Abstract
Cell-intrinsic and extrinsic signals regulate the state and fate of stem and progenitor cells. Recent advances in metabolomics illustrate that various metabolic pathways are also important in regulating stem cell fate. However, our understanding of the metabolic control of the state and fate of progenitor cells is in its infancy. Using Drosophila hematopoietic organ: lymph gland, we demonstrate that Fatty Acid Oxidation (FAO) is essential for the differentiation of blood cell progenitors. In the absence of FAO, the progenitors are unable to differentiate and exhibit altered histone acetylation. Interestingly, acetate supplementation rescues both histone acetylation and the differentiation defects. We further show that the CPT1/whd (withered), the rate-limiting enzyme of FAO, is transcriptionally regulated by Jun-Kinase (JNK), which has been previously implicated in progenitor differentiation. Our study thus reveals how the cellular signaling machinery integrates with the metabolic cue to facilitate the differentiation program.
Research organism: D. melanogaster
eLife digest
Stem cells are special precursor cells, found in all animals from flies to humans, that can give rise to all the mature cell types in the body. Their job is to generate supplies of new cells wherever these are needed. This is important because it allows damaged or worn-out tissues to be repaired and replaced by fresh, healthy cells.
As part of this renewal process, stem cells generate pools of more specialized cells, called progenitor cells. These can be thought of as half-way to maturation and can only develop in a more restricted number of ways. For example, so-called myeloid progenitor cells from humans can only develop into a specific group of blood cell types, collectively termed the myeloid lineage.
Fruit flies, like many other animals, also have several different types of blood cells. The fly’s repertoire of blood cells is very similar to the human myeloid lineage, and these cells also develop from the fly equivalent of myeloid progenitor cells. These progenitors are found in a specialized organ in fruit fly larvae called the lymph gland, where the blood forms. These similarities between fruit flies and humans mean that flies are a good model to study how myeloid progenitor cells mature.
A lot is already known about the molecules that signal to progenitor cells how and when to mature. However, the role of metabolism – the chemical reactions that process nutrients and provide energy inside cells – is still poorly understood. Tiwari et al. set out to identify which metabolic reactions myeloid progenitor cells require and how these reactions might shape the progenitors’ development into mature blood cells.
The experiments in this study used fruit fly larvae that had been genetically altered so that they could no longer perform key chemical reactions needed for the breakdown of fats. In these mutant larvae, the progenitors within the lymph gland could not give rise to mature blood cells. This showed that myeloid progenitor cells need to be able to break down fats in order to develop properly.
These results highlight a previously unappreciated role for metabolism in controlling the development of progenitor cells. If this effect also occurs in humans, this knowledge could one day help medical researchers engineer replacement tissues in the lab, or even increase our own bodies’ ability to regenerate blood, and potentially other organs.
Introduction
Recent studies have highlighted how metabolism regulates the state and fate of stem cells (Ito and Suda, 2014; Shyh-Chang et al., 2013; Shyh-Chang and Ng, 2017). Besides catering to the bioenergetic demands of a cell, metabolic intermediates can also alter the fate of stem cells via epigenetic mechanisms like histone modifications (Atlasi and Stunnenberg, 2017; Saraiva et al., 2010). Studies on diverse stem cell scenarios, primarily in Hematopoietic Stem Cells (HSCs), have established that at various developmental stages, stem cells have different metabolic requirements (Kohli and Passegué, 2014; Suda et al., 2011). Nevertheless, the metabolic demand of progenitors, the immediate descendants of stem cells, is yet to be fully elucidated.
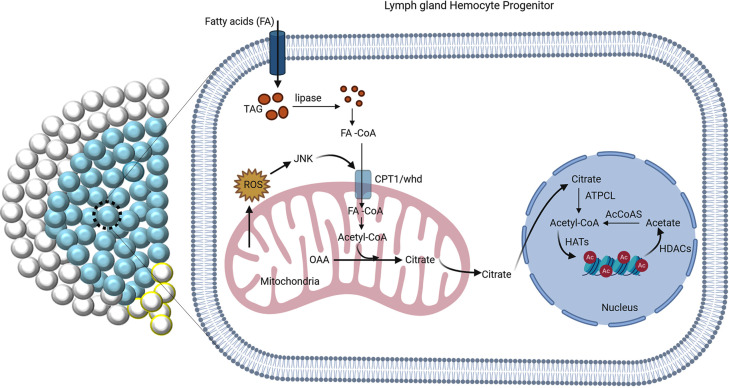
Studies to date have evidenced that glucose metabolism impacts the onset and magnitude of HSC induction (Harris et al., 2013; Shyh-Chang and Ng, 2017), as well as HSC specification (Oburoglu et al., 2014). Another major metabolic state that is active in stem and progenitor cells is fatty acid oxidation (FAO) (Ito et al., 2012; Knobloch et al., 2017; Lin et al., 2018; Wong et al., 2017; Ito et al., 2012). Enzymatic activities of the members of FAO lead to the shortening of fatty acids and the production of acetyl-CoA in mitochondria. The acetyl-CoA thus produced can not only generate NADH and FADH2 through TCA cycle but also can be utilized in acetylation of various proteins including histones (Fan et al., 2015; Houten and Wanders, 2010; McDonnell et al., 2016; Wong et al., 2017).
The primary goal of this study was to ascertain whether FAO regulates any aspect of the hemocyte progenitors in the Drosophila larval hematopoietic organ, lymph gland. The lymph gland is a multilobed structure consisting of a well-characterized anterior lobe (primary lobe) and uncharacterized posterior lobes (Figure 1A, Banerjee et al., 2019). The core of the primary lobe houses the progenitor populations and is referred to as the medullary zone (MZ), while the differentiated cells define the outer cortical zone (CZ, Figure 1A'). In between these two zones, lies a rim of differentiating progenitors or intermediate progenitors (IPs). The blood progenitors of late larval lymph gland are arrested in G2-M phase of cell cycle (Sharma et al., 2019), have high levels of ROS (Owusu-Ansah and Banerjee, 2009), lack differentiation markers, are multipotent (Jung et al., 2005) and are maintained by the hematopoietic niche/posterior signaling center, PSC (Krzemień et al., 2007; Lebestky et al., 2003; Mandal et al., 2007). The primary lobe has been extensively used to understand intercellular communication relevant to progenitor maintenance (Gao et al., 2013; Giordani et al., 2016; Gold and Brückner, 2014; Hao and Jin, 2017; Krzemień et al., 2007; Krzemien et al., 2010; Lebestky et al., 2003; Mandal et al., 2007; Mondal et al., 2011; Morin-Poulard et al., 2016; Sinenko et al., 2009; Small et al., 2014; Yu et al., 2018). Although these studies have contributed significantly toward our understanding of cellular signaling relevant for progenitor homeostasis, the role of cellular metabolism in regulating the state and fate of blood progenitors remains to be addressed.
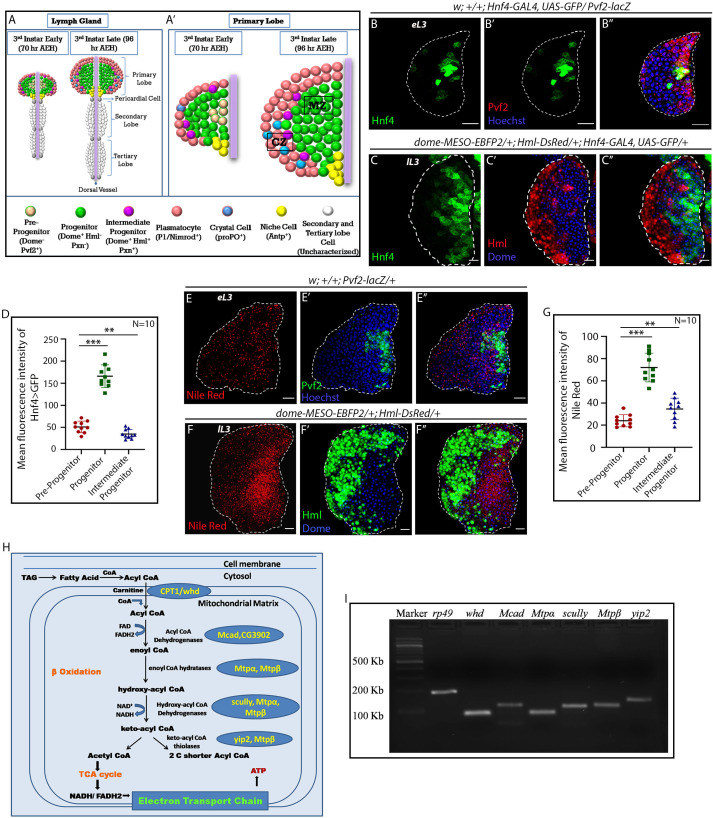
Figure 1. FAO genes are expressed in hemocyte progenitors of lymph gland.
Age and genotype of the larvae are mentioned in respective panels. (A–A') Model of lymph gland of third early and third late instar stages depicting anterior primary lobes and posterior lobes. (A’). Primary lobe showing different subpopulations: Pvf2+ Dome- pre-progenitor, Dome+ progenitors and Dome+ Pxn+ Hml+ Intermediate progenitors (IPs) in early third and late third instar larval stages. Progenitors are present in the core of the primary lobe called the medullary zone (MZ), and differentiated cells (Plasmatocytes and crystal cells) are present in the outer zone called cortical zone (CZ). (B–B'') Expression of Hnf4-GAL4 > UAS-GFP in Pvf2+ pre-progenitors of the early third instar lymph gland. (C–C'') Expression of Hnf4-GAL4 > UAS-GFP in Dome+ progenitors and Dome+ Hml+ Intermediate progenitors (IPs) shown in dome-MESO-EBFP2/+; Hml-DsRed/+ genotype. (D). Quantitative analysis of B–C''- reveals that the Dome+ progenitors have higher levels of Hnf4 expression. p-Value for Hnf4-GAL4 > UAS-GFP expression in Dome+ progenitors is 9.55 × 10−9 compared to control Pvf2+ pre-progenitors. p-Value for Hnf4-GAL4 > UAS-GFP expression for Dome+ Hml+ IPs is 7.34 × 10−3 compared to control Pvf2+ pre-progenitors. (E–E'') Nile red staining in Pvf2+ pre-progenitors of early third instar stage lymph gland. (F–F'') Expression of Nile red in Dome+ progenitors and Dome+ Hml+ Intermediate progenitors (IPs) shown in dome-MESO-EBFP2/+; Hml-DsRed/+ genotype (Dome+: blue, Hml+: green). (G). Quantitative analysis of E–F'' shows higher levels of neutral lipids in the Dome+ progenitors. Compared to control Pvf2+ pre-progenitors, p-Values for nile red expression in Dome+ progenitors is 1.39 × 10−7 and Dome+ Hml+ IPs pre-progenitors is 9.11 × 10−3. Five optical sections of 1 µm thickness from the middle of the Z-stack were merged into a single section. (H) Schematic representation of FAO and the constituent enzymes. (I) Transcripts of β-oxidation enzymes, whd, Mcad, Mtpα, scully, Mtpβ, and yip2 (Refer to H) can be detected in the third late instar lymph gland. eL3 and lL3 refer to the early and late instar lymph glands. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 1—figure supplement 1. Temporal analysis of pre-progenitors in the lymph gland and mitochondrial analysis in Dome+ progenitors.
Figure 1—figure supplement 2. FAO components are expressed in hemocyte progenitor subpopulations in the lymph gland.
Here, we show that the G2-M arrested hemocyte progenitors of the Drosophila larval lymph gland rely on FAO for their differentiation. While the loss of FAO prevents their differentiation, upregulation of FAO in hemocyte progenitors by either genetic or pharmacological means leads to precocious differentiation. More importantly, acetate supplementation restores the histone acetylation and differentiation defects of the progenitor cells observed upon loss of FAO. Our genetic and molecular analyses reveal that FAO acts downstream to the Reactive Oxygen Species (ROS) and c-Jun N-terminal Kinase (JNK) axis, which is essential for triggering the differentiation of these progenitors (Owusu-Ansah and Banerjee, 2009). In this study, we, therefore, provide the unknown link that connects cellular signaling and metabolic circuitry essential for differentiation of the blood progenitors.
Results
Genes involved in FAO pathway are expressed in the hemocyte progenitors of late third instar lymph gland
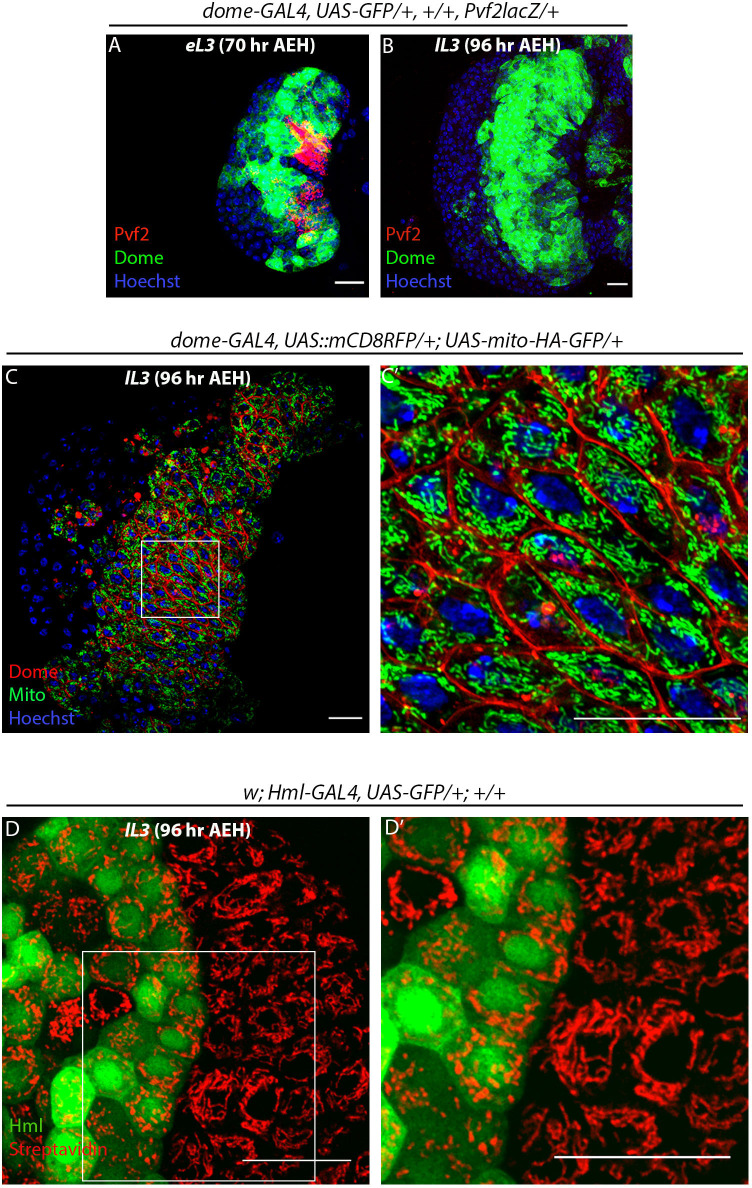
Drosophila hemocyte progenitors in the lymph gland proliferate in the early larval stages (Jung et al., 2005; Mondal et al., 2011). Eventually, they undergo a G2-M arrest in late third instar (Sharma et al., 2019). Studies have identified that lymph gland hemocyte progenitors of the primary lobe can be grouped into three subpopulations: Dome- pre-progenitors, Dome+ progenitors, and Dome+ Pxn+ Hml+ Intermediate progenitors (Banerjee et al., 2019). These progenitor subpopulations will be henceforth referred to as pre-progenitors, progenitors, and IPs, respectively. The pre-progenitors can also be visualized by Pvf2 expression in first, second, and early third instar larval lymph gland (Ferguson and Martinez-Agosto, 2017, Figure 1—figure supplement 1A–B). Since in the late third instar lymph gland, only progenitors and IPs are present (Figure 1A'), we analyzed the early third instar lymph gland to characterize the pre-progenitors.
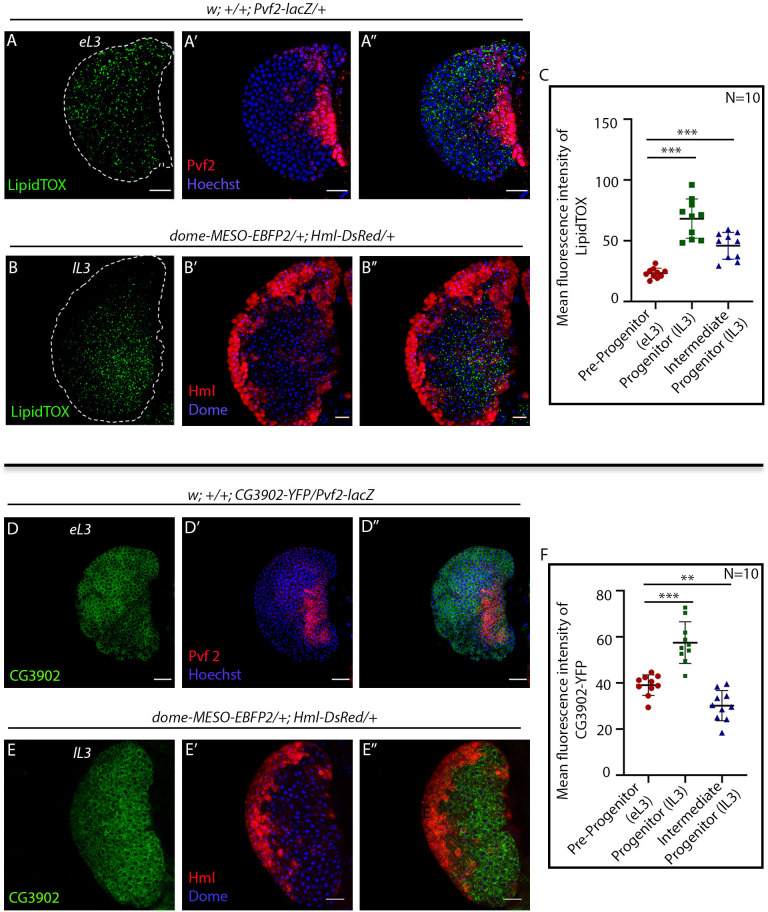
To ascertain the involvement of FAO, if any, in hemocyte progenitors of larval lymph gland, we monitored the expression of Hepatocyte Nuclear Factor 4 (Hnf4) (Palanker et al., 2009), an essential gene of larval FAO and lipid mobilization. As evident from Figure 1B–D, G2–M arrested progenitors (visualized by dome-MESO-EBFP2+) express high levels of Hnf4 > GFP compared to pre-progenitors (visualized by Pvf2 expression) and IPs (Dome+ Hml+). Interestingly, neutral lipids (visualized by Nile red staining: Figure 1E–G) are conspicuous in late third instar blood progenitors (dome-MESO-EBFP2+), compared to the pre-progenitors (Figure 1E–E'' and G) and IPs (Figure 1F–F'' and G ).The lipid enrichment in the late progenitors is further evident upon LipidTOX (validated marker for neutral lipids) labeling (Figure 1—figure supplement 2A–C). Based on the presence of relatively high levels of lipid droplets in a non-lipid storage tissue and the expression of Hnf4 in the progenitor cells, we speculated a developmental role of FAO in these cells. This prompted us to check for the expression of other genes involved in FAO (Palanker et al., 2009, Figure 1H). Figure 1I shows the expression of the rate-limiting enzyme of FΑO, withered (whd, Drosophila homolog of CPT1: Carnitine palmitoyltransferase 1) along with Mcad (medium-chain acyl-CoA dehydrogenase), Mtpα (mitochondrial trifunctional protein α subunit: Long-chain-3-hydroxyacyl-CoA dehydrogenase), scully (3-hydroxyacyl-CoA dehydrogenase), Mtpβ (mitochondrial trifunctional protein β subunit: Long-chain-3-hydroxyacyl-CoA dehydrogenase) and yip2 (yippee interacting protein 2: acetyl-CoA acyltransferase) in the late lymph gland. Elevated levels of expression of acyl-Coenzyme A dehydrogenase (CG3902) is also seen in the progenitors as compared to the pre-progenitors and IPs (Figure 1—figure supplement 2D–F).
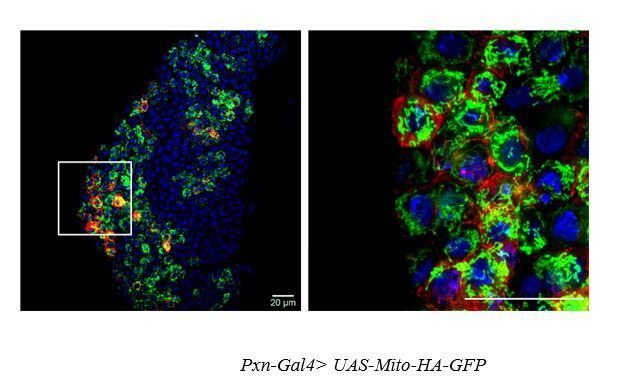
Major aspects of FAO takes place in the mitochondria where fat moiety is broken down to generate acetyl-CoA, NADH, and FADH2 (Bartlett and Eaton, 2004), we, therefore, looked at the status of mitochondria in the progenitors as well as the differentiated hemocytes. The presence of an abundant reticular network of mitochondria is evident in the progenitors (dome-GAL4 >UAS-mito-HA-GFP, Figure 1—figure supplement 1C–C', and Video 1). However, for reasons unknown to us HmlΔ-GAL4 is unable to drive UAS-mito-HA-GFP, therefore, we used streptavidin labeling to visualize the mitochondrial status in the differentiated hemocytes. Interestingly, differentiated hemocytes (HmlΔ-GAL4 > UAS-GFP) show less reticular mitochondria (labeled by Strepatvidin-Cy3, Chowdhary et al., 2017; Hollinshead et al., 1997, Figure 1—figure supplement 1D–D') in comparison to the progenitors (Hml>GFP negative) thereby indicating a preference for FAO in the hemocyte progenitors.
Video 1. Mitochondrial distribution in the progenitors (red, Dome+) visualized by UAS-mito-HA-GFP.
Put together; the above results implicate FAO as the metabolic state of the Dome+ cells of the primary lobe of the lymph gland. These observations, in turn, encouraged us to investigate the importance of FAO in maintaining the state and fate of these progenitors during development.
Loss of FAO affects hemocyte progenitor differentiation
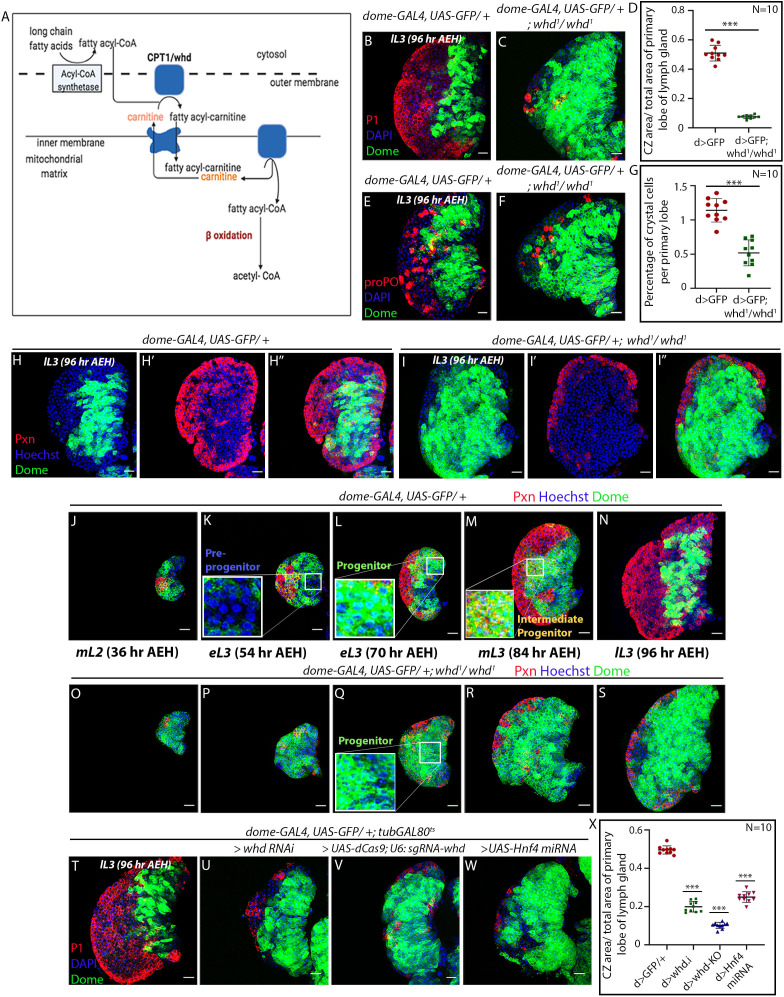
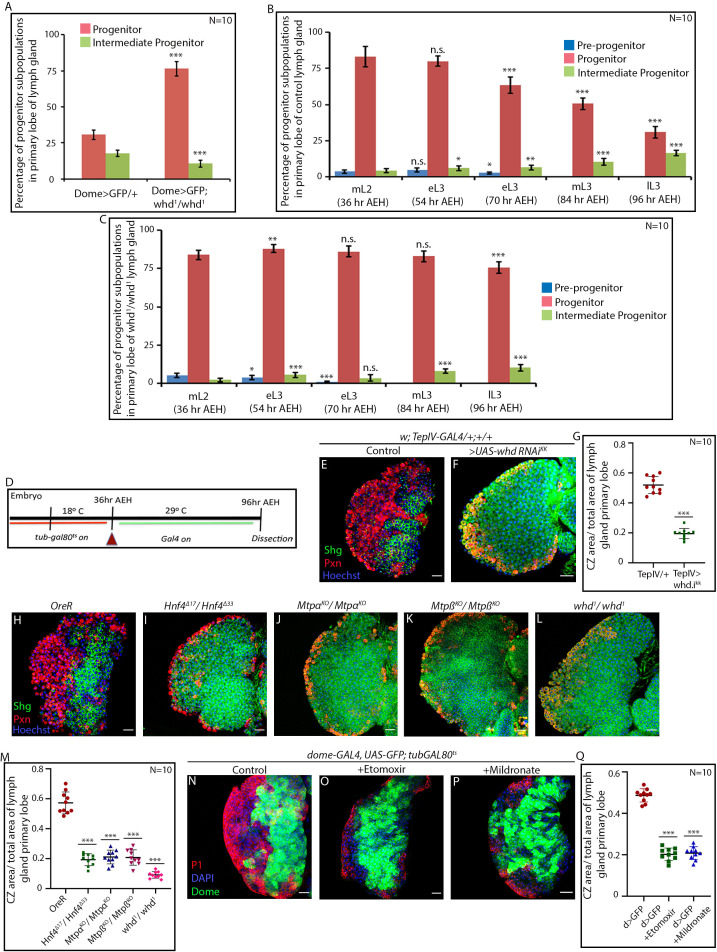
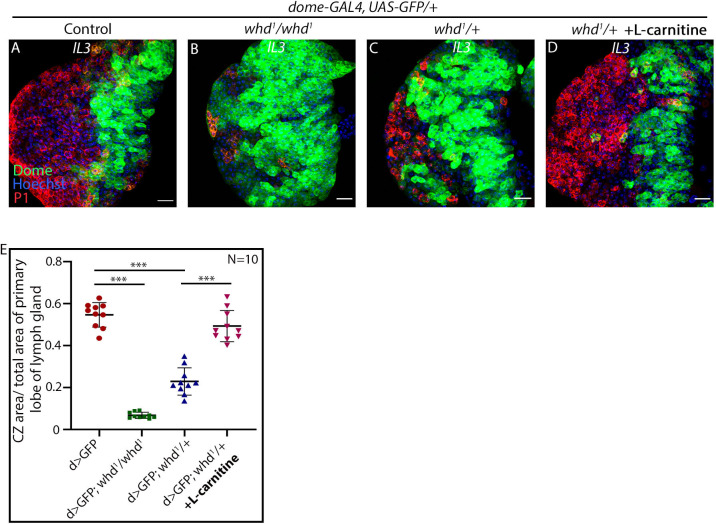
Drosophila ortholog of CPT1, withered (whd, Figure 2A), is a rate-limiting enzyme for FAO (Strub et al., 2008). The loss of function of CPT1/whd, therefore, blocks mitochondrial FAO (Schreurs et al., 2010). To investigate the role of FAO in late hemocyte progenitors, we employed a null allele of withered, whd1 (Strub et al., 2008). The primary lobes of whd1 homozygous lymph gland have abundant Dome+ progenitors but drastically reduced number of differentiated hemocytes (P1: plasmatocytes Figure 2B–C and D; proPO: Crystal cells, Figure 2E–F and G), and Intermediate progenitors (Dome+ Pxn+, Figure 2H–I'' and Figure 2—figure supplement 1A) compared to control.
Figure 2. Loss of fatty acid β-oxidation affected differentiation of hemocyte progenitors of the lymph gland.
(A) Schematic representation of fatty acid β-oxidation within the mitochondria of a cell. (B–D) Compared to control (B) decrease in differentiation (red, reported by P1 immunostaining) and increment in progenitor number (dome > GFP) is observed in the lymph gland of a homozygous null allele of whd (C). (D) Quantitative analysis of B–C reveals a significant increment in the number of Dome+ progenitors. p-Value for dome-GAL4, UAS-GFP; whd1/whd1=2.67×10−10 compared to control. (E–G) Compared to control (E) decrease in crystal cell number (red, reported by proPO immunostaining) and increment in the progenitor cell population (dome > GFP) is observed in the lymph gland of the homozygous null allele of whd (F). (G). Quantitative analysis of results from E–F shows a significant drop in the number of crystal cells. p-Value for dome-GAL4, UAS-GFP; whd1/whd1=4.38×10−7 compared to control. (H–I'') The hemocyte progenitor subpopulation dynamics (red, reported by Pxn immunostaining and green marking dome > GFP) of Dome+ progenitors and Dome+ Pxn+ (IPs) in the late third instar lymph gland of control (H–H'') and homozygous null allele of whd (I–I''). (J–S) Spatio-temporal analysis of hemocyte progenitor subpopulations of Dome- pre-progenitors, Dome+ progenitors, and Dome+ Pxn+ (IPs) (red, reported by Pxn immunostaining and green marking dome > GFP) observed in the lymph gland of control (J–N) and homozygous null allele of whd (O–S). Insets in K, L, and M show pre-progenitors, progenitors and intermediate progenitors respectively in control and inset in Q shows abundant progenitors in the homozygous null allele of whd. (T–X) Compared to control (T) decrease in differentiation (red, reported by P1 immunostaining) and increase in progenitor number (dome > GFP) is observed in lymph gland upon progenitor specific RNAi based down-regulation of whd (U) CRISPR-Cas9 based knock-out of whd (V) and miRNA based knockdown of Hnf4 (W). (X) Quantitative analysis of the results from T–W, illustrating the significant increase in Dome+ progenitors upon targeted loss of FAO. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-whd RNAi = 2.84×10−15 compared to control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-dCas9; U-6: sgRNA-whd = 3.84×10−19. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-Hnf4.miRNA =6.04×10−14. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 2—figure supplement 1. Fatty acid β-oxidation is essential for lymph gland progenitor differentiation.
Figure 2—figure supplement 2. Model depicting the posible role of Hnf4 and FAO in hemocyte progenitor differentiation.
Detailed temporal analysis of the dynamics of progenitor subpopulation during normal development, as well as upon loss of whd1 (Figure 2J–S), was next carried out. In sync with an earlier report (Ferguson and Martinez-Agosto, 2017), our analysis reveals that Dome- pre-progenitors (Figure 2K) are present in the developing lymph gland until the early third instar (Figure 2L and Figure 2—figure supplement 1B). Beyond this timeline, the subsets that populates the lymph gland are Dome+ progenitors (Figure 2M–N and Figure 2—figure supplement 1B), and Dome+ Pxn+ IP cells (Figure 2M–N and Figure 2—figure supplement 1B). Interestingly, in whd1 mutant lymph glands, while the pre-progenitors are present till the early third instar stage (Figure 2P and Figure 2—figure supplement 1C), there is an abundance of progenitors (Figure 2Q–S) with a small number of IP cells (Figure 2R–S). Quantification of the above results reflects that in whd1 mutant the progenitors are rather stalled instead of undergoing a natural transition to IP cells with time (Figure 2—figure supplement 1C). During normal development, the first sign of differentiation (as evidenced by Pxn expression) occurs around 36 hr AEH, and by 96 hr AEH, there is a prominent cortical zone defined by the differentiating cells.
In contrast, the cortical zone is drastically reduced due to lack of differentiation in the whd1 mutant lymph gland. The timed analysis also revealed that the defect seen in differentiation in this mutant has an early onset (36 hr AEH, Compare Figure 2O with Figure 2J). These observations implicate that the lack of FAO dampens the differentiation process of Dome+ progenitors.
Since the differentiation of progenitors is affected, we next performed an RNAi-mediated downregulation of whd by the TARGET system (McGuire et al., 2004; Figure 2—figure supplement 1D).
Progenitor-specific downregulation (dome-GAL4, UAS-GFP; tubGAL80ts20; UAS-whd RNAi) results in a halt in differentiation, as evidenced by an increase in the area of dome > GFP and a concomitant decline in adjoining CZ (visualized by differentiated plasmatocyte (Nimrod: P1, Figure 2U) compared to control (Figure 2T). Upon activation of whd RNAi from a different source (VDRC) by another independent progenitor specific driver TepIV-GAL4 (Figure 2—figure supplement 1E–G), a similar result is obtained. Additionally, progenitor-specific knockout of whd by CRISPR/Cas9 system (Hsu et al., 2014) supports the above results (Figure 2V and X).
Likewise, knockdown of the key player in lipid metabolism Hnf4 results in a decline in the differentiation of the progenitors, endorsing the role of FAO in progenitor differentiation (Figure 2W and X, Figure 2—figure supplement 2). Lymph glands from a hetero-allelic combination of dHNF4 (dHNF4Δ17/dHNF4Δ33: null allele of Hnf4) (Palanker et al., 2009), exhibits an abundant progenitor pool (Shg: DE-Cadherin) coupled with the reduction in differentiated cells (Pxn, compare Figure 2—figure supplement 1I with 1H, and Figure 2—figure supplement 1M), further denoting that FAO disruption indeed leads to compromised differentiation of progenitors.
To further verify our observations, we next analyzed the homozygous mutant of two essential enzymes of β−oxidation: Mtpα (mitochondrial trifunctional protein α subunit: Long-chain-3-hydroxyacyl-CoA dehydrogenase), and Mtpβ (mitochondrial trifunctional protein β subunit: Long-chain-3-hydroxyacyl-CoA dehydrogenase) (Kishita et al., 2012). Primary lobes from homozygous Mtpα[KO] and Mtpβ[KO] loss of function has a large progenitor pool (Shg: DE-Cadherin) at the expense of differentiated cells (Pxn) (compare Figure 2—figure supplement 1J–K with H), a phenotype similar to the whd1 (Figure 2—figure supplement 1L and M). DE-cadherin enrichment in the FAO loss of function progenitors is also indicative of high-maintenance signals (Gao et al., 2014).
In addition to genetic knockdown and classical loss-of-function analyses, we performed pharmacological inhibition of FAO in Drosophila larvae (Figure 2—figure supplement 1N–Q). Larvae grown in food supplemented with FAO inhibitors, Etomoxir (Lopaschuk et al., 1988), and Mildronate also demonstrate a more than two-fold reduction in the differentiation of the progenitors of the primary lobe.
Collectively, our genetic and pharmacological studies illustrate the cell-autonomous role of FAO in the differentiation of blood progenitors of the lymph gland.
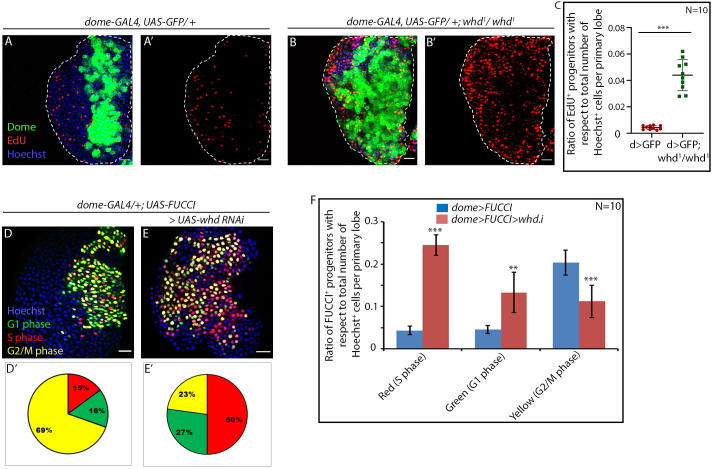
Loss of FAO causes an increase in progenitor proliferation
Previous study from our laboratory demonstrated that G2-M arrest is a hallmark of the otherwise proliferating progenitors prior to differentiation (Sharma et al., 2019). In contrast, we found that whd1 homozygous progenitors exhibit a stark increase in EdU incorporation implicating their highly proliferating status upon loss of FAO when compared to age-isogenised controls (Figure 3A–C). To have a further insight into the cell cycle status, we expressed Fly-FUCCI-fluorescent ubiquitination-based cell cycle indicator, specifically in the progenitors. This indicator employs two fluorescent probes: the first probe is an E2F moiety fused to GFP, which is degraded by Cdt2 during the S-phase. This construct allows the visualization of G1, G2, and M-phase cells by GFP expression. The second probe is a fusion of Red Fluorescent Protein (mRFP.nls) and CycB moiety. It is degraded by the anaphase-promoting complex/cyclosome (APC/C) during midmitosis, thereby reporting the cell in S, G2, and M-phase. Together this system allows the visualization of cells in G1, S, and G2/early mitosis by green, red, and yellow signals, respectively (Zielke et al., 2014).
Figure 3. Loss of fatty acid β-oxidation causes an increase in proliferation of hemocyte progenitors of the lymph gland primary lobe.
Genotype of the larvae are mentioned in respective panels. (A–C) The difference in proliferation status (reported by EdU incorporation) in the lymph gland of third late instar control larvae (A–A') compared to whd null mutant (B–B'). (C). Quantitative analysis of the results from A–B' illustrates a significant increase in proliferation in whd1 Dome+ progenitors. p-Value for dome-GAL4, UAS-GFP; whd1/whd1=1.71×10−6 compared to control. (D–F) Difference in cell cycle status (reported by Fly-FUCCI) in the lymph gland of third late instar control larvae (D) compared to the progenitor-specific RNAi-based down-regulation of whd (E). (D'–E'): Pie chart depicting the fraction of G1 (green), S(red), and G2/M (yellow) progenitors in J–K. (F) Quantitative analysis of the results from D–E. p-Value for red cells in dome-GAL4, UAS-Fly-FUCCI; UAS-whd RNAi = 1.41×10−11, p-Value for green cells in dome-GAL4, UAS-Fly-FUCCI; UAS-whd RNAi = 2×10−4, p-Value for yellow cells in dome-GAL4, UAS-Fly-FUCCI; UAS-whd RNAi = 1.5×10−5 compared to control. ns.=not significant, Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 3—figure supplement 1. Loss of fatty acid β-oxidation caused higher redox levels and an increase in maintenance factor of hemocyte progenitors of the lymph gland.
We found that, instead of being in a G2-M arrest, the hemocyte progenitors in whd mutant are actively proliferating, as evidenced by more cells in S-phase (red) compared to the control (Figure 3D–D' with E-E'). Quantitative analyses reveal more than three-fold increase in the number of cells in the S phase with a concomitant drop in G2-M arrested progenitors (compare Figure 3D' with E' and F).
Together these results assert that FAO disruption in proliferating progenitors doesn’t allow them to halt at G2-M and subsequently differentiate.
Failure in differentiation of hemocyte progenitors upon loss of FAO is not due to decline in ROS levels
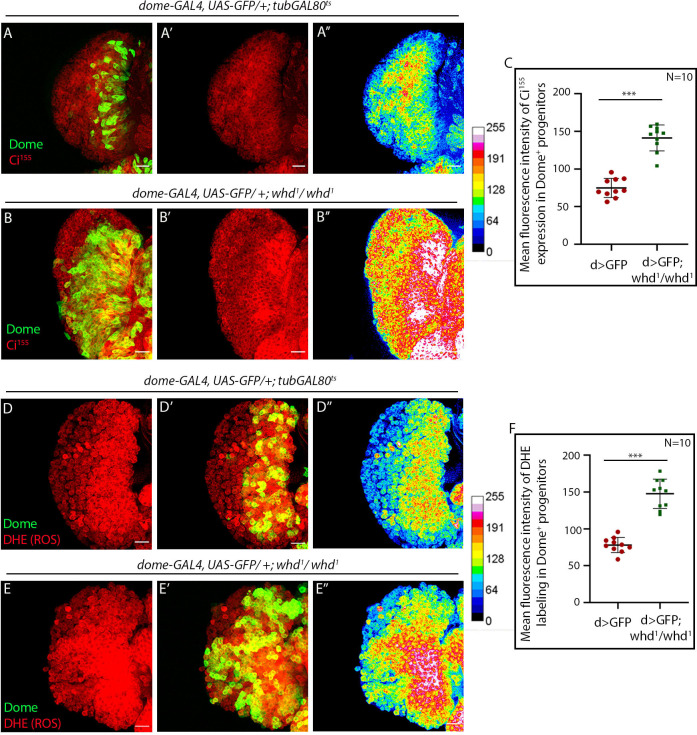
The perturbation of differentiation prompted us to look at the status of both differentiation and maintenance factors, per se in these hemocyte progenitors. Although the progenitor pool in the larval lymph gland is heterogenous (Baldeosingh et al., 2018; Banerjee et al., 2019; Sharma et al., 2019), our timed analysis indicates that the majority of the progenitors populating the late third instar lymph gland expresses dome. Hedgehog signaling has been implicated in the maintenance of the dome expressing progenitors (Baldeosingh et al., 2018; Mandal et al., 2007; Sharma et al., 2019; Tokusumi et al., 2010). Hematopoietic niche/PSC releases Hh, which leads to the expression of the Hh signal transducer Cubitus interruptus (Ci155[Alexandre et al., 1996]) in the progenitors. The homozygous whd1 lymph gland progenitors express a higher level of Ci155 compared to control (compare Figure 3—figure supplement 1B-B'' with A-A'' and quantitated in Figure 3—figure supplement 1C) correlating with higher proliferation and less differentiation (Figure 2B–D). This observation, along with enrichment of DE-cadherin in FAO mutants (Figure 2—figure supplement 1H–M), endorses high-maintenance signal in the progenitors.
Reactive oxygen species (ROS) is the major signal attributed to the differentiation of the hemocyte progenitors of the lymph gland (Owusu-Ansah and Banerjee, 2009). High levels of developmentally generated ROS trigger Jun Kinase (JNK) signaling, which sets these progenitors toward the differentiation program (Owusu-Ansah and Banerjee, 2009). Since homozygous whd1 progenitors fail to differentiate, we rationalized that this might be due to the drop in the differentiation signal ROS.
To probe this possibility, we analyzed the levels of ROS in whd1 lymph glands by dihydroxy ethidium (DHE) staining. Quite strikingly, whd1 homozygous progenitors exhibited elevated levels of ROS (Compare Figure 3—figure supplement 1D–D'' with 3E-E'' and quantified in Figure 3—figure supplement 1F). A similar observation of increased ROS in CPT1 knockdown endothelial cells has been reported in another study (Kalucka et al., 2018). Therefore, we can infer that the halt in differentiation observed in the Ci155 enriched progenitors of homozygous whd1 is not an outcome of compromised ROS level.
Collectively, these results indicate that despite achieving a high ROS level than the control, the progenitors are unable to move into differentiation, indicating that FAO might act downstream to ROS.
Upregulation of FAO causes precocious G2-M arrest and differentiation of blood progenitors
Due to the central role of carnitine in fat metabolism, it is often used as a supplement for enhancing fat oxidation (Pekala et al., 2011; Wall et al., 2011). Several studies have concluded that FAO can be upregulated in the cells by L-carnitine supplementation (Pekala et al., 2011; Sahlin, 2011; Wall et al., 2011).
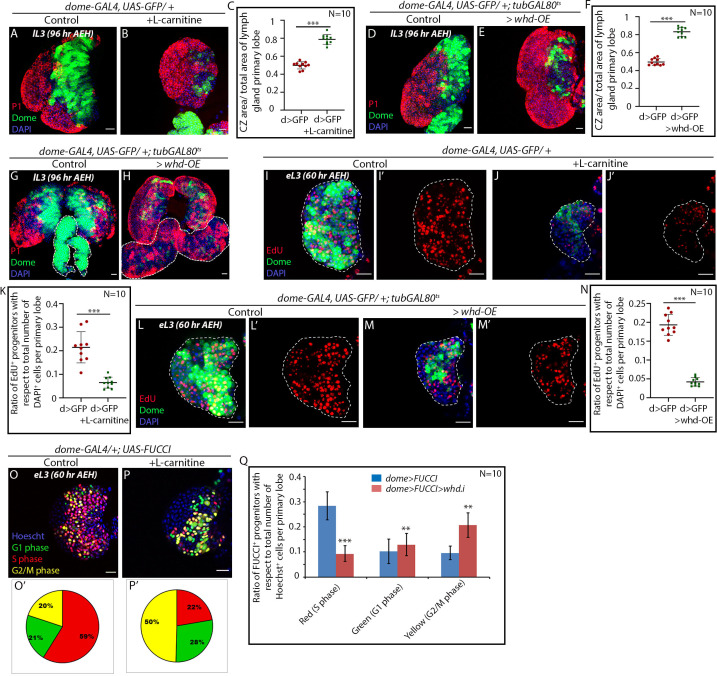
Our loss-of-function genetic analyses illustrated that FAO is essential for the differentiation of hemocyte progenitors. Whether it is sufficient for the progenitor differentiation was addressed by feeding L-carnitine supplemented food (100 mM concentration for 48 hr) to wandering third instar larvae. Compared to control (Figure 4A), the lymph gland from L-carnitine fed larvae (96 hr AEH) exhibits a drastic reduction in progenitor zone (visualized by dome > GFP) with a concomitant increase in differentiation (visualized by P1: Nimrod; Figure 4B–C). The increase in differentiation by L-carnitine supplementation is also apparent in whd1 heterozygous mutants. Feeding L-carnitine could rescue the differentitaion defects associated with whd1 heterozygous mutants (Compare Figure 4—figure supplement 1A–E).
Figure 4. FAO upregulation results in precocious differentiation and G2 arrest in hemocyte progenitors.
Age and genotype of the larvae are mentioned in respective panels. (A–C) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland of control (A) and L-carnitine supplemented (B) larvae. (C) Quantitative analysis of results from A–B showing increased differentiation upon L-carnitine supplementation. p-Value for dome-GAL4, UAS-GFP = 2.37×10−10 supplemented with L-carnitine compared to control. (D–F) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland of control (D) and CRISPR-Cas9 mediated whd overexpression (E) in larval hemocyte progenitors. (F) Quantitative analysis of result from D–E depicting a significant increase in differentiation upon overexpression of whd. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-whd-OE = 5.82×10−12 compared to control. (G–H) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland secondary lobes (marked by the white dotted boundary) of control (G) and CRISPR-Cas mediated whd overexpression (H) in larval hemocyte progenitors. (I–K) Proliferation status (marked by EdU) in third early instar hemocyte progenitors (dome > GFP) of control (I–I') and L-carnitine supplemented (J–J') larvae. (K) Quantitative analysis of results from I–J' reveals a decline in the number of proliferating Dome+ progenitors upon FAO overexpression. p-Value for dome-GAL4, UAS-GFP fed with L-carnitine = 2.87×10−5 compared to control. (L–N) The decline in proliferation status (marked by EdU) in third early instar hemocyte progenitors of CRISPR-Cas9 mediated whd overexpression (M–M') compared to (dome > GFP) of control (L–L'). (N) Quantitative analysis of result from L–M'. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > whd-OE=2.28×10−9 compared to control. (O–P) Alteration in cell cycle status (reported by Fly-FUCCI) in the lymph gland of third late instar larvae grown in L-carnitine supplemented food (P) compared to control (dome > UAS-FUCCI) (O). (O'–P'): Pie chart depicting the fraction of G1 (green), S (red), and G2/M (yellow) progenitors in (O-P). (Q) Quantitative analysis of the results from O–P, illustrating the increase in G2-M upon FAO overexpression. p-Value for red cells in L-carnitine supplemented dome-GAL4, UAS-FUCCI = 1.78×10−7, p-Value for green cells in L-carnitine supplemented dome-GAL4, UAS-FUCCI; UAS-whd RNAi = 2.16×10−1. p-Value for yellow cells in L-carnitine supplemented dome-GAL4, UAS- FUCCI; UAS-whd RNAi = 1.71×10−5 compared to control. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 4—figure supplement 1. L-carnitine supplementation rescues differentiation defect.
As a genetic correlate, whd was overexpressed by a Cas9-based transcriptional activator (BDSC68139) (Ewen-Campen et al., 2017) in the hemocyte progenitors following the scheme in Figure 2—figure supplement 1D. Overexpression of whd indeed results in an increase in the differentiation of hemocyte progenitors (compare Figure 4D with Figure 4E–F). It is interesting to note that the otherwise undifferentiated reserve progenitors of the secondary lobes also differentiate upon whd overexpression (marked by a dotted white line in Figure 4G–H).
We next employed dual fly-FUCCI construct, and EdU labeling to assay the cell cycle cell status of the FAO upregulated progenitors. The early third instar lymph glands from L-carnitine fed larvae exhibit a radical decline in EdU incorporation compared to the proliferating progenitors of the control larvae of similar age (compare Figure 4I–I' with Figure 4J-J' and quantified in Figure 4K). As a genetic correlate, we overexpressed whd in the progenitors, which also led to a decline in EdU incorporation (Figure 4L–N). Our FUCCI analysis reveals an abundance of G2-M progenitors in the early third instar larvae reared in L-carnitine supplemented food compared to control samples. Thus, less EdU incorporation due to upregulated FAO resulted in an early onset of G2-M arrest in the progenitors (compare Figure 4O–O' with Figure 4P–P' and quantified in Figure 4Q).
Put together; our results reveal that upon FAO upregulation, the progenitor experiences a precocious G2-M halt in their cell cycle. During normal development, the late progenitors also undergo a G2-M halt before they differentiate. We, thus, infer that FAO is imperative for the differentiation of lymph gland progenitors.
FAO loss in hemocyte progenitors leads to sustained glycolysis
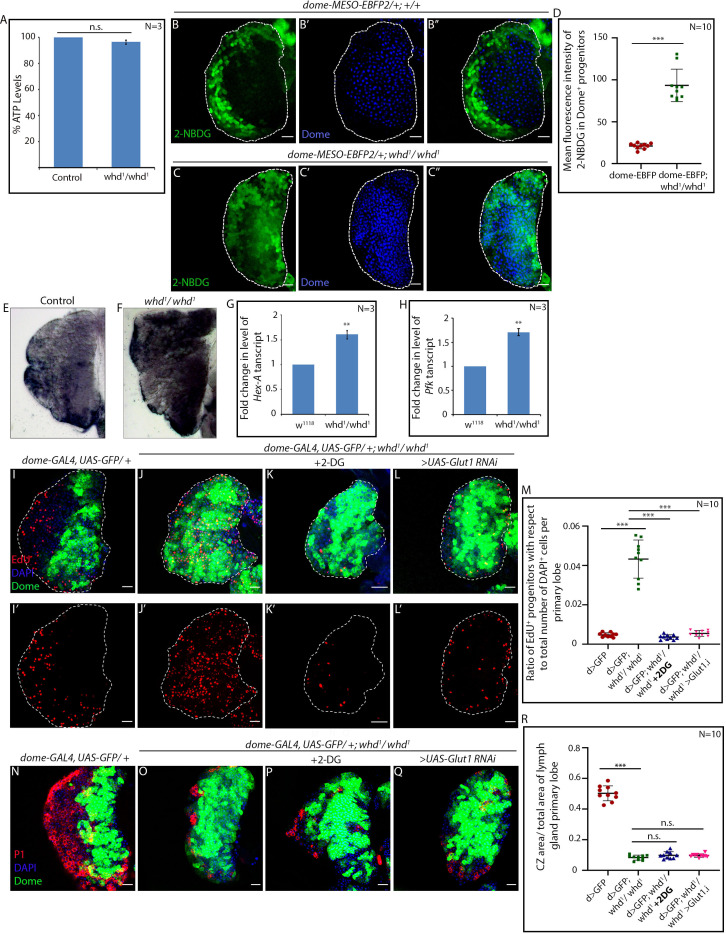
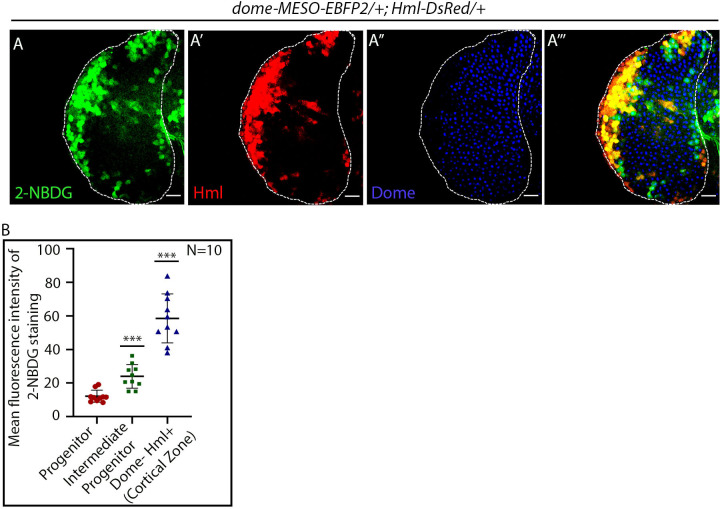
Next, we investigated whether a compromise in the intracellular energy source (ATP) is the reason behind the differentiation defect seen in the FAO mutants. Quite intriguingly, ATP levels of homozygous whd1 larvae are comparable to similarly aged control (Figure 5A). This unaltered ATP level is in sync with our observation of higher proliferation observed in homozygous whd1 hemocyte progenitors (Figure 3B–B'). Since higher proliferation is driven by elevated glycolysis in different scenarios (Lunt and Vander Heiden, 2011), we hypothesized that loss of FAO might push the progenitors towards a higher glycolytic index. We performed an in vivo glucose uptake assay employing fluorescent derivative of glucose, 2-NBDG (Zou et al., 2005) in the late third instar lymph gland of both control and FAO mutant. In control, late third instar Dome+ progenitors exhibit low glucose uptake (Figure 5B–B'' and Figure 5D) compared to the higher uptake detectable in the peripheral hemocyte population marked by Hml (Figure 5—figure supplement 1A–A''' and quantified in Figure 5—figure supplement 1B). In sharp contrast, higher glucose uptake is evident in the FAO mutant progenitors (Figure 5C–C'' and quantified in Figure 5D). In concordance with the above result, in vivo lactate dehydrogenase assay (Abu-Shumays and Fristrom, 1997) also revealed a high glycolytic index prevalent in the lymph glands of homozygous whd1 (Figure 5E–F).
Figure 5. FAO loss in hemocyte progenitors led to sustained glycolysis.
(A) ATP levels in control and whd1/whd1 whole larvae. p-Value of whd1/whd1compared to control = 5.327×10−2. (B–D) Glucose incorporation (marked by 2-NBDG uptake) levels in control dome-MESO-EBFP2/+ (B–B'') and dome-MESO-EBFP2/+; whd1/whd1 (C–C'') lymph glands. (D). Quantitative analysis of results from B–C demonstrating a significant increase in glucose uptake in the whd1/whd1 progenitors. p-Value for dome-MESO-EBFP2/+; whd1/whd1 = 6.09×10-7 compared to control. (E–F) Increased lactate dehydrogenase in-vivo enzymatic staining assay of whd1/whd1 lymph gland (F) compared to control (E). (G) Fold change in the level of Hex-A mRNA expression in control w1118 and whd1/whd1 lymph glands. p-Value of whd1/whd1 = 6.379×10−3 compared to control. (H) Fold change in the level of Pfk mRNA expression in control w1118 and whd1/whd1 lymph glands. p-Value of whd1/whd1 = 3.739×10−3 compared to control. (I–M) Proliferation status (marked by EdU) in control dome > GFP (I–I'), dome > GFP; whd1/whd1 (J–J'), 2-DG fed dome > GFP; whd1/whd1 (K–K') and dome > GFP; whd1/whd1; UAS-Glut1 RNAi (L–L') lymph glands. (M). Quantitative analysis of results from I–L'. p-Value for dome > GFP; whd1/whd1 = 4.37×10−7 compared to control and p-value for dome > GFP; whd1/whd1 = 3.25×10−7 fed with 2-DG compared to non-fed dome > GFP; whd1/whd1. p-Value for dome > GFP; whd1/whd1; UAS-Glut1 RNAi = 4.53×10−7 compared to non-fed dome > GFP; whd1/whd1. (M–P) Comparison of differentiation (marked by P1) levels in control dome > GFP (M), dome > GFP; whd1/whd1 (N) and 2-DG fed dome > GFP; whd1/whd1 (O) lymph glands. (P). Quantitative analysis of results from M–O show decline in proliferation upon 2-DG feeding. p-Value for dome > GFP; whd1/whd1 = 4.43×10−11 compared to control and p-Value for dome > GFP; whd1/whd1 = 8.6×10−2 fed with 2-DG compared to non-fed dome > GFP; whd1/whd1. p-Value for dome > GFP; whd1/whd1; UAS-Glut1 RNAi = 5.9×10−2 compared to non-fed dome > GFP; whd1/whd1. n.s. = not significant. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 5—figure supplement 1. 2-NBDG assay in the lymph gland primary lobe.
Moreover, in the whd1 lymph gland, the transcript levels of HexA (Hexokinase A) and Pfk (Phosphofructokinase), the enzymes involved in the two irreversible steps of glycolysis: exhibit a 1.6 fold and 1.7 fold increase in their expression, respectively (Figure 5G–H). Together these results reveal that upon FAO disruption, lymph gland progenitors adopt high-glucose utilization/metabolism.
Based on the above observations, we inferred that higher proliferation and differentiation defects observed in hemocyte progenitors with compromised FAO might be due to the surge in glucose uptake/metabolism. Upon rearing whd1 homozygous larvae in food supplemented with glycolysis inhibitor 2-Deoxy-D-glucose (2-DG), the otherwise hyper-proliferating hemocyte progenitors (EdU, Figure 5J) demonstrate a significant drop in EdU incorporation to a level that is comparable to control (compare Figure 5K with Figure 5I, and quantified in Figure 5M). Although the glycolytic block by feeding 2-DG rescues the cell cycle status, the abrogated differentiation observed in homozygous whd1 hemocyte progenitors is not restored (compare Figure 5P with Figure 5O and N and quantified in Figure 5R). Inhibition of glucose uptake by genetic perturbation of glucose transporter Glut1 in progenitor specific manner in the FAO mutant also endorses the above result (Figure 5L–L' and M, and Figure 5Q–R).
From these observations, it is evident that the surge in glucose metabolism encountered by the hemocyte progenitors upon FAO loss is responsible for their altered cell cycle. However, the glycolytic surge upon FAO disruption is unable to initiate progenitor differentiation. Collectively, the above results indicate that FAO plays a critical role in regulating the differentiation of hemocyte progenitors.
FAO loss in progenitors causes an altered histone acetylation
Acetyl-CoA generated from FAO, apart from serving as a substrate for the Krebs cycle, is essential for the acetylation of various proteins, including histones. We, therefore, wondered that disruption of FAO in whd1 hemocyte progenitors might also result in altered histone acetylations, which may, in turn, result in cell cycle and differentiation defects.
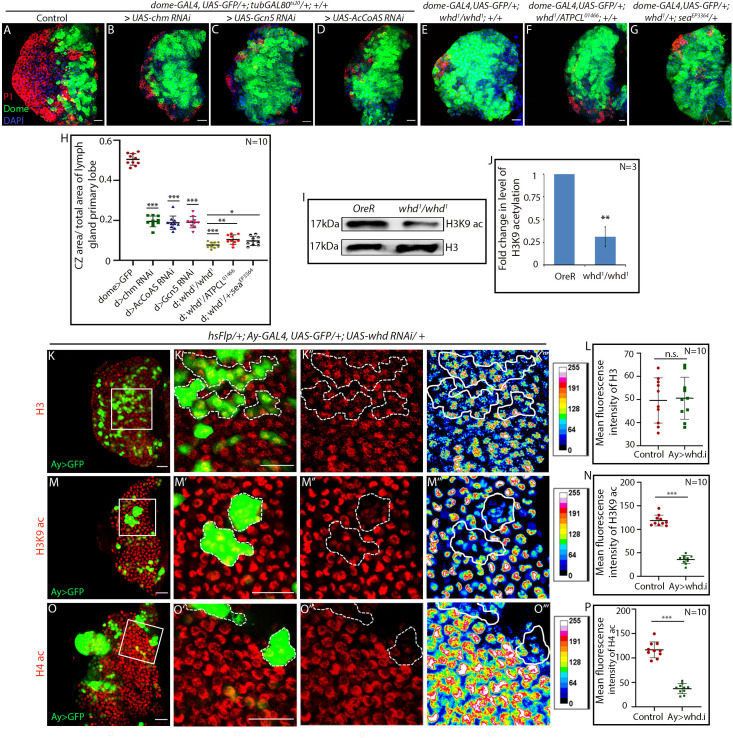
Histone acetylation mediated by Histone Acetyl Transferases (HATs) directly controls the expression of differentiation factor, thereby regulating germline stem cell differentiation (McCarthy et al., 2018; Xin et al., 2013). To ascertain whether HATs play a similar role in hemocyte progenitor differentiation, progenitor-specific RNAi-mediated knockdown of HATs function was done following the timeline, as shown in Figure 2—figure supplement 1D. Quite strikingly, loss of Histone Acetyl Transferase (HAT) genes, Gcn5 (Carré et al., 2005) and chm (chameau) (Grienenberger et al., 2002; Miotto et al., 2006) phenocopy the differentiation defect seen in the hemocyte progenitors of FAO loss of function (Figure 6A–C). Additionally, downregulation of Acetyl Coenzyme A synthase/AcCoAS (the Drosophila orthologue of ACSS2) (Mews et al., 2017) results in a phenotype identical to HAT or FAO loss (Figure 6D) confirming the essential role of acetylation in hemocyte progenitor differentiation.
Figure 6. Hemocyte progenitors of HAT and FAO loss of function exhibits altered histone acetylation.
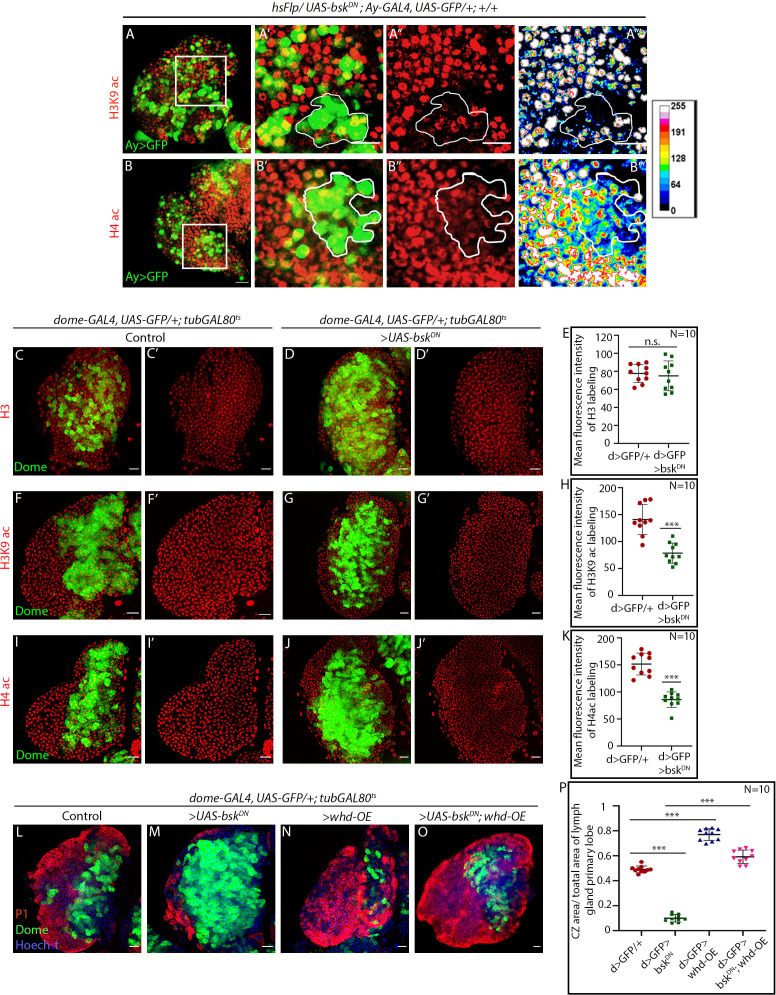
(A–H) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland of control (A) with progenitor-specific downregulation of (B) chm, (C) Gcn5, (D) AcCoAS and (E) whd1/whd1, (F) transheterozygote of whd and ATPCL (whd1/ATPCL01466) and transheterozygote of whd and sea (whd1/seaEPEP3364) (G). (H) Quantitative analyses of the results from A–G. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-chm RNAi = 2.267×10−15 compared to control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-Gcn5 RNAi = 1.990×10−14 compared to control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-AcCoAS RNAi = 2.601×10−15 compared to control. p-Value for dome-GAL4, UAS-GFP; whd1/whd1 = 1.400×10−15 compared to control. p-Value for dome-GAL4, UAS-GFP; whd1/ATPCL01466 = 6.835×10−3 compared to control dome-GAL4, UAS-GFP; whd1/whd1. p-Value for dome-GAL4, UAS-GFP; whd1/seaEP3364 = 2.974×10−2 compared to control dome-GAL4, UAS-GFP; whd1/whd1. (I–J) Western blot analysis of H3K9 acetylation level in control OreR and whd1/whd1 larvae with H3 as a loading control (I). (J). Quantitative analysis of H3K9 acetylation level in I. p-Value for whd1/whd1 = 8.056×10−3 compared to control OreR. (K–P) Clonal analysis of histone acetylation in the GFP-positive hs-Flp/Ay-GAL4 based clonal patches (GFP indicates cells where the whd function is knocked down). Immunostaining with H3 (K–L), H3K9 acetylation (M–N), and H4 pan acetylation (O–P) antibodies. (L). Quantitative analyses of H3 acetylation level in K–K'''. p-Value for hs-Flp/Ay-GAL4. UAS-GFP; UAS-whd RNAi = 8.188×10−1 compared to control. (N). Quantitative analysis of H3K9 acetylation level in (M–M'''). p-Value for hsFlp/+; Ay-GAL4. UAS-GFP; UAS-whd RNAi = 2.238×10−12 compared to control. (P). Quantitative analysis of H4 acetylation level in O–O'''. p-Value for hsFlp/Ay-GAL4. UAS-GFP, UAS-whd RNAi = 1.083×10−9 compared to control. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm n.s. = not significant.
Figure 6—figure supplement 1. Hemocyte progenitors of whd1 loss of function exhibit altered histone acetylation.
Next, we performed an epistatic interaction of whd1 allele with other major acetyl-CoA related genes. Citrate transporter SLC25A1 or scheggia/sea in Drosophila (Carrisi et al., 2008) exports Krebs cycle metabolite citrate from the mitochondria to the cytoplasm to generate acetyl-CoA. In the cytosol, citrate gets converted to acetyl-CoA by ATP citrate lyase (ACLY encoded by Drosophila orthologue, ATPCL). Trans-heterozygous loss-of-function allelic combinations of whd1 with either ATPCL01466 (Figure 6F) or seaEP3364 (Figure 6G) phenocopy differentiation defects seen in whd1 homozygous lymph gland (Figure 6E). Above set of genetic correlations reveal that alteration in histone acetylation affects hemocyte progenitor differentiation (quantified in Figure 6H).
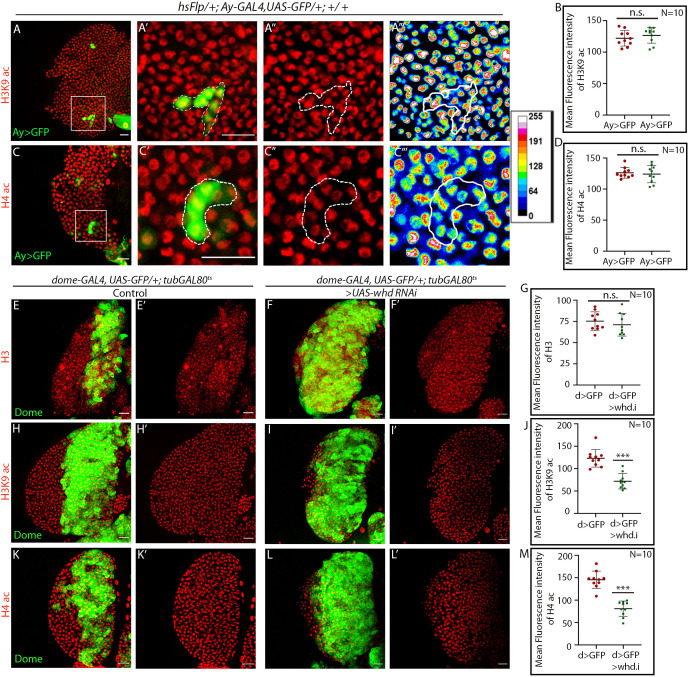
Knockdown of the rate-limiting enzyme of FAO:CPT1, leads to a reduced level of H3K9 acetylation in lymphatic endothelial cells (Wong et al., 2017). We wondered whether similar acetylation defect occurs in whd1 homozygous mutant larvae. Immunoblot analysis of extracted histones with antibody against acetylated anti-H3K9 reveals that whd1 homozygous larvae have low levels of H3K9 acetylation (Figure 6I–J) when compared to control. However, the level of histone H3 is comparable to that of control.
Whether the tissue of our interest also reflects this decline in acetylation level, H3K9 acetylation labeling was performed in mosaic clones using hsFlp/Ay-GAL4 mediating RNAi knockdown of Drosophila CPT1 orthologue whd. The clonal patches positively marked with GFP (where whd has been downregulated) show a significant drop in H3K9 acetylation levels (Figure 6M–N) compared to surrounding hemocyte progenitors. However, histone H3 labeling in both mutant and control clonal patches are comparable (Figure 6K–L) and serve as a control. This observation, along with the western blot analyses, reveals the occurrence of H3K9 acetylation defects in FAO loss of function. Likewise, histone H4 acetylation visualized by pan anti-H4 acetylation antibody reveals a drastic drop in whd knockdown clonal patches (Figure 6O–P). Both the expression of H3K9 and pan H4 acetylation remains unaltered in mock/wild type clones (Figure 6—figure supplement 1A–D). Further, upon progenitor specific downregulation of whd function, a decline in the level of both H3K9 acetylation (compare Figure 6—figure supplement 1H–H' with Figure 6—figure supplement 1I–I' and J) and pan H4 acetylation (Figure 6—figure supplement 1K–K’ with Figure 6—figure supplement 1L–L' and M) is evident. In all these scenarios, histone H3 labeling remains unaffected (Figure 6—figure supplement 1E–G).
Above molecular and genetic analyses demonstrate that the downregulation of FAO in the hemocyte progenitors leads to a decline in histone acetylation. The next step was to correlate whether the differentiation defects of hemocyte progenitors in FAO loss of function is a consequence of altered histone acetylation levels.
Acetate supplementation rescues differentiation defects of FAO mutant hemocyte progenitors
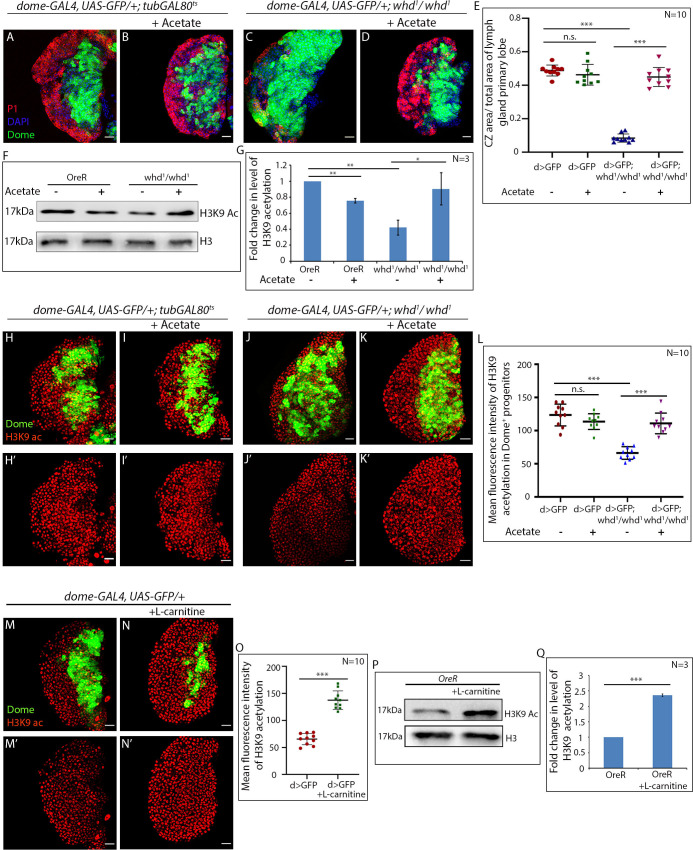
Histone acetylation in eukaryotes relies on acetyl-Coenzyme A (acetyl-CoA). It has been established earlier that compromised histone acetylation levels can be restored by supplementing acetate (Carrisi et al., 2008; Wellen et al., 2009; Wong et al., 2017). The supplemented acetate is converted to acetyl-CoA, which restores the endogenous histone acetylation in a cell. We wondered whether replenishing the H3K9 acetylation levels in whd1 by acetate supplementation (50 mM, supplemented fly food post first instar) can rescue the differentiation defect seen in those lymph glands.
Intriguingly, acetate feeding does not affect progenitor differentiation in control, whereas it rescues the differentiation defects seen in homozygous whd1 hemocyte progenitors (Figure 7A–E). At the molecular level, we observed that acetate supplementation to whd1 mutant larvae leads to a restoration of the H3K9 acetylation level (Figure 7F–G), which might lead to the rescue of the differentiation defect (Figure 7A–E). In order to probe this possibility, the lymph gland from acetate fed larvae were dissected and assayed for the status of H3K9 acetylation level. Figure 7H–L reveals that acetate supplementation indeed restores the compromised acetylation status in the whd1 lymph gland (compare Figure 7J–J' with Figure 7K–K').
Figure 7. Acetate supplementation rescues differentiation defects of FAO mutant hemocyte progenitors.
(A–E) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland of control (A) dome > GFP supplemented with acetate (B) dome > GFP; whd1/whd1 (C) and dome > GFP; whd1/whd1 supplemented with acetate (D). (E). Quantitative analysis of results from A–D. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 = 2.718×10-1 fed with acetate compared to control dome-GAL4, UAS-GFP; tubGAL80ts20. p-Value for dome-GAL4, UAS-GFP; whd1/whd1 = 3.18×10−16 compared to control dome-GAL4, UAS-GFP; tubgal80ts20. p-Value for dome-GAL4, UAS-GFP; whd1/whd1 = 2.576×10−10 fed with acetate compared to control dome-GAL4, UAS-GFP; tubGAL80ts20. (F–G) Western blot analysis of H3K9 acetylation levels in control OreR and whd1/whd1 larvae supplemented with acetate and non-fed controls with H3 as a loading control. (G) Quantitative analysis of H3K9 acetylation levels in F. p-Value for OreR = 4.589×10−3 supplemented with acetate compared to non-fed control OreR. p-Value for non-fed whd1/whd1 = 8.001×10−3 compared to non-fed control OreR. p-Value for acetate supplemented whd1/whd1 = 3.582×10−2 compared to non-fed control whd1/whd1. (H–L) Acetate supplementation restores H3K9 acetylation status in the whd1/whd1 lymph gland (H-Iʹ). (L) Quantitative analysis of acetylation level in control, whd mutant, and whd mutant fed on acetate. p-Value for acetate supplemented dome-GAL4, UAS-GFP = 1.38×10−1 compared to non-fed control. p-Value for dome-GAL4, UAS-GFP; whd1/whd1 = 1.276×10−7 compared to dome-GAL4, UAS-GFP. p-Value for acetate supplemented dome-GAL4, UAS-GFP; whd1/whd1 = 1.31×10−6 compared to non-fed control dome-GAL4; UAS-GFP; whd1/whd1. (M–O) Comparison of H3K9 acetylation level in Dome+ progenitors of L-carnitine fed larvae (N–N') with non-fed control (M–M'). (O) Quantitative analysis of H3K9 acetylation levels in M–N'. p-Value for dome-GAL4, UAS-GFP = 1.079×10−8 supplemented with L-carnitine compared to non-fed control dome-GAL4, UAS-GFP. (P–Q) Western blot analysis of H3K9 acetylation levels in OreR larvae supplemented with L-carnitine and non-fed controls with H3 as a loading control. Quantitative analysis of H3K9 acetylation levels in N. p-Value for OreR = 3.17×10−4 supplemented with L-carnitine compared to non-fed control OreR. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm n.s. = not significant.
Conversely, upregulation of FAO by L-carnitine feeding leads to elevated H3K9 acetylation in the lymph gland (compare Figure 7M–M' with Figure 7N–N' and quantified in Figure 7O). Likewise, the level of H3K9 acetylation in L-carnitine fed larvae demonstrates a significant upregulation when compared to age-isogenised non-fed control larvae (Figure 7P–Q).
These results establish that the hemocyte progenitors require FAO mediated histone acetylation for their differentiation.
JNK signaling regulates FAO in the hemocyte progenitors
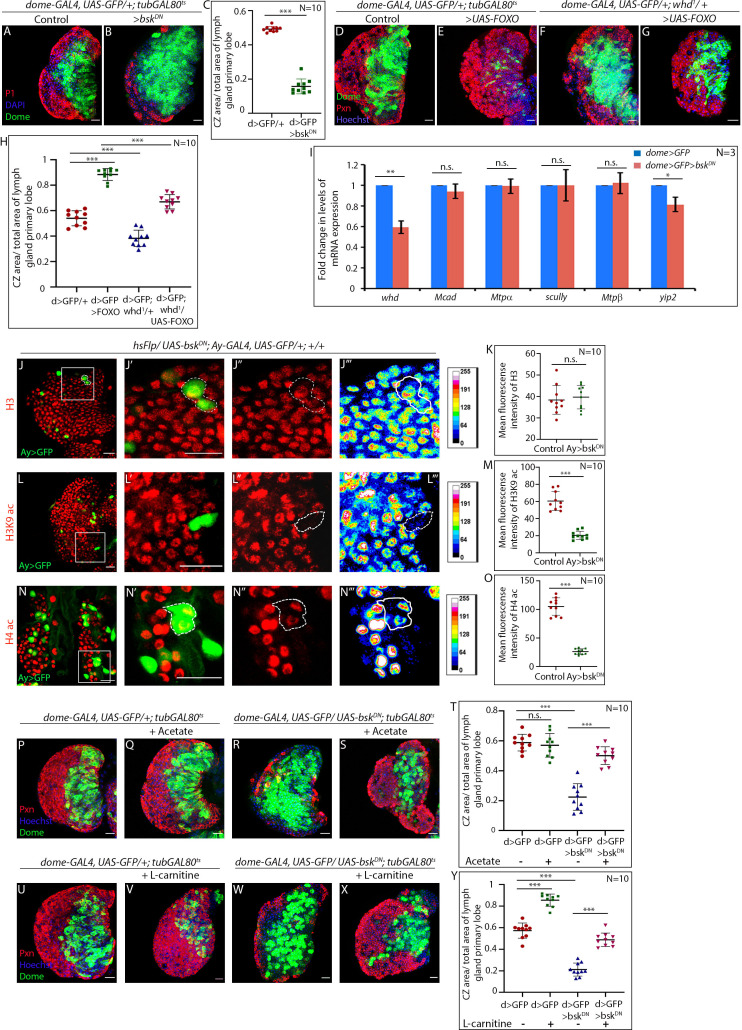
Next, we attempted to understand how the FAO-mediated metabolic circuitry collaborates with the known differentiation signals of hemocyte progenitors. Jun-Kinase and dFOXO (Forkhead box O) mediated signal has been previously implicated for hemocyte progenitor differentiation (Owusu-Ansah and Banerjee, 2009). Analogous to whd1 lymph glands, expression of a dominant-negative allele of basket (bsk, Drosophila orthologue of Jun-Kinase) in the progenitors results in stalled differentiation (Figure 8A–C). On the other hand, overexpression of FOXO, pushes the progenitor fate towards precocious differentiation (Owusu-Ansah and Banerjee, 2009; Figure 8D–E and Figure 8H). However, genetic removal of one copy of whd is sufficient enough to prevent the precocious differentiation as observed in progenitor-specific overexpression of FOXO (Figure 8F–H). These results illustrate an unappreciated link between the differentiation signals and FAO in the lymph gland progenitors.
Figure 8. JNK regulates FAO in hemocyte progenitors of larval lymph gland.
(A–C) Comparison of differentiation (marked by P1) levels in dome > GFP lymph gland of control (A), and bsk/JNK knockdown in hemocyte progenitors by dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN (B). (C). Quantitative analysis of the differentiation level from A–B reveals a significant increase in the Dome+ progenitor zone and a decrease in differentiation. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=5.84×10−11 compared to control. (D–H) Differentiation levels (red, marked by Pxn) in overexpression of FOXO by dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-FOXO (E) is significantly increased compared to control (D). The increased differentiation in FOXO overexpression background is significantly rescued by one copy of the null allele of whd (G). (F). The differentiation level in one copy null allele of whd. (H). Quantitative analysis of the differentiation level from D–G reveals a significant increment in Pxn+ differentiated cell area in FOXO overexpression from Dome+ progenitors, which is significantly rescued by one copy null allele of whd. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-FOXO =5.77×10−11 compared to control. p-Value for dome-GAL4, UAS-GFP; whd1/+ = 2.11×10−5 compared to control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-FOXO/whd1 = 3.84×10−9 compared to dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-FOXO. (I) Real-time expression analysis of fatty acid oxidation enzymes, whd, Mcad, Mtpα, scully, Mtpβ, and yip2 from dome > GFP and dome > GFP > UAS-bskDN lymph glands. The expression of whd shows a significant drop ~41% in dome > GFP > UAS-bskDN compared to control dome > GFP. p-Value for whd expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=7.06×10−3 compared to control. p-Value for Mcad expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=6.71×10−1 compared to control. p-Value for Mtpα expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=8.95×10−1 compared to control. p-Value for scully expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=9.73×10−1 compared to control. p-Value for Mtpβ expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=7.7×10−1 compared to control. p-Value for yip2 expression in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=2.42×10−2 compared to control. (J–O) Clonal analysis of histone acetylation in GFP-positive hsFlp/Ay-GAL4 based clonal patches expressing a dominant-negative form of bsk and immunostaining with H3 (J–J'''), H3K9 acetylation (L–L''') and H4 pan acetylation (N–N''') antibodies. (K). Quantitative analysis of H3 acetylation level in J–J'''. p-Value for hsFlp/Ay-GAL4. UAS-GFP, UAS-bskDN = 6.32×10−1 compared to control. (M). Quantitative analysis of H3K9 acetylation level in L–L'''. p-Value for hsFlp/Ay-GAL4. UAS-GFP; UAS-bskDN = 1.911×10−7 compared to control. (O). Quantitative analysis of H4 acetylation level in N–N'''. p-Value for hs-Flp/Ay-GAL4. UAS-GFP, UAS-bskDN = 8.22×10−9 compared to control. (P–T) Stalled differentiation levels (red, marked by Pxn) in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN (R) is significantly rescued in larvae reared in fly food supplemented with acetate (S). The differentiation level in control (P) dome-GAL4, UAS-GFP; tubGAL80ts20 remain unaltered upon acetate feeding (Q). (T). Quantitative analysis of the differentiation level from P–S reveals a significant rescue of differentiated cells upon acetate supplementation in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN lymph glands. p-Value for acetate supplemented dome-GAL4, UAS-GFP; tubGAL80ts20 = 5.655×10−1 compared to non-fed control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=1.32×10−8 compared to control dome-GAL4, UAS-GFP; tubGAL80ts20. p-Value for acetate fed dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN=4.73×10−7 compared to non-fed dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS bskDN. (U–Y) The differentiation level (red, marked by Pxn) in control (U) dome-GAL4, UAS-GFP; tubGAL80ts20 increases upon L-carnitine feeding (V). Defect in differentiation levels (red, marked by Pxn) in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS bskDN (W) is significantly rescued in larvae reared in fly food supplemented with L-carnitine (X). (Y). Quantitative analysis of the differentiation level from U–X, reveals a significant rescue of differentiated cells upon L-carnitine supplementation in dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN lymph glands. p-Value for L-carnitine supplemented dome-GAL4, UAS-GFP; tubGAL80ts20 = 1.69×10−8 compared to non-fed control. p-Value for dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN =4.5×10−10 compared to control dome-GAL4, UAS-GFP; tubGAL80ts20. p-Value for L-carnitine fed dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS-bskDN =8.307×10−9 compared to non-fed dome-GAL4, UAS-GFP; tubGAL80ts20 > UAS -bskDN. Individual dots represent biological replicates. Values are mean ± SD, asterisks mark statistically significant differences (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test). Scale bar: 20 µm.
Figure 8—figure supplement 1. JNK regulates FAO in hemocyte progenitors of the larval lymph gland.
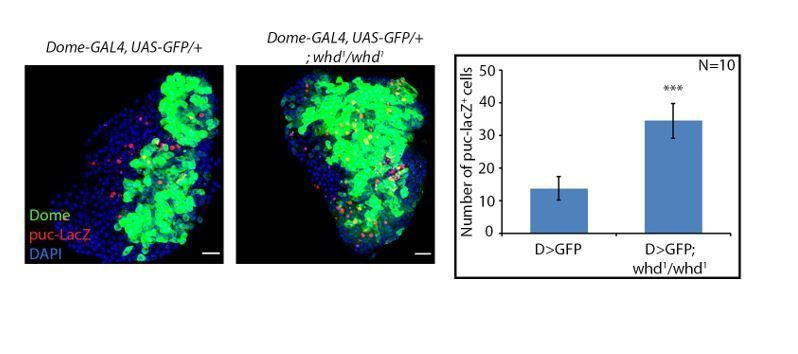
Next, we addressed whether JNK signaling regulates the expression of genes of FAO. The transcription of CPT1/whd, Mcad, Mtpα, scully, Mtpβ, and yip2 was assayed upon down-regulation of bsk from hemocyte progenitors. The transcript level of CPT1/whd indicates a ~ 41% drop (Figure 8I) while the expression of rest of the enzymes either exhibited a mild drop (~18% in yip2) or no significant alteration upon loss of bsk from the progenitors. This observation established that JNK controls the transcription of CPT1/whd, thereby regulating FAO.
Interestingly, clonal analyses of bsk/JNK knockdown in hemocyte progenitors reveals a drop in the levels of H3K9 acetylation (Figure 8L–M and Figure 8—figure supplement 1A–A''') and H4 pan acetylation (Figure 8N–O and Figure 8—figure supplement 1B–B''') similar to whd downregulated clonal patches (Figure 6M–P). Additionally, expression of bskDN in progenitor-specific manner brings about a conspicuous downregulation of both H3K9 acetylation (Figure 8—figure supplement 1F–H) and H4 pan acetylation (Figure 8—figure supplement 1I–K). However, in the above experiments, histone H3 labeling remains unaffected (Figure 8J–K and Figure 8—figure supplement 1C–E).
Since compromised acetylation leads to differentiation defect in whd1, which can be rescued upon acetate feeding, we wondered whether the block in differentiation upon JNK loss could be rescued similarly. Indeed, the hemocyte progenitors that lacked JNK (dome >bskDN) when reared in acetate supplemented food, demonstrate differentiation levels comparable to the similarly-aged control (Figure 8P–T).
Therefore, lack of histone acetylation encountered in JNK loss leads to the block in hemocyte progenitor differentiation. Moreover, whd transcription is under the regulation of JNK, which further endorses that JNK mediated regulation of FAO is crucial for differentiation.
If this is true, then the upregulation of FAO in JNK loss either by L-carnitine supplementation or by overexpression of whd should facilitate differentiation. Our results demonstrate that the upregulation of FAO in hemocyte progenitors that lacked JNK indeed elicits differentiation (Figure 8U–Y and Figure 8—figure supplement 1L–P).
Collectively, these results are in agreement with the fact that JNK regulates the differentiation of hemocyte progenitors by FAO-mediated histone acetylation.
Discussion
Along with cellular signaling network, stem/progenitor cell fate and state are directly governed by their metabolism in normal development and during pathophysiological conditions (Ito and Ito, 2016; Ito and Suda, 2014; Oginuma et al., 2017; Shyh-Chang et al., 2013; Shyh-Chang and Ng, 2017). How the metabolic circuitry works in sync with the cell signaling machinery to achieve cellular homeostasis is yet to be fully understood. Here, we show the developmental requirement of FAO in regulating the differentiation of hemocyte progenitors in Drosophila.
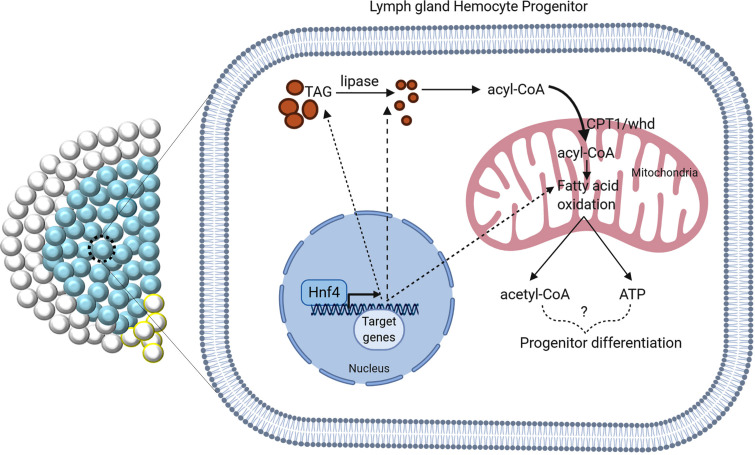
Our molecular genetic analyses reveal a signaling cascade that links ROS-JNK-FAO and histone modification essential for the differentiation of hemocyte progenitors (Figure 9). High ROS levels in the progenitors evoke differentiation program by triggering JNK and FOXO mediated signals (Owusu-Ansah and Banerjee, 2009). We show that activated JNK, in turn, leads to transcriptional induction of whd to facilitate the import of fat moiety into mitochondria for β-oxidation. Optimal level of acetyl-CoA, the end product of FAO, is critical for the acetylation of several proteins, including histones. Altering this pathway either at the level of JNK or FAO affects histone acetylation in a HAT dependent manner. On the other hand, it is quite possible that precocious differentiation of blood progenitors in the lymph gland of starved larvae (Shim et al., 2012) might be an outcome of starvation induced fat mobilization and increased FAO.
Figure 9. The regulation of FAO by JNK is critical for differentiation.
ROS-JNK link has been previously shown to be essential for differentiation (Owusu-Ansah and Banerjee, 2009). The G2-M arrested hemocyte progenitors employ β-oxidation for their differentiation. ROS–JNK circuit impinges on FAO to facilitate progenitor differentiation. JNK signaling transcriptionally regulates whd, the rate-limiting enzyme of FAO leading to the production of acetyl-CoA. Acetyl-CoA leads to acetylation of histones in the hemocyte progenitors, which is critical for their differentiation.
JNK signaling has been associated with histone acetylation in different biological processes (Miotto et al., 2006; Wu et al., 2008). In Drosophila, Fos, a transcriptional activator of JNK, interacts with Chm (HAT) and causes modification of histones. Our investigation reveals that indeed upon downregulation of JNK (Figure 8A–C) and Chm (Figure 6A–B), differentiation of hemocyte progenitors is halted. Further, we show that halt in differentiation upon JNK loss is associated with alteration of the acetylation profile of H3K9 and H4. Given the fact that JNK signaling regulates FAO, which in turn provides the acetyl moiety for histone acetylation, our study provides a new dimension in JNK’s role for histone acetylation in an FAO dependent manner. As a result, despite having high ROS levels, the hemocytes fail to differentiate if FAO is attenuated. Thus, the current work provides a metabolic link between JNK and epigenetic regulation of gene expression.
Our results show that upon disruption of FAO, hematopoietic progenitors adopt glycolysis to overcome the G2-M arrest but fail to initiate differentiation. Pharmacological and genetic inhibition of glycolysis in the FAO mutant restores their cell cycle defect but fails to facilitate their differentiation. The glycolytic surge in FAO mutants is not capable to take them through the differentiation process. This indicates that for the process of differentiation, the acetyl moiety derived from FAO plays a key role to facilitate hemocyte progenitor differentiation.
A recent study has demonstrated that alteration in acetyl-CoA levels can affect proteome and cellular metabolism by modulating intracellular crosstalk (Dieterich et al., 2019). It is intriguing to see that restoring acetylation level by the acetate supplementation is capable of rescuing hematopoietic defects in the lymph gland progenitors of FAO mutants. The acetate supplemented is converted into the end product of FAO: acetyl-CoA, the metabolite that is essential for histone acetylation. The involvement of acetyl-CoA in facilitating differentiation is further evidenced when on genetically downregulating AcCoAs (the major enzyme in acetyl-CoA generation) from the progenitor leads to a halt in their differentiation. Our study thus establishes that for hemocyte progenitor differentiation, the metabolic process FAO involves its metabolite acetyl-CoA for epigenetic modification. Earlier studies in diverse model systems have demonstrated that compromised in vivo histone acetylation defects can be rescued by acetate supplementation (Gao et al., 2016; Soliman et al., 2012). A similar finding in Drosophila hematopoiesis signifies the relevance of acetate supplementation across taxa. In light of this study, it would be interesting to see whether metabolite supplementation of FAO can modulate pathophysiological scenarios like certain forms of cancer which rely on fat oxidation.
FAO has been implicated in HSCs maintenance downstream of the PML-Peroxisome proliferator-activated receptor delta (PPARδ) pathway (Ito et al., 2012). Mechanistically, the PML-PPARδ-FAO pathway regulates HSC maintenance by controling asymmetric division. In FAO inhibition, HCSs undergo symmetric divisions, which lead to exhaustion and depletion of the stem cell pool resulting in their differentiation (Ito et al., 2012). Another study in mice shows that upon short term starvation, there is a decline in the number of HSC (Takakuwa et al., 2019). Since the HSC maintenance is FAO dependent (Ito et al., 2012), a loss in number might be attributed to heightened fat oxidation during starvation. Interestingly, metabolic dependence on FAO has been reported in mammalian neural stem cell (Knobloch et al., 2017), muscle stem cells (Ryall et al., 2015) and intestinal stem cells (Chen et al., 2020). Although endothelial precursors (Wong et al., 2017) is also known to be dependent on FAO, it remains to be seen whether FAO is a preferred metabolic requirement for progenitor differentiation.
The entire blood cell repertoire in Drosophila is engaged in innate immunity, maintenance of tissue integrity, wound healing, and heterogeneous stress responses, and is therefore functionally considered to be similar to myeloid cells in mammals (Banerjee et al., 2019; Gold and Brückner, 2014). Interestingly, several molecular mechanisms that regulate Drosophila lymph gland hematopoiesis are essential players in progenitor-based hematopoiesis in vertebrates (Banerjee et al., 2019; Gold and Brückner, 2014; Krzemien et al., 2010).
Based on the above conservations, it is reasonable to propose the requirement of FAO in progenitor differentiation described here will help us in understanding mammalian myeloid progenitor differentiation.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Drosophila melanogaster) | dome | Flybase:FB2020_01 | FLYB:FBgn 0043903 |
|
| Gene (Drosophila melanogaster) | Hml | Flybase:FB2020_01 | FLYB:FBgn 0029167 |
|
| Gene (Drosophila melanogaster) | Tep4 | Flybase:FB2020_01 | FLYB:FBgn 0031888 |
|
| Gene (Drosophila melanogaster) | CG3902 | Flybase:FB2020_01 | FLYB:FBgn 0036824 |
|
| Gene (Drosophila melanogaster) | Mtpα | Flybase:FB2020_01 | FLYB:FBgn 0041180 |
|
| Gene (Drosophila melanogaster) | Mtpβ | Flybase:FB2020_01 | FLYB:FBgn 0025352 |
|
| Gene (Drosophila melanogaster) | whd | Flybase:FB2020_01 | FLYB:FBgn 0261862 |
|
| Gene (Drosophila melanogaster) | Hnf4 | Flybase:FB2020_01 | FLYB:FBgn 0041180 |
|
| Gene (Drosophila melanogaster) | chm | Flybase:FB2020_01 | FLYB:FBgn 0028387 |
|
| Gene (Drosophila melanogaster) | Gcn5 | Flybase:FB2020_01 | FLYB:FBgn 0020388 |
|
| Gene (Drosophila melanogaster) | AcCoAS | Flybase:FB2020_01 | FLYB:FBgn 0012034 |
|
| Gene (Drosophila melanogaster) | Glut1 | Flybase:FB2020_01 | FLYB:FBgn 0264574 |
|
| Gene (Drosophila melanogaster) | ATPCL | Flybase:FB2020_01 | FLYB:FBgn 0020236 |
|
| Gene (Drosophila melanogaster) | sea | Flybase:FB2020_01 | FLYB:FBgn 0037912 |
|
| Gene (Drosophila melanogaster) | bsk | Flybase:FB2020_01 | FLYB:FBgn 0000229 |
|
| Genetic reagent(Drosophila melanogaster) | dome-GAL4 | BloomingtonDrosophilaStock Center | BDSC:81010; FLYB:FBti0022298; RRID:BDSC_81010 | FlyBase symbol: P{GawB}domePG14 |
| Genetic reagent (Drosophila melanogaster) | Hml-dsRed.Δ | Makhijani et al., 2011 | FLYB:FBgn 0041180 |
FlyBase symbol: P{Hml-dsRed.Δ} |
| Genetic reagent (Drosophila melanogaster) | HmlΔ-GAL4 | Sinenko and Mathey-Prevot, 2004 | FLYB: FBgn 0040877 |
FlyBase symbol: P{Hml-GAL4.Δ} |
| Genetic reagent (Drosophila melanogaster) | Pvf2-lacZ | Choi et al., 2008 | FLYB:FBtp0052107 | FlyBase symbol: P{Pvf2-lacZ.C} |
| Genetic reagent (Drosophila melanogaster) | TepIV-GAL4 | Kyoto Stock Center | DGGR:105442; FLYB:FBti0037434; RRID:DGGR_105442 |
FlyBase symbol: P{GawB}NP7379 |
| Genetic reagent (Drosophila melanogaster) | CG3902-YFP | Kyoto Stock Center | DGGR:115356; FLYB:FBti0143519; RRID:DGGR_115356 |
FlyBase symbol: PBac{566 .P.SVS-1}CG3902CPTI100004 |
| Genetic reagent (Drosophila melanogaster) | Mtpα[KO] | Kyoto Stock Center | DGGR:116261; FLYB:FBal0267653; RRID:DGGR_116261 |
FlyBase symbol: MtpαKO |
| Genetic reagent (Drosophila melanogaster) | Mtpβ[KO] | Kyoto Stock Center | DGGR:116262; FLYB:FBal0267654; RRID:DGGR_116262 |
FlyBase symbol: MtpβKO |
| Genetic reagent (Drosophila melanogaster) | UAS-whd RNAi [KK] | ViennaDrosophila RNAi Center | VDRC:v105400; FLYB:FBti0116709; RRID:FlyBase_FBst0477227 |
FlyBase symbol: P{KK100935}VIE-260B |
| Genetic reagent (Drosophila melanogaster) | OreR | BloomingtonDrosophilaStock Center | BDSC:5; FLYB:FBsn0000277; RRID:BDSC_5 | FlyBase symbol: Oregon-R-C |
| Genetic reagent (Drosophila melanogaster) | w[1118] | BloomingtonDrosophilaStock Center | BDSC:3605; FLYB:FBal0018186;RRID:BDSC_3605 | FlyBase symbol: w1118 |
| Genetic reagent (Drosophila melanogaster) | UAS-Hnf4.miRNA | BloomingtonDrosophilaStock Center | BDSC:44398; FLYB:FBti0152533;RRID:BDSC_44398 | FlyBase symbol: P{UAS-Hnf4.miRNA}attP16 |
| Genetic reagent (Drosophila melanogaster) | UAS-whd RNAi | BloomingtonDrosophilaStock Center | BDSC:34066; FLYB:FBal0263076; RRID:BDSC_34066 | FlyBase symbol: whdHMS00040 |
| Genetic reagent (Drosophila melanogaster) | UAS-FOXO.P | BloomingtonDrosophilaStock Center | BDSC:9575; FLYB:FBtp0017636; RRID:BDSC_9575 | FlyBase symbol: P{UAS-foxo.P} |
| Genetic reagent (Drosophila melanogaster) | Hnf4-GAL4 | BloomingtonDrosophilaStock Center | BDSC:47618; FLYB:FBti0136396; RRID:BDSC_47618 | FlyBase symbol: P{GMR50A12-GAL4}attP2 |
| Genetic reagent (Drosophila melanogaster) | UAS-FUCCI | BloomingtonDrosophilaStock Center | BDSC:55121; RRID:BDSC_55121 | FlyBase symbol: P{UAS-GFP.E2f1.1–230}32; P{UAS-mRFP1.NLS.CycB.1–266}19 |
| Genetic reagent (Drosophila melanogaster) | UAS-mito-HA-GFP | BloomingtonDrosophilaStock Center | BDSC:8442; FLYB:FBti0040803; RRID:BDSC_8442 | FlyBase symbol: P{UAS-mito-HA-GFP.AP}2 |
| Genetic reagent (Drosophila melanogaster) | UAS-chm RNAi | BloomingtonDrosophilaStock Center | BDSC:27027; FLYB:FBal0220716; RRID:BDSC_27027 | FlyBase symbol: chmJF02348 |
| Genetic reagent (Drosophila melanogaster) | UAS-Gcn5 RNAi | BloomingtonDrosophilaStock Center | BDSC:33981; FLYB:FBal0257611; RRID:BDSC_33981 | FlyBase symbol: Gcn5HMS00941 |
| Genetic reagent (Drosophila melanogaster) | UAS-AcCoAS RNAi | BloomingtonDrosophilaStock Center | BDSC:41917; FLYB:FBal0279313; RRID:BDSC_41917 | FlyBase symbol: AcCoASHMS02314 |
| Genetic reagent (Drosophila melanogaster) | UAS-Glut1RNAi | BloomingtonDrosophilaStock Center | BDSC:28645; FLYB:FBal0239561; RRID:BDSC_28645 | FlyBase symbol: Glut1JF03060 |
| Genetic reagent (Drosophila melanogaster) | ATPCL[01466] | BloomingtonDrosophilaStock Center | BDSC:11055; FLYB:FBal0007976; RRID:BDSC_11055 | FlyBase symbol: ATPCL01466 |
| Genetic reagent (Drosophila melanogaster) | sea[EP3364] | BloomingtonDrosophilaStock Center | BDSC:17118; FLYB:FBal0131420; RRID:BDSC_17118 | FlyBase symbol: seaEP3364 |
| Genetic reagent (Drosophila melanogaster) | UAS-bsk[DN] | BloomingtonDrosophilaStock Center | BDSC:6409; FLYB:FBti0021048; RRID:BDSC_6409 |
FlyBase symbol: P{UAS-bsk.DN}2 |
| Genetic reagent (Drosophila melanogaster) | UAS-mCD8::GFP | BloomingtonDrosophilaStock Center | BDSC:5137; FLYB:FBti0180511; RRID:BDSC_5137 | FlyBase symbol: P{UAS-mCD8::GFP.L}2 |
| Genetic reagent (Drosophila melanogaster) | UAS-mCD8::RFP | BloomingtonDrosophilaStock Center | BDSC:27400; FLYB:FBti0115747; RRID:BDSC_27400 | FlyBase symbol: P{UAS-mCD8.mRFP.LG}28a |
| Genetic reagent (Drosophila melanogaster) | U-6;sgRNA-whd-KO | BloomingtonDrosophilaStock Center | BDSC:77066; FLYB:FBal0335953; RRID:BDSC_77066 | FlyBase symbol: whdTKO.GS00854 |
| Genetic reagent (Drosophila melanogaster) | U-6;sgRNA-whd-OE | BloomingtonDrosophilaStock Center | BDSC:68139; FLYB:FBal0337690; RRID:BDSC_68139 | FlyBase symbol: whdTOE.GS00536 |
| Genetic reagent (Drosophila melanogaster) | whd[1] | BloomingtonDrosophilaStock Center | BDSC:441; FLYB:FBal0018515; RRID:BDSC_441 | FlyBase symbol: whd1 |
| Genetic reagent (Drosophila melanogaster) | Hnf4[Δ33] | BloomingtonDrosophilaStock Center | BDSC:43634; FLYB:FBal0240651; RRID:BDSC_43634 | FlyBase symbol: Hnf4Δ33 |
| Genetic reagent (Drosophila melanogaster) | Hnf4[Δ17] | BloomingtonDrosophilaStock Center | BDSC:44218; FLYB:FBal0240650; RRID:BDSC_44218 | FlyBase symbol: Hnf4Δ17 |
| Genetic reagent (Drosophila melanogaster) | tubGAL80[ts20] | BloomingtonDrosophilaStock Center | BDSC:7109; FLYB:FBti0027796; RRID:BDSC_7109 | FlyBase symbol: P{tubP-GAL80ts}20 |
| Genetic reagent (Drosophila melanogaster) | hsFlp | BloomingtonDrosophilaStock Center | BDSC:1929; FLYB:FBti0000784; RRID:BDSC_1929 | FlyBase symbol: P{hsFLP}12 |
| Genetic reagent (Drosophila melanogaster) | Ay-GAL4, UAS-GFP | BloomingtonDrosophilaStock Center | BDSC:4411; FLYB:FBti0012290;FBti0003040RRID:BDSC_4411 | FlyBase symbol: P{AyGAL4}25; P{UAS-GFP.S65T}Myo31DFT2 |
| Antibody | anti-P1 (Mouse monoclonal) | Kurucz et al., 2007 | Cat# NimC1, RRID:AB_2568423 | IF(1:50) |
| Antibody | anti-Pxn (Mouse) | Nelson et al., 1994 | IF(1:400) | |
| Antibody | anti-proPO (Rabbit polyclonal) | Jiang et al., 1997 | IF(1:1000) | |
| Antibody | anti-DE-cadherin (Rat polyclonal) | Developmental Studies Hybridoma Bank | Cat# DE-cad, RRID:AB_2314298 | IF(1:50) |
| Antibody | anti-Ci155(Rat polyclonal) | Developmental Studies Hybridoma Bank | Cat# 2A1, RRID:AB_2109711 |
IF(1:2) |
| Antibody | anti-GFP (Rabbit polyclonal) | Invitrogen | Cat# A-11122, RRID:AB_221569 |
IF(1:100) |
| Antibody | anti-H3 (Rabbit polyclonal) | Cell Signaling Technologies | Cat# 9927, RRID:AB_330200 |
IF(1:400), WB(1:1000) |
| Antibody | anti-H3K9 acetylation (Rabbit polyclonal) | Cell Signaling Technologies | Cat# 9927, RRID:AB_330200 |
IF(1:300), WB(1:1000) |
| Antibody | anti-H4 pan acetylation (Rabbit polyclonal) | Cell Signaling Technologies | Cat# 06–598, RRID:AB_2295074 |
IF(1:500) |
| Chemical compound, drug | Sodium butyrate | EMD Millipore | 19–137 | |
| Chemical compound, drug | Nicotinamide | Sigma-Aldrich | 72345 | |
| Chemical compound, drug | Etomoxir | Cayman Chemicals | Cay11969 | 5 µM |
| Chemical compound, drug | Mildronate | Cayman Chemicals | Cay15997 | 100 µM |
| Chemical compound, drug | L-carnitine hydrochloride | Sigma-Aldrich | C0283 | 100 mM |
| Chemical compound, drug | 2-DG | Sigma-Aldrich | D8375 | 100 mM |
| Chemical compound, drug | Sodium acetate | Sigma-Aldrich | 71196 | 50 mM |
| Chemical compound, drug | 2-NBDG | Invitrogen | N13195 | 0.25 mM |
| Chemical compound, drug | LipidTOX | Molecular Probes | H34477 | 1:1000 |
| Chemical compound, drug | Streptavidin-Cy3 | Molecular Probes | SA1010 | 1:200 |
| Chemical compound, drug | Nile red | Molecular Probes | N1142 | 0.5 ug/mL |
| Chemical compound, drug | DHE (Dihydroxy Ethidium) | Molecular Probes | D11347 | 0.3 µM |
| Sequence-based reagent | Pfk_F | This paper | PCR primers | ATCGTATTTTGGCTTGCCGC |
| Sequence-based reagent | Pfk_R | This paper | PCR primers | CCAGAGAGATGACCACTGGC |
| Sequence-based reagent | Hex_F | This paper | PCR primers | CTGCTTCTAACGGACGAACAG |
| Sequence-based reagent | Hex_R | This paper | PCR primers | GCCTTGGGATGTGTATCCTTGG |
| Sequence-based reagent | whd_F | This paper | PCR primers | GGCCAATGTGATTTCCCTGC |
| Sequence-based reagent | whd_R | This paper | PCR primers | TGCCCTGAACCATGATAGGC |
| Sequence-based reagent | Act5C_F | This paper | PCR primers | ACACATTTTGTAAGATTTGGTGTGT |
| Sequence-based reagent | Act5C_R | This paper | PCR primers | CCGTTTGAGTTGTGCTGT |
| Sequence-based reagent | Mcad_F | This paper | PCR primers | GGCCTGGATCTCGATGTGTT |
| Sequence-based reagent | Mcad_R | This paper | PCR primers | GATCACAGGAGTTTGGCCCAG |
| Sequence-based reagent | Mtpα_F | This paper | PCR primers | ATCACTGTTGGTGACGGACC |
| Sequence-based reagent | Mtpα_R | This paper | PCR primers | CTGCAGCAGTCTGATGGCTT |
| Sequence-based reagent | scully_F | This paper | PCR primers | GATCAAGAACGCCGTTTCCC |
| Sequence-based reagent | scully_R | This paper | PCR primers | CAGATCGGCCAGGATCACG |
| Sequence-based reagent | Mtpβ_F | This paper | PCR primers | CAGGCACTCGCTTTTGTCAT |
| Sequence-based reagent | Mtpβ_R | This paper | PCR primers | CCTGGCAATGTTGGAGGTCT |
| Sequence-based reagent | yip2_F | This paper | PCR primers | TCTGCCGCAACCAAAGGTAT |
| Sequence-based reagent | yip2_R | This paper | PCR primers | TTAAGACCGGCAGCATCCAG |
| Software, algorithm | Fiji | Fiji | RRID:SCR_002285 | |
| Software, algorithm | Photoshop CC | Adobe | RRID:SCR_014199 | |
| Software, algorithm | Imaris | Bitplane | RRID:SCR_007370 | |
| Commercial assay or kit | Click-iT EdU plus (DNA replication kit) | Invitrogen | C10639 | |
| Commercial assay or kit | ATP bioluminescence kit HSII | Sigma | 11699709001 | |
| Commercial assay or kit | Histone extraction kit | Abcam | ab113476 | |
| Commercial assay or kit | RNAeasy Mini Kit | Qiagen | 74104 |
Fly stocks
The fly stocks used were dome-GAL4, dome-MESO-EBFP2, Hml-DsRed (K. Bruckner), HmlΔ-GAL4 (S. Sinenko) Pvf2-LacZ (M. A. Yoo), TepIV-GAL4, CG3902-YFP, Mtpα[KO], Mtpβ[KO] (DGRC, Kyoto), UAS-whd RNAiKK (VDRC, Vienna), OreR, w1118, UAS-Hnf4.miRNA (Lin et al., 2009), UAS-whd RNAiHMS00040 (Manzo et al., 2018), UAS-FOXO.P, Hnf4-GAL4GMR50A12 (Tokusumi et al., 2017), UAS-FUCCI, UAS-mito-HA-GFP, UAS-chm-RNAiJF02348 (Dietz et al., 2015), UAS-Gcn5-RNAiHMS00941 (Janssens et al., 2017), UAS-AcCoAS RNAiHMS02314 (Eisenberg et al., 2014), UAS-Glut1 RNAiJF03060 (Charlton-Perkins et al., 2017), ATPCL01466, seaEP3364, UAS-bskDN, UAS-mCD8::GFP, U-6;sgRNA-whdTKO.GS00854, U-6;sgRNA-whdTOE.GS00536, whd1, Hnf4Δ33, Hnf4Δ17, tub-GAL80ts20, hsFlp and Ay-GAL4, UAS-GFP (BDSC, Bloomington Drosophila Stock Center).
Following genotypes were recombined for the current study:
w; +/+; Hnf4-GAL4GMR50A12, UAS-mCD8::GFP
dome-GAL4, UAS::mCD8RFP/FM7; +/+; +/+
dome-GAL4, UAS-GFP/FM7; whd1/whd1; +/+
w; U6:sgRNA-whd/U6:sgRNA-whd; UAS-dCas9/UAS-dCas9
dome-GAL4/FM7; UAS-FUCCI/Cyo; +/+
w; P{y[+t7.7] v[+t1.8]=TOE.GS00536}attP40/CyO; UAS-dCas9/UAS-dCas9
dome-MESO-EBFP2/+; whd1/whd1; +/+
hsFlp/hsFlp; Ay-GAL4, UAS-GFP/Ay-GAL4, UAS-GFP; +/+
UAS-bskDN/UAS bskDN; P{y[+t7.7] v[+t1.8]=TOE.GS00536}attP40/CyO; UAS-dCas9/UAS-dCas9
All Stocks and crosses were maintained at 25°C, except for those used in RNAi based and GAL4-UAS expression experiments. In those cases, crosses were maintained at 29°C. For GAL80ts experiments, crosses were initially maintained at 18°C for 5 days (equivalent to 60 hr at 25°C), and then shifted to 29°C till dissection (Figure 2—figure supplement 1D).
For synchronization of larvae, flies were allowed to lay eggs for 2 hr and newly hatched larvae within 1 hr interval were collected and transferred onto fresh food plates and aged for specified time periods at 25°C.
Metabolic supplements and inhibitors
Fatty acid β-oxidation inhibitors: Etomoxir (Cayman Chemicals, Cay11969, inhibitor of CPT1) and Mildronate (Cayman Chemicals, Cay15997, inhibitor of carnitine biosynthesis and transport) were used at a concentration of 5 µM and 100 µM respectively mixed in fly food and fed to larvae from 48 hr AEH and analysis of lymph gland was done in late third instar stages. L-carnitine hydrochloride (Sigma-Aldrich, C0283) at a concentration of 100 mM has been used to augment FAO by allowing the entry of palmitic acid into the mitochondria. L-carnitine was used at 100 mM concentrations in fly food and fed to larvae for 48 hr in third instar analysis and for 24 hr in second instar analysis. Glycolytic inhibitor: 2-DG (2-Deoxy-D-Glucose (2-Deoxyglucose) (Sigma-Aldrich, D8375) used at a concentration of 100 mM mixed in fly food and fed to larvae for 48 hr in third instar analysis and for 24 hr in second instar analysis. Sodium acetate (Sigma-Aldrich, 71196) supplement was used at a concentration of 50 mM and fed to larvae from second instar 36 hr AEH onwards and analysis was done in late third instar stages. Similar aged larvae fed on vehicle controls served as control larvae. For all feeding experiments control larvae had same vehicle control level mixed in fly food. Fly food mixed with permissible food dye was fed to the control and experimental larvae and larvae with abundant food intake were picked for the experimental analysis.
Clonal analysis using flp-out clone using Ay-GAL4 system
Generation of clones was done by the Ay-GAL4 system that combines the technique of Flippase (Flp)/FRT system and the GAL4/UAS system (Ito et al., 1997). In this system, the Act5C promoter GAL4 fusion gene is interrupted by a FRT cassette containing yellow (y+) gene. Heat shock treatment activates the Flp gene which in-turn excises the FRT cassette between the Act5C promoter and GAL4 sequence. This activates the expression of Act5C-GAL4 in cells. To induce UAS-whd RNAi and UAS-bskDN clones, mid second instar larvae of genotypes: hsFlp; Ay-GAL4, UAS-GFP; UAS-whd RNAi and hsFlp/UAS-bskDN; Ay-GAL4, UAS-GFP were subjected to heat shock for 90 min at 37°C, respectively. Post heat shock, larvae were transferred to 25°C to recover for 2 hr, then to express the respective knockdown constructs, larvae were reared at 29°C till dissection.
Immunohistochemistry and imaging
The primary antibodies used in this study includes mouse anti-P1 (Kurucz et al., 2007), rabbit anti-Pxn (J. Fessler), rabbit anti-proPO (M. Kanost), rat anti-DE Cadherin (Cat# DE-cad (DE-cadherin), RRID:AB_2314298, 1:50, DSHB), rat anti-Ci155 (Cat# 2A1, RRID:AB_2109711, 1:2, DSHB), rabbit anti-GFP (Cat# A-11122, RRID:AB_221569, 1:100, Invitrogen), rabbit anti-H3 (Cat# 9927, RRID:AB_330200, 1:400, Cell Signaling Technologies), rabbit anti-H3K9 acetylation (Cat# 9927, RRID:AB_330200, 1:300, Cell Signaling Technologies), rabbit anti-H4 pan acetylation (Cat# 06–598, RRID:AB_2295074, 1:500, Merck-Millipore). The following secondary antibodies mouse Cy3 (Cat# 115-165-166, RRID:AB_2338692), mouse FITC (Cat# 715-096-151, RRID:AB_2340796), rabbit Cy3 (Cat# 711-165-152, RRID:AB_2307443), rabbit FITC (Cat# 111-095-003, RRID:AB_2337972), rat Cy3 (Cat# 712-165-153, RRID:AB_2340667) from Jackson Immuno-research Laboratories were used at 1:400.
Lymph gland from synchronized larvae of required developmental age was dissected in cold PBS (1X Phosphate Buffer Saline, pH-7.2) and fixed in 4% Paraformaldehyde (PFA) for 45 min (Mandal et al., 2007) at room temperature (RT) on a shaker. Tissues were then permeabilized by 0.3% PBT (0.3% triton-X in 1X PBS) for 45 min (3 × 15 min washes) at RT. Blocking was then done in 10% NGS, for 30–45 min at RT. Tissues were next incubated in the respective primary antibody with appropriate dilution in 10% NGS overnight at 4°C. Post incubation in primary antibody, tissues were washed thrice in 0.3% PBT for 15 min each. This was followed by incubation of tissues in secondary antibody overnight at 4°C. The tissues were then subjected to four washes in 0.3% PBT for 15 min each, followed by incubation in DAPI solution (Invitrogen) for 1 hr at RT. Excess DAPI was washed off from the tissues by 1X PBS before mounting in Vectashield (Vector Laboratories).
Immunohistochemical analysis of histone acetylation in lymph gland
Immunostaining for specific histone acetylations were performed with a slight modification of the above protocol. Lymph gland from synchronized larvae was dissected in ice cold PBS with deacetylate inhibitors (Sodium butyrate (10 mM, EMD Millipore, 19–137) and Nicotinamide (10 mM, Sigma-Aldrich, 72345) and fixed in 4% PFA prepared in ice-cold 1X PBS (pH 7.2) for 5 hr at 4°C. Tissue were then permeabilized by 0.3% PBT for 45 min. Blocking was done with 5% BSA made in 1X PBS. Primary antibody and secondary antibody incubation solutions were made in 5% BSA in 1X PBS and subsequent washings were done with 0.1% PBT.
Immunohistochemical analysis of DE-cadherin expression in lymph gland
To detect the DE-cadherin expression, lymph glands were incubated in DE-Cadherin antibody (1:50 in PBS) before fixation (Langevin et al., 2005) for 1 hr at 4°C. Tissues were then fixed in 4% PFA prepared in ice cold 1X PBS (pH 7.2) for 5 hr at 4°C. Then, tissues were washed thrice with 0.3% PBT for 30 min. Secondary antibody incubation, washes, and mounting were performed following the standard protocol (Sharma et al., 2019).
Streptavidin-Cy3 labeling of mitochondria
Larvae were dissected in cold PBS followed by fixation in 4% PFA overnight at 4°C. This was followed by permeabilization with 0.1% PBT (0.1% triton-X in 1x PBS) for 45 min at RT and incubation in Streptavidin-Cy3 in 1:200 dilution (Molecular Probes, 434315) in 1XPBS for 1 hr at RT in dark. Post incubation samples were, washed thrice in PBS for 30 min. Lymph glands were then mounted in Vectashield and imaged in Zeiss LSM 780 confocal microscope.
EdU labeling
Click-iT EdU plus (5- ethynyl-2’- deoxyuridine, a thymidine analog) kit (Invitrogen, C10639) plus was used to perform DNA replication assay. Lymph glands were dissected and incubated in EdU solution (1:1000 in PBS) for 40 min at RT for EdU incorporation. Next fixation was done in 4% PFA prepared in 1X PBS (pH 7.2) for 45 min at RT. Tissue were then permeabilized by 0.3% PBT (0.3% triton-X in 1X PBS) for 45 min at RT. Blocking was then done in 10% NGS, for 30–45 min at RT. To detect the incorporated EdU in cells, azide-based fluorophore were used as described in manufacturer protocol. EdU-labeled cell counting was done using spot detection function in Imaris Software.
2-NBDG assay
The protocol was slightly modified after (Zou et al., 2005). Larvae were dissected in ice-cold PBS and incubated in PBS with 0.25 mM 2-NBDG (Invitrogen, N13195) for 45 min at RT, washed twice in PBS for 5 min, fixed 45 min in 4% PFA and washed twice for 10 min in PBS. All washes and the fixation were done with ice-cold PBS (4°C). Lymph glands were speedily dissected and mounted in Vectashield and were imaged immediately with a Zeiss LSM 780 confocal microscope.
Lactate dehydrogenase assay
Lactate dehydrogenase in vivo staining was modified from Abu-Shumays and Fristrom, 1997. Lymph glands of wandering third instar larvae were dissected in cold 1X PBS (pH8). Samples were fixed for 25 min in 0.5% glutaraldehyde in 1X PBS at room temperature, followed by four washes in 1X PBS for 15 min each. Staining was performed at 37°C in a solution of 0.1M NaPO4 (pH 7.4), 0.5 mM lithium lactate, 2.75 mM NAD+, 0.5 mg/ml NBT (Nitro blue tetrazolium) with 0.025 mg/ml PMS (Phenazine methosulfate). Reaction was stopped by washing in cold 1X PBS having pH 7.5. The samples were washed in four 1X PBS washes of 5 min each and immediately mounted and imaged.
LipidTOX staining
Larvae were dissected in cold PBS followed by fixation in 4% PFA for 1 hr at RT, permeabilized by 0.1% PBT (0.1% triton-X in 1X PBS) for 45 min at RT. It was then incubated in 1X LipidTOX (diluted from 1000X stock provided by the manufacturer; Molecular Probes, H34477) in PBS for 1 hr at RT in dark, washed thrice in PBS for 30 min. Lymph glands were then mounted in Vectashield and imaged in Leica SP8 confocal microscope.
Nile red staining
Larvae were dissected in cold PBS followed by fixation in 4% PFA for 1 hr at RT, permeabilized by 0.1% PBT (0.1% triton-X in 1X PBS) for 45 min at RT and incubated in 0.5 ug/mL Nile red (Molecular Probes, N1142) in PBS for 1 hr at RT in dark, washed thrice in PBS for 30 min. Lymph glands were mounted in Vectashield and imaged in Leica SP8 confocal microscope.
Detection of ROS
Larvae were dissected in Schneider’s medium (Gibco, 21720001) followed by incubation in 0.3 µM DHE (Molecular Probes, D11347) in Schneider’s medium for 8 min at room temperature in dark. This was followed by two washes in 1X PBS for 5 min each; a brief fixation was done with 4% PFA for 10 min followed by two quick 1X PBS washes. Tissues were then mounted in Vectashield and imaged in Zeiss LSM 780 confocal microscope.
Imaging and statistical analyses
Images were captured as confocal Z-stacks in Zeiss LSM 780, Leica SP8 confocal, and Olympus Flouview FV10i microscopes. Same confocal imaging settings were employed for image acquisition of control and experimental samples related to an experiment. Each experiment was repeated with appropriate controls at least three times to ensure reproducibility of the results. Data expressed as mean+/-Standard Deviation (SD) of values from three sets of independent experiments in GraphPad. Each dot in GraphPad represents a data point. Graphs plotted in EXCEL have Error Bars representing the Standard Deviation while graphs plotted in GraphPad employs Error Bars as mean+/-Standard Deviation. At least 10 images were analyzed per genotype, and statistical analyses performed employed two-tailed Student’s t-test. Raw data related to statistical analysis are attached in the source file of each figure along with graphs plotted in excel.
p-Value of <0.05;<0.01 and<0.001, mentioned as *, **, *** respectively are considered as statistically significant while n.s. = not significant.
Quantitative analysis of differentiation index in lymph gland
To measure the differentiation index of the primary lobe of lymph glands, middle confocal Z-stacks of a lymph gland image covering the Medullary Zone (MZ) were merged into a single section using ImageJ/Fiji (NIH) software as previously described (Shim et al., 2012). The merged section reflects the differentiated cell and hemocyte progenitor area clearly. For images with more than one fluorophore channel, each channel was separately analyzed. To measure differentiation index, P1-positive area was recalibrated into an identical threshold by using the Binary tool (Process–Binary–Make binary, Image J). Wand tool was used to capture the area with identical threshold whereas the size was measured using the Measure tool (Analyse–Measure). To measure the total area of one primary lobe of lymph gland, recalibration of the total area was then done by the Threshold tool until it was overlaid with identical threshold colour. Wand tool was used for selecting the total area for measurement. Differentiation index/fraction was estimated by dividing the size of the P1/Pxn positive area by the total size of the primary lobe. At least 10 lymph glands were analyzed per genotype, and two-tailed Student’s t-test was done to evaluate the statistical significance.
Quantification of the number of EdU+, FUCCI+ and progenitor subpopulations
Counting the number of EdU+ and FUCCI+ progenitors in lymph glands was done as described earlier (Sharma et al., 2019), using spot detection and surface tool in Imaris software and normalized by total number of nuclei per primary lobe of lymph gland. Different progenitor sub-populations in lymph glands were counted using the surface and spot detection function in Imaris as illustrated in detail (Sharma et al., 2019) and normalized by total number of cells (nuclei) in primary lobe and represented as percentage of progenitors in each primary lobe. Using surface tool, surface is created over Dome+ progenitors and nuclear label channel is masked in Dome+ surface. By utilizing the spot detection tool, the number of nuclei is counted in Dome+ surface. Similarly, number of nuclei (DAPI/Hoechst) is counted in another surface created over Pxn+ cells. Next, in Dome+ surface, Pxn+ channel is masked. A surface is created over Dome+ Pxn+ region and nuclear channel is masked in this surface. Using spot detection tool, number of Dome+ Pxn+ IP nuclei are counted and its percentage can be calculated from the total number of nuclei in the primary lobe of lymph gland.
ATP assay
ATP assay was performed with three biological replicates from late third instar larvae. Whole larvae were homogenized in ATP assay Lysis buffer (Costa et al., 2013). The samples were boiled at 95°C for 5 min and diluted 1:100 in dilution buffer provided in ATP bioluminescence kit HSII (Sigma, 11699709001). Further assay was performed as per manufacturer protocol in Glomax 96 microwell Luminometer (Promega). Standard curve was generated and ATP concentrations were calculated. The ATP concentration was normalized with protein concentration and expressed in percentage to plot the graph in EXCEL.
Histone extraction and detection by western blotting
Histone from late third instar larvae were extracted using Histone extraction kit (Abcam, ab113476) following manufacturer protocol and quantitated by Bradford reagent (Biorad, 5000006). Equal amount of protein of each genotype was run on 4–12% SDS-PAGE and transferred to PVDF membrane (Millipore, IPVH00010). Blots were developed using Luminata Crescendo Western HRP substrate (Millipore, WBLUR0500) in LAS2000 blot imaging instrument. Primary antibodies used rabbit anti-H3 (Cat# 9927, RRID:AB_330200, 1:1000, Cell Signaling Technologies), rabbit anti-H3K9 acetylation (Cat# 9927, RRID:AB_330200, 1:1000, Cell Signaling Technologies). Secondary antibody rabbit anti-IgG-HRP (Cat# A00098-1 mg, RRID:AB_1968815, 1:5000, GenScript) was used. The band intensity was measured in Image J and normalized with histone H3 as loading controls. Analysis was done using three biological replicates.
Quantitative RT-PCR
Extraction of RNA from lymph gland was performed from late third instar larvae of each genotype using TRIzol (Invitrogen, 15596018) followed by RNAeasy Mini Kit (Qiagen, 74104) according to the manufacturer’s instructions. cDNA was prepared using the Verso cDNA Synthesis Kit (Thermo Scientific, AB1453B). To quantitate transcripts, qPCR was done using iTaq Universal SYBR Green Supermix (Biorad, 1725124) on a CFX96 Real-Time system/C1000 thermal Cycler (Biorad). Drosophila Actin5C was used as internal control.
Analysis was done using at least three biological replicates.
Acknowledgements
We thank I Ando, U Banerjee and M Kanost for reagents. We thank all members of the two labs for their valuable inputs. Thanks to Parvathy Ramesh for her help in imaging with Olympus Flouview FV10i. We thank IISER Mohali’s Confocal Facility, Bloomington Drosophila Stock Center, at Indiana University, DGRC (Kyoto), VDRC (Vienna) and Developmental Studies Hybridoma Bank, University of Iowa for flies and antibodies. Models ‘Created with BioRender.com’. DBT/Wellcome-Trust India Alliance Senior Fellowship [IA/S/17/1/503100] to LM and Institutional support to SM and CSIR funding to SKT for this study duly acknowledged.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Lolitika Mandal, Email: lolitika@iisermohali.ac.in.
K VijayRaghavan, National Centre for Biological Sciences, Tata Institute of Fundamental Research, India.
K VijayRaghavan, National Centre for Biological Sciences, Tata Institute of Fundamental Research, India.
Funding Information
This paper was supported by the following grants:
Wellcome Trust/DBT India Alliance IA/S/17/1/503100 to Lolitika Mandal.
CSIR to Satish Kumar Tiwari.
Indian Institute of Science Education and Research Mohali to Lolitika Mandal, Sudip Mandal.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Formal analysis, Validation, Investigation, Visualization.
Formal analysis, Methodology, Writing - review and editing.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Additional files
Data availability
All data generated or analyzed during this study are included in the manuscript and supporting files. Source data files have been provided for all Figures (that includes GraphPad or excel representations of the quantitative analyses).
References
- Abu-Shumays RL, Fristrom JW. IMP-L3, A 20-hydroxyecdysone-responsive gene encodes Drosophila lactate dehydrogenase: structural characterization and developmental studies. Developmental Genetics. 1997;20:11–22. doi: 10.1002/(SICI)1520-6408(1997)20:1<11::AID-DVG2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes & Development. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- Atlasi Y, Stunnenberg HG. The interplay of epigenetic marks during stem cell differentiation and development. Nature Reviews Genetics. 2017;18:643–658. doi: 10.1038/nrg.2017.57. [DOI] [PubMed] [Google Scholar]
- Baldeosingh R, Gao H, Wu X, Fossett N. Hedgehog signaling from the posterior signaling center maintains U-shaped expression and a prohemocyte population in Drosophila. Developmental Biology. 2018;441:132–145. doi: 10.1016/j.ydbio.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Girard JR, Goins LM, Spratford CM. Drosophila as a Genetic Model for Hematopoiesis. Genetics. 2019;211:367–417. doi: 10.1534/genetics.118.300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett K, Eaton S. Mitochondrial beta-oxidation. European Journal of Biochemistry. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- Carré C, Szymczak D, Pidoux J, Antoniewski C. The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis. Molecular and Cellular Biology. 2005;25:8228–8238. doi: 10.1128/MCB.25.18.8228-8238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrisi C, Madeo M, Morciano P, Dolce V, Cenci G, Cappello AR, Mazzeo G, Iacopetta D, Capobianco L. Identification of the Drosophila melanogaster mitochondrial citrate carrier: bacterial expression, reconstitution, functional characterization and developmental distribution. Journal of Biochemistry. 2008;144:389–392. doi: 10.1093/jb/mvn076. [DOI] [PubMed] [Google Scholar]
- Charlton-Perkins MA, Sendler ED, Buschbeck EK, Cook TA. Multifunctional glial support by semper cells in the Drosophila retina. PLOS Genetics. 2017;13:e1006782. doi: 10.1371/journal.pgen.1006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Vasoya RP, Toke NH, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X, Verzi MP. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology. 2020;158:985–999. doi: 10.1053/j.gastro.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary S, Tomer D, Dubal D, Sambre D, Rikhy R. Analysis of mitochondrial organization and function in the Drosophila blastoderm embryo. Scientific Reports. 2017;7:5502. doi: 10.1038/s41598-017-05679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, Loh SH, Martins LM. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson's disease. Cell Death & Disease. 2013;4:e467. doi: 10.1038/cddis.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich IA, Lawton AJ, Peng Y, Yu Q, Rhoads TW, Overmyer KA, Cui Y, Armstrong EA, Howell PR, Burhans MS, Li L, Denu JM, Coon JJ, Anderson RM, Puglielli L. Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk. Nature Communications. 2019;10:3929. doi: 10.1038/s41467-019-11945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KN, Di Stefano L, Maher RC, Zhu H, Macdonald ME, Gusella JF, Walker JA. The Drosophila Huntington's disease gene ortholog dhtt influences chromatin regulation during development. Human Molecular Genetics. 2015;24:330–345. doi: 10.1093/hmg/ddu446. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Küttner V, Bhukel A, Mariño G, Pietrocola F, Harger A, Zimmermann A, Moustafa T, Sprenger A, Jany E, Büttner S, Carmona-Gutierrez D, Ruckenstuhl C, Ring J, Reichelt W, Schimmel K, Leeb T, Moser C, Schatz S, Kamolz LP, Magnes C, Sinner F, Sedej S, Fröhlich KU, Juhasz G, Pieber TR, Dengjel J, Sigrist SJ, Kroemer G, Madeo F. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metabolism. 2014;19:431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen-Campen B, Yang-Zhou D, Fernandes VR, González DP, Liu LP, Tao R, Ren X, Sun J, Hu Y, Zirin J, Mohr SE, Ni JQ, Perrimon N. Optimized strategy for in vivo Cas9-activation in Drosophila. PNAS. 2017;114:9409–9414. doi: 10.1073/pnas.1707635114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chemical Biology. 2015;10:95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GB, Martinez-Agosto JA. The TEAD family transcription factor scalloped regulates blood progenitor maintenance and proliferation in Drosophila through PDGF/VEGFR receptor (Pvr) signaling. Developmental Biology. 2017;425:21–32. doi: 10.1016/j.ydbio.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Drosophila E-cadherin functions in hematopoietic progenitors to maintain multipotency and block differentiation. PLOS ONE. 2013;8:e74684. doi: 10.1371/journal.pone.0074684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Simon L, Fossett N. Antioxidants maintain E-cadherin levels to limit Drosophila prohemocyte differentiation. PLOS ONE. 2014;9:e107768. doi: 10.1371/journal.pone.0107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lin SH, Ren F, Li JT, Chen JJ, Yao CB, Yang HB, Jiang SX, Yan GQ, Wang D, Wang Y, Liu Y, Cai Z, Xu YY, Chen J, Yu W, Yang PY, Lei QY. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nature Communications. 2016;7:11960. doi: 10.1038/ncomms11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani G, Barraco M, Giangrande A, Martinelli G, Guadagnuolo V, Simonetti G, Perini G, Bernardoni R. The human smoothened inhibitor PF-04449913 induces exit from quiescence and loss of multipotent Drosophila hematopoietic progenitor cells. Oncotarget. 2016;7:55313–55327. doi: 10.18632/oncotarget.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold KS, Brückner K. Drosophila as a model for the two myeloid blood cell systems in vertebrates. Experimental Hematology. 2014;42:717–727. doi: 10.1016/j.exphem.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienenberger A, Miotto B, Sagnier T, Cavalli G, Schramke V, Geli V, Mariol MC, Berenger H, Graba Y, Pradel J. The MYST domain acetyltransferase chameau functions in epigenetic mechanisms of transcriptional repression. Current Biology. 2002;12:762–766. doi: 10.1016/S0960-9822(02)00814-X. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jin LH. Dual role for Jumu in the control of hematopoietic progenitors in the Drosophila lymph gland. eLife. 2017;6:e25094. doi: 10.7554/eLife.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Esain V, Frechette GM, Harris LJ, Cox AG, Cortes M, Garnaas MK, Carroll KJ, Cutting CC, Khan T, Elks PM, Renshaw SA, Dickinson BC, Chang CJ, Murphy MP, Paw BH, Vander Heiden MG, Goessling W, North TE. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinshead M, Sanderson J, Vaux DJ. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. Journal of Histochemistry & Cytochemistry. 1997;45:1053–1057. doi: 10.1177/002215549704500803. [DOI] [PubMed] [Google Scholar]
- Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. Journal of Inherited Metabolic Disease. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Medicine. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ito K. Metabolism and the control of cell fate decisions and stem cell renewal. Annual Review of Cell and Developmental Biology. 2016;32:399–409. doi: 10.1146/annurev-cellbio-111315-125134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nature Reviews Molecular Cell Biology. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens DH, Hamm DC, Anhezini L, Xiao Q, Siller KH, Siegrist SE, Harrison MM, Lee C-Y. An Hdac1/Rpd3-Poised circuit balances continual Self-Renewal and rapid restriction of developmental potential during asymmetric stem cell division. Developmental Cell. 2017;40:367–380. doi: 10.1016/j.devcel.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Ma C, Kanost MR. Subunit composition of pro-phenol oxidase from Manduca sexta: molecular cloning of subunit ProPO-P1. Insect Biochemistry and Molecular Biology. 1997;27:835–850. doi: 10.1016/S0965-1748(97)00066-0. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Brüning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi LC, Schoors S, Harjes U, Vriens K, Pilz GA, Chen R, Cubbon R, Thienpont B, Cruys B, Wong BW, Ghesquière B, Dewerchin M, De Bock K, Sagaert X, Jessberger S, Jones EAV, Gallez B, Lambrechts D, Mazzone M, Eelen G, Li X, Fendt SM, Carmeliet P. Quiescent endothelial cells upregulate fatty acid β-Oxidation for vasculoprotection via redox homeostasis. Cell Metabolism. 2018;28:881–894. doi: 10.1016/j.cmet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Kishita Y, Tsuda M, Aigaki T. Impaired fatty acid oxidation in a Drosophila model of mitochondrial trifunctional protein (MTP) deficiency. Biochemical and Biophysical Research Communications. 2012;419:344–349. doi: 10.1016/j.bbrc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Pilz G-A, Ghesquière B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Reports. 2017;20:2144–2155. doi: 10.1016/j.celrep.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli L, Passegué E. Surviving change: the metabolic journey of hematopoietic stem cells. Trends in Cell Biology. 2014;24:479–487. doi: 10.1016/j.tcb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemień J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Developmental Biology. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Márkus R, Zsámboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, Zettervall CJ, Hultmark D, Andó I. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila Plasmatocytes. Current Biology : CB. 2007;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaïche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Developmental Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes & Development. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Huang Y, Lee T. Nuclear receptor unfulfilled regulates axonal guidance and cell identity of Drosophila mushroom body neurons. PLOS ONE. 2009;4:e8392. doi: 10.1371/journal.pone.0008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Liu F, Shi P, Song A, Huang Z, Zou D, Chen Q, Li J, Gao X. Fatty acid oxidation promotes reprogramming by enhancing oxidative phosphorylation and inhibiting protein kinase C. Stem Cell Research & Therapy. 2018;9:47. doi: 10.1186/s13287-018-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circulation Research. 1988;63:1036–1043. doi: 10.1161/01.RES.63.6.1036. [DOI] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annual Review of Cell and Developmental Biology. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo E, O'Conner AG, Barrows JM, Shreiner DD, Birchak GJ, Zarnescu DC. Medium-Chain fatty acids, Beta-Hydroxybutyric acid and genetic modulation of the carnitine shuttle are protective in a Drosophila Model of ALS Based on TDP-43. Frontiers in Molecular Neuroscience. 2018;11:182. doi: 10.3389/fnmol.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A, Deiulio A, Martin ET, Upadhyay M, Rangan P. Tip60 complex promotes expression of a differentiation factor to regulate germline differentiation in female Drosophila. Molecular Biology of the Cell. 2018;29:2933–2945. doi: 10.1091/mbc.E18-06-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell E, Crown SB, Fox DB, Kitir B, Ilkayeva OR, Olsen CA, Grimsrud PA, Hirschey MD. Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell Reports. 2016;17:1463–1472. doi: 10.1016/j.celrep.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems inDrosophila. Science's STKE : Signal Transduction Knowledge Environment. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Mews P, Donahue G, Drake AM, Luczak V, Abel T, Berger SL. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. 2017;546:381–386. doi: 10.1038/nature22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto B, Sagnier T, Berenger H, Bohmann D, Pradel J, Graba Y. Chameau HAT and DRpd3 HDAC function as antagonistic cofactors of JNK/AP-1-dependent transcription during Drosophila metamorphosis. Genes & Development. 2006;20:101–112. doi: 10.1101/gad.359506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Poulard I, Sharma A, Louradour I, Vanzo N, Vincent A, Crozatier M. Vascular control of the Drosophila haematopoietic microenvironment by slit/Robo signalling. Nature Communications. 2016;7:11634. doi: 10.1038/ncomms11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. The EMBO Journal. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oburoglu L, Tardito S, Fritz V, de Barros SC, Merida P, Craveiro M, Mamede J, Cretenet G, Mongellaz C, An X, Klysz D, Touhami J, Boyer-Clavel M, Battini J-L, Dardalhon V, Zimmermann VS, Mohandas N, Gottlieb E, Sitbon M, Kinet S, Taylor N. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15:169–184. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, Pourquié O. A gradient of glycolytic activity coordinates FGF and wnt signaling during elongation of the body axis in amniote embryos. Developmental Cell. 2017;40:342–353. doi: 10.1016/j.devcel.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 Regulates Lipid Mobilization and β-Oxidation. Cell Metabolism. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala J, Patkowska-Sokola B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S, Librowski T. L-carnitine-metabolic functions and meaning in humans life. Curr Drug Metab. 2011;12:667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Dell'Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, Sartorelli V. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. Boosting fat burning with carnitine: an old friend comes out from the shadow. The Journal of Physiology. 2011;589:1509–1510. doi: 10.1113/jphysiol.2011.205815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva NZ, Oliveira CS, Garcia JM. Histone acetylation and its role in embryonic stem cell differentiation. World Journal of Stem Cells. 2010;2:121–126. doi: 10.4252/wjsc.v2.i6.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obesity Reviews. 2010;11:380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Ghosh S, Geetha AR, Mandal S, Mandal L. Cell Adhesion-Mediated actomyosin assembly regulates the activity of cubitus interruptus for hematopoietic progenitor maintenance in Drosophila. Genetics. 2019;212:1279–1300. doi: 10.1534/genetics.119.302209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nature Cell Biology. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes & Development. 2017;31:336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Developmental Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Mathey-Prevot B. Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 2004;23:9120–9128. doi: 10.1038/sj.onc.1208156. [DOI] [PubMed] [Google Scholar]
- Small C, Ramroop J, Otazo M, Huang LH, Saleque S, Govind S. An unexpected link between notch signaling and ROS in restricting the differentiation of hematopoietic progenitors in Drosophila. Genetics. 2014;197:471–483. doi: 10.1534/genetics.113.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman ML, Smith MD, Houdek HM, Rosenberger TA. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. Journal of Neuroinflammation. 2012;9:51. doi: 10.1186/1742-2094-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub BR, Parkes TL, Mukai ST, Bahadorani S, Coulthard AB, Hall N, Phillips JP, Hilliker AJ. Mutations of the withered (whd) gene in Drosophila melanogaster confer hypersensitivity to oxidative stress and are lesions of the carnitine palmitoyltransferase I (CPT I) gene. Genome. 2008;51:409–420. doi: 10.1139/G08-023. [DOI] [PubMed] [Google Scholar]
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Takakuwa T, Nakashima Y, Koh H, Nakane T, Nakamae H, Hino M. Short-Term fasting induces cell cycle arrest in immature hematopoietic cells and increases the number of naïve T cells in the bone marrow of mice. Acta Haematologica. 2019;141:189–198. doi: 10.1159/000496096. [DOI] [PubMed] [Google Scholar]
- Tokusumi Y, Tokusumi T, Stoller-Conrad J, Schulz RA. Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development. 2010;137:3561–3568. doi: 10.1242/dev.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, Brahier MS, Lam V, Stoller-Conrad JR, Kroeger PT, Schulz RA. Screening and Analysis of Janelia FlyLight Project Enhancer-Gal4 Strains Identifies Multiple Gene Enhancers Active During Hematopoiesis in Normal and Wasp-Challenged Drosophila Larvae. G3: Genes, Genomes, Genetics. 2017;7:437–448. doi: 10.1534/g3.116.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. The Journal of Physiology. 2011;589:963–973. doi: 10.1113/jphysiol.2010.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, García-Caballero M, Missiaen R, Huang H, Brüning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hägerling R, Moral-Dardé V, Wyns S, Lippens M, Jessberger S, Fendt SM, Luttun A, Noel A, Kiefer F, Ghesquière B, Moons L, Schoonjans L, Dewerchin M, Eelen G, Lambrechts D, Carmeliet P. The role of fatty acid β-oxidation in lymphangiogenesis. Nature. 2017;542:49–54. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang X, Nauta HJ, Lin Q, Li J, Fang L. JNK1 regulates histone acetylation in trigeminal neurons following chemical stimulation. Biochemical and Biophysical Research Communications. 2008;376:781–786. doi: 10.1016/j.bbrc.2008.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T, Xuan T, Tan J, Li M, Zhao G, Li M. The Drosophila putative histone acetyltransferase enok maintains female germline stem cells through regulating Bruno and the niche. Developmental Biology. 2013;384:1–12. doi: 10.1016/j.ydbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Yu S, Luo F, Jin LH. The Drosophila lymph gland is an ideal model for studying hematopoiesis. Developmental & Comparative Immunology. 2018;83:60–69. doi: 10.1016/j.dci.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Zielke N, Korzelius J, van Straaten M, Bender K, Schuhknecht GFP, Dutta D, Xiang J, Edgar BA. Fly-FUCCI: a versatile tool for studying cell proliferation in complex tissues. Cell Reports. 2014;7:588–598. doi: 10.1016/j.celrep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent Indicator for direct glucose uptake measurement. Journal of Biochemical and Biophysical Methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]