Abstract
A new coronavirus, named severe acute respiratory syndrome–coronavirus-2 by the WHO, has rapidly spread around the world since its first reported case in late December of 2019 from Wuhan, the People’s Republic of China. As of mid-April 2020, this virus has affected more than 180 countries and territories, infecting more than 1,650,000 individuals and causing over 100,000 deaths. With approximately 20 million new cases globally per year, cancer affects a substantial portion of the population. Individuals affected by cancer are more susceptible to infections owing to coexisting chronic diseases (cardiovascular, pulmonary, and diabetes), overall poor health status, and systemic immunosuppressive states caused by both cancer and the anticancer treatment. As a consequence, patients with malignancies, especially those with lung cancer who develop coronavirus disease 2019, experience more difficult outcomes. A recent multicenter study carried out by the Hubei Anti-Cancer Association has also documented that patients with lung cancer had an increased risk of death, intensive care unit requirement, risk of presenting severe or critical symptoms, and use of invasive mechanical ventilation. Here, we present two representative cases of patients with lung cancer and coronavirus disease 2019 without respiratory compromise and with atypical and severe skin manifestations—findings that could be influenced by the long-term use of anti–programmed cell death protein 1 antibody.
Keywords: Anti–PD-1 therapy, COVID-19, Lung cancer, Erythema multiforme, Urticarial, Vasculitis
Introduction
In December 2019, the first four cases of an acute respiratory syndrome of unknown cause (now known as coronavirus disease 2019 [COVID-19] caused by severe acute respiratory syndrome–coronavirus-2 [SARS-CoV-2]) were reported among people linked to a local seafood market in Wuhan City, Hubei Province, the People’s Republic of China.1 Since then, SARS-CoV-2 has infected more than 1,600,000 people in 184 countries, generating over 100,000 deaths as mid April 2020.2
Epidemiologic studies revealed that approximately 80% of patients had mild symptoms, 14% developed severe conditions, and 5% fell critically ill.3 Severity increases with age, and the case fatality rate is higher among individuals with underlying chronic conditions, including cancer.4 Patients with cancer seem to have a higher risk of severe clinical events (mechanical ventilation requirement or intensive care unit admission) than the general population.
The most frequent COVID-19–related symptoms are fever (88.4%), cough (71.7%), fatigue (60.3%), dyspnea (44.2%), myalgias (35.7%), and chills (26%).5 At the moment, the only existing record of dermatologic disorders of COVID-19 comes from Lombardy, Italy, where 18 of 88 patients (20.4%) had some cutaneous involvement,5 including erythematous rash, widespread urticaria, and chickenpox-like vesicles. The trunk was the main region involved; itching was low or absent and lesions usually healed in a few days.6
Among patients with NSCLC treated with anti–programmed cell death protein (ligand) 1 (anti–PD[L]-1) therapy, cutaneous immune-related adverse events (irAEs) occur in approximately 40% and include morbilliform eruptions, pruritus, eczema, psoriasis, lichenoid dermatitis, bullous pemphigoid, sarcoidosis, vitiligo, dermatomyositis, and lupus-like reactions.7 Cutaneous irAEs primarily develop early within 5 weeks of treatment initiation,8 though a recent meta-analysis9 suggested that PD-1 inhibitor–associated cutaneous irAEs may have late onsets.
Here, we present two cases of patients with NSCLC in long-term treatment with immunotherapy, an intervention that achieved a marked response and control of the disease. COVID-19 infection was recently documented in both patients with minimal respiratory manifestations but extensive cutaneous involvement—an extensive urticarial pattern in one case and multiform erythema in the other. Treatment, skin biopsy findings, and the role of various serum markers are also discussed.
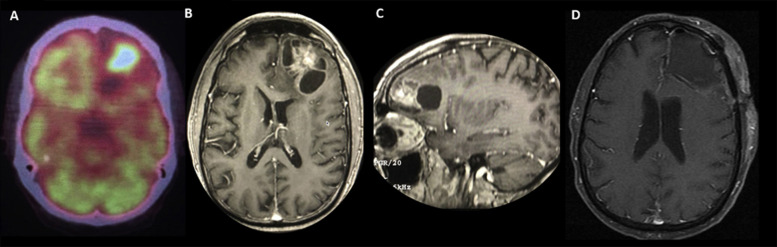
Case Description
Case number 1
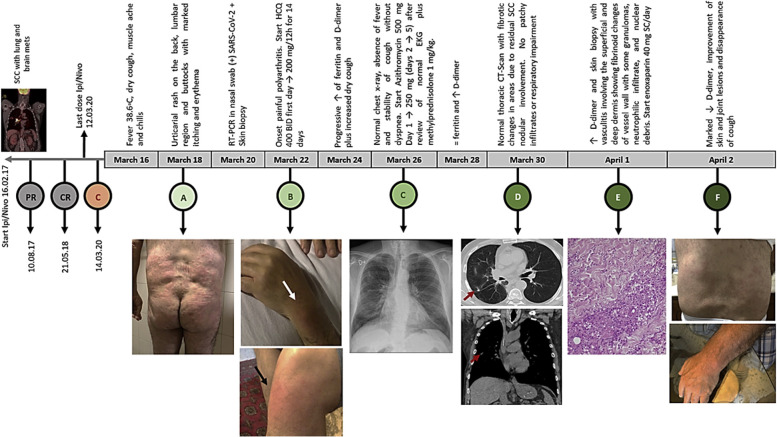
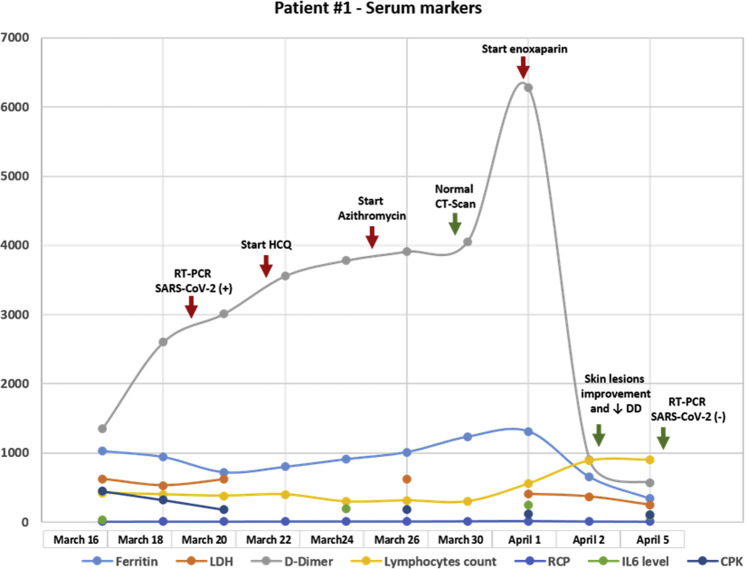
In March 2017, a 62-year-old man, a current smoker, was diagnosed with a T4N2M1b G3 stage IV squamous cell lung carcinoma with pleuropulmonary involvement. Next-generation sequencing analysis evidenced a high tumor mutational burden (14 Muts/Mb), p16INK4aR47f4, CUL3 splice site265-2A>C, NFE2L2G15R, RANBP2R1126W, TERC amplification, and TP53 R158L. The PD-L1 tumor proportion score was 40%. He started treatment with ipilimumab (1 mg/kg every 6 wk) plus nivolumab (3 mg/kg every 2 wk). After 6 months, he received an immunotherapy combo with excellent tolerance except for mild hypothyroidism requiring hormone-replacement therapy. After a partial response, he underwent intensity-modulated radiation therapy (60 Gy) on the residual disease. Subsequently, and after findings of a complete response in the thorax (May 21, 2018), immunotherapy was continued until January 2019. At this time, a brain magnetic resonance imaging was done and revealed the presence of a solitary left frontal lesion with a cystic component. He underwent a complete neurosurgical resection on January 8, 2019 (Supplementary Fig. 1) followed by stereotactic radiosurgery and ipilimumab/nivolumab restart, maintaining a complete extracranial and brain response until March 2020. After 2 days of the last immunotherapy dose, the patient had contact with a flu-symptomatic person on March 14, 2020. After 48 hours, he started to have a fever, fatigue, myalgia, and chills (Fig. 1 ), followed by the appearance of urticarial papular lesions with minimal erythema located in the lower dorsal, lumbar, and gluteal region. The lesions tended to be persistent and left a small purplish residue. In addition, the patient had a burning sensation of the compromised skin, severe joint pain, and reactive polyarthritis. The only respiratory symptoms were nasal congestion with minimal pharyngeal exudation and mild dry cough. On March 20, 2020, the presence of SARS-CoV-2 was confirmed, and a skin biopsy was performed (Supplementary Fig. 2). To assess prognosis and disease severity, taking into account the absence of respiratory complications, serial ferritin, D-dimer (DD), and interleukin (IL)-6, in addition to antinuclear antibodies (ANAs) and C4 (to discard differential diagnoses), were evaluated.10 Ferritin (940 ng/mL) and DD (2600 ng/dL) were noted to be elevated (Fig. 2 ), and hydroxychloroquine was started (400 mg twice daily on day 1 and then 200 mg twice daily for 14 d). From then on, a progressive elevation of DD and ferritin was documented to a maximum of 6.278 ng/dL and 1.310 ng/mL, respectively. Because there were no changes in respiratory findings, azithromycin (500 mg on day 1 and 250 mg on days 2–5) plus methylprednisolone 1 mg/kg were added on March 26, 2020. In addition, owing to elevated DD, enoxaparin 40 mg/day subcutaneously was added, particularly after receiving the skin pathology report, which revealed urticarial vasculitis with signs of microangiothrombosis. By March 30, 2020, the cough and most of the dominant skin lesions had disappeared; the chest computed tomography scan was normal. ANAs and complement C4 were normal as before, as were clotting times and fibrinogen. Serial evaluation of IL-6 levels by enzyme-linked immunosorbent assay revealed only a slightly elevated value of 246 pg/mL on April 1, 2020 (range 6.25–200 pg/mL, Human IL-6 ELISA Kit, DiaCLONE, Paris, France), and throughout the 18-day follow-up period, there was lymphopenia that became less evident as of April 5, 2020 (lymphocytes count 900/mm3). At the time of writing, the patient is asymptomatic with no injury to the skin or joints.
Figure 1.
Severe urticarial rash in addition to the elevation of DD in a patient with SCC of the lung treated with Ipi/Nivo after becoming infected with SARS-CoV-2 (ipi 1 mg/kg every 6 wks plus Nivo 3 mg/kg every 15 days). (C-red) contact with a patient infected with COVID-19; (A) beginning of the cutaneous manifestations; (B) onset painful polyarthritis; (C) normal chest radiograph; (D) normal chest CT-scan; (E) skin biopsy; (F) current joint and skin status. COVID-19, coronavirus 2019; CR, complete response; CT, computed tomography; DD, D-dimer; EKG, electrocardiogram; HCQ, hydroxychloroquine.; Ipi, ipilimumab; Nivo, nivolumab; PR, partial response; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome–coronavirus-2; SC, subcutaneous; SCC, squamous cell carcinoma.
Figure 2.
Detailed analysis of the evolution of blood markers in relation to the time of infection in case 1. CT, computed tomography; HCQ, hydroxychloroquine; RT-PCR, reverse transcription polymerase chain reaction; DD, D-dimer; LDH, lactate dehydrogenase; RCP, reactive C protein; IL6, interleukin 6; CPK, creatine phosphokinase; SARS-CoV-2, severe acute respiratory syndrome–coronavirus-2.
Case number 2
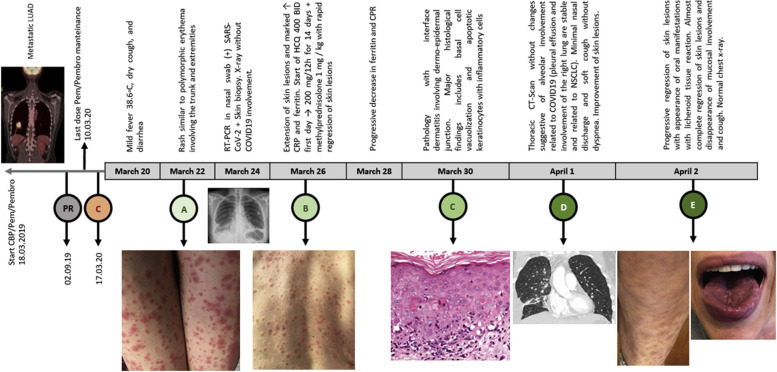
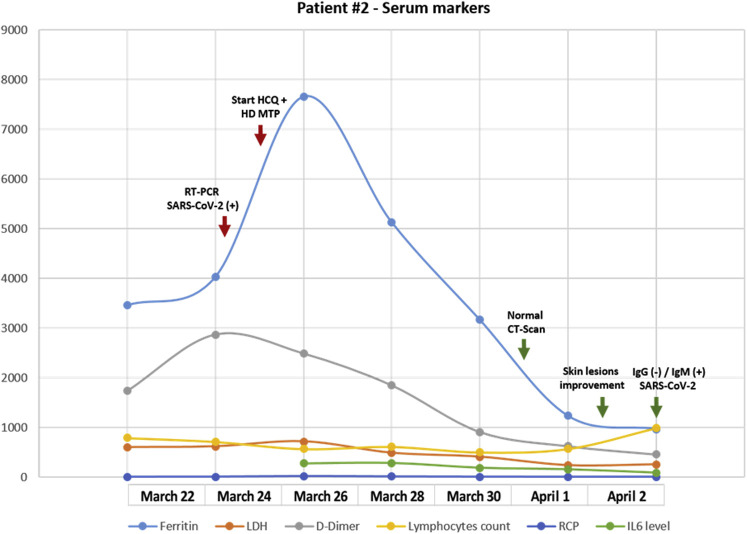
In February 2019, a 58-year-old woman, a former smoker, presented with terminal dysphagia and dry cough without dyspnea. Disease staging with positron emission tomography/computed tomography scan and brain magnetic resonance imaging revealed a mass measuring 36 × 34 mm located in the posterior basal segment of the right lower lobe, minimal pleural effusion, several ipsilateral pulmonary nodules, and three small brain lesions at the right frontal lobe. Lung biopsy revealed lung adenocarcinoma with Napsin expression that was negative for ALK, EGFR, ROS1, HER2, BRAF, and NTRK. PD-L1 tumor proportion scores ranged from 1% to 49%. On March 18, 2019, she started treatment with carboplatin/pemetrexed/pembrolizumab with a partial response. At the end of the induction phase, the two residual brain metastases were treated with stereotactic radiosurgery followed by pemetrexed/pembrolizumab maintenance; adverse effects included mild fatigue and hypothyroidism requiring hormone-replacing therapy. On March 17, 2020, she had direct contact with a patient with nonspecific flu-like symptoms, who subsequently tested positive for SARS-CoV-2. On March 20, 2020, she started to have diarrhea without mucus or blood (four stools/day) associated with fever and dry cough without dyspnea. After 2 days, she noted the progressive appearance of several lesions with targetoid appearance—a central zone of pallor with erythematous peripheral rim was evident (Fig. 3). Some manifested with a violaceous appearance with no true central clearing were associated with late-onset painful oral ulcers. On March 24, 2020, we confirmed SARS-CoV-2 infection from the nasopharyngeal swab through reverse transcriptase–polymerase chain reaction. In parallel, a skin biopsy was also taken. Given the progression of dermatologic lesions and a normal chest radiograph, she started hydroxychloroquine (400 mg twice daily on day 1 and 200 mg twice daily for 10 d), hydroxyzine (25 mg twice daily), desloratadine (5 mg/day), and methylprednisolone (1 mg/kg/day)—interventions that achieved rapid control of clinical findings. In addition, a progressive reduction in ferritin and C-reactive protein levels was observed without revealing any significant elevation of IL-6 (Fig. 4 ).
Figure 3.
Skin lesions suggestive of polymorphic erythema after acquiring SARS-CoV-2 infection during treatment with Pem/Pembro for metastatic lung adenocarcinoma. CBP (AUC 5)/Pem 500 mg/m2/day/Pembro 200 mg every 21 days for 4 cycles then Pem/Pembro for 31 cycles. (C-red) contact with a patient infected with COVID-19; (A) beginning of the cutaneous manifestations; (B) remarkable extension of skin lesions, elevation of biomarkers and start of HCQ; (C) pathology with interface dermatitis; (D) normal chest radiograph; (E) progressive regression of skin lesions and appearance of oral ulcers, which became the basis for starting methylprednisolone. CBP, carboplatin; COVID-19, coronavirus 2019; CT, computed tomography; HCQ, Hydroxychloroquine; LUAD, lung adenocarcinoma; Pem, pemetrexed; Pembro, pembrolizumab; PR, partial response; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome–coronavirus-2.
Figure 4.
A detailed analysis of the evolution of blood markers in relation to the time of infection in case 2. CT, computed tomography; SARS-CoV-2, severe acute respiratory syndrome–coronavirus-2; RT-PCR, reverse transcription polymerase chain reaction; LDH, lactate dehydrogenase; RCP, reactive C protein; IL6, interleukin 6.
Discussion
The two cases presented here had completed at least 1 year of treatment with an anti–PD-1 antibody, an intervention that achieved a major response. Neither of the two had previous skin manifestations, nor had they received other treatments until the onset of COVID-19. It is worth noting that, in patients with cancer, the diagnosis of cutaneous viral manifestations can be challenging owing to the frequency of skin adverse events of anticancer therapies.
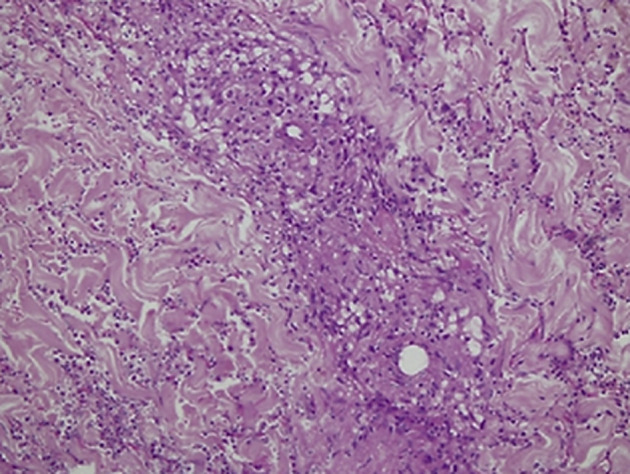
Our first case developed an urticarial reaction in relation to COVID-19. Development of secondary urticaria has been associated with infections, medications, Hymenoptera stings, hematological malignancies, and immunotherapy.11 Our clinical evaluation suggested the coexistence of dermatographism and cholinergic urticaria, an event that could have been favored by the basal hyperreactivity of T-lymphocytes induced by the anti–PD-1/CTLA-4 costimulus. The pathology assessment revealed dual changes with urticarial tissue reaction, dilated lymphatics and dermal edema, and indirect blood vessel damage with mild extravasation of red blood cells into the adjacent dermis. In addition, there were fibrinoid changes of the vessel wall with some granulomas and neutrophilic infiltrate (Supplementary Fig. 2). Despite being an acute event, the findings were associated only with COVID-19, given the normal levels of ANAs and C4.
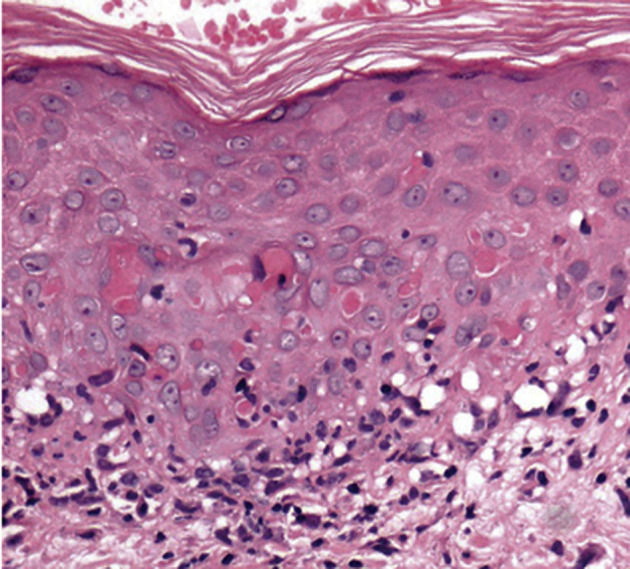
Our second case presented several lesions with a targetoid morphology having a peripheral rim of erythema and a central zone of pallor. Some manifest a dusky or violaceous appearance with no true central clearing and associated with the appearance of late-onset oral ulcers. Skin biopsy revealed necrotic keratinocytes within the epidermis and an interface dermatitis consistent with erythema multiforme (Supplementary Fig. 3) rapidly controlled with antihistamines, hydroxychloroquine, and the use of high doses of steroids. In erythema multiforme, there is a strong basal cell expression of ICAM-1 with evident cell surface accentuation and also pockets of suprabasal expression with cell surface accentuation. These patterns are associated with different factors that trigger cytokine release in different locations, especially tumor necrosis factor–α, IL-6, and interferon-γ12—proteins that are clearly associated with the cytokine storm generated by COVID-19 and were not associated with skin patterns of cutaneous toxicity from pemetrexed.13
In contrast to these case reports, patients with COVID-19 who received immunotherapy had the highest death rates or chances of developing critical symptoms.14 This discrepancy may be related to the low number of patients included in this report; however, our cases did not present major respiratory symptoms but rather atypical and severe dermatologic findings. Furthermore, both patients had low levels of IL-6, which is an important predictor of COVID-19–associated fatality.15 Owing to the marked elevation of DD, one of the patients received low-molecular-weight heparin, as recently recommended.16 Marked DD elevation has been recently reported as one of the predictors of mortality in patients infected with COVID-19.17
In contrast, skin manifestations such as urticaria have been reported in at least 19% of COVID-19 cases. It is worth mentioning that this report was largely based on a cohort of patients who presented with classic symptoms or were suspected cases.18 In the absence of respiratory symptoms and previous immunotherapy treatment, one could assume that our patient’s disease behavior was completely different.
Despite the controversy, and in the absence of severe pulmonary compromise with a unique cutaneous tropism of COVID-19 in our patients, steroids were offered in both cases. Overall, no unique reason exists to expect that patients with COVID-19 infection will benefit from corticosteroids, especially in the setting of acute respiratory distress syndrome; however, in the presence of other manifestations, an alternative perspective may be useful.
At this moment, the possibility of modulating diverse inflammatory manifestations derived from COVID-19 in patients with cancer (many of them exposed to immunotherapy) remains open. Among others, we will see the usefulness of the convalescent serum and the use of low doses of cyclophosphamide, baricitinib, ruxolitinib, and fedratinib—the last ones with potential in patients with dermatologic manifestations.
Footnotes
Drs. Rolfo and Cardona contributed equally to this work.
Disclosure: Dr. Rolfo reports receiving grants from Merck Sharp & Dohme, AstraZeneca, Archer, Inivata, Merck Serono, Lung Cancer Research Foundation-Pfizer, and Mylan; and nonfinancial support from Oncopass, Guardant Health, and Biomark Inc. outside of the submitted work. Dr. Cardona reports receiving personal fees from AbbVie, Celldex, Roche, Merck Sharp & Dohme, Novartis, AstraZeneca, Bristol-Myers Squibb, Foundation Medicine, Boehringer Ingelheim, and the Foundation for Clinical and Applied Cancer Research; and other fees from Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, Boehringer Ingelheim, and Foundation Medicine outside of the submitted work. Dr. Pino reports receiving personal fees from AstraZeneca, Roche, and Merck Sharp & Dohme outside of the submitted work. Dr. Viola reports receiving personal fees from AstraZeneca and other fees from Mundipharma, Boehringer Ingelheim, and AstraZeneca outside of the submitted work. Dr. Russo reports receiving personal fees from AstraZeneca outside of the submitted work. Dr. Rojas reports receiving personal fees and other fees from Bristol-Myers Squibb; grants and personal fees from Boehringer Ingelheim and AstraZeneca; grants, personal fees, and other fees from Roche and Merck Sharp & Dohme; and personal fees from Novartis outside of the submitted work. Dr. Arrieta reports receiving personal fees from Merck Sharp & Dohme, Roche, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, and Pfizer; and grants from AstraZeneca, Roche, and Merck Sharp & Dohme outside of the submitted work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2020.06.019.
Supplementary Data
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed July 28, 2020.
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidaway P. COVID-19 and cancer: what we know so far. Nat Rev Clin Oncol. 2020;17:336. doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunan D., Brassey J., Mahtani K., Heneghan C. COVID-19 signs and symptoms tracker. The Centre for Evidence-Based Medicine, The University of Oxford. https://www.cebm.net/covid-19/covid-19-signs-and-symptoms-tracker/ Accessed April 18, 2020.
- 6.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 7.Sibaud V., Meyer N., Lamant L., Vigarios E., Mazieres J., Delord J.P. Dermatologic complications of anti–PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 8.Weber J.S., Hodi F.S., Wolchok J.D. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 9.Belum V.R., Benhuri B., Postow M.A. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpos E., Ntanasis-Stathopoulos I., Elalamy I. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stepaniuk P., Vostretsova K., Kanani A. Review of cold-induced urticaria characteristics, diagnosis and management in a Western Canadian allergy practice. Allergy Asthma Clin Immunol. 2018;14:85. doi: 10.1186/s13223-018-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correia O., Delgado L., Barbosa I.L., Campilho F., Fleming-Torrinha J. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58–62. doi: 10.1067/mjd.2002.120473. [DOI] [PubMed] [Google Scholar]
- 13.Piérard-Franchimont C., Quatresooz P., Reginster M.-A., Piérard G.E. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769–772. doi: 10.3892/ol.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020:e2141. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galván Casas C., Català A., Carretero Hernández G. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]