Abstract
Ebola virus (EBOV) is one of the most pathogenic viruses in humans which can cause a lethal hemorrhagic fever. Understanding the cellular entry mechanisms of EBOV can promote the development of new therapeutic strategies to control virus replication and spread. It has been known that EBOV virions bind to factors expressed at the host cell surface. Subsequently, the virions are internalized by a macropinocytosis-like process, followed by being trafficked through early and late endosomes. Recent researches indicate that the entry of EBOV into cells requires integrated and functional lipid rafts. Whilst lipid rafts have been hypothesized to play a role in virus entry, there is a current lack of supporting data. One major technical hurdle is the lack of effective approaches for observing viral entry. To provide evidence on the involvement of lipid rafts in the entry process of EBOV, we generated the fluorescently labeled Ebola virus like particles (VLPs), and utilized single-particle tracking (SPT) to visualize the entry of fluorescent Ebola VLPs in live cells and the interaction of Ebola VLPs with lipid rafts. In this study, we demonstrate the compartmentalization of Ebola VLPs in lipid rafts during entry process, and inform the essential function of lipid rafts for the entry of Ebola virus. As such, our study provides evidence to show that the raft integrity is critical for Ebola virus pathogenesis and that lipid rafts can serve as potential targets for the development of novel therapeutic strategies.
Keywords: Ebola viruses, Single particle tracking, Lipid raft, Cell entry, Pathogenesis
HIGHLIGHTS
Scientific question
This study investigated the entry process of EBOV through lipid rafts in live cells using the single virus tracking technique.
Evidence before this study
Recent researches have revealed that EBOV were internalized into host cells by a macropinocytosis-like process, which required integration and function of lipid rafts. However, there has no convincing data to show the dynamic interaction between EBOV virions and lipid rafts on plasma membrane during EBOV entry of cells.
New findings
In this study, we generated the fluorescently labeled Ebola virus like particles (VLPs) which has striking morphological similarity with live filoviruses. By employing the single particle tracking technique we visualized the dynamic internalization of Ebola VLPs into live cells through lipid rafts. This is the first real time live imaging evidence for the lipid raft mediated endocytosis process for Ebola virus at a single virus level.
Significance of the study
Our study provides, for the first time, live imaging evidence for the important roles plasma membrane lipid rafts played during EBOV entry into cells. This lipid rafts mediated macropinocytosis-like process can be potential targets for future therapeutic strategies.
Alt-text: Unlabelled Box
1. Introduction
The Ebola virus (EBOV) within the family Filoviridae is one of the most pathogenic viruses in humans and non-human primates which can cause a severe hemorrhagic fever that rapidly progresses and has case fatality rates reaching 90% [1]. At present, neither currently approved vaccines nor antiviral therapeutics are available to combat the virus. Ebola virus disease was endemic in Sub-Saharan Africa, and the largest ever outbreak that started in 2013 spread across Guinea, Liberia, and Sierra Leone. The virus was then imported into the United States and Europe, triggering a global public health threat [2,3]. In an urgent situation such as this, a thorough understanding of the interaction mechanisms between EBOV and host cells is essential for the development of novel and effective prophylactic and therapeutic strategies against the virus [4].
EBOV is a single-stranded negative-sense RNA virus [5]. The structure of enveloped EBOV virion consists of a nucleocapsid complex, surrounding matrix, and coating envelope [5]. The nucleocapsid complex is formed by viral RNA genome packed with nucleoprotein NP, viral proteins VP35 and VP30, and the polymerase (L) [6]. The surrounding matrix beneath envelope comprises viral proteins VP40 and VP24, and maintains the structural integrity of the virion [5]. On the lipid envelope coating virion surface, glycoprotein GP forms trimers and can mediate attachment and entry into host cells [7]. Among the 7 encoded proteins, NP, L, VP40, VP35, VP30, VP24, GP, VP40 is the most abundantly virion protein. It plays a central role in maintaining the structure of the virion and virus assembly and budding [5]. Expression of the matrix protein VP40 alone is sufficient to produce virus-like particles (VLPs) that have indistinguishable morphology to the actual Ebola virus [8]. The GP is a type I transmembrane protein post-translationally cleaved by a furin protease into GP1 and GP2 subunits. GP1 subunit is responsible for interaction with its cellular receptor, whereas GP2 subunit is involved in the process of virus-host cell membrane fusion [9]. The recombinant expressed virus-like particles composed of two proteins VP40 and GP can mediate the whole virus entry process, including attachment, internalization, and membrane fusion [10].
Cellular entry of Ebola viruses is known at first to bind to surface molecules on host cells, and after attachment, the virions are internalized by a macropinocytosis-like process and subsequently trafficked through early and late endosomes [11]. While many studies have already investigated the mechanism of EBOV entry and revealed some important features on this [10,12], critical host factors on plasma membrane which mediate the initial interaction and internalization remain to be elucidated. There are distributed specific microdomains on the cellular membrane that are rich in cholesterol and sphingolipids, and detergent-insoluble with low density known as lipid rafts [13]. These lipid rafts act as functional platforms for multiple cellular functions, such as modulate cell signaling, and mediate virus trafficking [14]. Plasma membrane lipid rafts associated molecules can respond to extracellular stimuli and initiate signaling pathway by oligomerization when partitioned and concentrated in aggregated lipid rafts, which are sparsely distributed in small patches on plasma membrane without stimuli [15]. The compartmentalization of signaling molecules in clustered lipid rafts may help signals accumulate to the required threshold at physiological concentrations of the stimuli [16]. Partitioning in lipid rafts may also be perceived as a measure to perform functions in a more specific and economic manner while keeping distinct pathways spatially separated [13]. Recent researches have showed that the entry of EBOV requires functional rafts [17], revealed the EBOV glycoprotein GP and matrix protein VP40 localized to plasma membrane rafts, and determined a critical role for rafts in the assemble and release of EBOV [17,18].
While the function of lipid rafts in virus entry has previously been reported, a majority of these studies were biochemical studies or those which used fluorescent protein labeling of fixed cells. They could not portray a live dynamic interaction between the virions and host cells during the entry process. Live cell imaging of single viral particles allows the visualization of viral entry in live cells in real time [19,20]. To provide evidence on whether lipid rafts are involved in the entry process of Ebola virus, we utilized single-particle tracking (SPT) based on real-time confocal fluorescence microscope to track the entry of Ebola VLPs and investigate the interaction of Ebola VLPs with lipid-rafts. We generated the fluorescently labeled Ebola VLPs, comprising EBOV glycoprotein GP and the matrix protein VP40 fused with enhanced GFP protein. Using the single-particle tracking technique, the dynamics of Ebola virus entry was imaged in live cells in real time. The live-cell imaging approach also enabled an in-depth analysis of interactions between Ebola VLPs and lipid rafts on the plasma membrane. In this study, we demonstrate the compartmentalization of Ebola virus like particles in lipid rafts during entry process. Our findings also show that the interruption of lipid rafts can block internalization of Ebola virus into cells. Thus, our study provides further evidence to show that the functional integrity of lipid rafts on plasma membrane is critical for the pathogenicity of Ebola virus and that lipid rafts can serve as potential targets for therapeutic interventions.
2. Materials and methods
2.1. Cell lines and antibody
Vero cells and 293T cells were grown in Dulbecco's Modified Eagle's medium (DMEM) (Gibco, US) supplemented with 10% fetal bovine serum (Gibco), 100 units/ml of penicillin,100 μg/ml streptomycin at 37 °C in 5% CO2. The anti-GP antibody of Ebola Virus was donated by Prof. Jinghua Yan from the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS).
2.2. Generation of fluorescent virus-like particles
Fluorescent Ebola-VLPs were generated based on the genome sequence of wild type Zaire EBOV strains-Mayinga (GenBank No.: AF086833.2), by co-transfecting 12 μg pcDNA3.1-eGFP-VP40 and 6 μg pcDNA3.1-GP plasmids into 293T cells in the 10-cm plates using Lipofectamine® 2000 Transfection Reagent (Invitrogen). Six hours post-transfection, the supernatant was transferred into DMEM with 10% FBS and 1% penicillin and streptomycin. Forty eight hours post-transfection, the cultures were centrifuged at 1200 rpm and the VLP-containing supernatant was collected. The supernatant containing VLPs were further centrifuged through a 20% sucrose cushion at 25,000 rpm for 2.5 h at 4 °C. The pellet containing VLPs was re-suspended in ice-cold NTE buffer (10 mM Tris (pH 7.5),100 mM NaCl,1 mM EDTA). Then the containing-VLPs buffer was dialyzed in ice-cold NTE buffer at 4 °C overnight. The fluorescent VLPs were stored in aliquots away from light.
2.3. Identification of fluorescent VLPs
Fluorescent VLPs were stained negatively with 2% Phosphotungstic acid (PTA), and the morphology of VLPs was examined by electron microscope (Hitachi, H-7000FA, Japan). The fluorescent VLPs were put into a 2 cm-plate with glass bottom and dried, then fixed by 4% paraformaldehyde at room temperature for 15 min. After washing with phosphate buffered saline (PBS), the fluorescent VLPs were cultured with mouse anti-GP monoclonal antibodies (produced in our lab) at 4 °C overnight. After reactions with the Alexa-555 labeled rabbit anti-mouse second antibody (Life Sciences), the VLPs were examined under the fluorescence microscope Delta Vision personal DV (Applied Precision) to identify the co-localization of immunofluorescence stained by anti-GP antibody (red) and inherent fluorescence of VLPs (Green) to confirm the composition of the fluorescent Ebola-VLPs.
2.4. VLPs entry assay
Vero cells were cultured in DMEM with 10% FBS and 1% P/S in 6 wells of 20 mm-plates with glass bottoms, and the fluorescent VLPs were added into each well. After adding the fluorescent VLPs, the lipid raft of cell membrane was labeled with the Cholera Toxin B Subunit (Recombinant), Alexa Fluor® 594 Conjugate (Thermo Fisher Scientific, US) at 5, 10, 15, 20, 30, and 60 min. The labeling was for 10 min on ice and away from light. After washing with PBS, the cells were fixed with 4% paraformaldehyde for 40 min and washed again with PBS. Then the cell nucleus was labeled with Hoechst33342 (Beyotime, China) at room temperature for 10 min and washed with PBS. In the entry inhibition assays, the lipid raft inhibitor methyl-β-cyclodextrin (m-β-CD, Aladdin reagent) was diluted to a working concentration of 10 mM using the cell culture medium and pretreated Vero cells for 10 min at room temperature before being cultured with fluorescent VLPs. The images were taken under the PE Ultra VIEW VoX double disc living cell fluorescence confocal microscope. For experiments observing the entry of VLPs in time gradients, we randomly chose 5 views and counted the number of viruses absorbing on cell membrane and the number of cells entering into cells at different time points in the inhibitor treated group and the untreated group.
2.5. Single-particle tracking of Ebola VLPs entry
UltraVIEW VoX double disc live cell fluorescence confocal microscope (PerkinElmer, Co) was used for dynamic observation of single Ebola VLP entry into living cells. The interactions between VLP and lipid rafts of cell membrane were observed in the entry process. Vero cells were cultured in a 20-mm plate with glass bottoms, and purified fluorescent Ebola VLPs were added and incubated to allow adherence for 30 min. Following this, 1 μg/ml Alexa Fluor®-594 Conjugated Cholera Toxin B Subunit (Recombinant) was added into culture. The Cholera Toxin B Subunit can selectively label lipid rafts by binding with the pentasaccharide chain of ganglioside GM1 embedded in plasma membrane lipid rafts [21]. After reacting on ice for 10 min to label lipid rafts of live cell membrane, the supernatant was discarded and the precooling culture medium was added. The real-time dynamic images of VLPs and cell membrane lipid rafts were analyzed in a cell culture environmental chamber (Tokai Hit, 37 °C, 5% CO2) under the UltraVIEW VoX confocal microscope with living cells' workstation.
3. Results
3.1. Morphological similarity of Fluorescent Ebola VLPs to filoviruses
The morphology of fluorescent Ebola VLPs, which comprised GP and VP40 fused with enhanced GFP (eGFP) at the N terminus, was determined using electron microscopy. Most of the purified particles displayed a filamentous morphology similar to filoviruses (Fig. 1A). The VLPs had a similar diameter range of 70–80 nm and length range of 1,000–2,000 nm as Ebola viruses in cell cultures. In addition, the VLPs were coated with 5–10 nm surface projections or “spikes” characteristic of EBOV (Fig. 1A). The envelope layer was clearly formed along the length of the filamentous particles. The fluorescent Ebola VLPs showed inherent bright green fluorescence under the microscope (Fig. 1B). Furthermore, immunofluorescence staining of the VLPs with anti-Ebola GP antibodies showed that the inherent eGFP fluorescence was readily detected, and the majority of eGFP fluorescence were overlaid with the fluorescence from the antibody against the envelope protein GP (Fig. 1C).
Fig. 1.
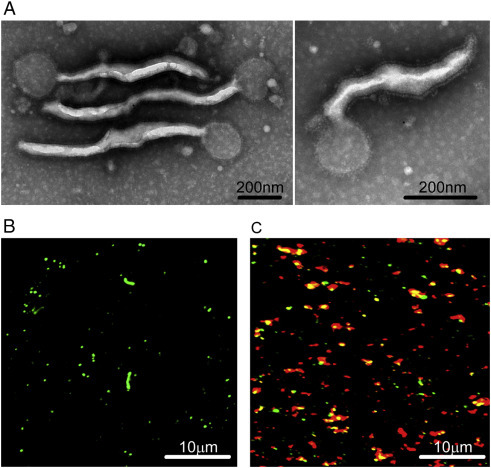
Identification of fluorescent Ebola VLPs. (A) The morphology of fluorescent Ebola VLPs visualized under electron microscope. (B) The fluorescence of Ebola VLPs fused with eGFP examined using fluorescence microscope. (C) The immunofluorescence of Ebola VLPs fused with eGFP tag (green) stained by Alexa-555 conjugated anti-GP antibody (red) examined using fluorescence microscope.
3.2. Co-localization of Ebola VLPs with lipid-rafts on cell membrane
To visualize the interaction between Fluorescent Ebola VLPs and lipid rafts on cell membrane, we co-cultured Ebola VLPs and Vero cells and tracked the interaction at different time points during the culture. Using confocal microscopy, we found that at all time points there were VLPs co-localized with large patches of lipid rafts on the cell membrane which were targeted with fluorescently labeled cholera toxin B subunit, thus confirming the raft association of Ebola VLPs on intact cells (Fig. 2A). We counted the particles that adhered on cells and internalized into cells, and found that after 30 mins there were increased number of particles associated with cells (Fig. 2B), while the ratio of particles associated with the cell membrane to particle internalized inside cells decreased after 30 mins (Fig. 2C). These findings suggested that the entry of EBOV mainly occurred around 30 mins after adding viral particles into cell cultures, and the entry process of EBOV was associated with lipid rafts on cell membrane.
Fig. 2.
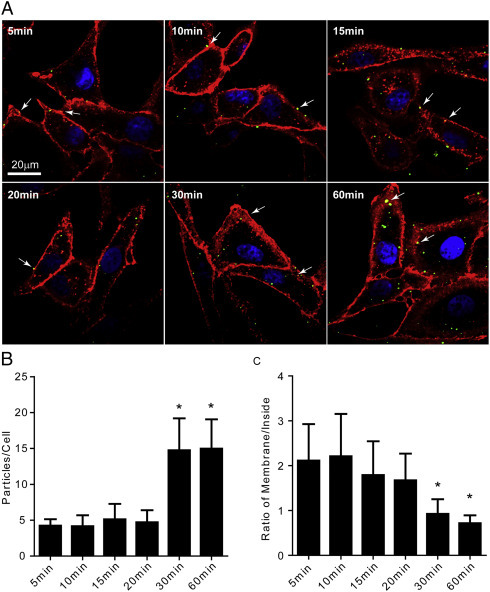
Interaction of Ebola VLPs with lipid-rafts at different time points. (A) Purified fluorescent Ebola VLPs (green) were added into Vero cells, and at the indicated time points the cells were stained with the Alexa Fluor® 594 conjugated Cholera Toxin Subunit B (CTB) to label the lipid rafts on cell membrane (red). Then the cells were fixed and visualized under confocal microscope. (B) The graph shows the average number of Ebola VLPs per cell at different time points. The average number of Ebola VLPs associated with each cell was calculated from 100 cells randomly chosen from 5 different views at each time point. * indicates P < 0.05. (C) The graph shows the ratio of Ebola VLPs on cell membrane to Ebola VLPs internalized inside of cells. The ratio was calculated from 100 cells randomly chosen from 5 different views at each time point. * indicates P < 0.05.
3.3. Prohibition of internalization of Ebola VLPs by Interruption of lipid rafts
To understand the importance of lipid-rafts in the internalization process of Ebola VLPs, Vero cells were pre-treated with methyl-β-cyclodextrin (MCD) that depletes cholesterol from the membrane. After 30 mins of culturing, the cellular internalization of Ebola VLPs was drastically reduced in cells that had been treated with MCD (Fig. 3A). The internalization percentage of VLPs in MCD pretreated Vero cells were around 20%, significantly lower than in MCD untreated Vero cells (Fig. 3B). Thus, the findings confirmed that the functional lipid rafts were critical to the internalization of Ebola VLPs into host cells.
Fig. 3.
Interrupting the function of lipid-rafts inhibited the internalization of Ebola VLPs. (A) Vero cells were pretreated with MβCD and then co-cultured with Ebola VLPs for 30 mins. Compared to the MβCD untreated control group, there were much less Ebola VLPs (green) entering into cells. Lipid-rafts were stained red. (B) The graph shows that the average internalization percentage in the MβCD pre-treated group was significantly higher than in the control group. * indicates P < 0.05.
3.4. Visualization of the interactions between Ebola VLPs and lipid rafts on cell membranes by Single-particle tracking
To better visualize the interaction between Ebola VLPs and lipid rafts on cell membrane, we used the single particle tracking technique to image the internalization of Ebola VLPs into live cells (Fig. 4A). As shown in Fig. 4B and Movie S1 (Supplementary data), a particle moved towards the cell surface and attached onto a membrane consisted of lipid rafts (time 54:30–55:00) domain. Then, the particle moved along the edge of the membrane (time 55:08–55:15), and was gradually moved into a tiny pocket-like depression (time 55:23–57:23), which is a typical lipid raft structure. Finally, the viral particle merged into the lipid raft domains and internalized inside the cell (time 58:00–58: 38). The live imaging of single Ebola particle internalization clearly showed that the Ebola virus entered cells through the membrane domain that consisted of lipid rafts (Movie S1 in Supplementary data).
Fig. 4.
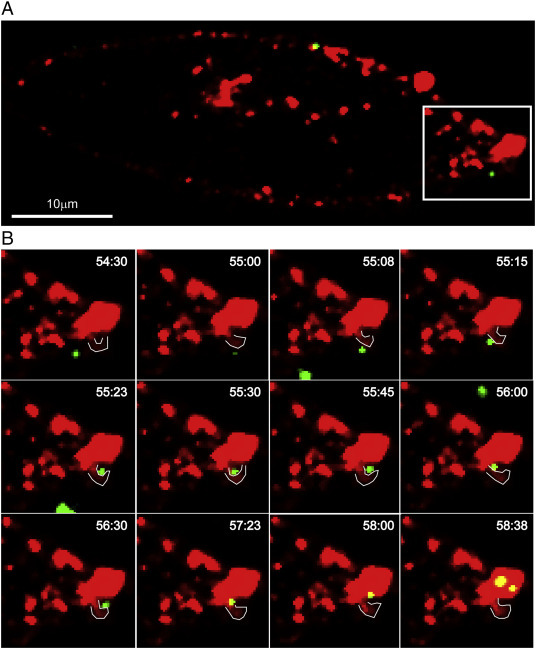
Single particle tracking entry of Ebola VLPs. Real-time imaging of endocytic entries of EBOV-eGFP particles into Vero cells. (A) Dynamic co-localization of EBOV-eGFP (Green) and Lipid rafts (Red) in a Vero cell was examined under real-time confocal microscope for tracking the entry process of Ebola VLPs. The area for focused observation was highlighted by white frame. (B) The graphs show the snapshots of a movie to show the movement of a Ebola VLP from outside the cells to the inside of the cell. The white lines show the edge of a pseudopod on membrane.
4. Discussion
Understanding the interactions between Ebola virus and the host cells is essential for successful development of effective prophylactic and therapeutic strategies. Host cell factors exploited by Ebola viruses for viral amplification constitute potential targets for antiviral intervention. Blocking the factors required for the initial step in viral replication and its entry into target cells is of particular interest. In this study, by using single particle tracking and fluorescently labeled Ebola VLP which has striking morphological similarity with live filoviruses, and incorporates both Ebola GP and matrix protein VP40, we visualized the dynamic internalization of Ebola VLPs in live cells, and uncovered the lipid raft mediated endocytosis process for Ebola virus at a single virus level in real time.
Membrane fusion is a common feature for the entry of enveloped viruses and can be mediated in different ways [22], either by direct fusion with the cell plasma membrane or by entering the endocytic pathway [23]. As previously reported in earlier studies, filoviruses first bind to the cell surface and are then internalized by a macropinocytosis-like process after its interaction with cell surface receptors or other co-factors. Then, the EBOV fusion requires priming the viral GP by the cysteine proteases cathepsin L and B inside the late endosome. Following this, the primed GP can then trigger fusion by exposure of its domain to bind the endosome-receptors. Recent studies demonstrate that the endosome-residing membrane protein Niemann-Pick C1(NPC1) is the endosome-receptor that binds to the primed GP, and the NPC1-C domain is the binding partner of the primed GP [24,25].The mechanisms of EBOV virions to enter cells and then traffick through early and late endosomes have been studied extensively [[26], [27], [28]]. Thus, the internalization of EBOV virions into cells are critical for further trafficking through endosomes and the endocytic membrane fusion. Previous studies showed that EBOV can enter host cells through either macropinocytosis-like endocytosis or endocytosis, depending on target cells. The macropinocytosis-like endocytosis of EBOV is independent of clathrin, caveolae, and dynamin, and dependent of actin and lipid raft [[27], [28], [29]]. The endocytosis of EBOV is clathrin, caveolae, and dynamin dependent [30]. Mannose-binding lectin (MBL), a prototypic soluble calcium-dependent (C-type) lectin, was reported to mediate lipid raft dependent macropinocytosis of EBOV. Contrary to the filovirus canonical endocytic pathway, the pathway for macropinocytosis of EBOV requires less actin function or early endosomal processing [31]. Our studies showed that the cellular internalization of EBOV VLPs comprising GP and VP40 had decreased when Vero cells had been pretreated with β-MCD. Our results provide evidence that the macropinocytosis-like endocytosis of EBOV VLPs is dependent on lipid rafts, in agreement with previous findings using wild type virus [28].
The lipid raft microdomains on plasma membrane are enriched with virus receptors and co-receptors, which may be advantageous for viruses to enter into host cells, and provide a specific site for initiating the attachment and membrane fusion of many viruses, including Ebola virus [18] [17]. In prominence, Ebola virus has a broad cell tropism and could infect many cell types, including monocytes, macrophages, dendritic cells and epithelial cells. Ebola viruses initiate infection by binding multiple adherent molecules on host cells, such as DC-SIGN (dendritic-cell-specific ICAM3-grabbing non-integrin), L-SIGN (liver and lymph node SIGN) [25,32]. In addition, several integrins such as integrinαV, T cell immunoglobulin and mucin domain 1 (TIM-1) proteins, and tyrosine protein kinase receptor 3 (TYRO3) family members (Axl, Dtk, Mer) which are also enriched in lipid rafts, have been implicated in Ebola-GP mediated cell entry [[33], [34], [35]]. More precisely, the Ebola glycoprotein GP and the matrix protein VP40 were also shown to locate in lipid rafts and associate with the entry and release of EBOV [17,18]. However, previous studies mostly showed the interaction of EBOV with lipid rafts using biochemical experiments, or immunofluorescence staining of fixed cells. There has been no direct live image evidence of the entry of EBOV into cells through lipid rafts. In our study relying on the high-resolution live imaging technique, we are the first to visualize the dynamic interaction of EBOV VLPs with plasma membrane and the entry of EBOV through large patches of lipid-rafts. Although we could not visualize the interaction between EBOV and the host cell attachment factors, integrins, TIM-1, and TYRO3 family members, we propose that the entry of EBOV through lipid rafts will potentiate the signaling through these sensor molecules to coordinate the intracellular traffic of virions through endosomes and within membrane fusion, and activate multiple pathways. Besides being in the plasma membrane, cholesterol-sphingolipid rafts are also present in late endosomal or lysosomal membranes. It is interesting to note that the lipid rafts are associated with endosomal residing NPC1 protein in late endosomes, where this protein is the binding partner of EBOV GP for endosomal membrane fusion, and is also enhanced by cholesterol loading [36]. Furthermore, NPC1 can modulate the efficient recycle of endocytosed LDL-cholesterol to the plasma membrane, which seems to reciprocally operate with membrane transport machinery [37]. Thus the flow of lipid raft components from plasma membrane to late endosomes may affect the function of NPC-1 and impact the membrane fusion process of EBOV degradative compartments. To further understand the molecular basis of the altered cellular functions of NPC during the endosomal raft accumulation and changing of lipid composition in late endosomes, more detailed investigations are needed.
An important aspect of this study is the visualization of Ebola trafficking in live cells by using the SPT; whereas previous biochemical data and image data indicated that lipid rafts play a role in entry of Ebola virus, they did not examine the entry of intact virus particles with both GP and VP40 in living cells [18]. Live cell imaging of single virion particles allows the visualization of the interaction of virus and cellular structure in real time. Herewith, single particle tracking shows the detailed dynamics of interactions between Ebola VLPs and plasma membrane lipid rafts. Our findings suggest that functional lipid rafts are critical to the entry of Ebola virus, and the integrity of the molecular components in lipid rafts could be potential therapeutic targets. Further characterization of the lipid raft-located host molecules that bind to viral GP40 and VP40 during host-virus interaction by using other methods such as proteomic analysis, will help to identify critical molecule targets mediating the function of lipid rafts.
The following are the supplementary data related to this article.
A typical dynamic interaction process between Ebola VLPs (green) and lipid-rafts tiny pocket (red) during the entry of VLPs.
Supplementary Fig. 1.
The representative snapshots of the live imaging video showing the co-localization of EBOV VLPs (green) with plasma membrane lipid rafts (red).
Acknowledgments
Acknowledgements
This work was supported by the national key project for infectious disease control and prevention (Grant no 2018ZX10711-001) and the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB29050201).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
C. Jin and B. Che performed the research work and wrote the paper. Z. Guo, C. Li, Y. Liu, W. Wu assisted the laboratory experiments. D. Li and S. Wang supervised and discussed the data. M. Liang and C. Qiang designed the project and edited the manuscript. All authors approved the final manuscript.
Contributor Information
Zongqiang Cui, Email: czq@wh.iov.cn.
Mifang Liang, Email: liangmf@ivdc.chinacdc.cn.
References
- 1.Feldmann H., Geisbert T.W. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849–862. doi: 10.1186/s40249-019-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W.J. HIV prevalence in suspected Ebola cases during the 2014–2016 Ebola epidemic in Sierra Leone. Infect. Dis. Poverty. 2019;8(1):15. doi: 10.1186/s40249-019-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Languon S., Quaye O. Filovirus disease outbreaks: a chronological overview. Virology (Auckl) 2019;10 doi: 10.1177/1178122X19849927. doi: 10.1177/1178122X19849927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.William J., Liu W.S., Zhu Wuyang, Jin Cong, Zou Shumei, Wang Ji, Ke Yuehua, Li Xiaofeng, Liu Mi, Hu Tao, Fan Hang, Tong Yigang, Zhao Xiang, Chen Wenbin, Zhao Yuhui, Di Liu Gary Wong, Chen Chengchao, Geng Chunyu, Xie Weiwei, Jiang Hui, Kamara Idrissa Laybor, Kamara Abdul, Lebby Matt, Kargbo Brima, Qiu Xiangguo, Wang Yu, Liang Xiaofeng, Liang Mifang, Dong Xiaoping, Wu Guizhen, Gao George F., Shu Yuelong. Intra-host Ebola viral adaption during human infection. Biosafety and Health. 2019;1(1):14–24. doi: 10.1016/j.bsheal.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adu-Gyamfi E. The Ebola virus matrix protein penetrates into the plasma membrane: a key step in viral protein 40 (VP40) oligomerization and viral egress. J. Biol. Chem. 2013;288(8):5779–5789. doi: 10.1074/jbc.M112.443960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olejnik J. Intracellular events and cell fate in filovirus infection. Viruses. 2011;3(8):1501–1531. doi: 10.3390/v3081501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann H. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol Suppl. 1999;15:159–169. doi: 10.1007/978-3-7091-6425-9_11. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Conserved motifs within Ebola and Marburg virus VP40 proteins are important for stability, localization, and subsequent budding of virus-like particles. J. Virol. 2010;84(5):2294–2303. doi: 10.1128/JVI.02034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandran K. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308(5728):1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez O. A mutation in the Ebola virus envelope glycoprotein restricts viral entry in a host species- and cell-type-specific manner. J. Virol. 2013;87(6):3324–3334. doi: 10.1128/JVI.01598-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aleksandrowicz P. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J. Infect. Dis. 2011;204(Suppl. 3):S957–S967. doi: 10.1093/infdis/jir326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlmann F. Analysis of Ebola virus entry into macrophages. J. Infect. Dis. 2015;212(Suppl. 2):S247–S257. doi: 10.1093/infdis/jiv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manes S., del Real G., Martinez A.C. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3(7):557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 14.Simons K., Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 15.Harder T. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141(4):929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drevot P. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002;21(8):1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bavari S. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 2002;195(5):593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panchal R.G. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc. Natl. Acad. Sci. U. S. A. 2003;100(26):15936–15941. doi: 10.1073/pnas.2533915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q. Single-particle tracking of human immunodeficiency virus type 1 productive entry into human primary macrophages. ACS Nano. 2017;11(4):3890–3903. doi: 10.1021/acsnano.7b00275. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y. Real-time imaging of single HIV-1 disassembly with multicolor viral particles. ACS Nano. 2016;10(6):6273–6282. doi: 10.1021/acsnano.6b02462. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Altamirano M.M., Aguilar-Carmona I., Sanchez-Garcia F.J. Expression of GM1, a marker of lipid rafts, defines two subsets of human monocytes with differential endocytic capacity and lipopolysaccharide responsiveness. Immunology. 2007;120(4):536–543. doi: 10.1111/j.1365-2567.2006.02531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backovic M., Rey F.A. Virus entry: old viruses, new receptors. Curr. Opin. Virol. 2012;2(1):4–13. doi: 10.1016/j.coviro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison S.C. Viral membrane fusion. Virology. 2015;479-480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E.H. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012;31(8):1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H. Ebola viral glycoprotein bound to its endosomal receptor Niemann-pick C1. Cell. 2016;164(1–2):258–268. doi: 10.1016/j.cell.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulherkar N. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology. 2011;419(2):72–83. doi: 10.1016/j.virol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanbo A. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;6(9) doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed M.F. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6(9) doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez A. Analysis of filovirus entry into vero e6 cells, using inhibitors of endocytosis, endosomal acidification, structural integrity, and cathepsin (B and L) activity. J. Infect. Dis. 2007;196(Suppl. 2):S251–S258. doi: 10.1086/520597"10.1086/520597. [DOI] [PubMed] [Google Scholar]
- 30.Hunt C.L. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J. Virol. 2011;85(1):334–347. doi: 10.1128/JVI.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brudner M. Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS One. 2013;8(4):e60838. doi: 10.1371/journal.pone.0060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez C.P. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 2002;76(13):6841–6844. doi: 10.1128/jvi.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jemielity S. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimojima M., Ikeda Y., Kawaoka Y. The mechanism of Axl-mediated Ebola virus infection. J. Infect. Dis. 2007;196(Suppl. 2):S259–S263. doi: 10.1086/520594"10.1086/520594. [DOI] [PubMed] [Google Scholar]
- 35.Takada A. Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology. 2000;278(1):20–26. doi: 10.1006/viro.2000.0601. [DOI] [PubMed] [Google Scholar]
- 36.Neufeld E.B. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 1999;274(14):9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- 37.Lusa S. Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane. J. Cell Sci. 2001;114(Pt 10):1893–1900. doi: 10.1080/152165401317190851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A typical dynamic interaction process between Ebola VLPs (green) and lipid-rafts tiny pocket (red) during the entry of VLPs.


