularly excessively elevated in COVID-19, can also result in kidney injury and impair sodium and water metabolism, which may precipitate the worsening of HF [12].
Recent studies on the role of ACE2 in COVID-19 infection have reported that ACE2 levels are 50% higher in male patients with HF [[13], [14], [15]]. This finding explains the higher risk of poor prognosis, hospitalization, and death in this population in the event of occurrence of COVID-19. In this meta-analysis, the majority of the studies had higher number of male patients than female patients.
In patients with coexisting HF and diabetes, who are in a pro-inflammatory state, the membrane-bound ACE2 protein can be cleaved by ADAM17, resulting in the release of ACE2 into the interstitium and blood circulation [16]. This inflammatory response, which occurs in COVID-19 as an elevated immune response [17,18] might also explain the increase in mortality in patients with COVID-19 accompanied with HF. The excessive inflammatory response and oxidative stress in these patients predispose them to a more severe clinical course of COVID-19 [19]. This phenomenon is particularly significant in patients with preexisting HF since these patients suffer from decreased circulatory and physiological reserves. Older patients with HF commonly suffer from multiple comorbidities, deterioration in function of multiple organ systems, and functional decline [20,21].
ACE2 is an enzyme involved in the RAAS pathway; however, it differs from ACE in not being capable of converting angiotensin I into angiotensin II. Furthermore, as opposed to the vasoconstrictor property of angiotensin type 1 receptors, ACE2 via the ACE-2 receptors cleaves angiotensin II into angiotensin 1–7, which has vasodilatory properties [22].
Based on these explanations, it is easy for us to conclude regarding the dangers of upregulation of ACE2 as in patients being treated with renin-angiotensin system blockers (RASB). One study has even proposed ceasing administration of RASB in patients with COVID-19 on these drugs [23]. However, this does not seem to be the case, and several other authors have questioned this hypothesis [[24], [25], [26], [27]]. The rationale for this rebuttal is based upon several in vitro studies, which revealed that upon entry into alveolar cells using membrane-bound ACE-2 receptors, SARS-CoV-2 downregulates the expression and causes internalization of ACE2 receptors. The result of this process is a decrease in angiotensin-II to angiotensin (1–7) conversion and an increase in the availability of angiotensin II to bind to the AT1 receptor. This induces cellular inflammation, oxidative stress, vasoconstriction, fibrosis, and aldosterone activation, which may eventually lead to lung injury [[28], [29], [30], [31], [32]]. The use of RASB may serve as a protective measure against lung injury by the inhibition of angiotensin II synthesis using ACEIs and blockade of AT1R by ARBs. This would result in a skew of the RAAS towards angiotensin (1–7) and its subsequent interaction with Mas-receptors, which may minimize the risk of lung injury [29,33].
The result of this meta-analysis is similar to that of previous studies in which patients with preexisting heart HF demonstrated an elevated risk of poor outcome, hospitalization, and death [31–49] [34] [[35], [36], [37], [38], [39], [40], [41]] [[42], [43], [44], [45], [46], [47], [48], [49], [50]] [51,52].
Compared to other diseases, based on our pooled results, patients with HF suffering from SARS-CoV-2 infection have a threefold increased risk of in-hospital mortality compared to patients with HF who develop influenza, OR 3.46 [2.52, 4.75; p < 0.001] vs. OR 1.15 [1.03, 1.30; p = 0.02] and OR 1.66 [1.44–1.91; p < 0.001] [53,54]. However, when compared to patients infected with Middle East respiratory syndrome coronavirus (MERS-CoV), patients with preexisting HF who developed COVID-19 had a lower risk of mortality [OR 3.46; 2.52, 4.75; p < 0.001 vs OR, 12.981; 1.324, 127.313; p = 0.025] [55].
Studies have shown a poorer prognosis in patients with HF who develop influenza [53,54]. As a result of the decreased physiological reserve, influenza virus infection in patients with HF is associated with a severe course and significant hemodynamic compromise, which often requires cardiac support [56]. Influenza vaccination in these patients is associated with a reduced risk of all-cause and cardiovascular death, furthermore receiving >1 influenza vaccine is associated with 18% reduction in both all-cause and cardiovascular death (all-cause death: 0.82; 95% CI,0.81–0.84; p < 0.001; cardiovascular death: 0.82; 95% CI, 0.81–0.84; p < 0.001), in the event of influenza infection, patients with heart failure may fail to cope with the increased metabolic demand which is caused by the infection itself, as a result, decompensation or exacerbation in heart failure symptoms may occur [57]. Patients with HF are currently classified as a high-priority group to be vaccinated with the influenza vaccine [58]. Based on these findings, once an effective vaccine for SARS-CoV-2 infection becomes available, patients with HF should be prioritized to be vaccinated even in case of COVID-19.
We observed some limitations of this meta-analysis, such as the use of preprint studies included in this meta-analysis. Additionally, several studies have been conducted in the same cities/regions, posing a risk of subject overlap. Data regarding the difference between new-onset and worsening of HF in patients in the studies was rarely available. Data regarding staging of HF was also rarely found. Additionally, in patients with HF suffering from a severe course of COVID-19 infection, data regarding the use of vasopressors and inotropic agents as well as the incidence of cardiogenic shock was lacking.
5. Conclusion
In conclusion, patients with HF are at increased risk for poor outcomes such as hospitalization, and death from COVID-19. A significant difference in mortality between patients with and without HF was observed, those with HF showing higher mortality rates. These findings probably stem from the reduced physiological reserves in patients with HF. Mortality of patients with HF accompanied with SARS-CoV-2 infection is higher than that of patients with influenza, but lower than that of patients with MERS-CoV. Thus, if SARS-CoV-2 vaccine becomes available, patients with HF should be prioritized to be vaccinated.
CRediT authorship contribution statement
Emir Yonas:Conceptualization, Formal analysis, Methodology, Writing - original draft, Project administration.Idrus Alwi:Writing - review & editing.Raymond Pranata:Writing - review & editing, Formal analysis, Methodology.Ian Huang:Writing - review & editing.Michael Anthonius Lim:Writing - review & editing.Eddy Jose Gutierrez:Writing - review & editing.Muhammad Yamin:Writing - review & editing.Bambang Budi Siswanto:Writing - review & editing.Salim S. Virani:Conceptualization, Writing - review & editing.
Acknowledgments
Acknowledgements
None.
Funding
None.
Declaration of competing interest
The authors of this manuscript has no conflict of interest.
References
- 1.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) situation report – 95. [Google Scholar]
- 2.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320926899. 147032032092689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia — a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pranata R., Soeroto A.Y., Ian H., et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020 doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 5.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pranata R., Huang I., Lukito A.A., Raharjo S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. May 2020 doi: 10.1136/postgradmedj-2020-137884. postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 — systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. May 2020;104949 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pranata R., Tondas A.E., Huang I., et al. Potential role of telemedicine in solving ST-segment elevation dilemmas in remote areas during the COVID-19 pandemic. Am J Emerg Med. June 2020 doi: 10.1016/j.ajem.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Lim M.A., Huang I., Yonas E., Vania R., Pranata R. A wave of non-communicable diseases following the COVID-19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):979–980. doi: 10.1016/j.dsx.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie T.J., Shariatzadeh M.R. Community-acquired pneumonia requiring admission to an intensive care unit. Medicine (Baltimore) 2007;86(2):103–111. doi: 10.1097/MD.0b013e3180421c16. [DOI] [PubMed] [Google Scholar]
- 12.Corrales-Medina V.F., Musher D.M., Shachkina S., Chirinos J.A. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 13.Fagyas M., Úri K., Siket I.M., et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) II: albumin suppresses angiotensin converting enzyme (ACE) activity in human. Karamyan V, ed. PLoS One. 2014;9(4):e87844. doi: 10.1371/journal.pone.0087844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Úri K., Fagyas M., Mányiné Siket I., et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a Biomarker of Systolic Dysfunction in Human Hypertension and Heart Failure. Karamyan V, ed. PLoS One. 2014;9(4):e87845. doi: 10.1371/journal.pone.0087845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu K., Fang Y.-Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen K.B., Chodavarapu H., Porretta C., Robinson L.K., Lazartigues E. Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology. 2015;156(12):4411–4425. doi: 10.1210/en.2015-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. April 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasoni D., Italia L., Adamo M., et al. COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. May 2020 doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitale C., Spoletini I., Rosano G.M. Frailty in heart failure: implications for management. Card Fail Rev. 2018;4(2):104. doi: 10.15420/cfr.2018.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and Meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo K.B., McCullough P.A., Rangaswami J. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020;8(5) doi: 10.1016/S2213-2600(20)30156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown J.D. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020;8(5) doi: 10.1016/S2213-2600(20)30158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A.K., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tignanelli C.J., Ingraham N.E., Sparks M.A., et al. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020;8(5):e30–e31. doi: 10.1016/S2213-2600(20)30153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. April 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 29.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. March 2020 doi: 10.1056/NEJMsr2005760. NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dijkman R., Jebbink M.F., Deijs M., et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol. 2012;93(9):1924–1929. doi: 10.1099/vir.0.043919-0. [DOI] [PubMed] [Google Scholar]
- 31.Pranata R., Permana H., Huang I., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. June 2020 doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, d-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020 doi: 10.1177/0123456789123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahajan K., Gaur N. Letter in response to the article: comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers (Singh et al.) Diabetes Metab Syndr Clin Res Rev. 2020;14(5):723–724. doi: 10.1016/j.dsx.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Stewart Coats A.J., Zheng Z., et al. Management of heart failure patients with COVID-19. A joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. May 2020 doi: 10.1002/ejhf.1915. ejhf.1915. [DOI] [PubMed] [Google Scholar]
- 35.Argenziano M., Bruce S., Slater C., et al. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series. MedRxiv. 2020 doi: 10.1101/2020.04.20.20072116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker K., Hanrath A., Van Der Loeff I., et al. COVID-19 management in a UK NHS Foundation Trust with a high consequence infectious diseases Centre: a detailed descriptive analysis. MedRxiv. 2020 doi: 10.1101/2020.05.14.20100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caraballo C., McCullough M., Fuery M., et al. COVID-19 infections and outcomes in a live registry of heart failure patients across an integrated health care system. MedRxiv. 2020 doi: 10.1101/2020.04.27.20082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;1091(December 2019):m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings M., Baldwin M., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. MedRxiv. 2020 doi: 10.1101/2020.04.15.20067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebinger J., Achamallah N., Ji H., et al. Pre-existing traits associated with COVID-19 illness severity. MedRxiv. 2020 doi: 10.1101/2020.04.29.20084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garibaldi B., Fiksel J., Muschelli J., et al. Patient trajectories and risk factors for severe outcomes among persons hospitalized for COVID-19 in the Maryland/DC region. MedRxiv. 2020 doi: 10.1101/2020.05.24.20111864. [DOI] [Google Scholar]
- 42.Heng G, Zhu M, Du J, et al. Cardiac Structural and Functional Characteristics in Patients with Coronavirus Disease 2019: A Serial Echocardiographic Study. doi: 10.1101/2020.05.12.20095885 [DOI]
- 43.Inciardi R.M., Adamo M., Lupi L., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji W., Huh K., Kang M., et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in South Korea. MedRxiv. 2020 doi: 10.1101/2020.05.08.20095174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao X., Chen H., Wang B., et al. Critical care for severe COVID-19: a population-based study from a province with low case-fatality rate in China. MedRxiv. 2020 doi: 10.1101/2020.03.22.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paranjpe I., Russak A., De Freitas J., et al. Clinical characteristics of hospitalized Covid-19 patients in New York City. MedRxiv. 2020 doi: 10.1101/2020.04.19.20062117. [DOI] [Google Scholar]
- 47.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. May 2020:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reilev M., Kristensen K., Pottegaard A., et al. Characteristics and predictors of hospitalization and death in the first 9,519 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. MedRxiv. 2020 doi: 10.1101/2020.05.24.20111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi P., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the province of Reggio Emilia, Italy. MedRxiv. 2020 doi: 10.1101/2020.04.13.20063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanover C., Mizrahi B., Kalkstein N., et al. What factors increase the risk of complications in SARS-CoV-2 positive patients? A cohort study in a nationwide Israeli health organization. MedRxiv. 2020 doi: 10.1101/2020.05.07.20091652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin Y., Zhou S., Zhang X., et al. Critically ill patients with COVID-19 in China: a multicenter retrospective observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3562469. [DOI] [Google Scholar]
- 52.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panhwar M.S., Kalra A., Gupta T., et al. Effect of influenza on outcomes in patients with heart failure. JACC Hear Fail. 2019;7(2):112–117. doi: 10.1016/j.jchf.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Panhwar M.S., Kalra A., Gupta T., et al. Relation of concomitant heart failure to outcomes in patients hospitalized with influenza. Am J Cardiol. 2019;123(9):1478–1480. doi: 10.1016/j.amjcard.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 55.Alqahtani F.Y., Aleanizy F.S., Ali El Hadi Mohamed R., et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2019;147 doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sellers S.A., Hagan R.S., Hayden F.G., Fischer W.A. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respi Viruses. 2017;11(5):372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modin D., Jørgensen M.E., Gislason G., et al. Influenza vaccine in heart failure. Circulation. 2019;139(5):575–586. doi: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
- 58.Grohskopf L.A., Alyanak E., Broder K.R., Walter E.B., Fry A.M., Jernigan D.B. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices — United States, 2019–20 influenza season. MMWR Recomm Rep. 2019;68(3):1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]